1. Introduction
Rhenium is a rare-earth metal that is widely used in heat-resistant superalloys, platinum–rhenium catalysts, and prospective structural materials for rocket and space technology [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13]. The trend of providing various industries with high-tech products in the next 150 years will lead to the exhaustion of the proven reserves of rhenium in the Earth’s crust [
14]. To date, about 80% of Re is consumed in the production of superalloys, about 15% of Re is used as a catalyst in fuel production, and the remaining 5% of Re is used in alloy form, applied as electrical contacts, electromagnets, heating elements, mass spectrographs, semiconductors, thermocouples, vacuum pipes, etc. [
15]. The United States is the main consumer of rhenium, which is primarily used for heat-resistant alloy production; US consumption reached ~43,000 kg of Re in 2021, with a total global Re production of 59,000 kg per year. The global interest in Re is expected to grow, especially in the space industry. Rhenium is an important metal for different industrial areas, which is why having a stable rhenium production source has become a vital issue. Thus, Pratt & Whitney—an aerospace manufacturer—analyzed the market and came to the conclusion that their key operation of gas turbine engine design and production may be affected by rhenium shortages. Therefore, in 2014, the company bought 230,000 kg of Re to ensure a stable operation cycle [
12]. Currently, Re is produced as a by-product of pyrometallurgical molybdenum production. During this process, rhenium compounds are oxidized to volatile rhenium oxide, Re
2O
7. This oxide is collected, together with outgoing smoke gases, and extracted in the form of rhenium acid or ammonium perrhenate. Ammonium perrhenate is a raw product for further metallic rhenium production. Metallic rhenium is mainly produced by reduction using gaseous hydrogen at temperatures close to 1273 K [
16]. In this regard, a significant effort should be dedicated to identifying resource-saving technologies for the recycling of rhenium materials. The most promising secondary sources of rhenium were predicted [
1] to be KReO
4 and NH
4ReO
4 compounds or rhenium metal powder (as a result of its reduction by hydrogen). The method of hydrogen reduction has a number of disadvantages, such as complex instrumentation requirements, the usage of gaseous hydrogen at high temperatures, and the possibility of obtaining rhenium only in a powder form.
Molten salt electrolysis is a promising process for obtaining rhenium. It can enable both the production of metallic rhenium from its compounds and the compaction of metallic rhenium powders, yielding finished or semi-finished products with a density close to the theoretical one [
2]. The properties of molten salts are the basis of electrochemical technologies for obtaining metals [
17,
18]. It is widely known that, by using molten salts, it is possible to obtain metallic coatings with desired properties, including roughness, mechanical strength, specific surface, density, etc. [
19,
20].
KF–KBF
4–B
2O
3–KReO
4 melts are prospective salts for use in rhenium electrolytic reduction [
21,
22]. The possibility of obtaining compacted rhenium from KF–KBF
4–B
2O
3–KReO
4 melts in the area of the quasi-binary K
2B
2OF
6–KReO
4 melt, particularly under an air atmosphere, was demonstrated in [
21,
22]. It was found that the electrolysis of KF–KBF
4–B
2O
3–KReO
4 melts can be executed in an open atmosphere at temperatures up to 773 K [
21].
The fluoroborate melts’ liquidus temperature and the densities’ temperature dependence are required to develop the electrolytic rhenium-obtaining process. No corresponding data for fluoroborate melts were found.
The liquidus temperature determines the lowest temperature that can be used in electrolysis. Data on the melt densities are required for the technological calculations and equipment design. In addition to its practical significance, the liquidus temperature and density data are also important for the investigation of the KReO4 and KF–KBF4–B2O3 melts’ interaction.
Nevertheless, Kataev et al. presented data on the interaction of B
2O
3 with fluoride KF–AlF
3 and KF–NaF–AlF
3 molten salts [
23]. It was found that the interaction between individual components of the melts is realized according to the reaction:
Oxyfluoroborate, K
2B
2OF
6, can be obtained using reaction (1). The thermodynamic possibility of oxyfluoroborate formation has been verified and experimentally confirmed [
24]. The reaction that binds various boron complex compounds in the melts is presented as follows:
The results in [
24] show that the K
2B
2OF
6 melt is formed in the KF–KBF
4–B
2O
3 molten mixture in the form of oxyfluoroborate complexes according to reaction (2). The melting point of K
2B
2OF
6 is 628 K, and that of K
3B
3O
3F
6 is 705 K [
25].
Potassium perrhenate (KReO
4) is a rhenium chemical compound with a relatively low melting temperature of 828 K and a boiling temperature of 1643 K [
26].
The purpose of this work was to obtain the densities’ temperature dependence and to determine the liquidus temperature of the prospective KF–KBF4–B2O3–KReO4 melts. The KF–KBF4–B2O3 melt was chosen as a solvent because its composition corresponds stoichiometrically to the low-melting potassium oxyfluoroborate, K2B2OF6. The results obtained are required for determining the basics of rhenium-obtaining technology by the electrolysis of the KF–KBF4–B2O3–KReO4 melts.
2. Experimental Procedures
2.1. Molten Salt Preparation
The KF–KBF4–B2O3–KReO4 melts were prepared using individual substances: KF (Vekton Company, Saints-Petersburg, Russia, 99.95 wt.% concentration of the main substance), KBF4, B2O3, and KReO4 (Reakhim Company, Moscow, Russia, 99.90 wt.% concentration of the main substance).
The potassium perrhenate powder was dried at 423 K for 3 h in the air in a glassy carbon container. Potassium tetrafluoroborate was dried at 473 K for 2 h. B2O3 was remelted under vacuum and then used in the powdered form. The prepared substances were used for the melt preparation.
The substances were mixed to prepare a KF (37.28 wt. %)–KBF
4 (40.39 wt. %)–B
2O
3 (22.33 wt. %) mixture. The mixture was placed in the glassy carbon container. The container was placed inside a quartz retort. Then, it was heated to 773 K and kept in the molten state for 4 h. Thus, a stoichiometric melt corresponding to the K
2B
2OF
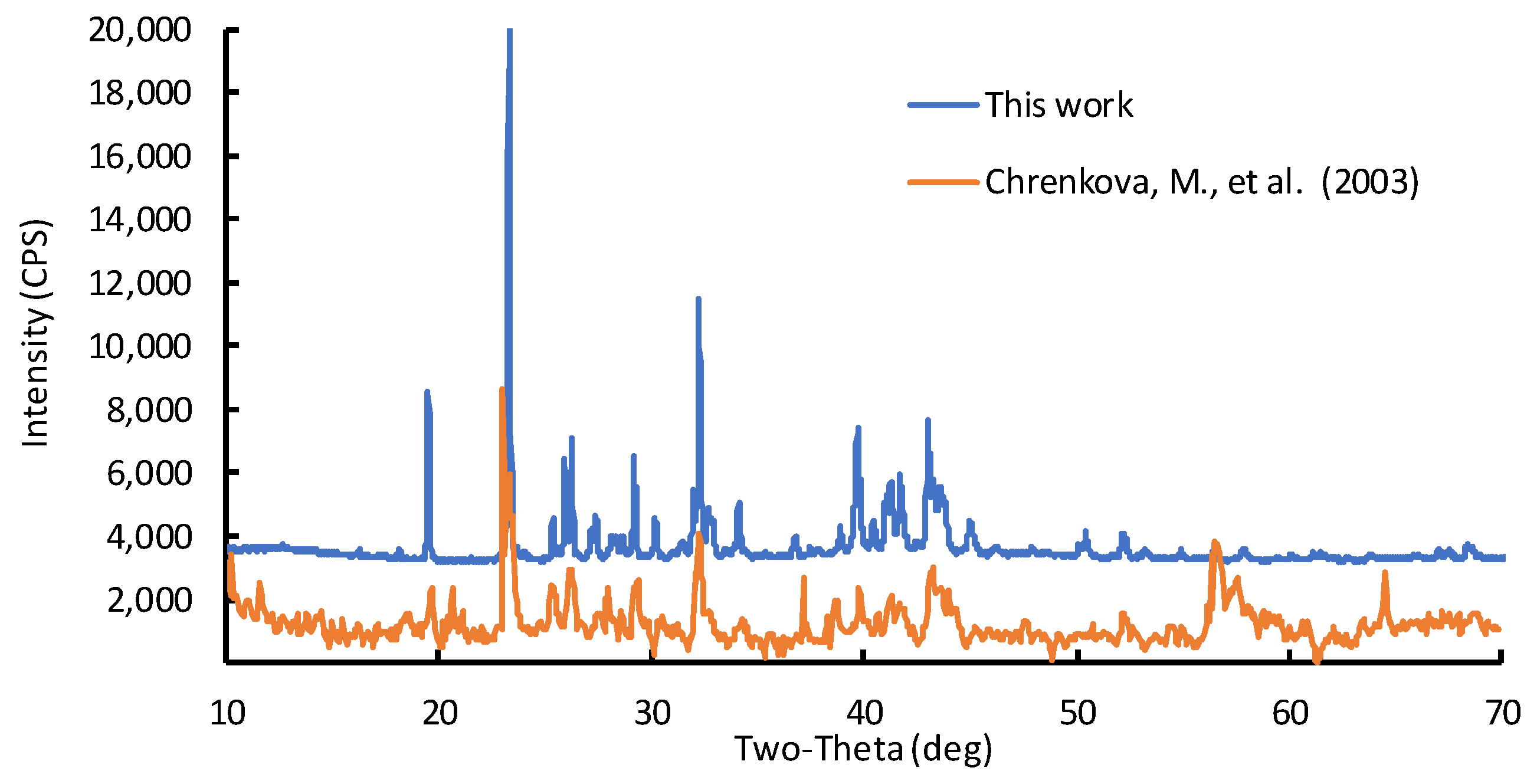
6 composition was synthesized. To confirm the phase composition of the resulting melt, an X-ray phase analysis was carried out using a Rigaku D/MAX-2200VL/PC X-ray diffractometer (Rigaku Corporation, Matsubara-cho, Akishima-shi, Tokyo, Japan).
Figure 1 shows the results of the K
2B
2OF
6 molten salt X-ray analysis.
The required content of the potassium perrhenate powder was added to the K
2B
2OF
6 melts to form the desired K
2B
2OF
6–KReO
4 melts. The prepared mixture was subjected to a chemical composition analysis, the results of which are presented in
Table 1.
The results of the X-ray analysis show that the initial K
2B
2OF
6 melt is monophase. The obtained results correspond to the experimental data reported in [
24].
2.2. Measuring Procedures
Density measurements are generally carried out according to the hydrostatic weighing method. The method involves measuring the change in a spherical platinum weight immersed in the melt [
27,
28,
29].
The experimental cell was a quartz retort plugged with a vacuum rubber stopper, which was connected to a Mettler AT20 electronic balance (Mettler-Toledo GmbH, Greifensee, Switzerland). The platinum weight (diameter of 6.8 × 10−3 m) was hung on a platinum wire, about 0.6 m long and 2 × 10−4 m in diameter, and was connected to the electronic balance.
A special lift was used to immerse the platinum weight in and extract it from the melt. All measurements were carried out in an argon atmosphere of 99.999% purity (UralCryoGas LLC, Ekaterinburg, Russia). The spherical platinum weight was successively weighed first in the gaseous atmosphere and then in the molten salts.
The densities of the molten salts were calculated according to Equation (3), where the difference between the weight’s mass in the gaseous atmosphere and in the melts is divided by the volume of the weight.
where
is the melt density, kg/m
3;
is the mass of the weight in the air, kg;
is the mass of the weight immersed in the melt, kg; and
is the volume of the Pt ball and is equal to 1.6485 × 10
−7 m
3.
The schematic of the measuring cell is presented in
Figure 2.
The density investigation of the K2B2OF6–KReO4 melts was carried out in a temperature range of 929–628 K. The glass–carbon crucible SU-2000 (DONKARB GRAFITE LLC, Chelyabinsk, Russia) was used as a container for the melts. The glass–carbon crucible had a volume of 1.1 × 10−4 m3 (dimensions: the basic diameter was 5 × 10−2 m; the top diameter was 7.3 × 10−2 m; the height was 6.9 × 10−2 m). The moving device was used to fix the platinum weight’s location with an accuracy of 10 μm along the crucible axis.
The density measurements for each melt composition were repeated at least 3 times. The obtained results of parallel measurements were averaged.
The preliminary experiments elucidated that K
2B
2OF
6–KReO
4 melts are layered systems. The results of the densities measurement can be represented in two positions. The top position is realized when the platinum weight is located about 5 × 10
−3 m above the melts’ surfaces. The bottom position is realized when the platinum weight is located about 5 × 10
−3 m higher than the crucible bottom. Changing the platinum weight’s position by 1–2 × 10
−3 m lower or higher resulted in densities’ temperature dependence being irreproducible when recorded at different temperatures. This fact could be explained by the influence of two non-mixed liquids on the platinum weight. It should be mentioned here that, when using this method, the interfacial forces acting on the Pt wire at the upper-liquid–gas and lower-liquid–upper-liquid interfaces [
30] cause a singularity.
The crystallization temperatures and the temperatures of phase transitions were obtained by performing a thermal analysis based on a heat release evaluation. The time dependence of the thermocouple EMF was recorded using an APPA 502 multimeter (APPA Technology Corp., New Taipei City, Taiwan) during both the heating and cooling of the melts. The temperature was automatically measured every second. The average cooling and heating rates were 4.8 and 7.1 K per minute, respectively. The differences in temperatures at the points of phase transitions obtained during heating/cooling cycles did not exceed the measurement error (~5 K).
Simultaneous thermal analysis (STA) was performed using a Netzsch STA 449 F3 Jupiter device (NETZSCH-Gerätebau GmbH, Wittelsbacherstr, Germany). STA included differential scanning calorimetry (DSC) and sample thermogravimetry (TG) performed simultaneously. All experiments were carried out under an inert argon atmosphere of 99.999% purity (UralCryoGas LLC, Ekaterinburg, Russia).
3. Results and Discussion
The study of the K
2B
2OF
6–KReO
4 melts was carried out in a temperature range of 307 K–873 K by STA.
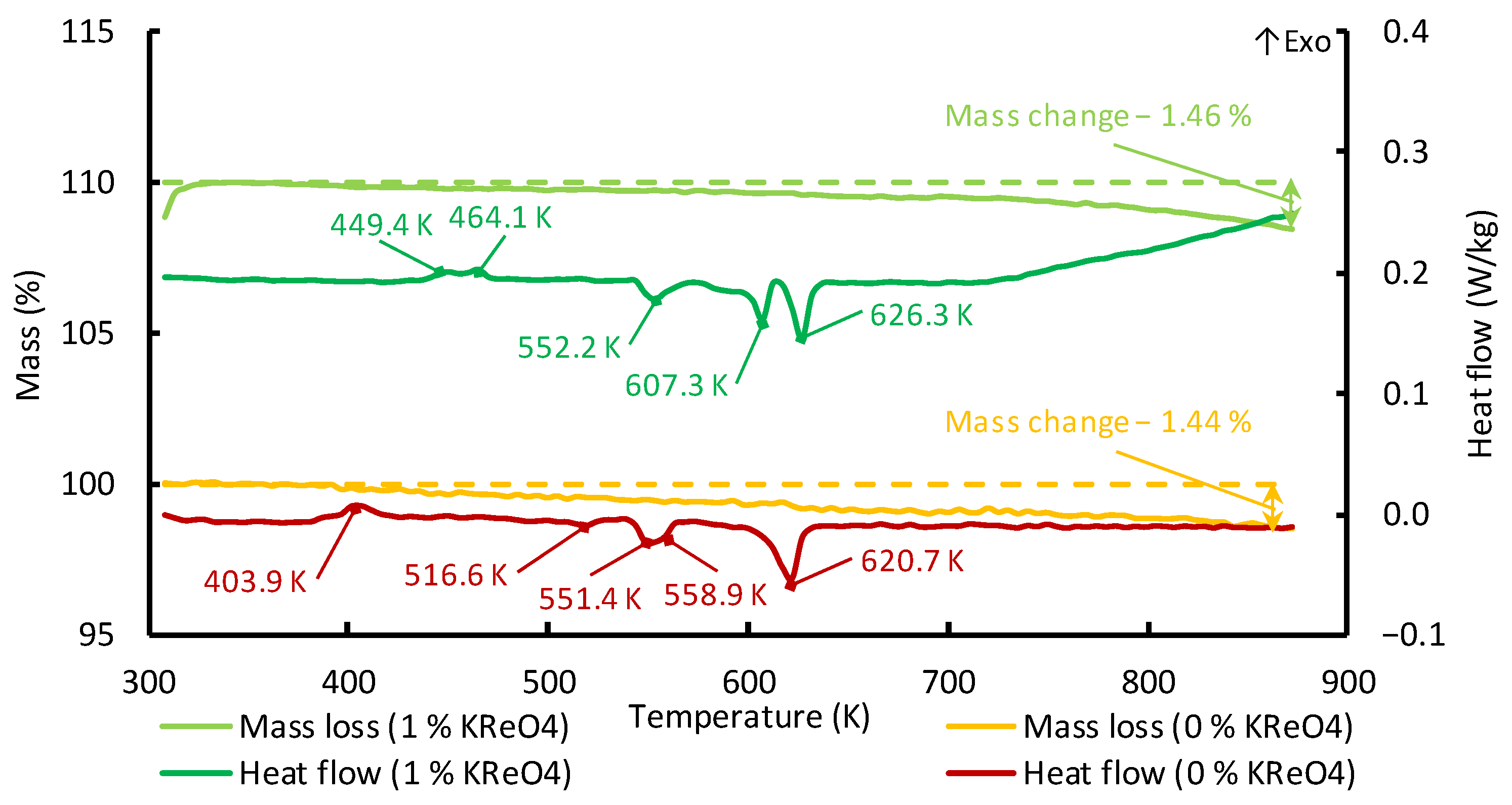
Figure 3 shows the data from the DSC and thermogravimetric analyses of the K
2B
2OF
6 melts.
According to
Figure 3, there are a number of phase transitions in solid K
2B
2OF
6–KReO
4 while heating to the melting point of K
2B
2OF
6. An exothermic heat release was observed at 403 K. This can be related to the grain recrystallization caused by the compaction of the crystal structure. A significant change in the heat flux was observed at 551.4–552.2 K. The phase transition temperature coincided with the predicted one for the formation of K
10B
38O
62 structural groups [
31]. It should be noted that the samples of the K
2B
2OF
6 melts, including those containing KReO
4, were X-ray amorphous. So, it is difficult to identify a specific phase transition in the solid state. It was found that the melting temperature of the K
2B
2OF
6 compound was 628 K. According to the differential scanning calorimetry data, the solidus temperature for the K
2B
2OF
6–(0–15 wt. %) KReO
4 melts was 628 K.
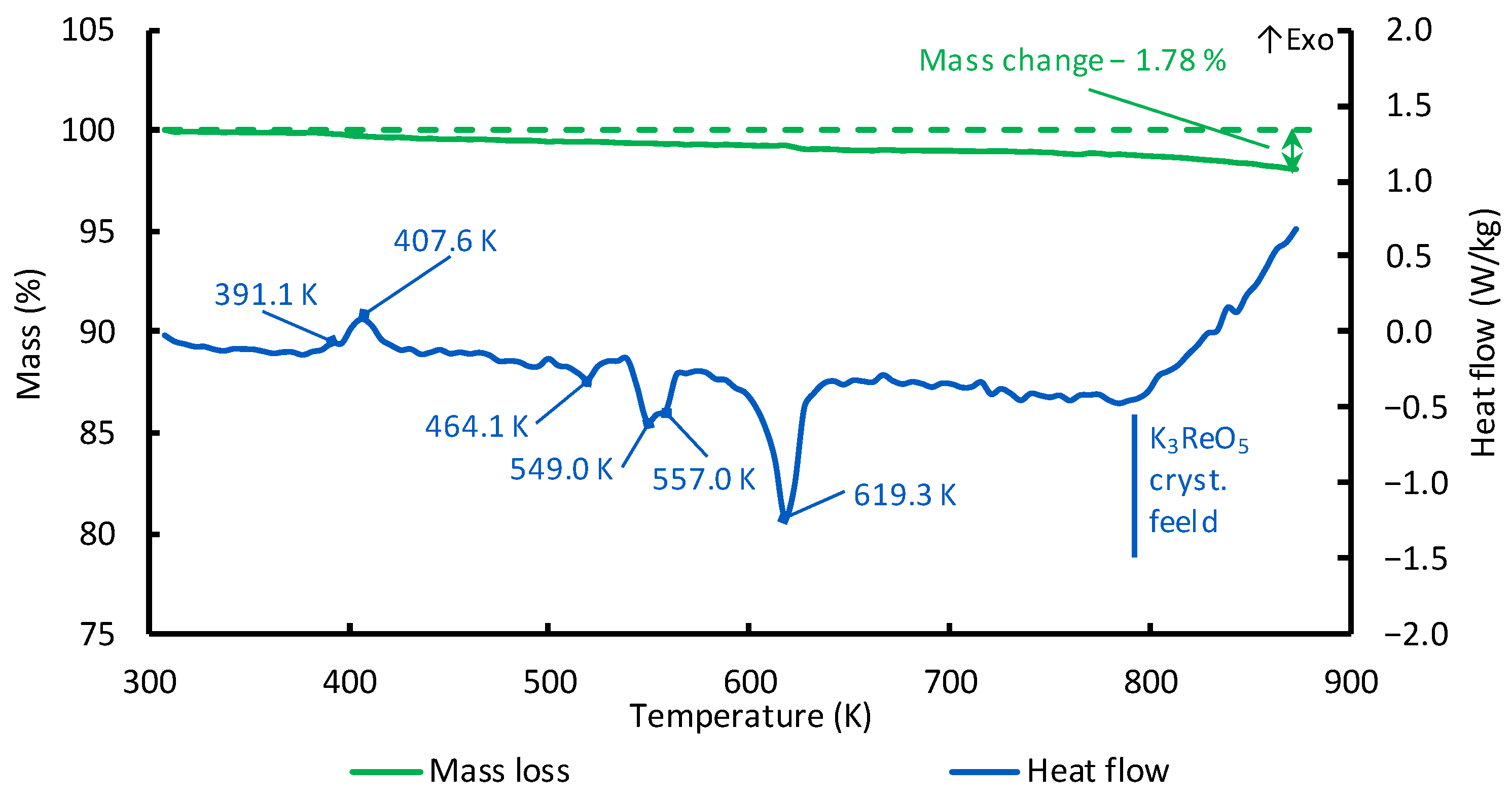
It was found that the addition of KReO
4 up to 6 wt.% to the K
2B
2OF
6 melts resulted in the appearance of an exothermic effect at a temperature of about 773 K, which was accompanied by an increase in the mass loss of the melts (
Figure 4). This fact is probably related to the crystallization area of the volatile Re
2O
7 and the more refractory K
3ReO
5 compound. The presence of rhenium compounds in the Re
+7 oxidation state in the K
2B
2OF
6–KReO
4 melts is in agreement with the results reported in [
32]. Presumably, the following reaction related to the release of rhenium oxide takes place in the melts:
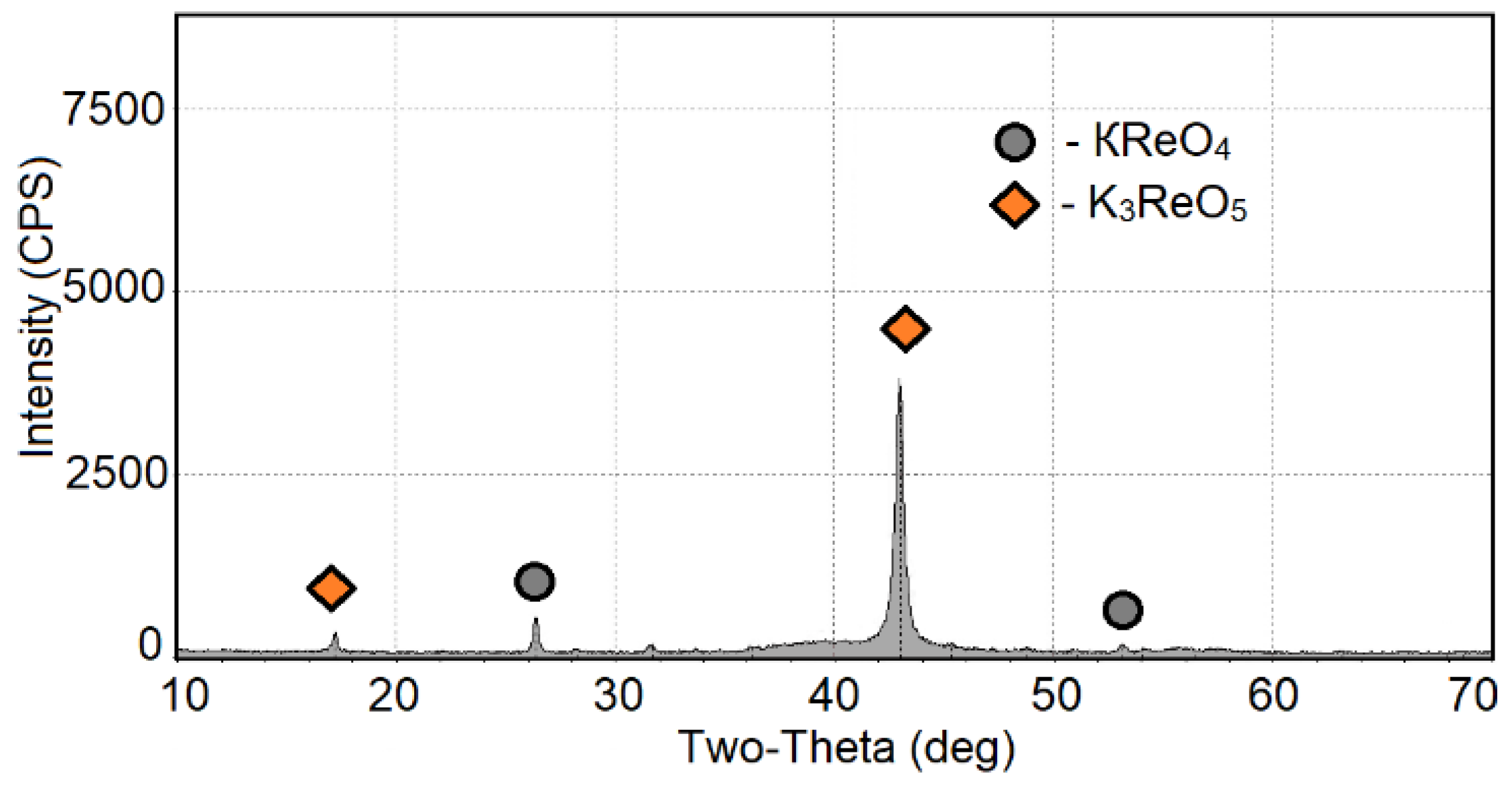
The presence of the K
3ReO
5 compound in the samples of the K
2B
2OF
6–KReO
4 melts was detected by X-ray phase analysis (
Figure 5). The data analysis showed that rhenium was present in two phases, namely, KReO
4 and K
3ReO
5, in the solidified melts [
33]. The K
2B
2OF
6 compound had a glass structure, and it did not have any individual peaks in the diffraction pattern. The presence of any other phases was not determined.
An increase in the content of KReO4 from 2 to 6 wt.% resulted in a decrease in the temperature of the exothermic heat release from 793 to 773 K. We associate this with the fact that the melt composition shifts to the area of the K3ReO5 crystallization in the K2B2OF6–KReO4 phase diagram.
All thermal effects can be systemized. The majority of them are associated with phase transitions in the solid states of K
2B
2OF
6 and K
2B
2OF
6–KReO
4, and additional investigations will be necessary in the future to determine their mechanisms (
Table 2).
The analyses of the thermogravimetric dependence indicate that the relative loss of the mass does not exceed 2.05 % for the K
2B
2OF
6 melts with the addition of 15 wt. % of KReO
4 at 873 K. The data obtained were used as a source for determining the liquidus temperatures of the K
2B
2OF
6–KReO
4 system.
Table 3 shows the liquidus temperatures of the K
2B
2OF
6–KReO
4 systems depending on the KReO
4 content. It is found that an increase in the KReO
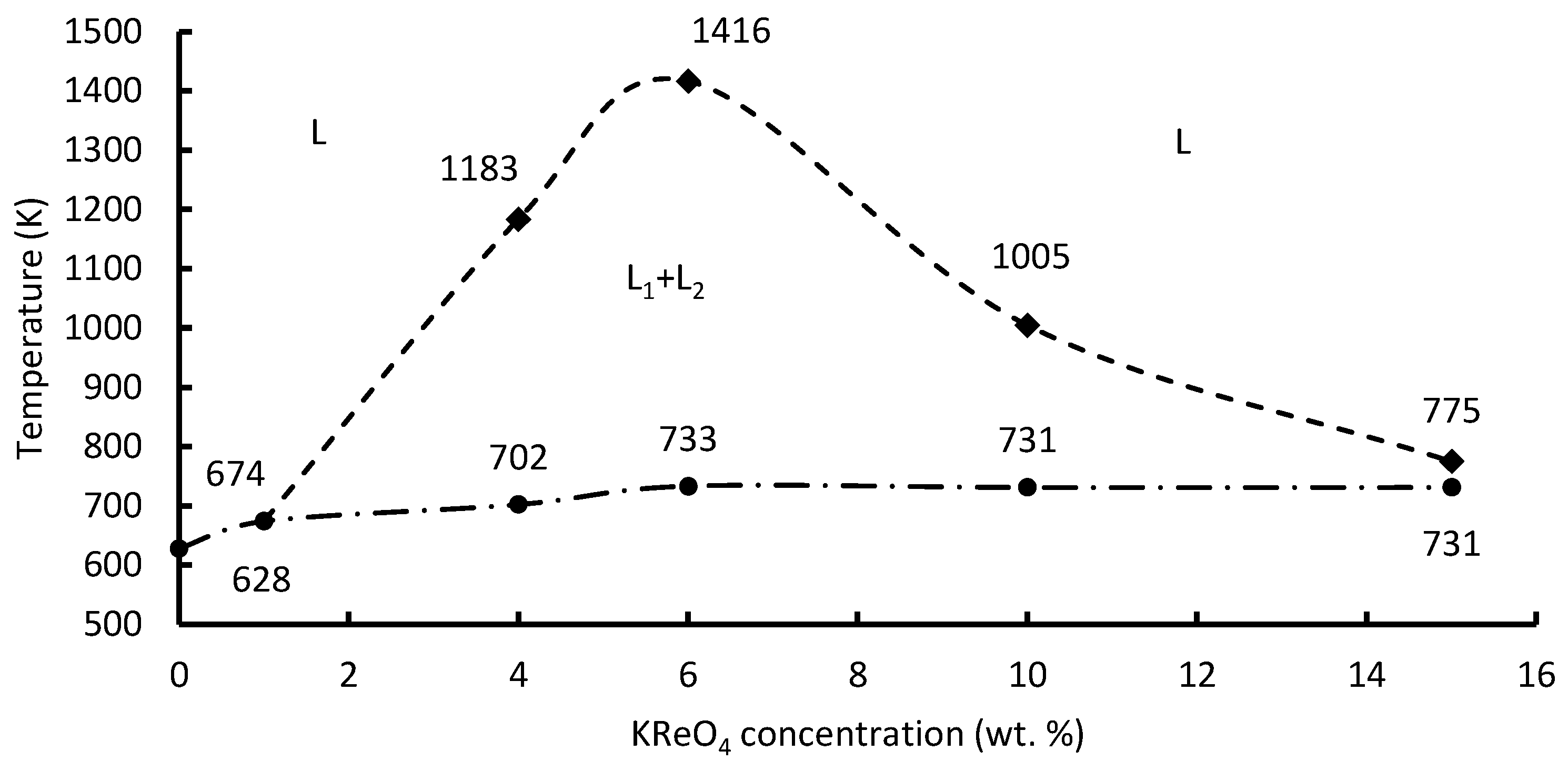
4 concentration up to 6 wt. % results in the liquidus temperature rising from 628 to 733 K. Increasing the potassium perrhenate concentration above 6 wt. % does not influence the liquidus temperature.
The immutability of the liquidus temperature of the K2B2OF6–KReO4 melts with increasing KReO4 content indicates the presence of a miscibility gap. Further measurements of the liquidus temperatures of the K2B2OF6–KReO4 melts at various melt-level points confirmed the existence of the phase separation. The liquidus temperature stabilization at a temperature of 733 K for melt compositions of K2B2OF6–(4–15 wt. %) KReO4 elucidated that the melt state was within the boundaries of the miscibility gap of the phase diagram.
The experimental data on the liquidus temperature were used to determine the temperature range of the density measurements of the K
2B
2OF
6–KReO
4 melts. The temperature dependence of densities was measured at two level points of the K
2B
2OF
6–KReO
4 melts: 1—immersed by 5 × 10
−3 m from the melt mirror (upper layer—“U”); 2—suspended about 5 × 10
−3 m from the bottom of the container with the melt (conditionally, the lower layer—“L”). It was discovered that the temperature dependence of the melt densities could be described by linear equations (
Table 2). It was found that the K
2B
2OF
6 and K
2B
2OF
6–1 wt. % KReO
4 melts (№. 1 and №. 2 in
Table 2) did not delaminate in the entire range of the temperatures studied.
Melts №. 3–№. 10 (
Table 3) delaminated. The densities of the upper and lower layers differed significantly. The temperature dependence of the densities of the K
2B
2OF
6–KReO
4 melts are shown in
Figure 6.
It was found that the addition of up to 1 wt. % KReO4 had a negligible influence on the density homogeneity of the K2B2OF6–KReO4 melts.
Phase separation was observed for the K
2B
2OF
6–KReO
4 melts with KReO
4 content above 4 wt.%. It could be seen from the experimental data that the densities of the upper and lower phases started to differ, and phases did not mix with up to 15 wt. % KReO
4. Such behavior is consistent with the data obtained by measuring the densities of the stratified molten salt systems [
22].
The behavior of the temperature dependence of the K
2B
2OF
6–KReO
4 (4–15 wt.) melts’ densities (compositions with phase separation) is in accordance with the results reported in [
34]. The densities’ temperature dependence can be approximated by linear functions.
The density of the heavier phase of K
2B
2OF
6–KReO
4 (4–10 wt.%) decreased more rapidly. This indirectly indicates the lower solubility of the heavy component in the light layer and its better solubility in the heavy layer. The densities of the upper and lower phases approached each other as the temperature and KReO
4 content increased.
Figure 7 shows the density isotherms of the K
2B
2OF
6–KReO
4 melts for the upper and lower layers at T = 820 K.
It was found that the melt density increased as the composition of the K
2B
2OF
6–KReO
4 (4–15 wt. %) melts changed. This is in agreement with the fact that a higher rhenium concentration (as a powerful complexing agent) increases the densities of the K
2B
2OF
6–KReO
4 melts. The densities of the upper and lower layers were found to vary. This indicates that the K
2B
2OF
6–KReO
4 (4–15 wt. %) melts are characterized by different values of critical temperature. The critical temperature is the upper boundary of the miscibility gap. At the same time, the trend changed significantly for the K
2B
2OF
6–15 wt. % KReO
4 melts (
Figure 7), and it indicates the convergence of phase densities to the critical point at lower temperatures.
Tkachev et al. reported that the value of the critical temperature T
Cr, i.e., the upper boundary of the miscibility gap, in a molten salt corresponded to the same density of two liquids within the boundaries of the miscibility gap. Thus, the critical temperature can be predicted by solving the system of linear equations (
Table 3) for one composition, provided that the densities of the upper and lower layers are equal to:
where
is the temperature, K;
is the upper layer’s density, g/cm
3;
is the lower layer’s density, g/cm
3;
,
are the linear equation coefficients of the density’s temperature dependence for the upper and lower layers.
We calculated the critical temperatures for the K
2B
2OF
6–KReO
4 (4–15 wt. %) molten systems. For that purpose, the system of equations of the densities’ temperature dependence (data of
Table 2) for the upper and lower layers was constructed. The solution to the system of equations was obtained under the condition of the equality of the upper and lower layers at the critical temperature T
cr.
The obtained values of critical temperature, as well as the liquidus and monotectic ones, can be summarized.
Figure 8 shows the liquidus, monotectic, and critical temperatures of the K
2B
2OF
6–KReO
4 (0–15 wt. %) melts.
The results of the critical temperature calculation indicate that the peak of the miscibility gap in the K2B2OF6–KReO4 melts corresponds to a temperature of about 1416 K at a potassium perrhenate concentration of 6 wt. %. The critical temperature dependence on the concentration of potassium perrhenate is extreme. An increase in the potassium perrhenate content up to 15 wt. % in the K2B2OF6–KReO4 melts causes the critical temperature to decrease down to 775 K. Thus, the miscibility gap of the K2B2OF6–KReO4 melts is within 1 wt. % and 15 wt. % of the KReO4 concentration.