Abstract
Induced pluripotent stem cell (iPSC)-derived mesenchymal stem cells (iMSCs) are amenable for use in a clinical setting for treatment of osteoarthritis (OA), which remains one of the major illnesses worldwide. Aside from iPSC-derived iMSCs, chondrocytes (iCHO) and extracellular vesicles (EV) are also promising candidates for treatment of OA. Manufacturing and quality control of iPSC-derived therapies is mainly manual and thus highly time consuming and susceptible to human error. A major challenge in translating iPSC-based treatments more widely is the lack of sufficiently scaled production technologies from seeding to fill-and-finish. Formerly, the Autostem platform was developed for the expansion of tissue-derived MSCs at scale in stirred tank bioreactors and subsequent fill-and-finish. Additionally, the StemCellDiscovery platform was developed to handle plate-based cultivation of adherent cells including their microscopic analysis. By combining the existing automation technology of both platforms, all required procedures can be integrated in the AutoCRAT system, designed to handle iPSC expansion, differentiation to iMSCs and iCHOs, pilot scale expansion, and formulation of iMSCs as well as extracellular vesicles and their purification. Furthermore, the platform is equipped with several in-line and at-line assays to determine product quality, purity, and safety. This paper highlights the need for adaptable and modular automation concepts. It also stresses the importance of ensuring safety of generated therapies by incorporating automated release testing and cleaning solutions in automated systems. The adapted platform concepts presented here will help translate these technologies for clinical production at the necessary scale.
1. Introduction
Osteoarthritis (OA) is a disease of the joint including but not limited to degeneration of cartilage and ligaments, synovial inflammation, formation of osteophytes and changes in subchondral bone [1]. OA remains one of the leading causes for disabilities, loss of function and chronic pain among the elderly, with a high socioeconomic burden [2]. The highly complex pathophysiology of OA impedes the development of effective therapies, with most common treatment options addressing symptom relief but not structural repair of the affected joint [3]. Mesenchymal stem cells (MSCs) have been generally recognized as a treatment option for OA [4], due to their immunomodulatory and chondrogenic differentiation potential [5,6]. The International Society for Cell Therapy defines MSCs as plastic adherent cells with a specific expression profile (CD105+, CD73+, CD90+, CD45−, CD34−, CD14−, CD11b−, CD79alpha−, CD19−, and HLA-DR−) [7] and the potential to differentiate into osteoblasts, adipocytes and chondroblasts in vitro [8]. Efforts to define criteria for iPSC derived mesenchymal stem cells (iMSCs) have also been initiated [9]. Additionally, iMSC derived extracellular vesicles (iEV) [10] exhibit regenerative properties [11]. Autologous chondrocyte implantation has been used to treat defined articular cartilage defects in the femorotibial area of human joints [12]. Cadaver derived juvenile chondrocytes did not elicit an immunologic response in vitro but have not been tested in vivo.
Despite clinical success, the lack of sophisticated production technology for large-scale isolation and expansion of iMSCs and their EVs inhibits the widespread application of these therapies. The most beneficial production strategy for MSCs is partly dependent on whether an autologous or allogeneic approach is used. This influences both the therapeutic effect as well as the technology used for MSC production [13]. Allogeneic therapy utilizing economies-of-scale seems to be more cost-effective, as several doses for several patients can be produced in one campaign. Manufacturing in larger batches is especially cost-effective for the expansion process [14,15], a main cost driver in MSC manufacturing making the more cost-effective approach at the moment [16,17]. Also, induced pluripotent stem cell (iPSC)-derived chondrocytes and their EV cargo have not been used as sources for cartilage defect repair. Currently, MSCs are mainly extracted from bone marrow, adipose tissue, or umbilical cords using different isolation methods [18,19] and expanded manually. The lack of standardization in sourcing of MSCs and their limited expansion potential impedes treatment approvals by authorities due to varying quality parameters. By manufacturing iMSCs from differentiated iPSCs, a more standardized, well-characterized cell population can be generated [20]. To increase standardization in cell differentiation and expansion, automation needs to be applied across the entire production process to guarantee a reproducible quality of cells. Incorporating new analytical technologies for process understanding and control is critical for sustained successful production campaigns. Additionally, several tests for microbial contamination must be undertaken for product release. These tests should be integrated with production to avoid unnecessary logistics and shorten release timelines. Relevant analytics include endotoxin testing and mycoplasma detection by quantitative polymerase chain reaction (qPCR). Additionally, to decrease the socioeconomic burden of OA and OA therapies, production of iMSCs needs to become more cost-effective. The high level of manual operation in iMSC, iEV, and iPSC-derived chondrocyte (iCHO) production hinders widespread application and production. Automation is a key technology not only for standardization of production but also to reduce costs.
2. Materials and Methods
The development of the AutoCRAT systems started with an analysis of the manual standard operating procedures for each process listed in Figure 1. For each process, it was evaluated whether hardware and software components were available on the automated system or whether a functionality needed to be added. Overall, commercial devices were integrated with custom automation solutions developed in-house to enable an end-to-end automated manufacturing process. The hardware on all systems is listed in Table 1, that formerly and newly integrated is listed in Table 1.
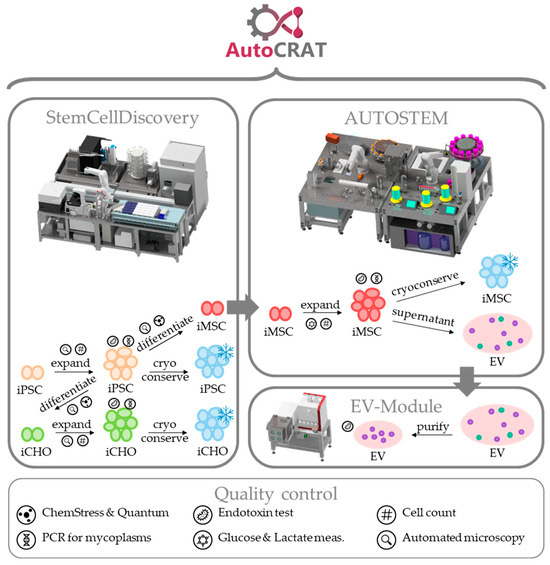
Figure 1.
Scheme of the AutoCRAT production processes and quality controls.

Table 1.
List of devices and stations for the StemCellDiscovery, Autostem and EV module. * devices are newly integrated for AutoCRAT.
3. Results—The AutoCRAT Platforms
For increased product quality as well as production efficiency, full automation of OA cell therapy manufacturing and quality control (QC) is required. Except for the input of resources/materials, the ejection of waste, and product and process supervision no human interference shall be required. This enables the processes to be executed aseptically in isolator-type platforms that are separated by sealed housing from the outer environment [21], resulting in a significantly reduced contamination risk. However, automation in the field of cell therapy manufacturing faces several challenges [22]. Firstly, most biological production and analysis processes contain steps, materials, or technologies that are difficult to execute by robots. Thus, new devices and solutions for these handling steps need to be developed and tested. Secondly, to design an automated manufacturing suite, the process parameters and settings (e.g., temperatures, forces, times quantities) must be very well defined. This includes a thorough and validated set of standard operating procedures (SOPs), which requires a high degree of overall process standardization. Thirdly, robotic handlers require defined geometries of disposables as it is challenging to design grippers suited to a high range of different geometries. Therefore, a low variety of disposables is beneficial for automation. Also, regulatory requirements and guidelines need to be considered, such as good manufacturing practice (GMP). Additionally, the best technologies for fast and reliable cell expansion have to be identified for the different process steps. The application of different manufacturing technologies, such as plate-based or bioreactor-based expansion of MSCs and their cost-effectiveness have been discussed extensively [17,23,24]. AutoCRAT aims to address these issues by developing an automated platform for production of cell therapies targeting OA. To address the heterogeneity [25,26,27] of OA cases, different therapy options will be produced on the same platform, as shown in Figure 1. Firstly, iPSC-derived iMSCs will be produced as a treatment option and as a source of iMSC-derived EVs, as a second treatment option. iCHOs will be produced as a third option. Expansion of iPSCs and differentiation to iMSCs and chondrocytes is carried out in plates, while iMSC expansion and EV production is carried out using microcarriers in stirred tank bioreactors. Establishing these procedures, differing in scale, from plate-based to bioreactor-based expansion, and biochemical needs, different media, and procedures for each cell type, requires a sophisticated automated platform with different interconnecting modules.
Ideally, a fully automated platform for manufacturing of allogeneic iPSCs, iMSCs, iMSC-derived EVs, and iCHOs should be one system interconnected with robotics and other automated handlers to avoid any hands-on time during the manufacturing campaign. However, to allow for widespread use of automation technology in the field, the adaptability to address evolving processes and product design has to be demonstrated. To show the adaptability of automation technology as well as address the challenges described above, two existing systems, the StemCellDiscovery [28] and the Autostem [29] platform were combined and expanded upon to form the AutoCRAT system. To allow for automated manufacturing of iPSCs, iCHOs and iMSC-derived EVs on existing systems, new processes have to be implemented. These processes include: QC assays for Endotoxin and Mycoplasma content, fingerprinting and Glucose (Glc)/Lactate (Lac) determination, cultivation and maintenance of iPSCs, differentiation of iPSCs to iMSCs and iCHOs, cultivation and maintenance of iCHOs, and expansion of iMSCs for EV manufacturing. These tasks will be divided among the platforms: the StemCellDiscovery, developed for high-throughput plate-based cultivation and screening of iMSCs, will be used for iPSC cultivation and maintenance, differentiation to iMSCs and iCHOs as well as QC assays thus integrating missing steps in the manufacturing process and increasing the analytical capability of the automated systems. The Autostem platform, developed for large-scale expansion and fill-and-finish of MSCs, will be used for iMSC and iEV production as well as EV manufacturing and Glc/Lac measurement during expansion [21]. Accordingly, several changes were made to both systems. The StemCellDiscovery was equipped with new analytical technology to increase process understanding and facilitate product release, as well as the means to process and store reagents and materials required for QC, cultivation, and maintenance processes. Autostem was also adapted to fit a third bioreactor for increased capacity of expansion and an option for EV manufacturing in a packed-bed bioreactor. Additionally, an EV module was developed to allow for automated purification of EVs.
3.1. StemCellDiscovery
As mentioned above, the StemCellDiscovery is originally a platform for the automated cultivation of iMSCs. It consists of an automated incubator, a liquid-handling unit (LHU) to enable a range of pipetting steps from 0.5 to 5 mL, a centrifuge, a microscope, a decapper to open and close 50 mL falcon tubes, an automated fridge to store these tubes at 4 °C, reservoirs for materials, and a centrally positioned robot for transporting cells and materials [28]. In AutoCRAT, the StemcellDiscovery is to be used for analytics, to automate differentiation of iPSCs to iMSCs and iCHOs, and to act as a seed train for the Autostem. To adapt the hardware to the new demands for automation a wide range of reagents has to be stored on the platform. To avoid unnecessary complexity when designing a platform, the variety in shape of used disposables in an automated platform is critical for its mechanical design. The StemCellDiscovery was originally designed to only handle 50 mL falcon tubes and micro-titer plates. For the different cultivation and quality control processes of iPSCs, iMSCs, and iCHOs, 5 mL and 2 mL vials are also required. The platform was enhanced by establishing storing positions and designing decapping devices for these vials. As some of these vials will need to be stored at a temperature of −20 °C, a new automatable freezer was developed and integrated into the control system.
The quality control processes required further changes and additions to the platform. Firstly, the cell products need to be analyzed for endotoxins. This is performed by using an endotoxin test, which is integrated at a position where it can be reached by both the liquid handling unit as well as the six-axis robotic arm. Additionally, the endotoxin tester uses specific cartridges to determine endotoxin content in a sample. These cartridges required additional storage positions and a modification of the robot grippers to transport them. Secondly, the cells will be tested for mycoplasma using a quantitative polymerase chain reaction (qPCR). Mycoplasma (gram-negative bacteria) can infect cells but must be absent in any therapeutic cell product. Some mycoplasma species are human pathogens and are a serious regulatory concern in medicinal products [30]. The mycoplasma assay requires PCR, for which the qTOWER device³ was chosen. This novel product was designed specifically for robotic access and remote control. Additionally, an automated plate sealer was also integrated (ALPS 3000, Thermo Fisher Scientific, Waltham, MA, USA). Thirdly, a cell analyzing process from the AutoCRAT partner ValitaCell was implemented: the ChemStress fingerprinting assay [31], which is used to characterize iMSCs. This process required replacing the existing plate reader (Infinite M200 Pro, Tecan, Männedorf, Switzerland) with the plate reader Spark® Cyto, which provides additional functionality for microscopic and spectroscopic cell analyses. This assay also requires high throughput pipetting at low volumes, thus the LHU was equipped with an additional 96-channel 1 mL pipetting head.
3.2. Autostem
Originally designed for the high-scale cultivation of MSCs, the Autostem platform is equipped with two stirred tank bioreactors. A pump station provides media changes, sampling and addition of reagents, in a range from 5 mL to several liters at a time. Secondly, the cells can be harvested and transferred for cryopreservation. Autostem consists of two areas with different cleanroom grades, a cleanroom GMP-grade A area (ISO 5) for open cell handling for filling of cells and a cleanroom grade D (ISO 8) area for expansion of cells in a closed bioreactor system [29,32,33]. Modification of the Autostem platform facilitates production of EVs, which are secreted by iMSCs and can be isolated from conditioned media. To this end, the bioreactor system BioFlo® 320 was added onto the platform. This system provides the option of cultivating cells in a packed-bed bioreactor for optimization of EV secretion [34]. To place the reactor and the reactor controller on the platform, the bioreactor area of the Autostem platform was redesigned and the new bioreactor integrated to fluid supply circuits. Both requirements were addressed by changing the pumping system of the platform. In its first design, the Autostem had a central pump station controlling all pumps and valves to feed media to the reactors, remove fluid waste, and harvest cells after cultivation. To decrease the footprint as well as dead volumes due to the long tubes, a decentralized concept for pumping was implemented. Eight pumps and fourteen valves were installed near the bioreactors or the stations they supply and thus could be placed onto formerly unused spaces between the devices. Aside from enhancing the platform for EV production, adding a third bioreactor to the system potentially allows for a 30% increase in cell manufacturing.
Furthermore, some improvements are implemented on the Autostem platform. Firstly, the hatch connecting the two areas with different cleanroom grades is enhanced by a ventilation system to prohibit air from the grade D area entering the grade A compartment and increasing the GMP compatibility of the platform. Secondly, the fill-and-finish process is improved by implementing the cooling of tubes and vials during filling while the cells are in contact with Dimethylsulfoxide (DMSO), which improves viability of the cells.
3.3. EV Module
EV isolation will be performed on a new platform specifically designed for this process. The EV module automatically purifies the EVs that are contained in the iMSC supernatant. This newly developed system initially required a frame to support all devices of the platform. The main device of the EV module is the FPLC (ÄKTA pure 150 M, Cytiva, Marlborough, MA, USA), that performs the automated purification process with supernatant from adjacent cell expansion vessels and sorts the purified solutions via a fraction collector into separated tubes. The FPLC on the EV module is a device for full automated processing by itself. Once connected to the cell supernatant and all relevant reagents and liquids, it performs all purification steps based on a pre-defined process program. The AutoCRAT partner, University Clinic Essen, has researched the efficacy of EVs [35,36] as well as evaluated scalable production processes [37], which subsequently were installed on the EV module. However, to be integrated into the automated AutoCRAT processing, it was extended by an additional pump to insert the supernatant into the FLPC. Furthermore, to remove products from the fraction collector without the risk of contamination, an isolation housing system was built around the FPLC fraction collector, which enables exchanging and filtering the air inside, before any product containing vials and flasks are opened.
The three systems are shown in Figure 2 with innovations highlighted in green. Interaction between these three separate systems was manual for the duration of the project. However, this approach allowed for the utilisation of existing technology to its fullest extent and combining it with new approaches to advance production technology for automated cell therapy manufacturing. In a constantly changing field of the process and product landscape, this is immensely important for development of automation technology to be sustainable.
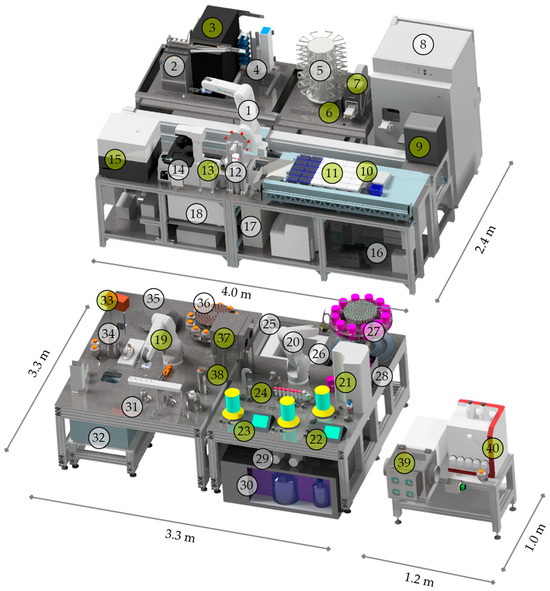
Figure 2.
Visual representation of the AutoCRAT platforms with dimensions: StemCellDiscovery with (1) robot, (2) cooled tube storage at 4 °C, (3) freezer tube storage at −20 °C, (4) plate storage, (5) pipette tip storage, (6) endotoxin tester cassette storage, (7) plate sealer, (8) incubator, (9) PCR, (10) endotoxin tester, (11) liquid handling unit, (12) decapper for 50 mL tubes, (13) decapper for 5 mL and 1 mL tubes, (14) high-speed microscope, (15) plate reader, (16) liquid waste, (17) solid waste, (18) centrifuge; Autostem with (19) and (20) robots, (21) Eppendorf bioreactor, (22) and (23) Applikon Bioreactors, (24) sampling station for cell counting, (25) freezer storage at −80 °C, (26) solid waste, (27) Nucleocounter cassette, cool container and 1 mL tube storage, (28) Nucleocounter, (29) preheaters, (30) cooled media and liquid waste storage at 4 °C, (31) decapper for centrifuge bottles, 5 mL and 1 mL tubes, (32) centrifuge, (33) pipette, (34) solid waste, (35) pipette tip storage, (36) centrifuge bottle and 5 mL tube storage, (37) hatch, (38) fluid transfer device; EV-module with (39) fraction collector and isolator, (40) fast protein liquid chromatography. Stations to be changed are highlighted in green.
3.4. Software
To enable end-to-end automation, the AutoCRAT platform is equipped with an integral software that assures automatic monitoring, execution, and documentation of all processes. The control software used, COPE, was developed at Fraunhofer IPT and has been successfully applied in several research projects for automated biotechnological platform development [38,39]. COPE enables flexible, service-oriented automation with every device equipped with a so-called COPE.agent, which functions as a driver for standardized communication to the centralized control unit. Due to the lack of standard interfaces for automatable devices in biotechnology [40], standardized communication is a key challenge for automation. In each COPE.agent, the capabilities of every device are modeled as independent services. With a drag-and-drop process creator these services can be easily combined in the desired process sequence. When COPE.agents are changed or added, COPE can reload them even during runtime. Disposable materials are logged in COPE with their properties (i.e., cell type, reagent type, volume) with a barcode for unique identification and tracked throughout the process. Apart from process related services, each station offers a service for error recovery. This allows pausing a process chain, resetting and then continuing the process.
Additionally, COPE can schedule different process sequences, so that they can be executed simultaneously with the software then computing the best timing and usage of devices to execute all processes as efficiently as possible. Furthermore, COPE monitors all materials and products on the three platforms including their status and position on the platform, corresponding measurements, and analysis values [41,42]. For the AutoCRAT project, two COPE instances are connected to allow for inter-platform operability between the Autostem and StemcellDiscovery and a number of new drivers were developed for the newly integrated devices. One aim of AutoCRAT is to enhance user-management, data storage, data export and code structure of all COPE programs to reach full good automated manufacturing practice (GAMP) compliancy. This also includes risk analysis to avoid failure.
3.5. Process Implementation
Aside from the physical and digital integration of all devices needed, translating processes from benchtop to automation comes with a unique list of challenges. Human operators are highly trained to perform the cell culture and quality processes while still being very flexible in their range of movements. When implementing automated processes, details that are routinely assessed by human operators such as resuspension of cells, mixing and gentle handling of cells, need to be defined and optimized for each step of the process to ensure its overall robustness and reliability. This includes the teaching of all robotic positions and paths. In AutoCRAT, this translation is achieved through cooperation with project partners with differing yet overlapping fields of expertise. Cell biology, biotechnology, medical and mechanical engineering as well as software development work on understanding, abstracting, and finally translating manual operation into a minutely defined automated process.
4. Discussion
The widespread commercial and clinical success of ATMPs such as iPSC derived cell OA treatments is greatly impeded by the lack of process understanding, existing production technology and end-to-end automation. When developing production technology, existing technology should not be overlooked but improved upon and incorporated. To the best of our knowledge, AutoCRAT is the first platform for fully automated cultivation of iPSCs, their differentiation to iMSCs and iCHOs and purification of cell-derived EVs, as well as the corresponding quality controls. To realize the automation, the already existing platforms StemCellDiscovery and Autostem were modified and enhanced. A new platform, the EV module, which performs production steps that cannot be added onto the other platforms, was developed. The need for manual labor to produce iPSCs, iMSCs, iCHOs, and EVs when using the automated platforms presented in this paper is far lower compared to fully manual production, which results in a high cost reduction. This is supported by Nießing et al. [43], who performed a thorough economic analysis with the StemCellFactory platform, which also produces iPSCs and has a similar design to the StemCellDiscovery [44]. It can be assumed that their results are transferrable to the AutoCRAT platforms. In total, the AutoCRAT system would be able to handle 95 plates simultaneously as well as three individual expansion processes at a time [28].
The transfer of cells and supernatant between the platforms is manual, due to space constraints in our current lab. Automation of these transfers is feasible, e.g., by using conveyor belts. However, in a subsequent version amenable for widespread use, all processes will be executed on one single system. Therefore, solutions for automated sterile transfer were not developed.
The AutoCRAT system shows how liquid handling tasks can be compartmentalized to achieve automated liquid handling across several orders of magnitude from 0.5 µL to 5 mL in a commercially available liquid handling unit in the StemCellDiscovery, through 5–50 mL using a custom automated serological pipette and a fluidic automation approach to achieve liquid handling, to 5 mL to 2 L at a time using a pump and valve system in the Autostem. Using these different approaches, automated liquid handling can cover the full extent of liquid handling tasks of a typical manual laboratory. This allows for highly integrated and parallel bioprocess management in the AutoCRAT platforms. Complex workflows for cell expansion, differentiation and quality control can be carried out in different vessels for different cell types at the same time. This would allow the platform operator a high degree of flexibility during manufacturing and release, while maintaining a high degree of standardization and robustness.
The key to long-term success of the application of such automated solutions is their ability to be adapted and expanded upon. Our approach in developing and implementing modular automated platforms with individual stations for different tasks, which are dynamically interconnected using robotic actuators as well as fluidic connectors, has proven to be a key approach for this challenge [28,29]. The AutoCRAT project also demonstrates how previous equipment and software can be adapted to new non-linear bioprocesses and product requirements with minimal effort. Additionally, the mechanical design of the platforms as well as the program structure and process definition tool of the control software COPE allow further updates of and changes to automated production.
One of the bottlenecks in iPSC-derived therapies for OA is the time-consuming and complex release testing. In AutoCRAT we are approached this by integrating endotoxin and mycoplasma testing into the manufacturing system to allow for testing in parallel with the manufacturing process. This is one step towards accelerated release but will require careful sample preparation and user management to keep manufacturing and quality control separate and prohibit any interference with the manufacturing process.
Beyond manufacturing, decontamination and cleaning have a huge impact on product quality and safety. The Autostem and StemcellDiscovery have a housing equipped with HEPA filters ensuring a laminar, filtered air flow throughout. However, initial cleaning processes are currently mostly performed manually. Due to its high impact on product safety, this step should be automated to avoid the risk of cleaning personnel contaminating the areas they aim to clean. Furthermore, in other processes in which viruses or other dangerous biological substances are used, cleaning personnel could be compromised by residues of these substances. In AutoCRAT automated wipe disinfection is explored as an option for mechanical cleaning of the platform and the automation of measurement of residual particles and contaminants is integrated to monitor this process.
Lastly, the applicability of the AutoCRAT products is not limited to OA therapies. MSC derived EVs have also shown to treat steroid-refractory acute graft-versus-host disease (acute GvHD) as well chronic kidney disease (CDK) [11]. Furthermore, studies indicate that iMSCs could show similar immunomodulatory abilities to MSCs [45].
5. Conclusions
The AutoCRAT system encompasses an end-to-end manufacturing process for iPSC-derived iMSCs, iCHO and their respective EVs and will facilitate the automated manufacturing of iPSCs, iMSCs, iCHOs, and EVs at scale. It also integrates analytical techniques, such as microscopy, cell count and viability measurement, qPCR, endotoxin assays, and the Chemstress Fingerprinting assay for continued quality control of all cell types and EVs. It demonstrates how existing automation technology can be adapted and advanced upon to be utilized to produce new therapeutic products.
The combined capacity of all modules will result in a comprehensive approach for advanced automated manufacturing of iPSC-derived therapies for widespread pain relief among patients.
Author Contributions
Conceptualization, L.H., writing—original draft preparation, L.H. and F.G.; writing—review and editing, L.H., F.G. and G.S.; visualization, F.G. and L.H.; supervision, B.N.; project administration, M.M.; funding acquisition, R.H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 874671. The materials presented and views expressed here are the responsibility of the author(s) only. The EU Commission takes no responsibility for any use made of the information set out.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Burns, L.C.; Ritvo, S.E.; Ferguson, M.K.; Clarke, H.; Seltzer, Z.; Katz, J. Pain catastrophizing as a risk factor for chronic pain after total knee arthroplasty: A systematic review. J. Pain Res. 2015, 8, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Yuan, S.; Zeng, Y.; Wang, C.; Yu, N.; Ding, C. New Trends in Pharmacological Treatments for Osteoarthritis. Front. Pharmacol. 2021, 12, 645842. [Google Scholar] [CrossRef]
- Pers, Y.-M.; Rackwitz, L.; Ferreira, R.; Pullig, O.; Delfour, C.; Barry, F.; Sensebe, L.; Casteilla, L.; Fleury, S.; Bourin, P.; et al. Adipose Mesenchymal Stromal Cell-Based Therapy for Severe Osteoarthritis of the Knee: A Phase I Dose-Escalation Trial. Stem Cells Transl. Med. 2016, 5, 847–856. [Google Scholar] [CrossRef]
- Lee, W.-S.; Kim, H.J.; Kim, K.-I.; Kim, G.B.; Jin, W. Intra-Articular Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Phase IIb, Randomized, Placebo-Controlled Clinical Trial. Stem Cells Transl. Med. 2019, 8, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Freitag, J.; Bates, D.; Wickham, J.; Shah, K.; Huguenin, L.; Tenen, A.; Paterson, K.; Boyd, R. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: A randomized controlled trial. Regen. Med. 2019, 14, 213–230. [Google Scholar] [CrossRef]
- Choudhery, M.S.; Mahmood, R.; Harris, D.T.; Ahmad, F.J. Minimum criteria for defining induced mesenchymal stem cells. Cell Biol. Int. 2022, 46, 986–989. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Sabapathy, V.; Kumar, S. hiPSC-derived iMSCs: NextGen MSCs as an advanced therapeutically active cell resource for regenerative medicine. J. Cell. Mol. Med. 2016, 20, 1571–1588. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, D.; Liu, Z.; Zhou, F.; Dai, J.; Wu, B.; Zhou, J.; Heng, B.C.; Zou, X.H.; Ouyang, H.; et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res. Ther. 2017, 8, 189. [Google Scholar] [CrossRef]
- Giebel, B.; Kordelas, L.; Börger, V. Clinical potential of mesenchymal stem/stromal cell-derived extracellular vesicles. Stem Cell Investig. 2017, 4, 84. [Google Scholar] [CrossRef]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef]
- Li, C.; Zhao, H.; Cheng, L.; Wang, B. Allogeneic vs. autologous mesenchymal stem/stromal cells in their medication practice. Cell Biosci. 2021, 11, 187. [Google Scholar] [CrossRef] [PubMed]
- Simaria, A.S.; Hassan, S.; Varadaraju, H.; Rowley, J.; Warren, K.; Vanek, P.; Farid, S.S. Allogeneic cell therapy bioprocess economics and optimization: Single-use cell expansion technologies. Biotechnol. Bioeng. 2014, 111, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Lipsitz, Y.Y.; Milligan, W.D.; Fitzpatrick, I.; Stalmeijer, E.; Farid, S.S.; Tan, K.Y.; Smith, D.; Perry, R.; Carmen, J.; Chen, A.; et al. A roadmap for cost-of-goods planning to guide economic production of cell therapy products. Cytotherapy 2017, 19, 1383–1391. [Google Scholar] [CrossRef]
- Malik, N.N.; Durdy, M.B. Chapter 7—Cell Therapy Landscape: Autologous and Allogeneic Approaches. In Translational Regenerative Medicine; Atala, A., Allickson, J.G., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 87–106. ISBN 978-0-12-410396-2. [Google Scholar]
- Jossen, V.; van den Bos, C.; Eibl, R.; Eibl, D. Manufacturing human mesenchymal stem cells at clinical scale: Process and regulatory challenges. Appl. Microbiol. Biotechnol. 2018, 102, 3981–3994. [Google Scholar] [CrossRef]
- Li, H.; Ghazanfari, R.; Zacharaki, D.; Lim, H.C.; Scheding, S. Isolation and characterization of primary bone marrow mesenchymal stromal cells. Ann. N. Y. Acad. Sci. 2016, 1370, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.T.; Reuveny, S.; Oh, S.K.-W. Human mesenchymal stem cell therapy for cartilage repair: Review on isolation, expansion, and constructs. Stem Cell Res. 2020, 44, 101738. [Google Scholar] [CrossRef]
- Jung, Y.; Bauer, G.; Nolta, J.A. Concise review: Induced pluripotent stem cell-derived mesenchymal stem cells: Progress toward safe clinical products. Stem Cells 2012, 30, 42–47. [Google Scholar] [CrossRef]
- Ochs, J.; Barry, F.; Schmitt, R.; Murphy, J.M. Advances in automation for the production of clinical-grade mesenchymal stromal cells: The AUTOSTEM robotic platform. Cell Gene Ther. Insights 2017, 3, 739–748. [Google Scholar] [CrossRef]
- Madrid, M.; Sumen, C.; Aivio, S.; Saklayen, N. Autologous induced pluripotent stem cell–based cell therapies: Promise, progress, and challenges. Current Protocols 2021, 1, e88. [Google Scholar] [CrossRef] [PubMed]
- Panchalingam, K.M.; Jung, S.; Rosenberg, L.; Behie, L.A. Bioprocessing strategies for the large-scale production of human mesenchymal stem cells: A review. Stem Cell Res. Ther. 2015, 6, 225. [Google Scholar] [CrossRef] [PubMed]
- Cherian, D.S.; Bhuvan, T.; Meagher, L.; Heng, T.S.P. Biological Considerations in Scaling up Therapeutic Cell Manufacturing. Front. Pharmacol. 2020, 11, 654. [Google Scholar] [CrossRef]
- Jørgensen, A.; Stengaard-Pedersen, K.; Simonsen, O.; Pfeiffer-Jensen, M.; Eriksen, C.; Bliddal, H.; Pedersen, N.W.; Bødtker, S.; Hørslev-Petersen, K.; Snerum, L.Ø.; et al. Intra-articular hyaluronan is without clinical effect in knee osteoarthritis: A multicentre, randomised, placebo-controlled, double-blind study of 337 patients followed for 1 year. Ann. Rheum. Dis. 2010, 69, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Beswick, A.D.; Wylde, V.; Gooberman-Hill, R.; Blom, A.; Dieppe, P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open 2012, 2, e000435. [Google Scholar] [CrossRef]
- Bjordal, J.M.; Ljunggren, A.E.; Klovning, A.; Slørdal, L. Non-steroidal anti-inflammatory drugs, including cyclo-oxygenase-2 inhibitors, in osteoarthritic knee pain: Meta-analysis of randomised placebo controlled trials. BMJ 2004, 329, 1317. [Google Scholar] [CrossRef]
- Ochs, J.; Biermann, F.; Piotrowski, T.; Erkens, F.; Nießing, B.; Herbst, L.; König, N.; Schmitt, R.H. Fully Automated Cultivation of Adipose-Derived Stem Cells in the StemCellDiscovery—A Robotic Laboratory for Small-Scale, High-Throughput Cell Production Including Deep Learning-Based Confluence Estimation. Processes 2021, 9, 575. [Google Scholar] [CrossRef]
- Ochs, J.; Hanga, M.P.; Shaw, G.; Duffy, N.; Kulik, M.; Tissin, N.; Reibert, D.; Biermann, F.; Moutsatsou, P.; Ratnayake, S.; et al. Needle to needle robot-assisted manufacture of cell therapy products. Bioeng. Transl. Med. 2022, 7, e10387. [Google Scholar] [CrossRef]
- Sugita, S.; Hono, A.; Fujino, S.; Futatsugi, Y.; Yunomae, Y.; Shimizu, N.; Takahashi, M. Detection of Mycoplasma Contamination in Transplanted Retinal Cells by Rapid and Sensitive Polymerase Chain Reaction Test. Int. J. Mol. Sci. 2021, 22, 12555. [Google Scholar] [CrossRef]
- Szaraz, P.; Mander, P.; Davies, S.; Gasner, A.; Clifford, J.; Librach, C. Multiplex Functional Testing of Bioreactor-Upscaled First Trimester Human Umbilical Cord Perivascular Cells (FTM HUCPVC) and Bone Marrow-Derived Mesencymal Stem Cells (BMSC) using the chemstress® fingerprinting assay, reveals hidden differences between cell therapy candidates. Cytotherapy 2020, 22, S159–S160. [Google Scholar] [CrossRef]
- ISO 14644-1:2015; NA 041-02-21 AA—Reinraumtechnik. Reinräume und Zugehörige Reinraumbereiche—Teil 1: Klassifizierung der Luftreinheit Anhand der Partikelkonzentration. Beuth: Berlin, Germany, 2016.
- European Commission. The Rules Governing Medicinal Products in the European Union Volume 4 EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use: Annex 1: Manufacture of Sterile Medicinial Products, C. In EudraLex; European Commission: Brussels, Belgium, 2022. [Google Scholar]
- Patel, D.B.; Luthers, C.R.; Lerman, M.J.; Fisher, J.P.; Jay, S.M. Enhanced extracellular vesicle production and ethanol-mediated vascularization bioactivity via a 3D-printed scaffold-perfusion bioreactor system. Acta Biomater. 2019, 95, 236–244. [Google Scholar] [CrossRef]
- Kordelas, L.; Schwich, E.; Dittrich, R.; Horn, P.A.; Beelen, D.W.; Börger, V.; Giebel, B.; Rebmann, V. Individual Immune-Modulatory Capabilities of MSC-Derived Extracellular Vesicle (EV) Preparations and Recipient-Dependent Responsiveness. Int. J. Mol. Sci. 2019, 20, 1642. [Google Scholar] [CrossRef]
- Witwer, K.W.; van Balkom, B.W.M.; Bruno, S.; Choo, A.; Dominici, M.; Gimona, M.; Hill, A.F.; de Kleijn, D.; Koh, M.; Lai, R.C.; et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J. Extracell. Vesicles 2019, 8, 1609206. [Google Scholar] [CrossRef]
- Staubach, S.; Bauer, F.N.; Tertel, T.; Börger, V.; Stambouli, O.; Salzig, D.; Giebel, B. Scaled preparation of extracellular vesicles from conditioned media. Adv. Drug Deliv. Rev. 2021, 177, 113940. [Google Scholar] [CrossRef]
- Jung, S.; Grunert, D.; Schmitt, R. Service-oriented Communication and Control System Architecture for Dynamically Interconnected Assembly Systems. In Tagungsband des 3. Kongresses Montage Handhabung Industrieroboter, [1. Auflage]; Schüppstuhl, T., Tracht, K., Franke, J., Eds.; Springer Vieweg: Berlin, Germany, 2018; pp. 223–229. ISBN 978-3-662-56713-5. [Google Scholar]
- Schüppstuhl, T.; Tracht, K.; Franke, J. (Eds.) Tagungsband des 3. Kongresses Montage Handhabung Industrieroboter; [1. Auflage]; Springer Vieweg: Berlin, Germany, 2018; ISBN 978-3-662-56713-5. [Google Scholar]
- Biermann, F.; Mathews, J.; Nießing, B.; König, N.; Schmitt, R.H. Automating Laboratory Processes by Connecting Biotech and Robotic Devices—An Overview of the Current Challenges, Existing Solutions and Ongoing Developments. Processes 2021, 9, 966. [Google Scholar] [CrossRef]
- Egri, P.; Csáji, B.C.; Kis, K.B.; Monostori, L.; Váncza, J.; Ochs, J.; Jung, S.; König, N.; Schmitt, R.; Brecher, C.; et al. Bio-inspired control of automated stem cell production. Procedia CIRP 2020, 88, 600–605. [Google Scholar] [CrossRef]
- Doulgkeroglou, M.-N.; Di Nubila, A.; Niessing, B.; König, N.; Schmitt, R.H.; Damen, J.; Szilvassy, S.J.; Chang, W.; Csontos, L.; Louis, S.; et al. Automation, Monitoring, and Standardization of Cell Product Manufacturing. Front. Bioeng. Biotechnol. 2020, 8, 811. [Google Scholar] [CrossRef] [PubMed]
- Nießing, B.; Kiesel, R.; Herbst, L.; Schmitt, R.H. Techno-Economic Analysis of Automated iPSC Production. Processes 2021, 9, 240. [Google Scholar] [CrossRef]
- Elanzew, A.; Nießing, B.; Langendoerfer, D.; Rippel, O.; Piotrowski, T.; Schenk, F.; Kulik, M.; Peitz, M.; Breitkreuz, Y.; Jung, S.; et al. The StemCellFactory: A Modular System Integration for Automated Generation and Expansion of Human Induced Pluripotent Stem Cells. Front. Bioeng. Biotechnol. 2020, 8, 580352. [Google Scholar] [CrossRef]
- Ng, J.; Hynes, K.; White, G.; Sivanathan, K.N.; Vandyke, K.; Bartold, P.M.; Gronthos, S. Immunomodulatory Properties of Induced Pluripotent Stem Cell-Derived Mesenchymal Cells. J. Cell. Biochem. 2016, 117, 2844–2853. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).