Effect of Cation Nonstoichiometry on Hydration and Charge Transport Processes in Yb-Doped SrZrO3 Perovskite-Type Proton Conductor for Ceramic Electrochemical Cells
Abstract
1. Introduction
2. Defect Chemistry of SrxZr0.95Yb0.05O3-δ
2.1. Stoichiometric Yb-Doped Strontium Zirconate
2.2. Sr-Deficient Yb-Doped Strontium Zirconate
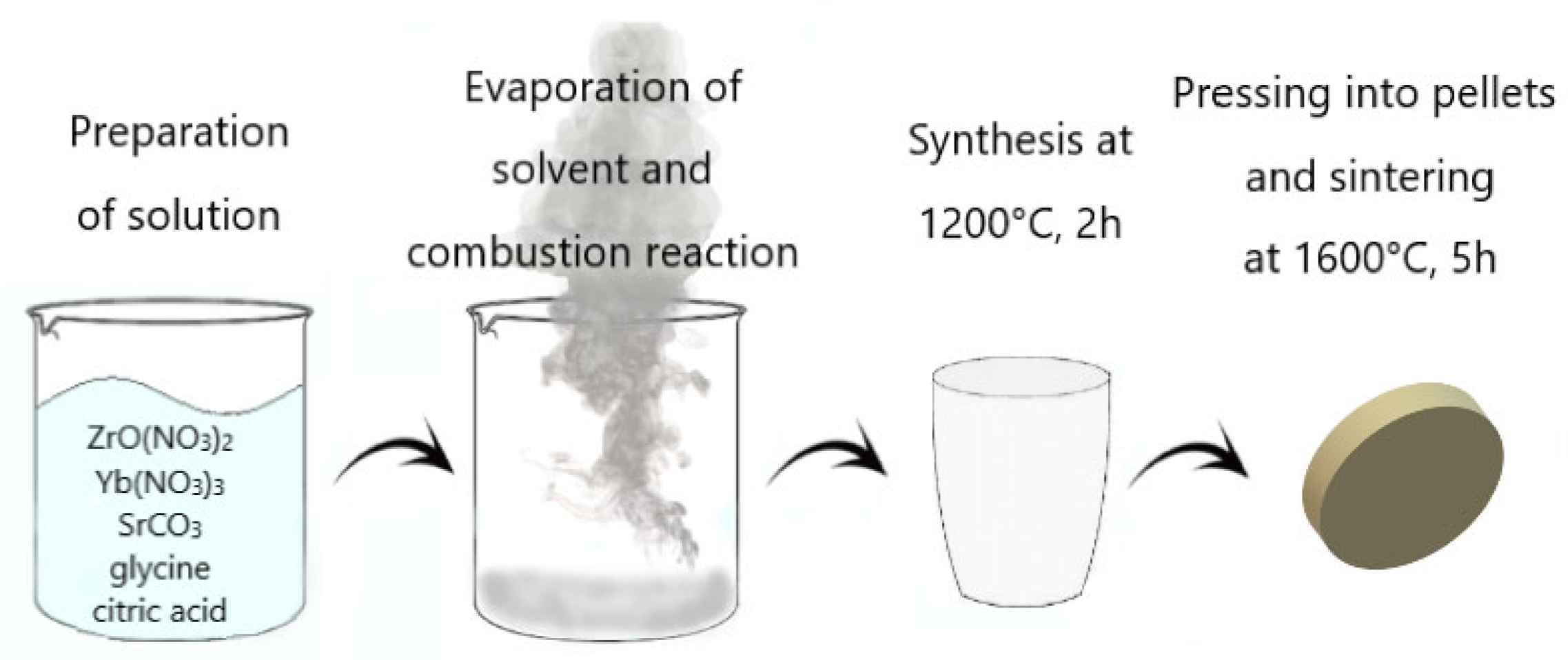
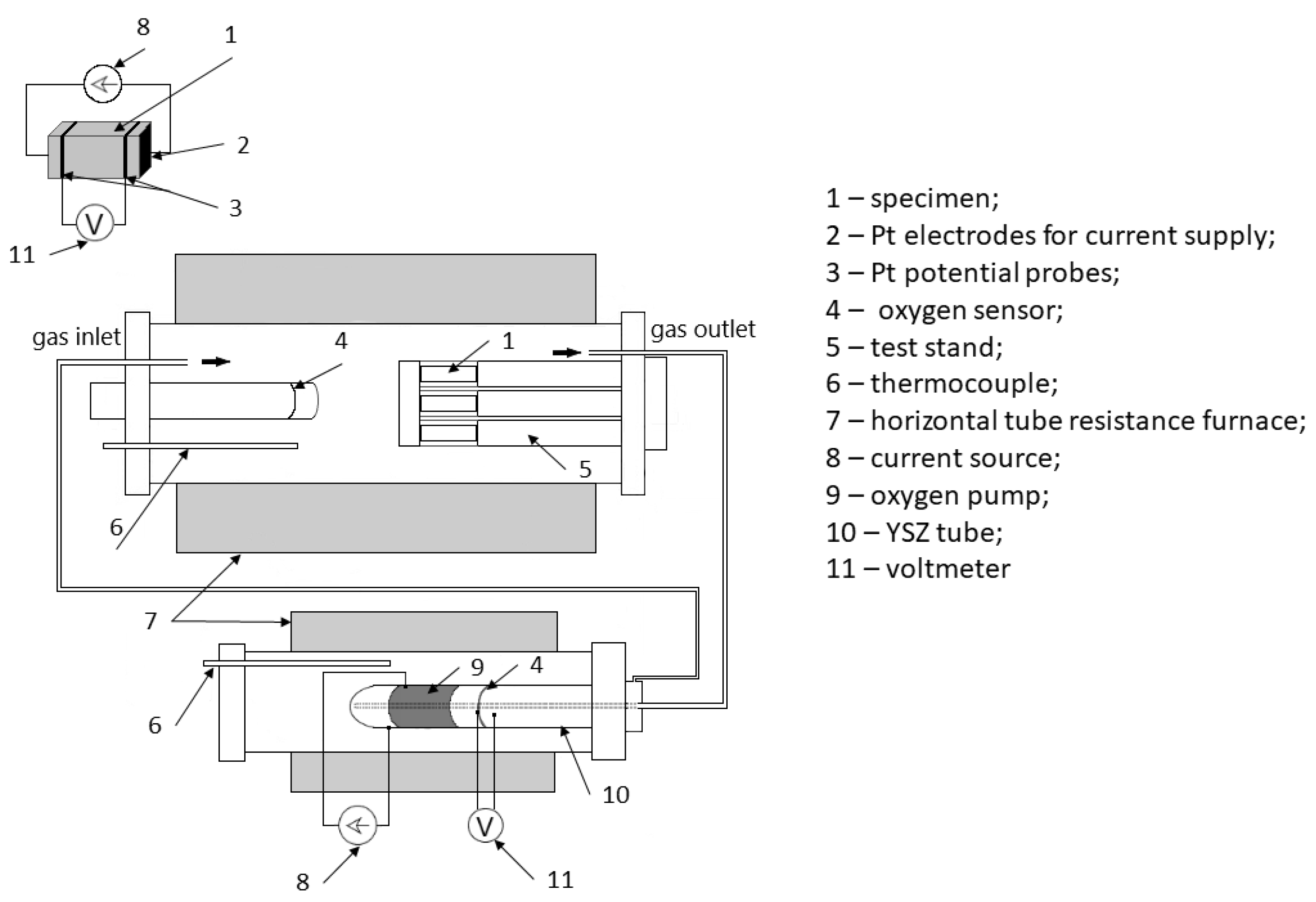
3. Materials and Methods
4. Results
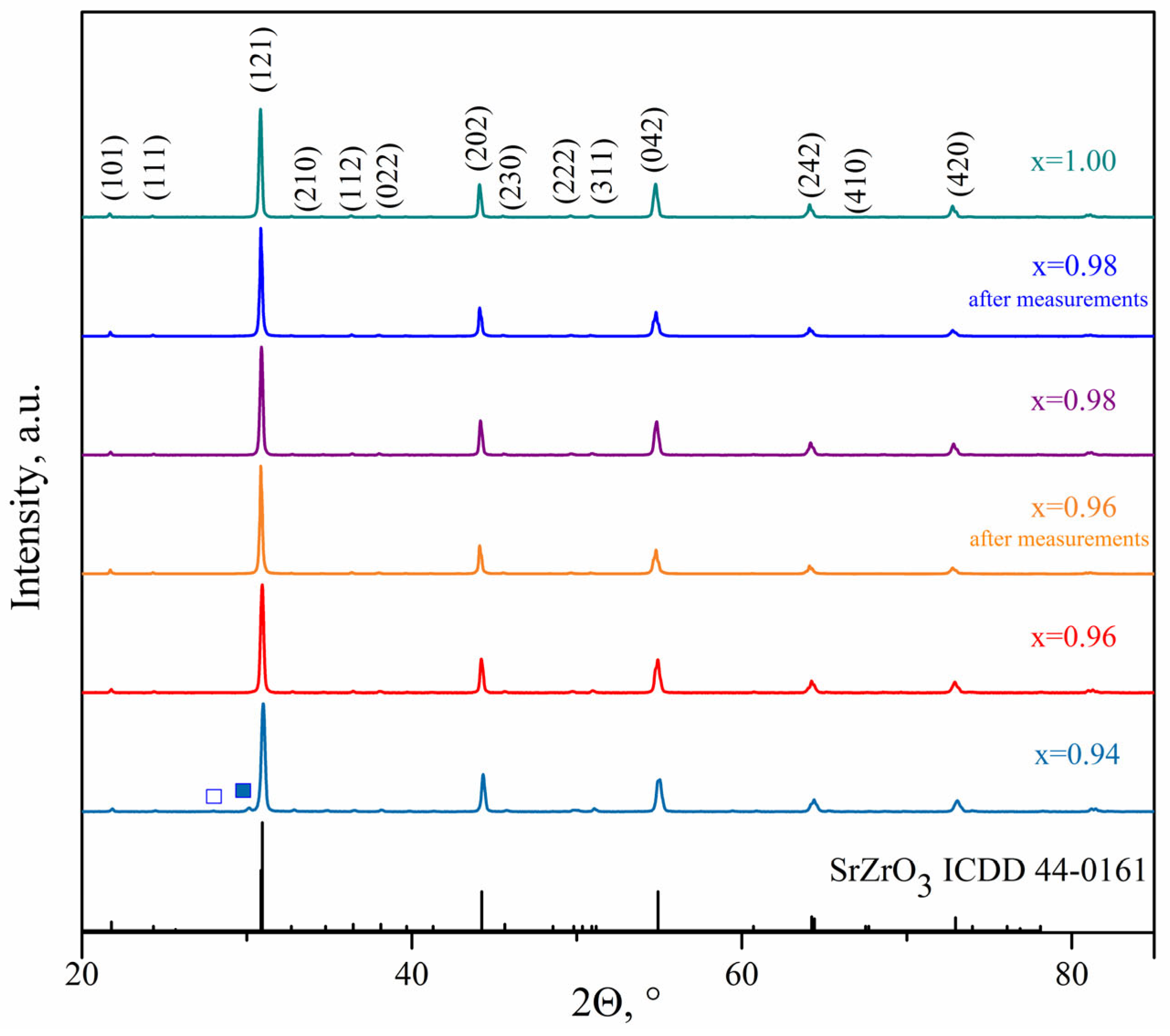
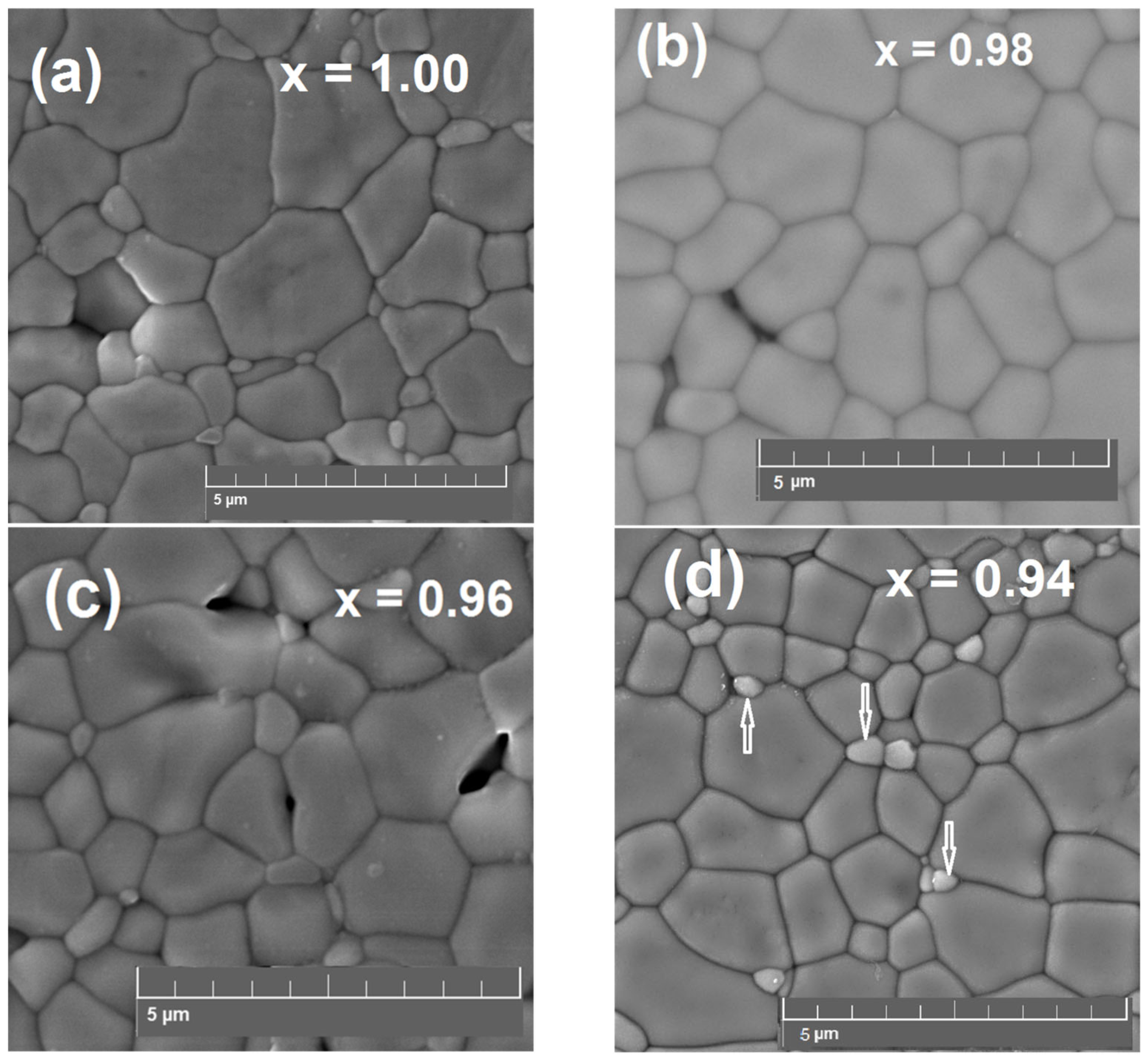
4.1. Phase Composition and Microstructure of SrxZr0.95Yb0.05O3-δ
4.2. Raman Spectra
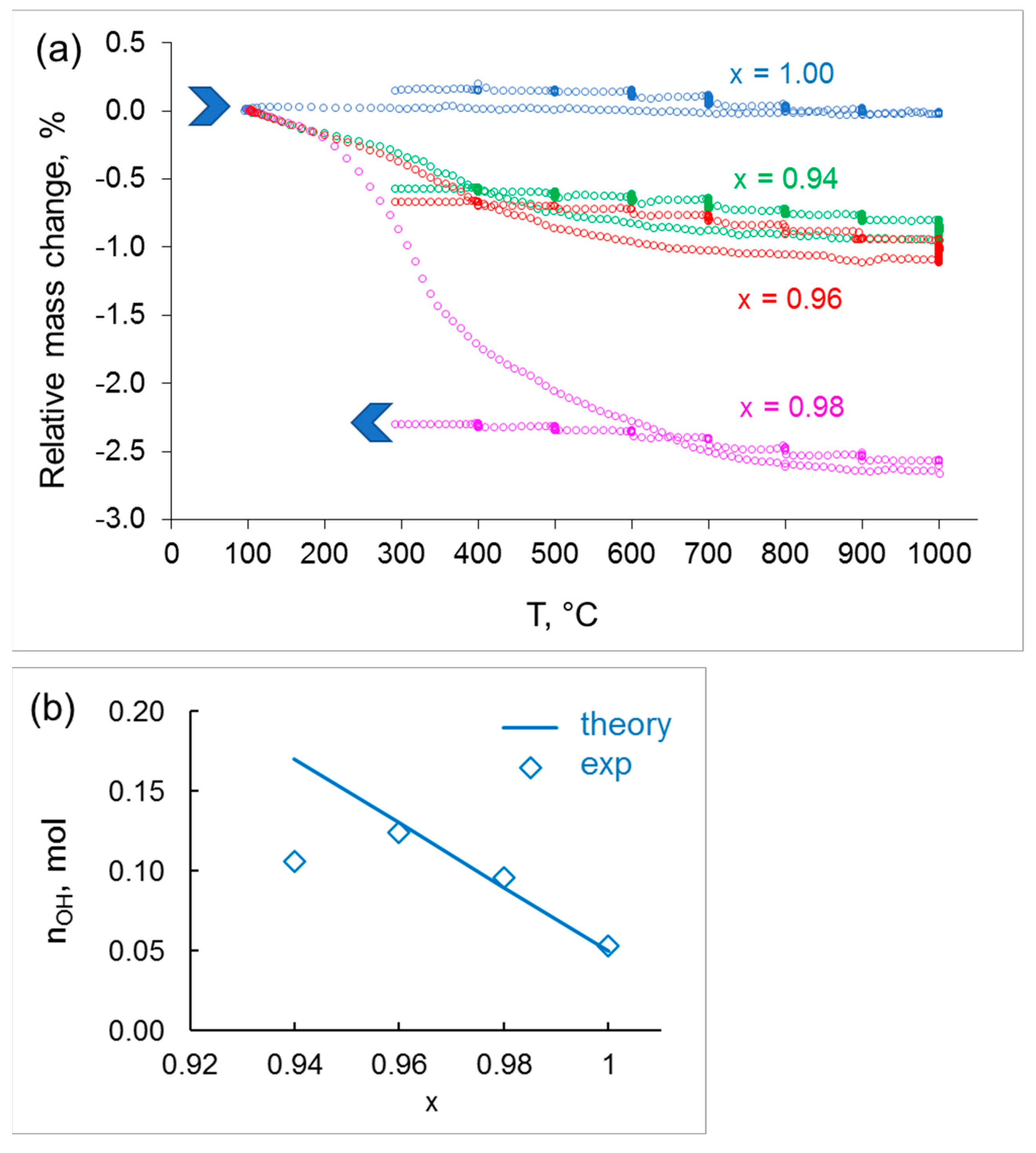
4.3. Thermogravimetry Analysis
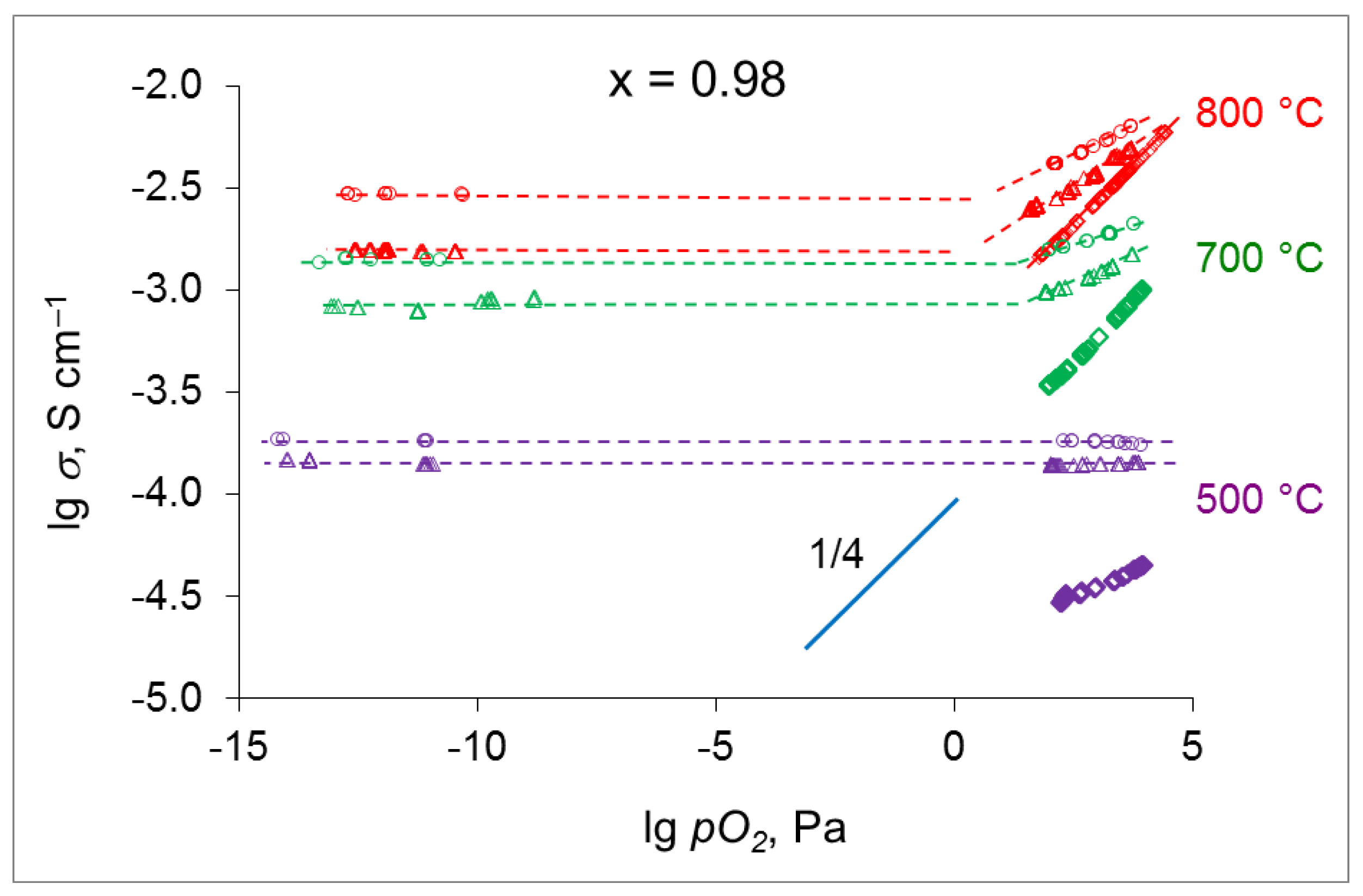
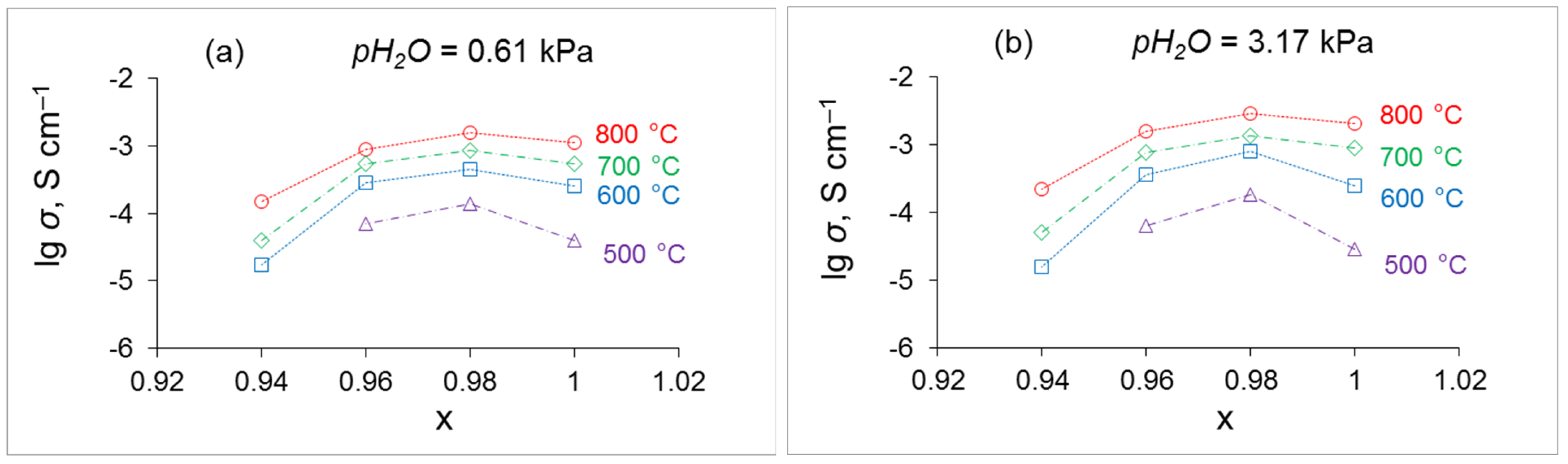
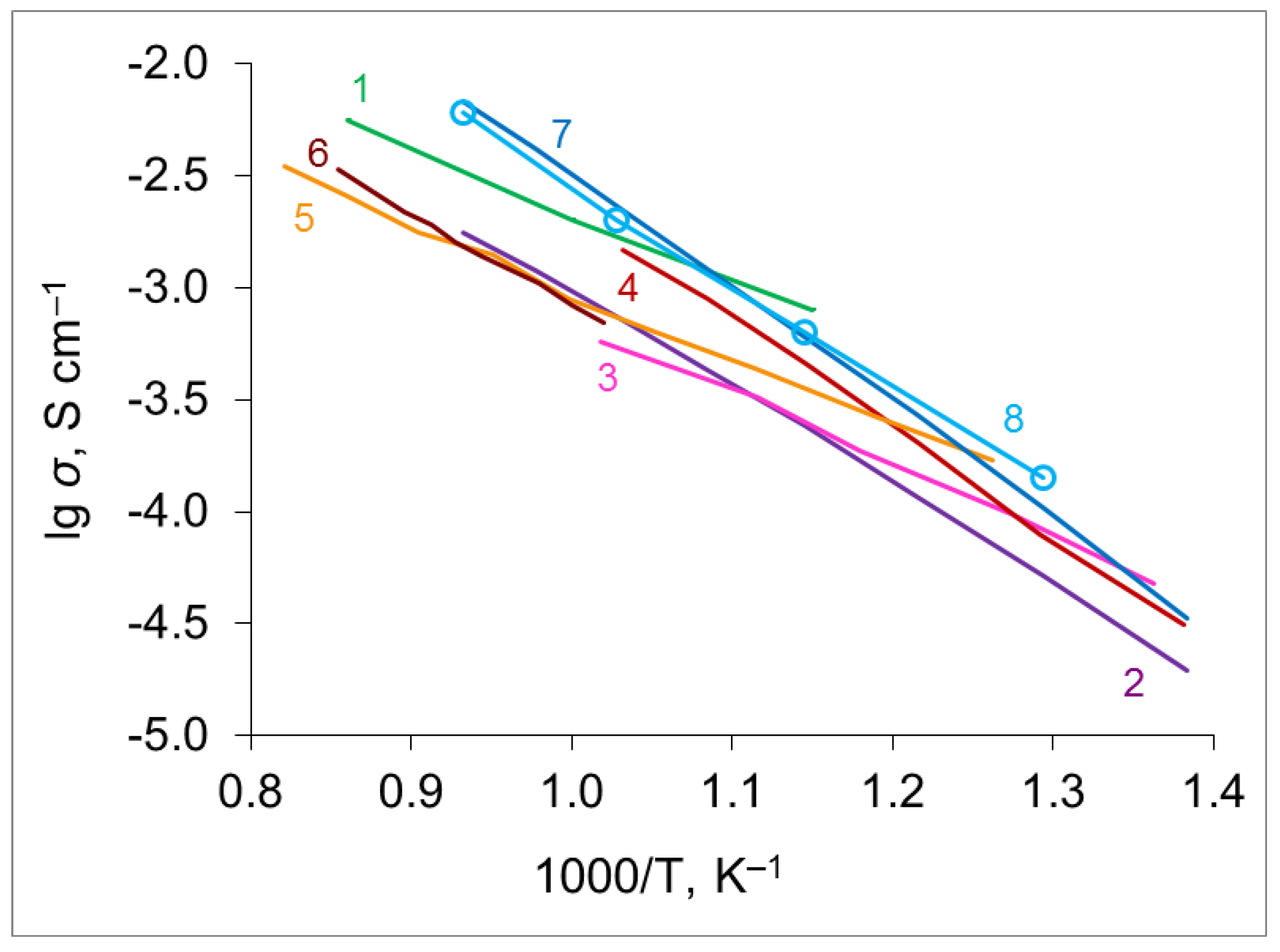
4.4. Conductivity of SrxZr0.95Yb0.05O3-d
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iwahara, H.; Esaka, T.; Uchida, H.; Maeda, N. Proton conduction in sintered oxides and its application to steam electrolysis for hydrogen production. Solid State Ion. 1981, 3–4, 359–363. [Google Scholar] [CrossRef]
- Iwahara, H.; Uchida, H.; Tanaka, S. High temperature type proton conductor based on SrCeO3 and its application to solid electrolyte fuel cells. Solid State Ion. 1983, 9–10, 1021–1025. [Google Scholar] [CrossRef]
- Iwahara, H.; Uchida, H.; Maeda, N. High temperature fuel and steam electrolysis cells using proton conductive solid electrolytes. J. Power Sources 1982, 7, 293–301. [Google Scholar] [CrossRef]
- Takahashi, T.; Iwahara, H. Solid state ionics: Proton conduction in perovskite type solid solutions. Rev. Chim. Min. 1980, 17, 243–253. [Google Scholar]
- Shimura, T.; Komori, M.; Iwahara, H. Ionic conduction in pyrochlore-type oxides containing rare earth elements at high temperature. Solid State Ion. 1996, 86–88, 685–689. [Google Scholar] [CrossRef]
- Labrincha, J.A.; Frade, J.R. Protonic conduction in La2Zr2O7-based pyrochlore materials. Solid State Ion. 1997, 99, 33–40. [Google Scholar] [CrossRef]
- Regalado Vera, C.Y.; Ding, H.; Peterson, D.; Gibbons, W.T.; Zhou, M.; Ding, D. A mini-review on proton conduction of BaZrO3-based perovskite electrolytes. J. Phys. Energy 2021, 3, 032019. [Google Scholar] [CrossRef]
- Hossain, S.; Abdalla, A.M.; Jamain, S.N.B.; Zaini, J.H.; Azad, A.K. A review on proton conducting electrolytes for clean energy and intermediate temperature-solid oxide fuel cells. Renew. Sustain. Energy Rev. 2017, 79, 750–764. [Google Scholar] [CrossRef]
- Shiraiwa, M.; Kido, T.; Fujii, K.; Yashima, M. High-temperature proton conductors based on the (110) layered perovskite BaNdScO4. J. Mater. Chem. A 2021, 9, 8607. [Google Scholar] [CrossRef]
- Dunyushkina, L.A.; Belyakov, S.A.; Filatov, N.M. Proton-conducting alkaline earth hafnates: A review of manufacturing technologies, physicochemical properties and electrochemical performance. J. Eur. Ceram. Soc. 2023, 43, 6681–6698. [Google Scholar] [CrossRef]
- Swierczek, K.; Zajac, W.; Klimkowicz, A.; Zheng, K.; Malikova, N.; Dabrowski, B. Crystal structure and proton conductivity in highly oxygen-deficient Ba1−xLax(In,Zr,Sn)O3−δ perovskites. Solid State Ion. 2015, 275, 58–61. [Google Scholar] [CrossRef]
- Guan, D.; Xu, H.; Zhang, Q.; Huang, Y.-C.; Shi, C.; Chang, Y.-C.; Xu, X.; Tang, J.; Gu, Y.; Pao, C.-W.; et al. Identifying a universal activity descriptor and a unifying mechanism concept on perovskite oxides for green hydrogen production. Adv. Mater. 2023. [Google Scholar] [CrossRef] [PubMed]
- Guan, D.; Zhong, J.; Xu, H.; Huang, Y.-C.; Hu, Z.; Chen, B.; Zhang, Y.; Ni, M.; Xu, X.; Zhou, W.; et al. A universal chemical-induced tensile strain tuning strategy to boost oxygen-evolving electrocatalysis on perovskite oxides. Appl. Phys. Rev. 2022, 9, 011422. [Google Scholar] [CrossRef]
- Hanif, M.B.; Rauf, S.; Motola, M.; Babar, Z.U.D.; Li, C.-J.; Li, C.-X. Recent progress of perovskite-based electrolyte materials for solid oxide fuel cells and performance optimizing strategies for energy storage applications. Mater. Res. Bull. 2022, 146, 111612. [Google Scholar] [CrossRef]
- Duan, C.; Kee, R.; Zhu, H.; Sullivan, N.; Zhu, L.; Bian, L.; Jennings, D.; O’Hayre, R. Highly efficient reversible protonic ceramic electrochemical cells for power generation and fuel production. Nat. Energy 2019, 4, 230–240. [Google Scholar] [CrossRef]
- Choi, S.; Davenport, T.C.; Haile, S.M. Protonic ceramic electrochemical cells for hydrogen production and electricity generation: Exceptional reversibility, stability, and demonstrated faradaic efficiency. Energy Environ. Sci. 2019, 12, 206–215. [Google Scholar] [CrossRef]
- Zajac, W.; Rusinek, D.; Zheng, K.; Molenda, J. Applicability of Gd-doped BaZrO3, SrZrO3, BaCeO3 and SrCeO3 proton conducting perovskites as electrolytes for solid oxide fuel cells. Cent. Eur. J. Chem. 2013, 11, 471–484. [Google Scholar] [CrossRef]
- Skubida, W.; Zheng, K.; Swierczek, K.; Michna, M.; Kondracki, L. Indium doping in SrCeO3 proton-conducting perovskites. J. Solid State Chem. 2020, 284, 121210. [Google Scholar] [CrossRef]
- Gorelov, V.P.; Balakireva, V.B.; Kuzmin, A.V. Charge transfer and defect structure in BaCeO3. Russ. J. Inorg. Chem. 2018, 63, 930–937. [Google Scholar] [CrossRef]
- Ryu, K.H.; Haile, S.M. Chemical stability and proton conductivity of doped BaCeO–BaZrO solid solutions. Solid State Ion. 1999, 125, 355–367. [Google Scholar] [CrossRef]
- Medvedev, D.A.; Lyagaeva, J.G.; Gorbova, E.V.; Demin, A.K.; Tsiakaras, P. Advanced materials for SOFC application: Strategies for the development of highly conductive and stable solid oxide proton electrolytes. Prog. Mater. Sci. 2016, 75, 38–79. [Google Scholar] [CrossRef]
- Shi, Z.; Sun, W.; Wang, Z.; Qian, J.; Liu, W. Samarium and yttrium codoped BaCeO3 proton conductor with improved sinterability and higher electrical conductivity. ACS Appl. Mater. Interfaces 2014, 6, 5175–5182. [Google Scholar] [CrossRef] [PubMed]
- Kreuer, K.D. Proton-Conducting Oxides. Annu. Rev. Mater. Res. 2003, 33, 333–359. [Google Scholar] [CrossRef]
- Pérez-Coll, D.; Heras-Juaristi, G.; Fagg, D.P.; Mather, G.C. Transport-number determination of a protonic ceramic electrolyte membrane via electrodepolarisation correction with the Gorelov method. J. Power Sources 2014, 245, 445–455. [Google Scholar] [CrossRef]
- Duan, C.; Huang, J.; Sullivan, N.; O’Hayre, R. Proton-conducting oxides for energy conversion and storage. Appl. Phys. Rev. 2020, 7, 011314. [Google Scholar] [CrossRef]
- Li, S.; Irvine, J.T.S. Non-stoichiometry, structure and properties of proton-conducting perovskite oxides. Solid State Ion. 2021, 361, 115571. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Hernandez-Sanchez, R.; Haile, S.M. Cation non-stoichiometry in yttrium-doped barium zirconate: Phase behavior, microstructure, and proton conductivity. J. Mater. Chem. 2010, 20, 8158–8166. [Google Scholar] [CrossRef]
- Dunyushkina, L.A.; Khaliullina, A.S.; Meshcherskikh, A.N.; Pankratov, A.A.; Osinkin, D.A. Effect of A-site nonstoichiometry on defect chemistry and electrical conductivity of undoped and Y-doped SrZrO3. Materials 2019, 12, 1258. [Google Scholar] [CrossRef]
- Sharova, N.V.; Gorelov, V.P. Effect of Cation Nonstoichiometry on the Properties of Solid Electrolyte BaxCe0.97Nd0.03O3–d (0.90 ≤ x ≤ 1.10). Russ. J. Electrochem. 2004, 40, 639–645. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, H.; Wang, L.; Li, Y.; He, Z.; Han, C.; Dai, L. The influence of Ba-site non-stoichiometry on the phase composition, sinterability, conductivity and chemical stability of BaxZr0.6Hf0.2Y0.2O3−d (0.9 ≤ x ≤ 1.03) proton conductors. Int. J. Hydrogen Energy 2021, 46, 10838–10849. [Google Scholar] [CrossRef]
- Shkerin, S.N.; Rudakova, A.V.; Bulanin, K.M.; Khaliullina, A.S.; Meshcherskikh, A.N.; Vovkotrub, E.G.; Dunyushkina, L.A. Raman spectroscopy of SrZrO3 based proton conducting electrolyte: Effect of Y-doping and Sr-nonstoichiometry. Int. J. Hydrogen Energy 2021, 46, 17007–17018. [Google Scholar] [CrossRef]
- Khaliullina, A.; Meshcherskikh, A.; Pankratov, A.; Dunyushkina, L. Effect of Sr deficiency on electrical conductivity of Yb-doped strontium zirconate. Materials 2022, 15, 4126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hu, Y.H. Progress in proton-conducting oxides as electrolytes for low-temperature solid oxide fuel cells: From materials to devices. Energy Sci. Eng. 2021, 9, 984–1011. [Google Scholar] [CrossRef]
- Kosacki, I.; Tuller, H.L. Mixed conductivity in SrCe0.95Yb0.05O3 protonic conductors. Solid State Ion. 1995, 80, 223–229. [Google Scholar] [CrossRef]
- Dunyushkina, L.A.; Gorelov, V.P. High temperature electrical behavior of CaTi1−xFexO3−δ (x = 0–0.5). Oxygen-ion, electronic and proton conductivity. Solid State Ion. 2013, 253, 169–174. [Google Scholar] [CrossRef]
- Huang, Y.; Qiu, R.; Lian, W.; Lei, L.; Liu, T.; Zhang, J.; Wang, Y.; Liu, J.; Huang, J.; Chen, F. Review: Measurement of partial electrical conductivities and transport numbers of mixed ionic-electronic conducting oxides. J. Power Sources 2022, 528, 231201. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crysstallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Kamishima, O.; Hattori, T.; Ohta, K.; Chiba, Y.; Ishigame, M. Raman scattering of single-crystal SrZrO3. J. Phys. Condens. Matter 1999, 11, 5355–5365. [Google Scholar] [CrossRef]
- Yang, Y.; Ning, X.; Luo, S.; Dong, F.; Li, L. Electron irradiation effects of SrZrO3 ceramic for radioactive strontium immobilization. Procedia Environ. Sci. 2016, 31, 330–334. [Google Scholar] [CrossRef]
- Colomban, P.; Slodczyk, A. Raman intensity: An important tool to study the structure and phase transitions of amorphous/crystalline materials. Opt. Mater. 2009, 31, 1759–1763. [Google Scholar] [CrossRef]
- Murphy, R.; Zhou, Y.; Zhang, L.; Soule, L.; Zhang, W.; Chen, Y.; Liu, M. A new family of proton-conducting electrolytes for reversible solid oxide cells: BaHfxCe0.8−xY0.1Yb0.1O3−δ. Adv. Funct. Mater. 2020, 30, 2002265. [Google Scholar] [CrossRef]
- Yang, W.; Han, C.; Li, Y.; Zhou, H.; Liu, S.; Wang, L.; He, Z.; Dai, L. Influence of rare-earth doping on the phase composition, sinterability, chemical stability and conductivity of BaHf0.8Ln0.2O3−δ (Ln = Yb, Y, Dy, Gd) proton conductors. Int. J. Hydrogen Energy 2021, 46, 35678–35691. [Google Scholar] [CrossRef]
- Wang, X.; Fei, M.; Zhou, C.; Li, W.; Wang, X.; Shen, X.; Liu, D.; Chen, W.; Chen, P.; Jiang, G.; et al. Enhanced proton conductivity and CO2-tolerance of intermediate-temperature protonic ceramic fuel cell with lanthanum tungstate-based composite cathode. Compos. Part B Eng. 2023, 253, 110565. [Google Scholar] [CrossRef]
- Sopiha, K.V.; Malyi, O.I.; Persson, C.; Wu, P. Suppression of surfaces states at cubic perovskite (001) surfaces by CO2 adsorption. Phys. Chem. Chem. Phys. 2018, 20, 18828–18836. [Google Scholar] [CrossRef] [PubMed]
- Tavender, S.M.; Johnson, S.A.; Balsom, D.; Parker, A.W.; Bisby, R.H. The carbonate, CO3−, in solution studied by resonance Raman spectroscopy. Laser Chem. 1999, 19, 311–316. [Google Scholar] [CrossRef]
- Hong, J.; Heo, S.J.; Singh, P. Water mediated growth of oriented single crystalline SrCO3 nanorod arrays on strontium compounds. Sci. Rep. 2021, 11, 3368. [Google Scholar] [CrossRef]
- Bagherisereshki, E.; Tran, J.; Lei, F.; AuYeung, N. Investigation into SrO/SrCO3 for high temperature thermochemical energy storage. Sol. Energy 2018, 160, 85–93. [Google Scholar] [CrossRef]
- Fontana, M.D.; Mabrouk, K.B.; Kauffmann, T.H. Raman spectroscopic sensors for inorganic salts. Spectrosc. Prop. Inorg. Organomet. Compd. 2013, 44, 40–67. [Google Scholar]
- Burikov, S.A.; Dolenko, T.A.; Fadeev, V.V. The Role of Fermi Resonance in Formation of Valence Band of Water Raman Scattering. Int. J. Opt. 2008, 2008, 204828. [Google Scholar] [CrossRef][Green Version]
- Balakireva, V.B.; Gorelov, V.P.; Dunyushkina, L.A.; Kuzmin, A.V. Impact of Humidity on Charge Transport in Proton-Conducting Perovskites AZr0.95Sc0.05O3−δ (A = Ca, Sr, Ba) Exposed to an Oxidative Atmosphere. Phys. Solid State 2019, 61, 515–522. [Google Scholar] [CrossRef]
- Gharbage, B.; Marques, F.M.B.; Frade, J.R. Protonic conduction in Sr1−y(Zr1−xDyx)O3−δ Ceramics. J. Eur. Ceram. Soc. 1996, 16, 1149–1156. [Google Scholar] [CrossRef]
- Yang, W.; Wang, L.; Li, Y.; Zhou, H.; He, Z.; Han, C.; Dai, L. An easily sintered, chemically stable indium and tin co-doped barium hafnate electrolyte for hydrogen separation. J. Alloys Compd. 2021, 868, 159117. [Google Scholar] [CrossRef]
- Zvonareva, I.A.; Mineev, A.M.; Tarasova, N.A.; Fu, X.Z.; Medvedev, D.A. High-temperature transport properties of BaSn1−xScxO3−δ ceramic materials as promising electrolytes for protonic ceramic fuel cells. J. Adv. Ceram. 2022, 11, 1131–1143. [Google Scholar]
- Pikalova, E.Y.; Bogdanovich, N.M.; Kuz’min, A.V. Composite Electrodes for Proton Conducting Electrolyte of CaZr0.95Sc0.05O3–d. Russ. J. Electrochem. 2017, 53, 752–760. [Google Scholar] [CrossRef]





| Model 1 Sr0.96Zr0.95Yb0.05O3 Pnma | Model 2 Sr0.91Yb0.05ZrO3 Pnma | Model 3 Sr0.935Yb0.025Zr0.975Yb0.025O3 Pnma | ||||||
|---|---|---|---|---|---|---|---|---|
| a, Å | 5.8190(1) | a, Å | 5.8190(3) | a, Å | 5.8190(1) | |||
| b, Å | 8.2047(1) | b, Å | 8.2048(8) | b, Å | 8.2046(9) | |||
| c, Å | 5.7946(2) | c, Å | 5.7945(2) | c, Å | 5.7946(2) | |||
| V, Å3 | 276.65(5) | V, Å3 | 276.65(7) | V, Å3 | 276.65(4) | |||
| Sr0.96 (4c) | x | 0.4753(5) | Sr0.91Yb0.05 (4c) | x | 0.4745(6) | Sr0.935Yb0.025 (4c) | x | 0.4755(7) |
| y | 0.25 | y | 0.25 | y | 0.2500(0) | |||
| z | 0.0054(6) | z | 0.0051(1) | z | 0.0051(7) | |||
| Occ | 0.48 | Occ | 0.48 | Occ | 0.48 | |||
| Zr0.95Yb0.05 (4a) | x | 0 | Zr (4a) | x | 0 | Zr0.975Yb0.025 (4a) | x | 0 |
| y | 0 | y | 0 | y | 0 | |||
| z | 0 | z | 0 | z | 0 | |||
| Occ | 0.5 | Occ | 0.5 | Occ | 0.5 | |||
| O1 (8c) | x | 0.2187(4) | O1 (8c) | x | 0.2159(8) | O1 (8c) | x | 0.2195(4) |
| y | 0.0266(4) | y | 0.0347(0) | y | 0.0267(8) | |||
| z | 0.2832(5) | z | 0.2859(0) | z | 0.2827(3) | |||
| Occ | 1 | Occ | 1 | Occ | 1 | |||
| O2 (4c) | x | 0.5188(3) | O2 (4c) | x | 0.5170(0) | O2 (4c) | x | 0.5205(5) |
| y | 0.25 | y | 0.25 | y | 0.2500(0) | |||
| z | 0.6093(7) | z | 0.5820(8) | z | 0.6162(2) | |||
| Occ | 0.5 | Occ | 0.5 | Occ | 0.5 | |||
| Rwp, % | 10.6 | Rwp, % | 10.7 | Rwp, % | 10.8 | |||
| Rexp, % | 10.59 | Rexp, % | 10.59 | Rexp, % | 10.60 | |||
| Rp, % | 8.81 | Rp, % | 8.66 | Rp, % | 8.91 | |||
| χ2 | 1.01 | χ2 | 1.02 | χ2 | 1.03 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaliullina, A.; Meshcherskikh, A.; Dunyushkina, L. Effect of Cation Nonstoichiometry on Hydration and Charge Transport Processes in Yb-Doped SrZrO3 Perovskite-Type Proton Conductor for Ceramic Electrochemical Cells. Processes 2023, 11, 2939. https://doi.org/10.3390/pr11102939
Khaliullina A, Meshcherskikh A, Dunyushkina L. Effect of Cation Nonstoichiometry on Hydration and Charge Transport Processes in Yb-Doped SrZrO3 Perovskite-Type Proton Conductor for Ceramic Electrochemical Cells. Processes. 2023; 11(10):2939. https://doi.org/10.3390/pr11102939
Chicago/Turabian StyleKhaliullina, Adelya, Anastasia Meshcherskikh, and Liliya Dunyushkina. 2023. "Effect of Cation Nonstoichiometry on Hydration and Charge Transport Processes in Yb-Doped SrZrO3 Perovskite-Type Proton Conductor for Ceramic Electrochemical Cells" Processes 11, no. 10: 2939. https://doi.org/10.3390/pr11102939
APA StyleKhaliullina, A., Meshcherskikh, A., & Dunyushkina, L. (2023). Effect of Cation Nonstoichiometry on Hydration and Charge Transport Processes in Yb-Doped SrZrO3 Perovskite-Type Proton Conductor for Ceramic Electrochemical Cells. Processes, 11(10), 2939. https://doi.org/10.3390/pr11102939








