Abstract
Recently, many studies have reported the properties and functionality of okra pectin. However, these studies are about green okra pods, and pectin in red okra pods, stems, and roots has not been reported. Therefore, this study aimed to optimize the red okra extraction method using response surface methodology (RSM) analysis and evaluate the effects of extraction time, temperature, solvent ratio, and pH on the extract yield and crude pectin content. Based on RSM analysis, 4.35 h, 98.04 °C, 23.34 solvent ratio, and pH 3.36 are the optimal parameters for extracting crude pectin from red okra, and the crude pectin content was predicted to be 40.83%. When red okra was extracted under these extraction conditions (4 h, 100 °C, 23 solvent ratio, and pH 3), the extraction yield was 45.26%, and the crude pectin content was 38.42%, which was similar to the yield obtained under the conditions derived from the RSM analysis. In addition, the pH control using hydrochloric acid was replaced with citric acid, and the changes in extract yield and crude pectin content were compared. When citric acid was used, the extract yield was 49.15% (8.6% increase), and the crude pectin content was 42.76 ± 2.56% (11.3% increase); compared to when hydrochloric acid was used, the yield increased. Finally, the standardization of red okra raw materials was determined by analyzing the extraction yield and crude pectin content by part, harvest time, and size of red okra using the established extraction method using citric acid. As a result, it was confirmed that the extraction yield obtained from the established extraction method was reached from the extraction of red okra fruits up to 12 cm in size that were harvested between July and November. Additionally, compared to the non-pectin fraction, the crude pectin fraction isolated from red okra pod extract showed significantly higher total phenolic content (TPC) and total flavonoid content (TFC). These findings, reported for the first time, may contribute to the development of processes to purify red okra pectin, functional evaluation studies of pectin, and potential applications of red okra extract in various industries and research.
1. Introduction
Okra (Abelmoschus esculentus (L.) Moench) is a flowering plant in the Malvaceae family and an integral part of diets in several African and Indian countries. Okra is now cultivated in many parts of the world, including Asia, the Middle East, and the southern United States [1,2,3,4]. Okra, which is also known as lady’s finger, bamyah, bamieh, kacang, gumbo, dharos, bhindi, bendi, or bamia in different regions, is traditionally used for medicinal purposes [2,5,6]. Okra has been used to treat various diseases, such as constipation, leukemia, diabetes, and jaundice [7]. Okra pods are rich in phenolic compounds and consist of hydroxycinnamic and quercetin derivatives [8]. In addition to having therapeutic uses, okra pods contain polyphenols and flavonoids, including quercetin, which exhibits powerful antioxidant activity. Interestingly, red okra contains higher levels of anthocyanins and quercetin than green okra [9]. The thick and slimy texture of okra water extracts is attributed to its polysaccharide content [10,11]. This thick mucilage in okra has been used as an emollient to relieve symptoms of diarrhea, dysentery, and gastric ulcers [7,12]. Recently, studies on antioxidant, antidiabetic, anti-inflammatory, wound healing, and Alzheimer’s prevention effects have been conducted [13]. Additionally, the mucilage of okra water extract has been reported to be a natural food emulsifier, thickener, and emulsion stabilizer [14,15,16,17]. The special mucilage in the cell wall material of okra is rich in pectin but not well used for pectin extraction in the food industry [18]. To date, the commercial sources of pectin are apple pulp (15–18%) and citrus peel (20–30%) [19,20,21].
Pectin is a multipurpose heteropolysaccharide of great importance in the food, pharmaceutical, and cosmetic industries. It is a complex polysaccharide mainly composed of α-1,4-D-galacturonic acid (Gal A) [16]. The main pectin domains recognized in plants are homogalacturonan (HG), rhamnogalacturonan I (RGI), rhamnogalacturonan II (RGII), xylogalacturonan (XGA), and apiogalacturonan (AGA). HG exclusively contains Gal A units, in which some carboxyl groups are esterified with methanol and/or acetyl groups. RGI consists of repeating residues of Gal A and α-1,2-L-rhamnose. RGII is composed of a backbone that consists of Gal A residues (the same as HG), but the backbone is highly branched, with side chains at C-2 and C-3. Additionally, xylogalacturonan (XGA) and apiogalacturonan (AGA) are often considered pectins because they contain the same backbone as HG [22]. The main structural component of green okra pectin polysaccharide also contains alternating units of rhamnose and galacturonic acid residues (RGI), and the disaccharide side chain consists of galactose attached to the O-4 of half of the lactose residues [23]. However, there is no report on the properties of red-okra-derived pectin.
The chemical and structural properties of pectin mainly depend on the following factors: the plant species [24], the ripening/biological stage [25], and the extraction method [26,27,28]. The extraction processes play an important role in generating high yields of bioactive compounds and maintaining quality. Therefore, optimizing the extraction method is essential for maximizing the yield and preserving the desired properties of the extracted compounds. Various factors, such as extraction time, temperature, solvent ratio, and pH, can affect the efficiency of the extraction process. Response surface methodology (RSM) is a statistical technique used to simultaneously optimize multiple factors by modeling the relationship between a variable and the response variable of interest. By using this approach, the optimal conditions resulting in the highest yield and quality of extracted bioactive compounds can be determined. In recent years, several studies have focused on methods to optimize various plant materials and improve the yield and quality of bioactive compounds [29,30,31]. Therefore, in this study, the red okra extraction method was optimized using RSM and by examining the effects of extraction time, temperature, solvent ratio, and pH on the extraction yield and crude pectin content. In addition, after the possibility of using alternative natural products was confirmed and an extraction method was established, the suitability of red okra raw materials for pectin extraction was identified. By establishing an optimal method for extracting red okra, this study helps improve the manufacturing process by increasing the yield and quality. In addition, the results may broaden the scope of applications to various industries. This could provide new opportunities for incorporating red okra into nutraceutical and cosmetic formulations.
2. Material and Methods
2.1. Material
Red okra seeds (Candle Fire hybrid F1 seed, non-GMO) and green okra seeds (Ever-Lucky F1 seed, non-GMO) were obtained from KNOWN-YOU SEED Co., Ltd. (Kaohsiung, Taiwan). Red okra was directly cultivated in Gangjin-gun, Jeollanamdo, Republic of Korea (34°35′20.4″ N, 126°48′04.2″ E), in 2022. The cultivation area was 800 m2, the plants were sown in June, and all red okra pods were harvested once a week from July to December. The total yield of red okra pods in an area of 800 m2 was measured monthly, as shown in Table 1. Green okra used as a control was grown in an area of 2 m2 and used in this study. The harvested red and green okra pods, stems, and roots were washed, cut into pieces of approximately 1 cm in size, and then dried for 30 h in hot air at 55 °C (DY-280H, Daeyoung E&B, Ansan, Republic of Korea). The dry yield of red okra pods was 9.80 ± 0.08%. After drying, the pods were pulverized into powder using a multipurpose grinder (DSCH-370, Deoksan Co., Ltd., Siheung-si, Republic of Korea) and stored at room temperature.

Table 1.
Harvest yield of red okra by season.
2.2. Extraction of Red Okra
The extraction time (Ex time, h), temperature (Temp, °C), solvent ratio (v/w ratio), and pH of 100 g of red okra powder were variously set to 19 conditions, as shown in Table 2. After extraction was performed under each condition, the mixture was filtered through a nonwoven fabric. After the filtered extract was centrifuged (8000 rpm, 10 min), the supernatant was taken. It was concentrated in a vacuum concentrator at 70 °C, and the concentrated extract was freeze-dried for 72 h. The overall extraction process is shown in Figure 1. The solvent used for extraction was distilled water. The pH of the extraction solvent was adjusted using hydrochloric acid (HCl). The yield of red okra extract was determined as follows [16,21]:
Here, M0 is the mass (g) of the red okra extract after drying, and M is the mass (g) of the red okra raw material.

Table 2.
Set of variable conditions for optimal process analysis of red okra pods through RSM analysis.
Table 2.
Set of variable conditions for optimal process analysis of red okra pods through RSM analysis.
| No. | Test Condition Group | Ex Time (h) | Temp (°C) | v/w Ratio | pH |
|---|---|---|---|---|---|
| 1 | Extraction time variation test group | 1 | 70 | 20 | 4 |
| 2 | 3 | 70 | 20 | 4 | |
| 3 | 5 | 70 | 20 | 4 | |
| 4 | 7 | 70 | 20 | 4 | |
| 5 | Extraction temperature fluctuation test group | 4 | 40 | 20 | 4 |
| 6 | 4 | 60 | 20 | 4 | |
| 7 | 4 | 80 | 20 | 4 | |
| 8 | 4 | 100 | 20 | 4 | |
| 9 | Solvent amount fluctuation test group | 4 | 70 | 12 | 4 |
| 10 | 4 | 70 | 18 | 4 | |
| 11 | 4 | 70 | 24 | 4 | |
| 12 | 4 | 70 | 30 | 4 | |
| 13 | pH fluctuation test group | 4 | 70 | 20 | 1 |
| 14 | 4 | 70 | 20 | 2 | |
| 15 | 4 | 70 | 20 | 3 | |
| 16 | 4 | 70 | 20 | 4 | |
| 17 | 4 | 70 | 20 | 5 | |
| 18 | 4 | 70 | 20 | 6 | |
| 19 | 4 | 70 | 20 | 7 |
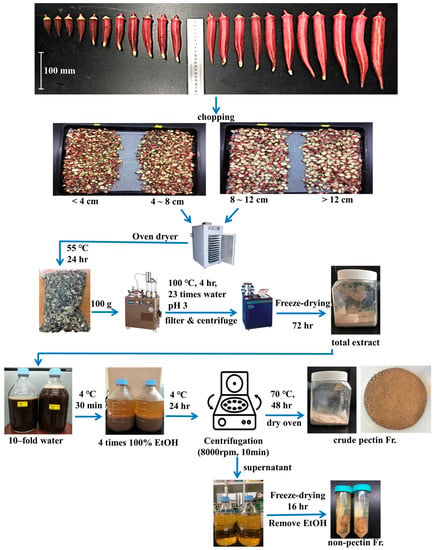
Figure 1.
Pretreatment process and extraction of crude pectin from red okra pods.
2.3. Extraction of Crude Pectin
The crude pectin content of red okra extract was analyzed by modifying the pectin extraction method of Lee et al. [32]. Tenfold distilled water was added to 1 g of the red okra water extract powder, and ultrasonic extraction (Power sonic 520, Hwashin Tech, Daegu, Republic of Korea, 700 W, 40 KHz) was performed for 1 h. After the solution was cooled in a 4 °C refrigerator for 30 min, 4 times the volume of ethanol (99.9%, Duksan, Ansan, Republic of Korea) cooled to 4 °C was added, shaken, and immersed at 4 °C for 24 h. After the precipitated crude pectin was centrifuged (8000 rpm, 10 min), the supernatant was discarded and dried in a 70 °C dry oven (HB-502S, Hanbaek Science Co., Bucheon, Republic of Korea) for 48 h to obtain crude pectin. The amount of crude pectin was determined as follows [16,21]:
where P0 is the mass (g) of dry red okra crude pectin, and P is the mass (g) of red okra extract.
2.4. Experimental Design
In this experiment, the extraction yield and crude pectin content were analyzed through RSM to optimize the crude pectin extraction conditions of red okra. Based on the principles of randomization, repetition, and blocking, four variables (extraction temperature (χ1), extraction time (χ2), solvent ratio (χ3), and pH (χ4)) were designed, and predicted values were derived mathematically. Independent variables (χi) were selected to be extraction time (1–7 h), temperature (40–100 °C), extraction solvent ratio (12–30 multiples), and pH (1–7). The variable (Yi) was used for regression analysis as extraction yield (%) and crude pectin content (%). The statistical analysis system (SAS) program (version 9.4, SAS Institute Inc., Cary, NC, USA) was used to confirm the canonical form by regression analysis. A rising ridge was generated in which the response value continuously increased with a stationary point outside the experimental range, or two inflection points were found within the experimental range. In the case of an abnormal saddle point, prediction and confirmation of the optimal point within the experimental range were obtained using ridge regression analysis.
2.5. Acid Treatment Conditions
Citric acid, a natural organic acid, was used in the extraction to replace dilute HCl in an acidic pH condition that was derived as the optimal point through RSM. The correlation between the pH change value and the amount of citric acid added was determined, and the linearity was verified by measuring the pH value according to the amount of citric acid added for each solvent ratio. The pectin purification yield was evaluated to compare the extraction yield obtained using HCl or citric acid and mutual equivalence.
2.6. Analysis of the Conditions for Optimal Raw Materialization
The pectin content of red okra was compared by harvest time, size, and part using the optimal extraction method (4 h, 100 °C, 23 solvent ratio, and pH 3). Red okra pods were harvested in July, August, September, October, and November and classified by harvest time, size (less than 4 cm, 4–8 cm, 8–12 cm, or more than 12 cm), and part (pod, root, stem) to obtain pectin. Through this method, the optimal raw materialization conditions were analyzed.
2.7. Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
The total polyphenol content was determined using a modified Folin–Ciocalteu’s reagent (FCR) method [33]. The sample was diluted with distilled water to a concentration of 1000 μg/mL, and the diluted sample and FCR were mixed 1:1 and reacted at room temperature for 3 min. Thereafter, 10% NaCO3 was added, and the mixture was reacted at room temperature for 1 h; the absorbance was measured at 760 nm using a UV–visible spectrometer. A calibration curve was prepared using gallic acid (10–60 µg/mL) as a standard (R2 = 0.999). The total flavonoid content was measured using an aluminum chloride colorimetric assay [34]. Samples were diluted with distilled water to a concentration of 1000 μg/mL. Then, 800 μL of 80% ethanol, 60 μL of 5% NaNO2, and 60 μL of 10% AlCl3 were added to 200 μL of the diluted sample. After the reaction, 400 μL of 1 M NaOH was added, and the absorbance was measured at 510 nm using a UV–visible spectrometer. A calibration curve was prepared using catechin (50–600 µg/mL) as a standard (R2 = 0.999).
2.8. Statistical Analyses
The data were statistically evaluated using Student’s t test, one-way ANOVA, or Duncan’s test with GraphPad Prism 5 version 5.01 for Windows (GraphPad, Inc., San Diego, CA, USA) or SAS software. Statistical significance was defined as p < 0.05. Data are presented as the mean ± standard deviation.
3. Results
3.1. Prediction of Optimal Extraction Conditions Using Response Surface Methodology (RSM) Analysis
The purpose of this study was to determine the optimal conditions for producing red okra extract, and independent variables, such as time, temperature, solvent ratio, and pH, were considered. A total of 19 samples under different conditions were prepared, and the extract yield and pectin content, which are dependent variables, were measured. The extraction yield was 27.72 to 55.97%, and the crude pectin content was 29.12 to 40.98% (Table 3). A second-order polynomial model was developed to represent the relationship between independent and dependent variables.
An analysis of variance (ANOVA) was performed to assess the goodness of fit of the model. In the secondary model, the R2 of the Y1 value was 0.5415, and the F value was 0.0855, which was not significant, but the p-value for lack of fit was 0.9923, confirming the suitability of the model. The Y2 value was not a significant result as the R2 value was 0.2832, and the F value was 0.4575, but the p-value for lack of fit was 0.0611, confirming the suitability of the model [35].

Table 3.
Measurement of extraction yield and crude pectin content of red okra pods according to experimental parameters.
Table 3.
Measurement of extraction yield and crude pectin content of red okra pods according to experimental parameters.
| No. | Factor | Response | ||||
|---|---|---|---|---|---|---|
| χ1 | χ2 | χ3 | χ4 | y1 | y2 1 | |
| 1 | 1 | 70 | 20 | 4 | 31.67 | 29.98 ± 1.81 |
| 2 | 3 | 70 | 20 | 4 | 36.27 | 31.63 ± 0.53 |
| 3 | 5 | 70 | 20 | 4 | 35.23 | 34.50 ± 1.09 |
| 4 | 7 | 70 | 20 | 4 | 36.09 | 32.53 ± 0.77 |
| 5 | 4 | 40 | 20 | 4 | 27.72 | 30.70 ± 3.70 |
| 6 | 4 | 60 | 20 | 4 | 36.91 | 37.38 ± 2.41 |
| 7 | 4 | 80 | 20 | 4 | 40.68 | 36.33 ± 3.84 |
| 8 | 4 | 100 | 20 | 4 | 44.78 | 40.26 ± 1.56 |
| 9 | 4 | 70 | 12 | 4 | 28.59 | 33.20 ± 2.43 |
| 10 | 4 | 70 | 18 | 4 | 35.78 | 32.60 ± 3.14 |
| 11 | 4 | 70 | 24 | 4 | 36.85 | 40.98 ± 2.96 |
| 12 | 4 | 70 | 30 | 4 | 39.59 | 34.52 ± 1.17 |
| 13 | 4 | 70 | 20 | 1 | 55.97 | 40.60 ± 1.45 |
| 14 | 4 | 70 | 20 | 2 | 38.76 | 25.75 ± 2.27 |
| 15 | 4 | 70 | 20 | 3 | 38.06 | 32.53 ± 2.12 |
| 16 | 4 | 70 | 20 | 4 | 32.66 | 31.54 ± 0.81 |
| 17 | 4 | 70 | 20 | 5 | 33.39 | 36.80 ± 2.79 |
| 18 | 4 | 70 | 20 | 6 | 33.58 | 30.20 ± 0.50 |
| 19 | 4 | 70 | 20 | 7 | 34.01 | 29.12 ± 4.09 |
χ1, extraction time (h); χ2, temperature (°C); χ3, solvent ratio; χ4, pH; y1, extraction yield (%); y2, crude pectin content (%). 1 Value shows the mean ± standard deviation (n = 3).
3.2. RSM-Based Regression Analysis to Predict Optimal Conditions for Extracting Red Okra Pods
Regression analysis based on the RSM was performed to predict the optimal extraction conditions. The critical point appeared as a minimum point, not a maximum point (Figure 2). Therefore, the optimal point was calculated by performing ridge analysis, and the optimal conditions for extracting red okra include an extraction time of 4.19 h, a temperature of 80.23 °C, a ratio (w/v) of 22.57, and a pH of 1.24. Under these conditions, the maximum yield of red okra extract was predicted to be 55.40% (Table 4).
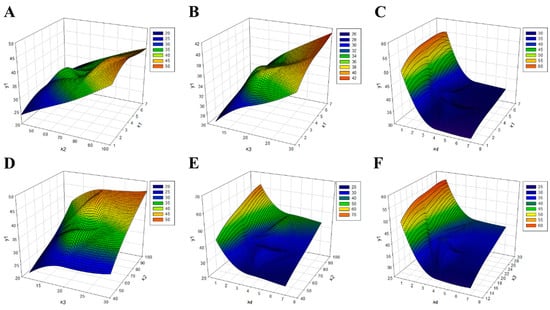
Figure 2.
Response surface diagram for yield optimization of the red okra extraction method. (A) y1 result based on χ1 and χ2 conditions, (B) y1 result based on χ1 and χ3 conditions, (C) y1 result based on χ1 and χ4 conditions, (D) y1 result based on χ2 and χ3 conditions, (E) y1 result based on χ2 and χ4 conditions, and (F) y1 result based on χ3 and χ4 conditions. χ1, extraction time (h); χ2, temperature (°C); χ3, solvent ratio; χ4, pH; y1, extraction yield (%).

Table 4.
Yield of red okra extract condition prediction level for the maximum response of variables through ridge analysis.
3.3. RSM-Based Regression Analysis to Predict Optimal Extraction Conditions of Crude Pectin from Red Okra Pods
RSM-based regression analysis was performed to predict the optimal crude pectin extraction conditions. Similar to the extraction conditions, the critical points of crude pectin appeared as saddle points, not maximum points (Figure 3). Therefore, the optimal point was calculated by performing ridge analysis on the condition of crude pectin. For crude pectin, a maximum yield of 40.83% was expected under the extraction conditions of 4.35 h, 98.04 °C, 23.34 ratio (w/v), and pH 3.36 (Table 5).
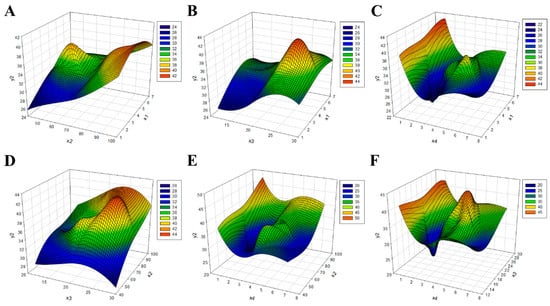
Figure 3.
Response surface diagram for the optimization of crude pectin content in the red okra extraction method. (A) y2 result based on χ1 and χ2 conditions, (B) y2 result based on χ1 and χ3 conditions, (C) y2 result based on χ1 and χ4 conditions, (D) y2 result based on χ2 and χ3 conditions, (E) y2 result based on χ2 and χ4 conditions, and (F) y2 result based on χ3 and χ4 conditions. χ1, extraction time (h); χ2, temperature (°C); χ3, solvent ratio; χ4, pH; y2, crude pectin content (%).

Table 5.
Crude pectin yield condition prediction level for the maximum response of variables through ridge analysis.
3.4. Experimental Verification of the Extraction Yield and Crude Pectin Content for RSM Expected Values
The optimal extraction yield and optimal crude pectin content conditions were compared, and significant differences were not observed for the extraction time, temperature, and solvent ratio but were observed for the pH conditions. The purpose of our research is to develop a process to derive optimal pectin content. Therefore, the pH 3 condition, which provides the optimal yield of crude pectin, was selected as the optimal manufacturing condition. After these optimal conditions were applied to a second-order polynomial model, the red okra extraction yield was predicted to be 40.28%, and the crude pectin content was predicted to be 41.38%. Reproducibility was verified with an extraction yield of 45.26% and a crude pectin content of 38.42 ± 0.24 during actual extraction (Table 6). Therefore, the optimal extraction conditions were ultimately determined to be 4 h, 100 °C, 23 solvent ratio, and pH 3.

Table 6.
Comparison of predicted and measured values of response variables within the range of optimal conditions.
3.5. Comparison of Pectin Content According to Acid Treatment Method
According to a study by Zhou et al., a higher pectin yield was found in thinned young apples when an organic acid, such as citric acid, was used compared to when an inorganic acid, such as hydrochloric acid or nitric acid, was used; these results were also verified [36]. Therefore, to determine the optimal acid method for treating pectin extracted from red okra, the extraction yield and pectin content were compared by treatment with hydrochloric acid, which was used in the optimal manufacturing process established in this study, and citric acid, an alternative organic acid. By examining correlations between the amount of citric acid treatment and amount of extraction solvent that increased while pH 3 conditions were maintained, it was determined that the increase was directly proportional (R2 = 0.9999) (Figure 4). The extract yield obtained from the hydrochloric acid treatment process was 45.26%, and the crude pectin content was 38.43 ± 0.24%. On the other hand, the extract yield obtained from the citric acid treatment process was 49.15% (8.6% increase), and the crude pectin content was higher at 42.76 ± 2.56% (11.3% increase) (Table 7). These results indicate that extract yield and crude pectin content increased when the citric acid treatment process was applied.
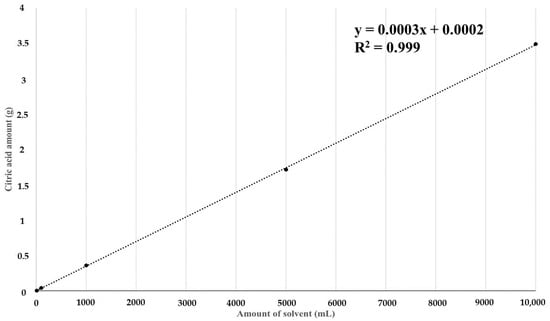
Figure 4.
Verification of linearity of citric acid content change according to solvent volume. The polynomial representing the amount of citric acid added according to the amount of solvent was y = 0.0003x + 0.0002, and, as a result of the analysis, the R2 value was 0.9999, showing a strong correlation.

Table 7.
Comparison of the extract yield and crude pectin content of red okra extract obtained from the hydrochloric acid treatment process and citric acid treatment process at pH 3.
3.6. Determining the Extraction Yield and Pectin Content of Red Okra by Part, Harvest Time, and Size
The pods, stems, and roots of red okra are edible. In addition, it is known that the active ingredients of the plant differ depending on the harvest time; similarly, the content and type of ingredients differ depending on the maturity of the fruit. Therefore, using the optimal extraction method, which was confirmed through RSM analysis and verification of extraction yield, red okra was extracted by part (pod, stem, root), harvest time (July–November), and size (less than 4 cm, 4–8 cm, 8–12 cm, and over 12 cm) and analyzed for extract yield and crude pectin content (Table 8). The extraction yield was measured by part, and the pods showed the highest extraction yield at 49.15%, and the crude pectin content was highest at 63.41% in the roots. However, the root showed the lowest extraction yield (30.10%), and, compared to the extract, the crude pectin content was low (19.09%). The highest crude pectin content ratio was confirmed in the pods at 21.01%. The extraction yield of pods was analyzed by harvest time, and high extraction yields of 49.54% and 48.75% were found in those harvested in July and November, respectively. The crude pectin content of red okra pods harvested in August, October, and November was relatively high, and the crude pectin content of red okra pods harvested in September was low, but the differences were not significant. The extraction yield by size of red okra pods showed similar yields of 47.83% to 49.93% for all sizes. However, red okra pods less than 4 cm in size showed the highest crude pectin content (50.81%).

Table 8.
Analysis of pectin content by raw material condition of red okra.
3.7. Comparison of TPC and TFC in Red Okra and Green Okra Extracts
According to a study by Huo et al., pectin extracted from passion fruit peel through RSM analysis showed high TPC and improved antioxidant effects [37]. Interestingly, using citric acid in this study increased the TPC and antioxidant effect of extracted pectin. Therefore, the total phenol content and total flavonoid content were compared in extracts using the optimal extraction method with citric acid from red okra pods and green okra pods (Figure 5). TPC and TFC measurements were analyzed by classifying okra pods smaller than 12 cm as immature and those larger than 12 cm as mature. The TPC level of green okra pod extract was 21.41 ± 0.38 mg/g in immature okra and 20.04 ± 0.31 mg/g in mature okra (Figure 5A). On the other hand, the TPC level of red okra pod extract was 58.57 ± 0.57 mg/g in immature okra and 56.37 ± 0.49 mg/g in mature okra, which was significantly higher than that of green okra (p = 0.0002). In addition, the TFC level of red okra pod extract was 28.68 ± 0.71 mg/g in immature okra and 28.12 ± 0.27 mg/g in mature okra, which was also significantly higher than the TFC level of green okra (Figure 5B). Interestingly, the TPC level of red okra pod extract was significantly higher in immature pod extract than in mature pod extract (p = 0.0363).
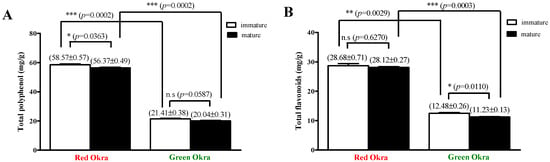
Figure 5.
Comparison of the total phenolic content (TPC) and total flavonoid content (TFC) of red okra and green okra extracts using the optimal processes. (A) Results obtained from comparing TPC levels of red okra or green okra extracts. (B) Results obtained from comparing TFC levels of red okra or green okra extracts. The results are expressed as the mean ± standard deviation (n = 5). * p < 0.05, ** p < 0.01, *** p < 0.001 compared between values marked by the line; ns, not significant.
3.8. Comparison of TPC and TFC in Crude Pectin and Non-Pectin Fractions Extracted from Red Okra Pods
As shown in Figure 5, it was confirmed that the TPC and TFC were higher in red okra extract than in green okra. Therefore, we tested the TPC and TFC in the crude pectin and non-pectin fractions of red okra pods (Figure 6). The crude pectin fraction showed significantly higher TPC and TFC than the non-pectin fraction. As shown in Figure 6A, in which the TPC of mature and immature pods is compared, it was confirmed that the non-pectin fraction was significantly higher in immature pods than in mature pods (p = 0.0008). The TPC in mature pods was significantly higher in the crude pectin fraction than that in immature pods (p = 0.0156). The TFC was also significantly higher in the crude pectin fraction than in the non-pectin fraction (Figure 6B). The TFC in the crude pectin fraction was significantly higher in mature pods than in immature pods (p = 0.0360).
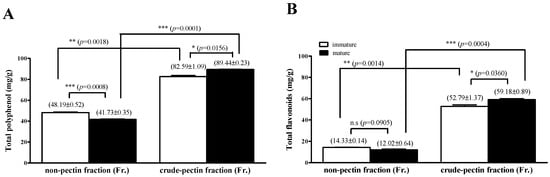
Figure 6.
Comparison of TPC and TFC in crude pectin and non-pectin fractions extracted from red okra pods. (A) Results obtained from comparing TPC levels of red okra crude pectin or non-pectin fractions. (B) Results obtained from comparing TFC levels of red okra crude pectin or non-pectin fractions. The results are expressed as the mean ± standard deviation (n = 5). * p < 0.05, ** p < 0.01, *** p < 0.001 compared between values marked by the line; ns, not significant.
4. Discussion
Pectin is a complex mixture of polysaccharides (mainly Gal A units) in higher plants, and the middle layer of a plant’s cell wall contains the greatest concentration of pectin. Citrus and mango peels are used as the main raw materials for pectin manufacturing, but these two main sources of pectin have long maturity periods [38,39]. To overcome these problems, okra pods, which have a relatively short plant maturation period, have recently emerged as an alternative source of pectin [40]. Therefore, many studies on green okra pectin have been conducted, including comparative studies on the properties of pectin according to genotypic variation of green okra [41] and RSM analysis to optimize the extraction of green okra pectin [16]. Extracts from red okra obtained through various extraction methods can also play an essential role in preventing and treating many diseases. According to several studies, ethanol extract of red okra pods has been reported to exhibit anticancer activity [42,43], and n-hexane extract has been reported to show a protective effect against liver damage [5]. However, these studies did not standardize the extraction method and ingredients, and, in particular, there are no studies on pectin from red okra. Therefore, through this study, we explored the optimal pectin extraction conditions through RSM analysis of red okra and determined the standardized optimal extraction method by verifying the extraction conditions. In addition, by applying optimal extraction conditions, red okra was analyzed based on its part, size, and harvest time to standardize raw materials and provide standard information for the extraction of red okra and pectin production.
First, we confirmed the conditions for optimal extraction and crude pectin extraction of red okra pods through RSM analysis. When the optimal extraction method derived through RSM analysis was applied, 55.40% of the red okra pods was extracted, and the crude pectin yield was 40.83% (Table 4 and Table 5). However, the extraction conditions for red okra pods and crude pectin extraction conditions were derived differently, and, since our final goal was to determine the optimal conditions for crude pectin extraction, these conditions were determined as simultaneous extraction conditions. Additionally, although pH 1 generated the optimal red okra extract, some difficulties were encountered; for example, equipment for strong acid treatments, a neutralization process, and wastewater treatment were necessary. Therefore, we finally determined that the optimal conditions for extracting red okra pods and crude pectin were 4 h, 100 °C, 23 solvent ratio, and pH 3. As a result, a 45.26% extraction yield and 38.42% crude pectin yield were verified (Table 6). We confirmed that our results were better than those obtained for the pectin content of green okra reported in the literature (11–15.7%) [44,45,46]. Chen et al. performed RSM analysis to determine the optimal conditions for pectin extraction from green okra, and the optimal conditions were determined to be pH 3.9, 64 min, 60 °C, and a solvent ratio of 42. Under these conditions, 2.71% pectin was obtained [16]. In our study, we successfully determined the optimal conditions for red okra extract production by considering the extract yield of red okra and the crude pectin content. Our findings will help improve the process of manufacturing red okra extract, providing potential benefits in a variety of applications.
Second, to improve the acid treatment process, we studied conditions that do not require a neutralization process, exhibit low toxicity, and are environmentally friendly. The acid treatment process is important in the process of extracting pectin, and acid treatment is especially essential for okra-derived pectin. Generally, pectin consists of three parts: the water-soluble, oxalic acid, and acid-soluble parts. The composition, structure, and biological functions of pectin are influenced by acid–base extraction conditions [47]. Interestingly, when the extraction yield and crude pectin yield of red okra pods were examined under pH 3 conditions using citric acid as an organic acid, the extraction yield (49.15%) increased by 8.6%, and the crude pectin content (42.76%) also increased by 11.3% compared to that obtained with hydrochloric acid. These results are consistent with a previous study in which the pectin extraction yield was high when green okra was extracted with citric acid [48]. Based on these results, it was concluded that the final extraction method should involve the citric acid treatment process. Citric acid extraction offers potential advantages in terms of sustainability and because environmentally friendly materials are used in the manufacturing process. Hydrochloric acid extraction is accompanied by the destruction of the crosslinking blocks and depolymerization of pectin. Citric acid partially demineralizes the crosslinking blocks, contributing to the release of macromolecular chains that do not have calcium pectate units [49]. However, a previous study reported that when green okra extract was treated with citric acid, the content of pectin increased, while the purity of pectin decreased [48]. Therefore, the purity and characteristics of pectin extracted from red okra with citric acid should be further studied.
Third, the quality of pectin, such as its extraction yield and purity, is greatly influenced by the extraction process, the maturity, plant part, and the harvest time [22]. The extraction yield and pectin content in different parts of red okra (pods, stems, and roots) were compared, and a high crude pectin content of 42.76% was extracted from the pods; in addition, compared to the extraction yield, the crude pectin content was also the highest at 21.01%. Red okra stems had the lowest extraction yield and pectin content, but, according to a study by Li et al., pectin derived from green okra stems exhibits a structure characteristic of the RG1 domain and shows excellent anti-fatigue effects, so the stems are also valuable [50]. The highest crude pectin content was extracted from the roots (63.41%), but, compared to the extraction yield, the pectin was low at 19.09. This result could result from the low extraction yield (30.10%) of red okra roots. Additionally, the pectin content results obtained in our study involved crude pectin and not pure pectin. Crude pectin, which showed a high content in the roots, may precipitate together during the pectin purification process due to the incorporation of other ingredients, such as starch and proteins. Therefore, when extracting pectin from red okra, pectin of higher purity can be obtained when pods are used rather than roots. Additionally, further research is needed on the purity and properties of crude pectin extracted using the crude pectin extraction method we determined. The crude pectin content was compared by harvest time and extraction yield, and the results showed that crude pectin content remained relatively constant from July to November except for in September. The low extraction yield and crude pectin content of red okra pods harvested in September may occur because September is a seasonal transition period from summer to fall in Korea, and the amount of sunlight and temperature change greatly during this period. However, the difference in extraction yields and crude pectin contents of red okra pods that were analyzed monthly was insignificant. Therefore, it can be concluded that fruit pods can be harvested from July to November without causing significant changes in extract yield or crude pectin content. The extraction yield and crude pectin content of red okra pods were compared by size, and pods shorter than 4 cm showed the highest extraction yield and crude pectin content, while pods longer than 12 cm showed the lowest extraction yield and crude pectin content. Although the highest extraction yield and crude pectin yield were observed in pods of less than 4 cm, collecting small red okra pods is an inconvenient harvesting method, and, from an economic perspective, supplying the raw materials needed for research and industry may be difficult. The highest extraction yield and crude pectin content were obtained when red okra pods of less than 12 cm were processed with our established extraction method. Our findings will be useful in selecting optimal raw materials for red okra extract production and optimizing the manufacturing process. Further research is needed to investigate the content of red okra pure pectin, physical properties of pectin, and other quality parameters. We believe these additional studies will provide a more comprehensive understanding of the potential applications and benefits of red okra pectin in various industries and research fields.
Fourth, the content and profile of phenolic and flavonoid components in plants are directly influenced by factors such as cultivar, maturity, and environmental conditions [51]. The interaction of these various factors influences the production of plant metabolites, which lead to the production of various bioactive compounds, such as various types of phenolic and flavonoid compounds [52]. Wu et al. reported that six types of phenolic compounds (catechin, isoquercitrin, protocatechuic acid, quercetin, quercetin-3-O-gentiobioside, and rutin), including flavonoids, were detected in okra fruits and that their contents varied depending on the cultivars [53]. Corresponding with our results, the difference in TPC and TFC between red and green okra possibly resulted from these factors, and the difference in content due to the degree of maturity may also result from changes in the plant’s metabolic system. We found that red okra pods contain polyphenols and flavonoids (Figure 5), which exhibit high antioxidant activity that can scavenge free radicals and reduce oxidative stress in cells [54]. Finally, as shown in Figure 1, the crude pectin from the red okra pods we isolated was characterized by a purple color. In a study by Zhang et al., 15 okra cultivars were compared, and two major anthocyanins, delphinidin 3-O-sambubioside and cyanidin 3-O-sambubioside, and six kinds of flavonol glycosides (most are quercetin-based) were detected only in the pods of four red okra cultivars, including Burgundy [55]. Interestingly, compared to the non-pectin fraction, the crude pectin fraction isolated from red okra pod extract showed significantly higher TPC and TFC (Figure 6). Many studies have reported that pectin has a strong binding effect with polyphenols due to its structural characteristics [56,57,58,59,60,61,62]. Pectin is strongly bonded due to high water interaction and hydrogen bonds between tannins [58]. Additionally, among polyphenols, anthocyanin is also bonded with pectin [57,60,61]. These bonds are due to intermolecular bonds between the carboxyl groups of pectin and polyphenols [62]. In particular, red okra pods are reported to contain xenobiotics (anthocyanins), which are responsible for the red color of the pods [9]. Additionally, it has been reported that red okra extract contains anthocyanins with higher antioxidant and quercetin contents than those of green okra [63]. Specifically, additional research is needed to increase the purity of crude pectin, evaluate the quality of pectin, and measure the molecular weight, physicochemical properties, and viscosity of pectin. Overall, these results improve our knowledge on the optimal process of pectin extraction and basic properties of okra pods; in addition, strong support is provided for the future development of okra pods as functional food and pharmaceutical raw materials.
5. Conclusions
The purpose of this study was to determine the optimal conditions for the production of red okra extract and to reveal how the acid treatment method affects pectin extraction. In the present study, it was revealed that organic acid extraction exhibited advantages, and the optimal extraction conditions were an extraction time of 4 h, a temperature of 100 °C, a ratio of 23 (w/v), and a pH of 3. In the results obtained for the red okra crude pectin content, it was confirmed that the content was the highest when extraction was performed with the pods of red okra harvested from July to November with a diameter of less than 12 cm. In addition, it was concluded that the red okra pod extract had higher TPC and TFC levels than the green okra extract, and the crude pectin fraction had higher TPC and TFC levels than the non-pectin fraction. Our study, reporting for the first time, clearly suggests the feasibility of pectin production using red okra. These results may contribute to the development of pectin purification processes, functional evaluation studies of pectin, and potential applications of red okra extract in various industries. Furthermore, the findings contribute to the manufacturing process and potential applications of red okra extract, which will provide a basis for further research in this field.
Author Contributions
Conceptualization, project administration, and writing—original draft, S.K.; data curation and formal analysis, S.a.S. and Y.K.; methodology, E.K., K.H.L., W.S.K. and K.J.K.; resources, D.L. and S.-y.C.; writing—review and editing, J.S.K. and T.-S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Jeonnam materials, parts, and root industry research and development agency one-stop support project (grant number: JNTP one-2022-01), funded by the Jeonnam Technopark. The funding body did not play a role in the study design, performance, data collection, or analysis nor in the decision to publish or preparation/writing of the manuscript.
Data Availability Statement
Not applicable.
Acknowledgments
We sincerely appreciate the other colleagues in our laboratory for their help and effort in this study.
Conflicts of Interest
The authors declare that they have no competing interest. Author Sunoh Kim and all coauthors are from the B&Tech company; the B&Tech company had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Sengkhamparn, N.; Sagis, L.M.C.; de Vries, R.; Schols, H.A.; Sajjaanantakul, T.; Voragen, A.G.J. Physicochemical properties of pectins from okra (Abelmoschus esculentus (L.) Moench). Food Hydrocoll. 2010, 24, 35–41. [Google Scholar] [CrossRef]
- Bai, L.; Zhu, P.; Wang, W.; Wang, M. The influence of extraction pH on the chemical compositions, macromolecular characteristics, and rheological properties of polysaccharide: The case of okra polysaccharide. Food Hydrocoll. 2019, 102, 105586. [Google Scholar] [CrossRef]
- Dimopoulou, M.; Ritzoulis, C.; Papastergiadis, E.S.; Panayiotou, C. Composite materials based on okra hydrocolloids and hydroxyapatite. Food Hydrocoll. 2014, 42, 348–354. [Google Scholar] [CrossRef]
- Zhu, X.; Xu, R.; Wang, H.; Chen, J.; Tu, Z. Structural Properties, Bioactivities, and Applications of Polysaccharides from Okra [Abelmoschus esculentus (L.) Moench]: A Review. J. Agric. Food Chem. 2020, 68, 14091–14103. [Google Scholar] [CrossRef] [PubMed]
- Wahyuinigsih, S.P.A.; Mwendolwa, A.A.; Winarni, D.; Angreini, R.W.; Mamuaya, B.K.K. Protective Effect of Red Okra (Abelmoschus esculentus (L.) Moench) Pods against Sodium Nitrite-Induced Liver Injury in Mice. Vet. Med. Int. 2021, 2021, 6647800. [Google Scholar]
- Esmaeilzadeh, D.; Razavi, B.M.; Hosseinzadeh, H. Effect of Abelmoschus esculentus (okra) on metabolic syndrome: A review. Phytother. Res. 2020, 34, 2192–2202. [Google Scholar] [CrossRef]
- Chopra, R.N.; Nayar, S.L.; Chopra, I.C. Glossary of Indian Medicinal Plants; Council of Scientific & Industrial Research: New Delhi, India, 1956; p. 133. [Google Scholar]
- Arapitsas, P. Identification and quantification of polyphenolic compounds from okra seeds and skins. Food Chem. 2008, 110, 1041–1045. [Google Scholar] [CrossRef]
- Irshad, M.; Debnath, B.; Mitra, S.; Arafat, Y.; Li, M.; Sun, Y.; Qiu, D. Accumulation of anthocyanin in callus cultures of red-pod okra [Abelmoschus esculentus (L.) Hongjiao] in response to light and nitrogen levels. Plant Cell Tissue Organ Cult. 2018, 134, 29–39. [Google Scholar] [CrossRef]
- Whistler, R.L.; BeMiller, J.N. Industrial Gums: Polysaccharides and Their Derivatives; Academic Press: San Diego, CA, USA, 1993. [Google Scholar]
- Ghori, M.U.; Alba, K.; Smith, A.M.; Conway, B.R.; Kontogiorgos, V. Okra extracts in pharmaceutical and food applications. Food Hydrocoll. 2014, 42, 342–347. [Google Scholar] [CrossRef]
- Alqasoumi, S.I. “Okra” Hibiscus esculentus L.: A study of its hepatoprotective activity. Saudi Pharm. J. 2012, 20, 135–141. [Google Scholar] [CrossRef]
- Sipahi, H.; Orak, D.; Reis, R.; Yalman, K.; Şenol, O.; Palabiyik-Yücelik, S.S.; Deniz, I.; Algul, D.; Guzelmeric, E.; Celep, M.E.; et al. A comprehensive study to evaluate the wound healing potential of okra (Abelmoschus esculentus) fruit. J. Ethnopharmacol. 2022, 287, 114843. [Google Scholar] [CrossRef] [PubMed]
- Ndjouenkeu, R.; Akingbala, J.O.; Oguntimein, G.B. Emulsifying properties of three African food hydrocolloids: Okra (Hibiscus esculentus), dika nut (Irvingia gabonensis), and khan (Belschmiedia sp.). Plant Foods Hum. Nutr. 1997, 51, 245–255. [Google Scholar] [CrossRef]
- Georgiadis, N.; Ritzoulis, C.; Sioura, G.; Kornezou, P.; Vasiliadou, C.; Tsioptsias, C. Contribution of okra extracts to the stability and rheology of oil-in-water emulsions. Food Hydrocoll. 2011, 25, 991–999. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.G.; Sun, H.J.; Wei, Z.-J. Pectin from ablemoschus esculentus: Optimaization of extraction and rheological properties. Int. J. Biol. Macromol. 2014, 70, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Kissiedu, K.O.; Agbenorhevi, J.K.; Datsomor, D.N. Optimization of sensory acceptability of milk chocolate containing okra pectin as emulsifier. Int. J. Food Prop. 2020, 23, 1310–1323. [Google Scholar] [CrossRef]
- Kontogiorgos, V.; Margelou, I.; Georgiadis, N.; Ritzoulis, C. Rheological characterization of okra pectins. Food Hydrocoll. 2012, 29, 356–362. [Google Scholar] [CrossRef]
- De la Hoz Vega, S.; Jaraba, B.V.; Mendoza, J.P.; Arrieta, A.A.; Quinones, L.O. Effect of precipitating solvents on the extraction of pectin from the pomelo albedo by acid hydrolysis. Contemp. Eng. Sci. 2018, 11, 3849–3855. [Google Scholar] [CrossRef]
- Vanitha, T.; Khan, M. Role of Pectin in Food Processing and Food Packaging. In Pectins—Extraction, Purification, Characterization and Applications; Martin, M., Ed.; Intechopen: London, UK, 2020; Volume 6, pp. 85–97. [Google Scholar]
- Masmoudi, M.; Besbes, S.; Chaabouni, M.; Robert, C.; Paquot, M.; Blecker, C.; Attia, H. Optimization of pectin extraction from lemon by-product with acidified date juice using response surface methodology. Carbohydr. Polym. 2008, 74, 185–192. [Google Scholar] [CrossRef]
- Zdunek, A.; Pieczywek, P.M.; Cybulska, J. The primary, secondary, and structures of higher levels of pectin polysaccharides. Compr. Rev. Food Sci. Food Saf. 2020, 20, 1101–1117. [Google Scholar] [CrossRef]
- Tomada, M.; Shimada, K.; Saito, Y.; Sugi, M. Plant mucilages. XXVI. Isolation and structural features of a mucilage, ‘‘okra mucilage’’, from the immature fruit of Abelmoschus esculentus. Chem. Pharm. Bull. 1980, 28, 2933–2940. [Google Scholar] [CrossRef]
- Mierczyńska, J.; Cybulska, J.; Zdunek, A. Rheological and chemical properties of pectin enriched fractions from different sources extracted with citric acid. Carbohydr. Polym. 2017, 156, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Cybulska, J.; Brzyska, A.; Zdunek, A.; Woliński, K. Simulation of Force Spectroscopy Experiments on Galacturonic Acid Oligomers. PLoS ONE 2014, 9, e107896. [Google Scholar] [CrossRef] [PubMed]
- Ignatyeva, G.N.; Melgarejo, P.J.; Picazo, S.M.; Álvarez, D.F. The use of high-performance liquid chromatography as screening technique for pectin and pectin substances of dietary fibers. J. Microbiol. Biotechnol. Food Sci. 2015, 4, 30–41. [Google Scholar] [CrossRef]
- Mao, Y.; Lei, R.; Ryan, J.; Rodriguez, F.A.; Rastall, B.; Chatzifragkou, A.; Winkworth-Smith, C.; Harding, S.E.; Ibbett, R.; Binner, E. Understanding the influence of processing conditions on the extraction of rhamnogalacturonan-I “hairy” pectin from sugar beet pulp. Food Chem. X 2019, 2, 100026. [Google Scholar] [CrossRef] [PubMed]
- Yapo, B.M. Pectin quantity, composition and physicochemical behaviour as influenced by the purification process. Food Res. Int. 2009, 42, 1197–1202. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Shi, N.; Zhang, Z.; Chen, Y.; Yan, M.; Li, Y. Response surface methodology optimization and HPLC-ESI-QTOF-MS/MS analysis on ultrasonic-assisted extraction of phenolic compounds from okra (Abelmoschus esculentus) and their antioxidant activity. Food Chem. 2023, 405, 134966. [Google Scholar] [CrossRef]
- Mohsen, E.H.K.; Hossein, J. Optimization of mucilage extraction from Sepestan fruit and evaluation of its physicochemical and antioxidant activity. J. Food Meas. Charact. 2022, 16, 4331–4344. [Google Scholar]
- Puligundla, P.; Lim, S. A Review of Extraction Techniques and Food Applications of Flaxseed Mucilage. Foods 2022, 11, 1677. [Google Scholar] [CrossRef]
- Lee, K.Y.; Choi, W.Y.; Park, G.H.; Jeong, Y.S.; Park, A.; Lee, Y.; Kang, D.H. Study on Marine Pectin Extraction and Its Antioxidant Activities from 14 Marine Algae under Different Extraction Solvents. J. Korean Soc. Food Sci. Nutr. 2020, 49, 677–685. [Google Scholar] [CrossRef]
- Folin, O.; Denis, W. On phosphotungstic-phosphomolybdic compounds as color reagents. J. Biol. Chem. 1912, 12, 239–243. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Hwang, A.S.; Jeon, C.W.; Kim, S.M.; Lee, J.E.; Rho, J.O. A Study on the Optimization of Manufacturing Conditions for Lotus Root Dry ChipsUsing Response Surface Methodology. J. East Asian Soc. Diet. Life 2022, 32, 159–168. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, D.; Xia, W.; Guo, Y.; Luo, Y.; Xue, J. Physicochemical and functional properties of RG-I enriched pectin extracted from thinned-young apples. Int. J. Biol. Macromol. 2023, 236, 123953. [Google Scholar] [CrossRef] [PubMed]
- Huo, D.; Dai, J.; Yuan, S.; Cheng, X.; Pan, Y.; Wang, L.; Wang, R. Eco-friendly simultaneous extraction of pectins and phenolics from passion fruit (Passiflora edulis Sims) peel: Process optimization, physicochemical properties, and antioxidant activity. Int. J. Biol. Macromol. 2023, 243, 125229. [Google Scholar] [CrossRef] [PubMed]
- Khule, N.R.; Mahale, N.B.; Shelar, D.S.; Rokade, M.M.; Chaudhari, S.R. Extraction of pectin from citrus fruit peel and use as natural binder in paracetamol tablet. Pharm. Lett. 2012, 4, 558–564. [Google Scholar]
- Malviya, R.; Kulkarni, G.T. Extraction and characterization of mango peel pectin as pharmaceutical excipient. Indian J. Nat. Prod. Resour. 2012, 2, 185–190. [Google Scholar]
- Prajapati, V.D.; Jani, G.K.; Moradiya, N.G.; Randeria, N.P. Pharmaceutical applications of various natural gums, mucilages and their modified forms. Carbohydr. Polym. 2013, 92, 1685–1699. [Google Scholar] [CrossRef]
- Owusu, F.W.A.; Boakye-Gyasi, M.E.; Entsie, P.; Bayor, M.T.; Ofori-Kwakye, K. Utilization of Pectin from Okra as Binding Agent in Immediate Release Tablets. BioMed Res. Int. 2021, 2021, 6002286. [Google Scholar] [CrossRef]
- Pramudya, M.; Dewi, F.R.P.; Wong, R.W.; Anggraini, D.W.; Winarni, D.; Wahyuningsih, S.P.A. Anti-cancer activity of an ethanolic extract of red okra pods (Abelmoschus esculentus L. Moench) in rats induced by N-methyl-N-nitrosourea. Vet. World 2022, 15, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Nisa, N.; Wahyuningsih, S.P.A.; Darmanto, W.; Purnama, P.R.; Dewi, F.R.P.; Soegiarti, T.; Karsari, D. Effect of the Ethanol Extract of Red Okra Pods (Abelmoschus esculentus (L.) Moench) to Inhibit Cervical Cancer Cells Growth through Cell Cycle-Associated Oncogenes. Scientifica 2022, 2022, 1094771. [Google Scholar] [CrossRef]
- Alba, K.; Kontogiorgos, V. Pectin at the oil-water interface: Relationship of molecular composition and structure to functionality. Food Hydrocoll. 2017, 68, 211–218. [Google Scholar] [CrossRef]
- Samavati, V. Polysaccharide extraction from Abelmoschus esculentus: Optimization by response surface methodology. Carbohydr. Polym. 2013, 95, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Kpodo, F.M.; Agbenorhevi, J.K.; Alba, K.; Bingham, R.J.; Oduro, I.N.; Morris, G.A.; Kontogiorgos, V. Pectin isolation and characterization from six okra genotypes. Food Hydrocoll. 2017, 72, 323–330. [Google Scholar] [CrossRef]
- Xiong, B.; Zhang, W.; Wu, Z.; Liu, R.; Yang, C.; Hui, A.; Huang, X.; Xian, Z. Preparation, characterization, antioxidant and anti-inflammatory activities of acid-soluble pectin from okra (Abelmoschus esculentus L.). Int. J. Biol. Macromol. 2021, 181, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Afotey, B.; Agbenorhevi, J.K.; De-Souza, L.D.K.; Logosu, J.K.; Kpodo, F.M.; Falade, K.O. Okra (Abelmoschus esculentus L.) pectin yield as influenced by particle size and extraction solvent. Food Chem. Adv. 2023, 3, 100339. [Google Scholar] [CrossRef]
- Lepilova, O.; Aleeva, S.; Koksharov, S.; Lepilova, E. Supramolecular structure of banana peel pectin and its transformations during extraction by acidic methods. Int. J. Biol. Macromol. 2023, 242 Pt 2, 124616. [Google Scholar] [CrossRef]
- Li, Y.; Deng, Y.; Li, Z.; Liu, Z.; Piao, M.; Cui, X. Composition, physicochemical properties, and anti-fatigue activity of water-soluble okra (Abelmoschus esculentus) stem pectins. Int. J. Biol. Macromol. 2020, 15, 2630–2639. [Google Scholar] [CrossRef]
- Santos, J.; Oliveira, M.B.P.P.; Ibanez, E.; Herrero, M. Phenolic profile evolution of dierent ready-to-eat baby-leaf vegetables during storage. J. Chromatogr. A 2014, 1327, 118–131. [Google Scholar] [CrossRef]
- Lin, Y.; Lu, M.-F.; Liao, H.-B.; Li, Y.-X.; Han, W.; Yuan, K. Content determination of the flavonoids in the dierent parts and dierent species of Abelmoschus esculentus L. by reversed phase-high performance liquid chromatograph and colorimetric method. Pharmacogn. Mag. 2014, 10, 278–284. [Google Scholar]
- Wu, D.-T.; Nie, X.-R.; Shen, D.-D.; Li, H.-Y.; Zhao, L.; Zhang, Q.; Lin, D.-R.; Qin, W. Phenolic Compounds, Antioxidant Activities, and Inhibitory Effects on Digestive Enzymes of Different Cultivars of Okra (Abelmoschus esculentus). Molecules 2020, 25, 1276. [Google Scholar] [CrossRef]
- Wahyuningsih, S.P.A.; Savira, N.I.I.; Anggraini, D.W.; Winarni, D.; Suhargo, L.; Kusuma, B.W.A.; Nindyasari, F.; Setianingsih, N.; Mwendolwa, A.A. Antioxidant and nephroprotective effects of okra pods extract (Abelmoschus esculentus L.) against lead acetate-induced toxicity in mice. Scientifica 2020, 2020, 4237205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, T.; Zhao, Q.; Xie, X.; Li, Y.; Chen, Q.; Cheng, F.; Tian, J.; Gu, H.; Huang, J. Comparative Transcriptome Analysis of the Accumulation of Anthocyanins Revealed the Underlying Metabolic and Molecular Mechanisms of Purple Pod Coloration in Okra (Abelmoschus esculentus L.). Foods 2021, 10, 2180. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.W.; Renard, C.M.G.C.; Rolland-Sabate, A.; Le Bourvellec, C. Exploring interactions between pectins and procyanidins: Structure-function relationships. Food Hydrocoll. 2021, 113, 106498. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Watrelot, A.A.; Ginies, C.; Imberty, A.; Renard, C.M.G.C. Impact of processing on the noncovalent interactions between procyanidin and apple cell wall. J. Agric. Food Chem. 2012, 60, 9484–9494. [Google Scholar] [CrossRef]
- Mamet, T.; Ge, Z.Z.; Zhang, Y.; Li, C.M. Interactions between highly galloylated persimmon tannins and pectins. Int. J. Biol. Macromol. 2018, 106, 410–417. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Zhao, W.; Gao, Z.; Wu, M.; Zhou, T.; Wu, C.; Zhou, K.; Han, X.; Zhou, Q. Hawthorn Juice Simulation System for Pectin and Polyphenol Adsorption Behavior: Kinetic Modeling Properties and Identification of the Interaction Mechanism. Foods 2022, 11, 2813. [Google Scholar] [CrossRef]
- Fernandes, A.; Brás, N.F.; Mateus, N.; de Freitas, V. Understanding the molecular mechanism of anthocyanin binding to pectin. Langmuir 2014, 30, 8516–8527. [Google Scholar] [CrossRef]
- Padayachee, A.; Netzel, G.; Netzel, M.; Day, L.; Zabaras, D.; Mikkelsen, D.; Gidley, M.J. Binding of polyphenols to plant cell wall analogues—Part 1: Anthocyanins. Food Chem. 2012, 134, 155–161. [Google Scholar] [CrossRef]
- Belitz, H.-D.; Grosch, W.; Shieberle, P. Food Chemistry, 4th ed.; Springer: Berlin, Germany, 2009. [Google Scholar]
- Anjani, P.P.; Damayanthi, E.; Rimbawan, R.; Handharyani, E. Antidiabetic potential of purple okra (Abelmoschus esculentus L.) extract in streptozotocin-induced diabetic rats. IOP Conf. Ser. Earth Environ. Sci. 2018, 196, 012038. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).