Pineapple Peel Flours: Drying Kinetics, Thermodynamic Properties, and Physicochemical Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparation
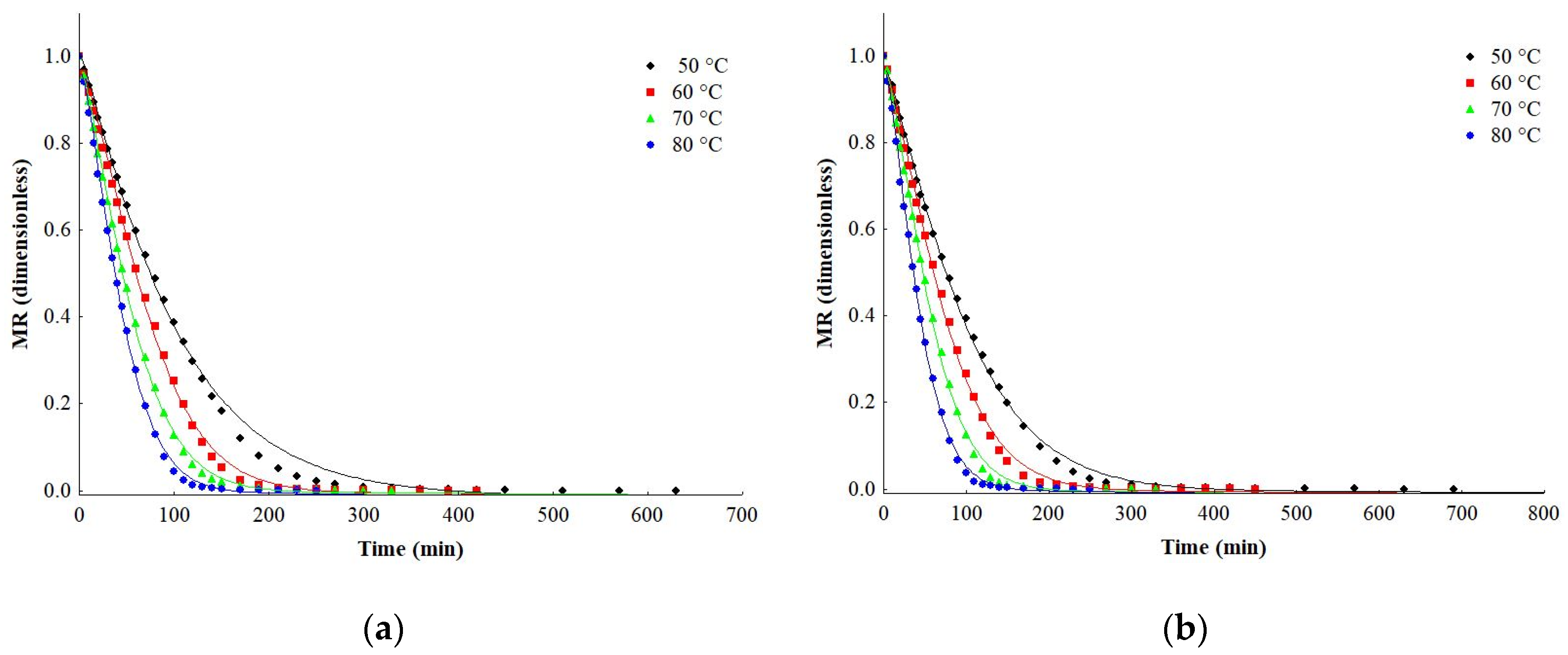
2.2. Drying
2.3. Effective Diffusivity
2.4. Thermodynamic Properties
2.5. Physicochemical Evaluation and Centesimal Composition of in Natura Peels and Flours
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IBGE—Instituto Brasileiro de Geografia e Estatística. Produção Agrícola Municipal, 2019; IBGE: Rio de Janeiro, Brazil, 2020. Available online: https://sidra.ibge.gov.br/Tabela/1612 (accessed on 21 June 2023).
- Brito Neto, J.F.; Pereira, W.E.; Sá Sobrinho, R.G.; Barbosa, J.A.; Santos, D.P. Aspectos produtivos da abacaxicultura familiar e comercial no Estado da Paraíba. Rev. Caatinga 2008, 21, 43–50. [Google Scholar]
- Sanya, C.A.K.; Chdare, F.J.; Hounhouigan, M.H.; Hotegni, N.V.F.; Gbaguidi, M.A.; Dekpemadoha, J.E.; Linnemann, A.R.; Hounhouigan, D.J. Effects of plant density and fertilizer formula on physicochemical and sensorial characteristics of pasteurized juice from Perolera sugarloaf pineapples grown in the long rainy season. NJAS-Wagening. J. Life Sci. 2020, 92, 100320. [Google Scholar] [CrossRef]
- Dorta, E.; Sogi, D.S. Value added processing and utilization of pineapple by-products. In Handbook of Pineapple Technology: Production, Postharvest Science, Processing and Nutrition; John Wiley & Sons, Ltd.: London, UK, 2017; pp. 196–220. [Google Scholar] [CrossRef]
- Badjona, A.; Adubofuor, J.; Amoah, I.; Diako, C. Valorisation of carrot and pineapple pomaces for rock buns development. Sci. Afr. 2019, 6, e00160. [Google Scholar] [CrossRef]
- Lopez-Nunez, J.S.; Salcedo-Mendonza, J.G.; Arteaga-Marquez, M.R.; Perez-Sierra, O.A. Effect of drying on the physicochemical and techno-functional properties of pineapple peel flour. Indian J. Sci. Technol. 2018, 11, 1–7. [Google Scholar] [CrossRef]
- Leite Filho, M.T.; Martins, J.H.; Mata, M.E.R.M.C.; Silva, A.L.C.; Martins, I.T.A. Drying kinetics of pineapple agro-industrial residues: A new approach. Braz. J. Dev. 2020, 6, 3928–3949. [Google Scholar] [CrossRef]
- Jusoh, N.; Othman, N.; Idris, A.; Nasruddin, A. Characterization of liquid pineapple waste as carbon source for production of succinic acid. J. Teknol. 2014, 69, 11–13. [Google Scholar] [CrossRef]
- Martins, J.G.P.; Matta Júnior, M.D.; Almeida, M.A.; Santos, T.; Spoto, M.H.F. Avaliação sensorial de bolo com resíduo de casca de abacaxi para suplementação do teor de fibras. Rev. Bras. Prod. Agroind. 2012, 14, 281–287. [Google Scholar] [CrossRef]
- Zziwa, A.; Jjagwe, J.; Kizito, S.; Kabenge, I.; Komakech, A.J.; Kayondo, H. Nutrient recovery from pineapple waste through controlled batch and continuous vermicomposting systems. J. Environ. Manag. 2021, 279, 111784. [Google Scholar] [CrossRef]
- Charoenphun, N. Utilization of pineapple residue for pineapple paste and gluten-freepie. J. Food Health Bioenviron. Sci. 2019, 12, 20–28. [Google Scholar]
- Baidhe, E.; Kigozi, J.; Mukisa, I.; Muyania, C.; Namubiru, L.; Kitarikawe, B. Unearthing the potential of solid waste generated along the pineapple drying process line in Uganda: A review. Environ. Chall. 2021, 2, 100012. [Google Scholar] [CrossRef]
- Joardder, M.U.H.; Mourshed, M.; Masud, M.H. Bound Water Removal Techniques. In State of Bound Water: Measurement and Significance in Food Processing; Joardder, M.U.H., Mourshed, M., Masud, M.H., Eds.; Springer International Publishing: Porto, Portugal, 2019; pp. 93–118. [Google Scholar]
- Araújo, K.T.A.; Silva, R.M.; Silva, R.C.; Figueirêdo, R.M.F.; Queiroz, A.J.M. Caracterização físico-química de farinhas. Rev. Bras. Agrotecnologia 2017, 7, 110–115. [Google Scholar]
- Santos, F.S.; Leite, D.D.F.; Figueirêdo, R.M.F.; Queiroz, A.J.M. Modelagem matemática da cinética de secagem da romã. Rev. Espac. 2017, 38, 27–37. [Google Scholar]
- Alexandre, H.V.; Silva, F.L.; Gomes, J.P.; Silva, O.S.D.; Carvalho, J.P.; Lima, E.E.D. Cinética de secagem do resíduo de abacaxi enriquecido. Rev. Bras. Eng. Agrícola Ambient. 2013, 17, 640–646. [Google Scholar] [CrossRef]
- Costa, J.M.C.; Freitas Felipe, É.M.; Maia, G.A.; Brasil, I.M.; Hernandez, F.F.H. Comparação dos parâmetros físico-químicos e químicos de pós alimentícios obtidos de resíduos de abacaxi. Rev. Ciência Agron. 2007, 38, 228–232. [Google Scholar]
- Silva, G.V.; Machado, B.A.S.; Oliveira, W.P.; Silva, C.F.G.; Quadros, C.P.; Druzian, J.I.; Ferreira, E.S.; Umsza-Guez, M.A. Effect of drying methods on bioactive compounds and antioxidant capacity in grape skin residues from the new hybrid variety “BRS Magna”. Molecules 2020, 25, 3701. [Google Scholar] [CrossRef]
- Oliveira, A.S. Elaboração de Farinha de Polpa, Casca e Cilindro Central de Abacaxi cv. Pérola para Produção de Bolo. 2016. Available online: http://dspace.sti.ufcg.edu.br:8080/jspui/handle/riufcg/14983 (accessed on 2 November 2023).
- Santos, J.A.B.; Resende, L.G.; Santos, B.S.; Travália, B.M.; Constant, P.B.; Pagani, A.A. Modelagem matemática da secagem de casca do fruto cambucá-preto (eugenia velutina berg.) utilizando diferentes sistemas de secador. Blucher Chem. Eng. Proc. 2015, 2, 1816–1822. [Google Scholar] [CrossRef]
- Azevedo, J.C.S. Características Bioativas, Funcionais e Efeito Protetor do Resíduo Desidratado de Camu-Camu (Myrciaria Dubia HBK (McVaugh)) Sobre Doenças Degenerativas Utilizando Modelos In Vivo C. elegans; Universidade Federal do Rio Grande do Norte: Natal, Brazil, 2015. [Google Scholar]
- Garcia, D.C.; Barros, A.C.S.A.; Peske, S.T.; Menezes, N.L. A secagem de sementes. Ciência Rural. 2004, 34, 603–608. [Google Scholar] [CrossRef]
- Celestino, S.M.C. Princípios de Secagem de Alimentos; Embrapa Cerrados: Planaltina, Brazil, 2010. [Google Scholar]
- Bontempo, L.H.S.; Castejon, L.V.; Santos, K.G. Secagem da casca de tangerina: Cinética e desempenho do secador solar convectivo. Res. Soc. Dev. 2020, 9, e44963458. [Google Scholar] [CrossRef]
- Silva, R.C.; Oliveira, E.N.A.; Feitosa, R.M.; Amadeu, L.T.S.; Araújo, K.T.A. Cinética de secagem, difusividade efetiva e caracterização de beterraba e cebola orgânicas. Rev. Bras. Agrotecnologia 2017, 7, 126–131. [Google Scholar]
- Araujo, W.D.; Goneli, A.L.D.; Corrêa, P.C.; Hartmann Filho, C.P.; Martins, E.A.S. Modelagem matemática da secagem dos frutos de amendoim em camada delgada. Ciência Agron. 2017, 48, 448–457. [Google Scholar] [CrossRef]
- Corrêa, P.C.; Oliveira, G.H.H.; Botelho, F.M.; Goneli, A.L.D.; Carvalho, F.M. Modelagem matemática e determinação das propriedades termodinâmicas do café (Coffea arabica L.) durante o processo de secagem. Rev. Ceres 2010, 57, 595–601. [Google Scholar] [CrossRef]
- CEAGESP. Programa Brasileiro Para Modernização da Horticultura: Normas de Classificação do Abacaxi; CQH. Documentos 24; Central de Qualidade em Horticultura: São Paulo, Brazil, 2003. [Google Scholar]
- Pantastico, E.B. Postharvest Physiology, Handling, and Utilization of Tropical and Subtropical Fruits and Vegetables; Avi Publishing Company: Westport, CT, USA, 1975. [Google Scholar]
- Nunes, W.S.; Lima, O.S.; Souza, E.G.; Junghans, D.T.; Matos, A.P.; Pereira, M.E.C. Qualidade de Frutos de Abacaxi ‘Pérola’ em Função do Tamanho e Estádio de Maturação na Colheita; Embrapa Mandioca e Frutas: Planaltina, Brazil, 2013. [Google Scholar]
- IAL—Instituto Adolfo Lutz. Métodos Físico-Químicos Para Análise de Alimentos; Instituto Adolfo Lutz: São Paulo, Brazil, 2008; p. 1020. [Google Scholar]
- Sharaf-Elden, Y.I.; Blaisdell, J.L.; Hamdy, M.Y. Um modelo de secagem de espigas de milho. Transacções ASAE 1980, 5, 1261–1265. [Google Scholar]
- Henderson, S.M. Progress in developing the thin layer drying equation. Trans. Am. Soc. Agric. Eng. 1974, 17, 1167–1168. [Google Scholar] [CrossRef]
- Midilli, A.; Kucuk, H.; Yapar, Z. A new model for single Layer drying. Dry. Technol. 2002, 20, 1503–1513. [Google Scholar] [CrossRef]
- Page, G.E. Factors Influencing the Maximum Rates of Air Drying Shelled Corn in Thin Layers. Ph.D. Thesis, Purdue University, West Lafayette, IN, USA, 1949. [Google Scholar]
- Henderson, S.M.; Pabis, S. Grain drying theory. II: Temperature effects on drying coefficients. J. Agric. Eng. Res. 1961, 6, 169–174. [Google Scholar]
- Yagcioglu, A.; Degirmencioglu, A.; Cagatay, F. Drying characteristics of laurel leaves under different conditions. In Proceedings of the 7th International Congress on Agricultural Mechanization and Energy, Adana, Turkey, 26–27 May 1999; pp. 565–569. [Google Scholar]
- Lewis, W.K. The rate of drying of solid materials. J. Ind. Eng. Chem. 1921, 13, 427–432. [Google Scholar] [CrossRef]
- Verma, L.R.; Bucklin, R.A.; Endan, J.B.; Wratten, F.T. Effects of drying air parameters on rice drying models. Trans. ASAE 1985, 28, 296–301. [Google Scholar] [CrossRef]
- Wang, C.Y.; Singh, R.P. Use of variable equilibrium moisture content in modeling rice drying. Trans. ASAE 1978, 11, 78–3001. [Google Scholar]
- Sánchez, L.; Peiró, J.M.; Castillo, H.; Pérez, M.D.; Ena, J.M.; Calvo, M. Kinetic parameters for denaturation of bovine milk lactoferrin. J. Food Sci. 1992, 57, 873–879. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Terra, J.; Antunes, A.M.; Bueno, M.I.M.S. Um método verde, rápido e simples para determinar o valor energético de farinhas e cereais matinais. Química Nova 2010, 33, 1098–1103. [Google Scholar] [CrossRef]
- Passos, K.E.; Bernardi, J.R.; Mendes, K.G. Análise da composição nutricional da Cesta Básica brasileira. Ciência Saúde Coletiva 2014, 19, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.A.S.; Azevedo, C.A.V. The Assistat Software Version 7.7 and its use in the analysis of experimental data. Afr. J. Agric. Res. 2016, 11, 3733–3740. [Google Scholar] [CrossRef]
- Barbosa, T.A.; Lobato, F.S. Determinação da cinética de secagem de produtos alimentícios usando algoritmos genéticos. Rev. Agric. Neotrop. 2016, 3, 28–37. [Google Scholar] [CrossRef]
- Olanipekun, B.F.; Tunde-Akintunde, T.Y.; Oyelade, O.J.; Adebisi, M.G.; Adenaya, T. A Mathematical modeling of thin-layer pineapple drying. J. Food Process. Preserv. 2015, 39, 1431–1441. [Google Scholar] [CrossRef]
- Santos, N.C.; Leite, D.D.F.; Câmara, G.B.; Barros, S.L.; Santos, F.S.; Soares, T.C.; Lima, A.R.N.; Soares, T.C.; Alburquerque, A.P.; Oliveira, M.N.; et al. Modelagem matemática da cinética de secagem de cascas da toranja (Citrus paradisi Macf.). Res. Soc. Dev. 2020, 9, 9. [Google Scholar] [CrossRef]
- Kara, C.; Doymaz, I. Thin layer drying kinetics of by-products from pomegranate juice processing. J. Food Process. Preserv. 2015, 39, 480–487. [Google Scholar] [CrossRef]
- Costa, C.F.; Corrêa, P.C.; Vanegas, J.D.B.; Baptestini, F.M.; Campos, R.C.; Fernandes, L.S. Mathematical modeling and determination of thermodynamic properties of jabuticaba peel during the drying process. Rev. Bras. Eng. Agrícola Ambient. 2016, 20, 576–580. [Google Scholar] [CrossRef]
- Nascimento, E.M.G.C.; Mulet, A.; Ascheri, J.L.R.; Carvalho, C.W.P.; Carcel, J.A. Effects of high-intensity ultrasound on drying kinetics and antioxidante properties of passion fruit peel. J. Food Eng. 2016, 170, 108–118. [Google Scholar] [CrossRef]
- Mphahlele, R.R.; Pathare, P.B.; Opara, U.L. Drying kinetics of pomegranate fruit peel (cv. Wonderful). Sci. Afr. 2019, 5, e00145. [Google Scholar] [CrossRef]
- Moreira, I.S.; Silva, L.M.M.; Castro, D.S.; Lima, J.P.; Sousa, F.C.; Almeida, F.A.C.; Silva, W.P.; Gomes, J.P.; Silva, C.M.D.P.S. Fruit of mandacaru: Kinetics of drying and physical-chemical characterization. J. Agric. Sci. 2018, 10, 461–470. [Google Scholar] [CrossRef]
- Santos, N.C.; Almeida, R.L.J.; Silva, L.R.I.; Muniz, C.E.S.; Pereira, T.S.; Silva, V.M.A.; Ribeiro, V.H.A.; Moreira, F.I.N.; Pinheiro, W.S.; Eduardo, R.S. Determinação dos parâmetros cinéticos durante o processo de secagem da casca de abacaxi. Res. Soc. Dev. 2020, 9, e06942794. [Google Scholar] [CrossRef]
- Ojediran, J.O.; Okonkwo, C.E.; Adeyi, A.J.; Adeyi, O.; Olaniran, A.F.; George, N.E.; Olayanju, A.T. Drying characteristics of yam slices (Dioscorea rotundata) in a convective hot air dryer: Application of ANFIS in the prediction of drying kinetics. Heliyon 2020, 6, e06942794. [Google Scholar] [CrossRef]
- Azeez, L.; Adebisi, S.A.; Oyedeji, A.O.; Adetoro, R.O.; Tijani, K.O. Bioactive compounds’ contents, drying kinetics and mathematical modelling of tomato slices influenced by drying temperatures and time. J. Saudi Soc. Agric. Sci. 2019, 18, 120–126. [Google Scholar] [CrossRef]
- Baptestini, F.M.; Corrêa, P.C.; Oliveira, G.H.H.; Almeida, L.F.J.; Vargas-Elpias, G.A. Constant and decreasing periods of pineapple slices dried by infrared. Rev. Bras. Ciências Agrária 2016, 11, 53–59. [Google Scholar] [CrossRef]
- Silva, I.L.; Silva, H.W.; Camargo, F.R.T.; Farias, H.F.L.; Freitas, E.F.M. Secagem e difusividade de sementes de melão. Rev. Ciências Agrárias 2018, 41, 309–315. [Google Scholar] [CrossRef]
- Zogzas, N.P.; Maroulis, Z.B.; Marinos-Kouris, D. Moisture diffusivity data compilation in foodstuffs. Dry. Technol. 1996, 14, 2225–2253. [Google Scholar] [CrossRef]
- Resende, O.; Oliveira, D.E.C.; Costa, L.M.; Ferreira Jínior, W.N. Drying kinetics of baru fruits (Dipteryx alata Vogel). Eng. Agrícola 2018, 38, 103–109. [Google Scholar] [CrossRef]
- Almeida, R.L.J.; Santos, N.C.; Alves, I.L.; André, A.M.M.C.N. Evaluation of thermodynamic properties and antioxidant activities of achachairu (Garcinia humilis) peels under drying process. Flavour Fragr. J. 2021, 36, 213–222. [Google Scholar] [CrossRef]
- Silva, H.W.; Rodovalho, R.S.; Velasco, M.F.; Silva, C.F.; Vale, L.S.R. Kinetics and thermodynamic properties related to the drying of ‘Cabacinha’ pepper fruits. Rev. Bras. Eng. Agrícola Ambient. 2016, 20, 174–180. [Google Scholar] [CrossRef]
- Cagnin, C.; Lima, M.S.; Silva, R.M.; Plácido, G.R.; Silva, M.A.P.; Freitas, S.M.; Oliveira, D.E.C. Garlic: Kinetic drying and thermodynamic properties. Biosci. J. 2017, 33, 905–913. [Google Scholar] [CrossRef]
- Oliveira, P.M.; Oliveira, D.E.C.; Resende, O.; Silva, D.V. Study of the drying of mesocarp of baru (Dipteryx alata Vogel) fruits. Rev. Bras. Eng. Agrícola Ambient. 2018, 22, 872–877. [Google Scholar] [CrossRef]
- Souza, J.L.F.; Oliveira, D.E.C.; Plácido, G.R.; Egea, M.B.; Caliari, M.; Silva, M.A.P. Thermodynamic and nutritional properties and drying kinetics of pequi (Caryocar brasiliense Cambess) mesocarp. Rev. Bras. Eng. Agrícola Ambient. 2019, 23, 655–661. [Google Scholar] [CrossRef]
- Corrêa, P.C.; Botelho, F.M.; Oliveira, G.H.H.; Goneli, A.L.D.; Resende, O.; Campos, S.C. Mathematical modeling of the drying process of corn ears. Acta Scientiarum. Agron. 2011, 33, 575–581. [Google Scholar] [CrossRef]
- Silva, F.P.; Siqueira, V.C.; Martins, E.A.S.; Miranda, F.M.N.; Melo, R.M. Thermodynamic properties and drying kinetics of Bauhinia forficata Link leaves. Rev. Bras. Eng. Agrícola Ambient. 2017, 21, 61–67. [Google Scholar] [CrossRef]
- Morais, M.F.; Santos, J.R.O.; Santos, M.P.; Santos, D.C.; Costa, T.N.; Lima, J.B. Modeling and thermodynamic properties of ‘bacaba’ pulp drying. Rev. Bras. Eng. Agrícola Ambient. 2019, 23, 702–708. [Google Scholar] [CrossRef]
- Tavone, L.A.S.; Nascimento, K.M.; Fachina, Y.J.; Madrona, G.S.; Bergamasco, R.C.; Scapim, M.R.S. Mathematical modeling and effect of thin-layer drying and lyophilization on antioxidant compounds from ultrasonic-assisted extracted Muntingia calaburapeels. Acta Scientiarum. Agron. 2021, 43, e50301. [Google Scholar] [CrossRef]
- Brasil. Ministério da Saúde. Agência Nacional de Vigilância Sanitária—ANVISA (2005). Regulamento Técnico Para Produtos de Cereais, Amidos, Farinhas e Farelos (Resolução—RDC nº 263, 22 de Setembro de 2005). Diário Oficial da República Federativa do Brasil. Available online: https://www.saude.rj.gov.br/comum/code/MostrarArquivo.php?C=MjIwMw%2C%2C (accessed on 24 June 2023).
- Almeida, R.L.J.; Santos, N.C.; Pereira, T.S.; Silva, V.M.A.; Silva, L.N.; Santos, S.B.F.; Cabral, M.B.; Silva, L.R.I.; Barros, E.R.; Ribeiro, V.H.A. Análises físico-química e microbiológicas de farinha elaborada do aproveitamento da casca de kiwi. Res. Soc. Dev. 2020, 9, e92932522. [Google Scholar] [CrossRef]
- Soares, D.J.; Moura Neto, L.G.; Freitas Júnior, E.M.; Alves, V.R.; Costa, Z.R.T.; Silva, E.M.; Nascimento, A.D.P. Desenvolvimento e caracterização de um shake produzido a partir de resíduos de frutos tropicais. Res. Soc. Dev. 2020, 9, e140942986. [Google Scholar] [CrossRef]
- Storck, C.R.; Basso, C.; Favarin, F.R.; Rodrigues, A.C. Qualidade microbiológica e composição de farinhas de resíduos da produção de suco de frutas em diferentes granulometrias. Braz. J. Food Technol. 2015, 18, 277–284. [Google Scholar] [CrossRef]
- Erkel, A.; Ávila, C.A.; Romeiro, M.M.; Santos, E.F.; Sarmento, U.C.; Novello, D. Utilização da farinha de casca de abacaxi em cookies: Caracterização físico-química e aceitabilidade sensorial entre crianças. Rev. Uniabeu 2015, 8, 272–288. [Google Scholar]
- Santos, C.C.S.; Guimarães, P.B.; Ramos, S.A.; Capobiango, M. Determinação da composição centesimal de farinha obtida a partir da casca de abacaxi. Sinapse Múltipla 2017, 6, 341–344. [Google Scholar]
- Brito, T.B.N.; Pereira, A.P.A.; Pastore, G.M.; Moreira, R.F.A.; Ferreira, M.S.L.; Fai, A.E.C. Chemical composition and physicochemical characterization for cabbage and pineapple by-products flour valorization. LWT-Food Sci. Technol. 2020, 124, 109028. [Google Scholar] [CrossRef]
- Santana Neto, D.C.; Onias, E.A.; Araújo, J.S.F.; Alves, A.M.A.; Silva, O.S. Avaliação do processo de enriquecimento proteico de resíduo de abacaxi. Rev. Verde Agroecol. Desenvolv. Sustentável 2017, 12, 95–99. [Google Scholar] [CrossRef]
- Damasceno, K.A.; Golçalves, C.A.A.; Pereira, G.S.; Costa, L.L.; Campagnol, P.C.B.; Almeida, P.L.; Arantes-Pereira, L. Development of cereal bars containing pineapplepeel flour (Ananas comosus L. Merril). J. Food Qual. 2016, 39, 417–424. [Google Scholar] [CrossRef]
- Vieira, D.M.; Barros, S.L.; Silva, V.M.A.; Santos, N.C.; Nascimento, A.P.S.; Melo, M.O.P. Elaboração de barra de cereal com resíduos secos de abacaxi e caju. Cad. Verde Agroecol. Desenvolv. Sustentável 2019, 9, e-6839. [Google Scholar] [CrossRef]
- Roberto, B.S.; Silva, L.P.; Macagnan, F.T.; Bizzani, M.; Bender, A.B.B. Qualidade nutricional e aceitabilidade de barras de cereais formuladas com casca e semente de goiaba. Rev. Inst. Adolfo Lutz 2015, 74, 39–48. [Google Scholar] [CrossRef]
- Garcia-Amezquita, L.E.; Tejada-Ortigoza, V.; Campanella, O.H.; Welti-Chanes, J. Influence of drying method on the composition, physicochemical properties, and prebiotic potential of dietary fibre concentrates from fruit peels. J. Food Qual. 2018, 2018, 9105237. [Google Scholar] [CrossRef]
- Reis, L.C.R.; Facco, E.M.P.; Salvador, M.; Flôres, S.H.; Rios, A.O. Characterization of orange passion fruit peel flour and its use as an ingredient in bakery products. J. Culin. Sci. Technol. 2020, 18, 214–230. [Google Scholar] [CrossRef]
- Silva, I.G.; Andrade, A.P.C.; Silva, L.M.R.; Gomes, D.S. Elaboração e análise sensorial de biscoito tipo cookie feito a partir da farinha do caroço de abacate. Braz. J. Food Technol. 2019, 22, 1–10. [Google Scholar] [CrossRef]
- Sousa, M.S.B.; Vieira, L.M.; Silva, M.J.M.; Lima, A. Caracterização nutricional e compostos antioxidantes em resíduos de polpas de frutas tropicais. Ciências Agrotecnologia 2011, 35, 554–559. [Google Scholar] [CrossRef]
- TACO. Tabela Brasileira de Composição de Alimentos, 4th ed.; NEPA-UNICAMP: Campinas, Brazil, 2011; p. 161. [Google Scholar]
- Bemfeito, C.M.; Carneiro, J.D.S.; Carvalho, E.E.N.; Coli, P.C.; Pereira, R.C.; Boas, E.V.B.V. Nutritional and functional potential of pumpkin (Cucurbita moschata) pulp and pequi (Caryocar brasiliense Camb.) peel flours. J. Food Sci. Technol. 2020, 57, 3920–3925. [Google Scholar] [CrossRef]
- Zanatta, C.L.; Schlabitz, C.; Ethur, E.M. Avaliação físico-química e microbiológica de farinhas obtidas a partir de vegetais não conformes à comercialização. Aliment. Nutr. 2010, 21, 459–468. [Google Scholar]
- Soquetta, M.B.; Stefanello, F.S.; Huerta, K.M.; Monteiro, S.S.; Rosa, C.S.; Terra, N.N. Characterization of physiochemical and microbiological properties, and bioactive compounds, of flour made from the skin and bagasse of kiwi fruit (Actinidia deliciosa). Food Chem. 2016, 199, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Leão, D.P.; Franca, A.S.; Oliveira, L.S.; Bastos, R.; Coimbra, M.A. Physicochemical characterization, antioxidant capacity, total phenolic and proanthocyanidin content of flours prepared from pequi (Caryocar brasilense Camb.) fruit by-products. Food Chem. 2017, 225, 146–153. [Google Scholar] [CrossRef]
- Nunes, J.S.; Lins, A.D.F.; Gomes, J.P.G.; Silva, W.P.; Silva, F.B. Influência da temperatura de secagem nas propriedades físico-química de resíduos abacaxi. Rev. Agropecuária Técnica 2017, 1, 41–46. [Google Scholar] [CrossRef]
- Santos, F.S.; Figueirêdo, R.M.F.; Queiroz, A.J.M.; Santos, D.C. Drying kinetics and physical and chemical characterization of white-fleshed ‘pitaya’ peels. Rev. Bras. Eng. Agrícola Ambient. 2017, 21, 872–877. [Google Scholar] [CrossRef]
- Nóbrega, E.M.; Oliveira, E.L.; Genovese, M.I.; Correia, R.T.P. The impact of hot air drying on the physical-chemical characteristics, bioactive compounds and antioxidant activity of acerola (Malphigia emarginata) residue. J. Food Process. Preserv. 2015, 39, 131–141. [Google Scholar] [CrossRef]
- Castro, D.S.; Oliveira, T.K.B.; Lemos, D.M.; Rocha, A.P.T.; Almeida, R.D. Efeito da temperatura sobre a composição físico-química e compostos bioativos de farinha de taro obtida em leito de jorro. Braz. J. Food Technol. 2017, 20, 1–5. [Google Scholar] [CrossRef]
- Brandão, M.C.C.; Maia, G.A.; Lima, D.P.; Parente, E.J.S.; Campello, C.C.; Nassu, R.T.; Feitosa, T.; Sousa, P.H.M. Análises físico-químicas, microbiológicas e sensoriais de pedúnculos de caju submetidos a desidratação osmótico-solar. Rev. Ciência Agron. 2003, 34, 139–145. [Google Scholar]
- Alves, F.M.S.; Machado, A.V.; Queiroga, K.H. Alimentos produzidos a partir de farinha de caju, obtida por secagem. Rev. Verde Agroecol. Desenvolv. Sustentável 2011, 6, 131–138. [Google Scholar]
- Queiroz, E.R.; Abreu, C.M.P.; Simao, A.A. Composição química e fitoquímica das farinhas da casca e da semente de lichias (Litchi chinensis Sonn) cultivar ‘Bengal’. Ciência Rural. 2015, 45, 329–334. [Google Scholar] [CrossRef]

| Model Name | Equation | Reference |
|---|---|---|
| Diffusion Approach | Sharaf-Elden et al. [32] | |
| Two Terms | Henderson [33] | |
| Two-term exponential | Sharaf-Elden et al. [32] | |
| Midilli | Midilli et al. [34] | |
| Page | Page [35] | |
| Henderson and Pabis | Henderson & Pabis [36] | |
| Logarithmic | Yagcioglu et al. [37] | |
| Newton | Lewis [38] | |
| Verma | Verma et al. [39] | |
| Wang and Singh | Wang & Singh [40] |
| Models | T (°C) | R2 | DQM | χ2 | EME |
|---|---|---|---|---|---|
| Diffusion Approach | 50 | 0.9992 | 0.0142 | 0.0002 | 0.0148 |
| 60 | 0.9986 | 0.0186 | 0.0004 | 0.0196 | |
| 70 | 0.9993 | 0.0128 | 0.0002 | 0.0135 | |
| 80 | 0.9989 | 0.0158 | 0.0003 | 0.0168 | |
| Two Terms | 50 | 0.9956 | 0.0327 | 0.0012 | 0.0347 |
| 60 | 0.9924 | 0.0434 | 0.0021 | 0.0464 | |
| 70 | 0.9940 | 0.0374 | 0.0016 | 0.0403 | |
| 80 | 0.9924 | 0.0417 | 0.0021 | 0.0453 | |
| Page | 50 | 0.9992 | 0.0143 | 0.0002 | 0.0147 |
| 60 | 0.9989 | 0.0166 | 0.0003 | 0.0171 | |
| 70 | 0.9995 | 0.0112 | 0.0001 | 0.0116 | |
| 80 | 0.9993 | 0.0131 | 0.0002 | 0.0136 | |
| Newton | 50 | 0.9935 | 0.0398 | 0.0016 | 0.0404 |
| 60 | 0.9888 | 0.0525 | 0.0028 | 0.0534 | |
| 70 | 0.9907 | 0.0465 | 0.0022 | 0.0473 | |
| 80 | 0.9890 | 0.0503 | 0.0026 | 0.0513 | |
| Henderson and Pabis | 50 | 0.9956 | 0.0327 | 0.0011 | 0.0336 |
| 60 | 0.9924 | 0.0434 | 0.0020 | 0.0448 | |
| 70 | 0.9940 | 0.0374 | 0.0015 | 0.0387 | |
| 80 | 0.9924 | 0.0417 | 0.0019 | 0.0434 | |
| Two Terms Exponential | 50 | 0.9932 | 0.0409 | 0.0018 | 0.0421 |
| 60 | 0.9886 | 0.0530 | 0.0030 | 0.0547 | |
| 70 | 0.9905 | 0.0470 | 0.0024 | 0.0487 | |
| 80 | 0.9886 | 0.0511 | 0.0028 | 0.0532 | |
| Logarithmic | 50 | 0.9968 | 0.0279 | 0.0008 | 0.0292 |
| 60 | 0.9944 | 0.0373 | 0.0015 | 0.0392 | |
| 70 | 0.9957 | 0.0316 | 0.0011 | 0.0333 | |
| 80 | 0.9949 | 0.0343 | 0.0013 | 0.0365 | |
| Midilli | 50 | 0.9983 | 0.0203 | 0.0005 | 0.0215 |
| 60 | 0.9993 | 0.0136 | 0.0002 | 0.0146 | |
| 70 | 0.9996 | 0.0097 | 0.0001 | 0.0105 | |
| 80 | 0.9995 | 0.0110 | 0.0001 | 0.0120 | |
| Verma | 50 | 0.9992 | 0.0142 | 0.0002 | 0.0148 |
| 60 | 0.9985 | 0.0191 | 0.0004 | 0.0200 | |
| 70 | 0.9907 | 0.0465 | 0.0024 | 0.0491 | |
| 80 | 0.9989 | 0.0157 | 0.0003 | 0.0167 | |
| Wang and Singh | 50 | 0.9625 | 0.0951 | 0.0099 | 0.0993 |
| 60 | 0.9795 | 0.0709 | 0.0056 | 0.0745 | |
| 70 | 0.9761 | 0.0744 | 0.0059 | 0.0771 | |
| 80 | 0.9822 | 0.0638 | 0.0044 | 0.0664 |
| Models | T (°C) | R2 | DQM | χ2 | EME |
|---|---|---|---|---|---|
| Diffusion Approach | 50 | 0.9994 | 0.0121 | 0.0002 | 0.0127 |
| 60 | 0.9988 | 0.0169 | 0.0003 | 0.0177 | |
| 70 | 0.9987 | 0.0180 | 0.0004 | 0.0190 | |
| 80 | 0.9992 | 0.0136 | 0.0002 | 0.0144 | |
| Two Terms | 50 | 0.9969 | 0.0272 | 0.0008 | 0.0288 |
| 60 | 0.9937 | 0.0394 | 0.0018 | 0.0420 | |
| 70 | 0.9918 | 0.0448 | 0.0023 | 0.0482 | |
| 80 | 0.9928 | 0.0405 | 0.0019 | 0.0441 | |
| Page | 50 | 0.9993 | 0.0129 | 0.0002 | 0.0133 |
| 60 | 0.9990 | 0.0155 | 0.0003 | 0.0160 | |
| 70 | 0.9991 | 0.0148 | 0.0002 | 0.0153 | |
| 80 | 0.9995 | 0.0109 | 0.0001 | 0.0113 | |
| Newton | 50 | 0.9954 | 0.0331 | 0.0011 | 0.0336 |
| 60 | 0.9904 | 0.0484 | 0.0024 | 0.0491 | |
| 70 | 0.9874 | 0.0553 | 0.0032 | 0.0563 | |
| 80 | 0.9891 | 0.0499 | 0.0026 | 0.0508 | |
| Henderson and Pabis | 50 | 0.9969 | 0.0272 | 0.0008 | 0.0279 |
| 60 | 0.9937 | 0.0394 | 0.0016 | 0.0406 | |
| 70 | 0.9918 | 0.0448 | 0.0022 | 0.0464 | |
| 80 | 0.9928 | 0.0405 | 0.0018 | 0.0422 | |
| Two Terms Exponential | 50 | 0.9951 | 0.0342 | 0.0012 | 0.0352 |
| 60 | 0.9899 | 0.0498 | 0.0026 | 0.0514 | |
| 70 | 0.9868 | 0.0565 | 0.0034 | 0.0585 | |
| 80 | 0.9889 | 0.0504 | 0.0028 | 0.0525 | |
| Logarithmic | 50 | 0.9978 | 0.0231 | 0.0006 | 0.0241 |
| 60 | 0.9952 | 0.0342 | 0.0013 | 0.0359 | |
| 70 | 0.9940 | 0.0381 | 0.0016 | 0.0403 | |
| 80 | 0.9950 | 0.0339 | 0.0013 | 0.0360 | |
| Midilli | 50 | 0.9995 | 0.0114 | 0.0001 | 0.0120 |
| 60 | 0.9993 | 0.0135 | 0.0002 | 0.0144 | |
| 70 | 0.9993 | 0.0130 | 0.0002 | 0.0140 | |
| 80 | 0.9996 | 0.0096 | 0.0001 | 0.0104 | |
| Verma | 50 | 0.9994 | 0.0122 | 0.0002 | 0.0127 |
| 60 | 0.9988 | 0.0169 | 0.0003 | 0.0178 | |
| 70 | 0.9987 | 0.0180 | 0.0004 | 0.0191 | |
| 80 | 0.9992 | 0.0137 | 0.0002 | 0.0145 | |
| Wang and Singh | 50 | 0.9498 | 0.1085 | 0.0124 | 0.1115 |
| 60 | 0.9739 | 0.0795 | 0.0067 | 0.0821 | |
| 70 | 0.9789 | 0.0713 | 0.0055 | 0.0739 | |
| 80 | 0.9773 | 0.0718 | 0.0056 | 0.0747 |
| Variety | Temperature (°C) | a | k | b | n |
|---|---|---|---|---|---|
| Jupi | 50 | 1.0136 | 0.0052 | −0.000025 | 1.1380 |
| 60 | 0.9695 | 0.0017 | −0.000016 | 1.4498 | |
| 70 | 0.9819 | 0.0036 | −0.000015 | 1.3729 | |
| 80 | 0.9768 | 0.0038 | −0.000029 | 1.4245 | |
| Pérola | 50 | 0.9808 | 0.0030 | −0.000010 | 1.2501 |
| 60 | 0.9757 | 0.0022 | −0.000014 | 1.3940 | |
| 70 | 0.9782 | 0.0024 | −0.000021 | 1.4558 | |
| 80 | 0.9840 | 0.0043 | −0.000023 | 1.4156 |
| Variety | Temperature (°C) | Effective Diffusivity (m2/s) | R2 |
|---|---|---|---|
| Jupi | 50 | 3.24 × 10−10 | 0.9699 |
| 60 | 4.29 × 10−10 | 0.9623 | |
| 70 | 5.62 × 10−10 | 0.9661 | |
| 80 | 7.01 × 10−10 | 0.9642 | |
| Pérola | 50 | 3.19 × 10−10 | 0.9740 |
| 60 | 4.21 × 10−10 | 0.9651 | |
| 70 | 5.55 × 10−10 | 0.9607 | |
| 80 | 7.33 × 10−10 | 0.9645 |
| Variety | D0 (m2/s) | Ea (kJ/mol) | R2 |
|---|---|---|---|
| Jupi | 3.08 × 10−6 | 24.60 | 0.9991 |
| Pérola | 5.55 × 10−6 | 26.25 | 0.9992 |
| Variety | T (°C) | ΔH (kJ/mol) | ΔS (kJ/mol K) | ΔG (kJ/mol) |
|---|---|---|---|---|
| Jupi | 50 | 21.9086 | −0.3511 | 135.3628 |
| 60 | 21.8255 | −0.3513 | 138.8749 | |
| 70 | 21.7424 | −0.3516 | 142.3896 | |
| 80 | 21.6592 | −0.3518 | 145.9066 | |
| Pérola | 50 | 23.5656 | −0.3462 | 135.4377 |
| 60 | 23.4825 | −0.3464 | 138.9009 | |
| 70 | 23.3993 | −0.3467 | 142.3666 | |
| 80 | 23.3162 | −0.3469 | 145.8347 |
| Parameter | Variety | Drying Temperature (°C) | ||||
|---|---|---|---|---|---|---|
| In Natura | 50 | 60 | 70 | 80 | ||
| Water content (% wb) | Jupi | 72.49 ± 0.32 bA | 9.57 ± 0.32 aB | 7.79 ± 0.27 aC | 7.80 ± 0.19 aC | 7.57 ± 0.46 aC |
| Pérola | 73.42 ± 0.21 aA | 8.67 ± 0.33 bB | 7.36 ± 0.16 aC | 7.33 ± 0.10 bC | 6.36 ± 0.09 bD | |
| Ashes (% db) | Jupi | 3.15 ± 0.09 bB | 3.52 ± 0.02 aA | 3.19 ± 0.08 bB | 3.30 ± 0.01 bB | 3.60 ± 0.15 aA |
| Pérola | 3.65 ± 0.07 aA | 3.56 ± 0.03 aA | 3.48 ± 0.07 aA | 3.47 ± 0.13 aA | 3.64 ± 0.13 aA | |
| Proteins (% db) | Jupi | 4.63 ± 0.01 bC | 4.77 ± 0.01 bA | 4.68 ± 0.01 bB | 4.68 ± 0.00 bB | 4.67 ± 0.00 bB |
| Pérola | 4.78 ± 0.03 aD | 5.20 ± 0.01 aA | 5.12 ± 0.01 aB | 5.12 ± 0.01 aB | 5.08 ± 0.01 aC | |
| Lipids (% db) | Jupi | 1.17 ± 0.02 bD | 2.22 ± 0.02 bBC | 2.26 ± 0.03 bB | 2.15 ± 0.03 bC | 2.35 ± 0.03 bA |
| Pérola | 1.67 ± 0.04 aC | 2.42 ± 0.03 aB | 2.87 ± 0.01 aA | 2.87 ± 0.06 aA | 2.88 ± 0.02 aA | |
| Carbohydrates (% db) | Jupi | 91.05 ± 0.13 aA | 89.49 ± 0.02 aC | 89.86 ± 0.07 aB | 89.87 ± 0.01 aB | 89.39 ± 0.12 aC |
| Pérola | 89.89 ± 0.08 bA | 88.83 ± 0.03 bB | 88.53 ± 0.09 bC | 88.54 ± 0.08 bC | 88.40 ± 0.14 bC | |
| TEV (kcal/ 100 g db) | Jupi | 393.21 ± 0.65 aC | 397.02 ± 0.10 aB | 398.53 ± 0.50 bA | 397.54 ± 0.29 bAB | 397.36 ± 0.28 bAB |
| Pérola | 393.76 ± 0.75 aC | 397.87 ± 0.43 aB | 400.46 ± 0.19 aA | 400.44 ± 0.83 aA | 399.83 ± 0.57 aA | |
| Parameter | Variety | Drying Temperature (°C) | ||||
|---|---|---|---|---|---|---|
| In Natura | 50 | 60 | 70 | 80 | ||
| Water activity (25 °C) | Jupi | 0.984 ± 0.001 aA | 0.291 ± 0.00 bB | 0.278 ± 0.00 aC | 0.241 ± 0.00 bD | 0.235 ± 0.00 bD |
| Pérola | 0.988 ± 0.001 aA | 0.305 ± 0.00 aB | 0.270 ± 0.00 bC | 0.252 ± 0.00 aD | 0.243 ± 0.001 aD | |
| Acidity (% citric ac. db) | Jupi | 2.66 ± 0.02 aBC | 2.59 ± 0.10 bC | 2.71 ± 0.10 bBC | 2.83 ± 0.10 bAB | 2.99 ± 0.10 bA |
| Pérola | 2.13 ± 0.05 bC | 3.09 ± 0.10 aB | 3.10 ± 0.00 aB | 3.27 ± 0.00 aB | 3.64 ± 0.10 aA | |
| Total sugars (g/100 g db) | Jupi | 45.10 ± 0.72 aE | 52.12 ± 1.27 aD | 54.92 ± 0.89 aC | 62.78 ± 0.16 aB | 71.73 ± 0.81 aA |
| Pérola | 35.49 ± 0.31 bD | 41.38 ± 0.09 bC | 41.95 ± 0.16 bC | 44.45 ± 0.09 bB | 48.81 ± 0.33 bA | |
| Reducing sugars (g/100 g db) | Jupi | 41.45 ± 0.65 aA | 40.99 ± 0.09 aA | 40.81 ± 0.08 aA | 36.72 ± 0.21 aB | 32.33 ± 0.97 aC |
| Pérola | 32.78 ± 0.46 bC | 36.84 ± 0.56 bA | 35.68 ± 1.04 bAB | 35.05 ± 0.27 bB | 30.50 ± 0.12 bD | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis, C.G.d.; Figueirêdo, R.M.F.d.; Queiroz, A.J.d.M.; Paiva, Y.F.; Amadeu, L.T.S.; Santos, F.S.d.; Ferreira, J.P.d.L.; Lima, T.L.B.d.; Andrade, F.S.; Gomes, J.P.; et al. Pineapple Peel Flours: Drying Kinetics, Thermodynamic Properties, and Physicochemical Characterization. Processes 2023, 11, 3161. https://doi.org/10.3390/pr11113161
Reis CGd, Figueirêdo RMFd, Queiroz AJdM, Paiva YF, Amadeu LTS, Santos FSd, Ferreira JPdL, Lima TLBd, Andrade FS, Gomes JP, et al. Pineapple Peel Flours: Drying Kinetics, Thermodynamic Properties, and Physicochemical Characterization. Processes. 2023; 11(11):3161. https://doi.org/10.3390/pr11113161
Chicago/Turabian StyleReis, Carolaine Gomes dos, Rossana Maria Feitosa de Figueirêdo, Alexandre José de Melo Queiroz, Yaroslávia Ferreira Paiva, Lumara Tatiely Santos Amadeu, Francislaine Suelia dos Santos, João Paulo de Lima Ferreira, Thalis Leandro Bezerra de Lima, Fabrícia Santos Andrade, Josivanda Palmeira Gomes, and et al. 2023. "Pineapple Peel Flours: Drying Kinetics, Thermodynamic Properties, and Physicochemical Characterization" Processes 11, no. 11: 3161. https://doi.org/10.3390/pr11113161
APA StyleReis, C. G. d., Figueirêdo, R. M. F. d., Queiroz, A. J. d. M., Paiva, Y. F., Amadeu, L. T. S., Santos, F. S. d., Ferreira, J. P. d. L., Lima, T. L. B. d., Andrade, F. S., Gomes, J. P., Silva, W. P. d., & Santos, D. d. C. (2023). Pineapple Peel Flours: Drying Kinetics, Thermodynamic Properties, and Physicochemical Characterization. Processes, 11(11), 3161. https://doi.org/10.3390/pr11113161







