The Influence of Draft Tubes on the Mass Transfer and Mixing Performance of a Pilot-Scale Internal-Loop Airlift Reactor
Abstract
:1. Introduction
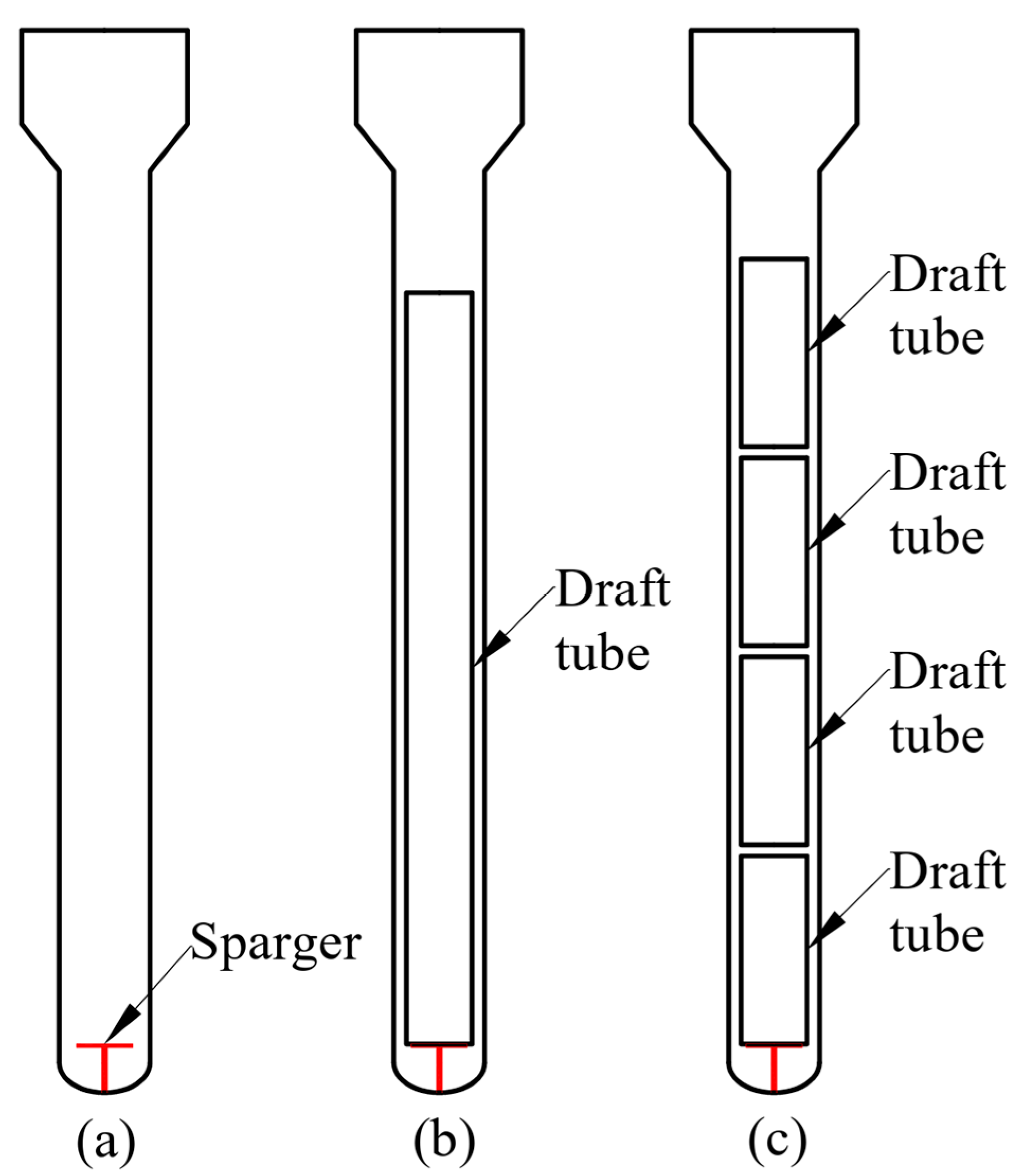
2. Experimental Work
3. Results and Discussion
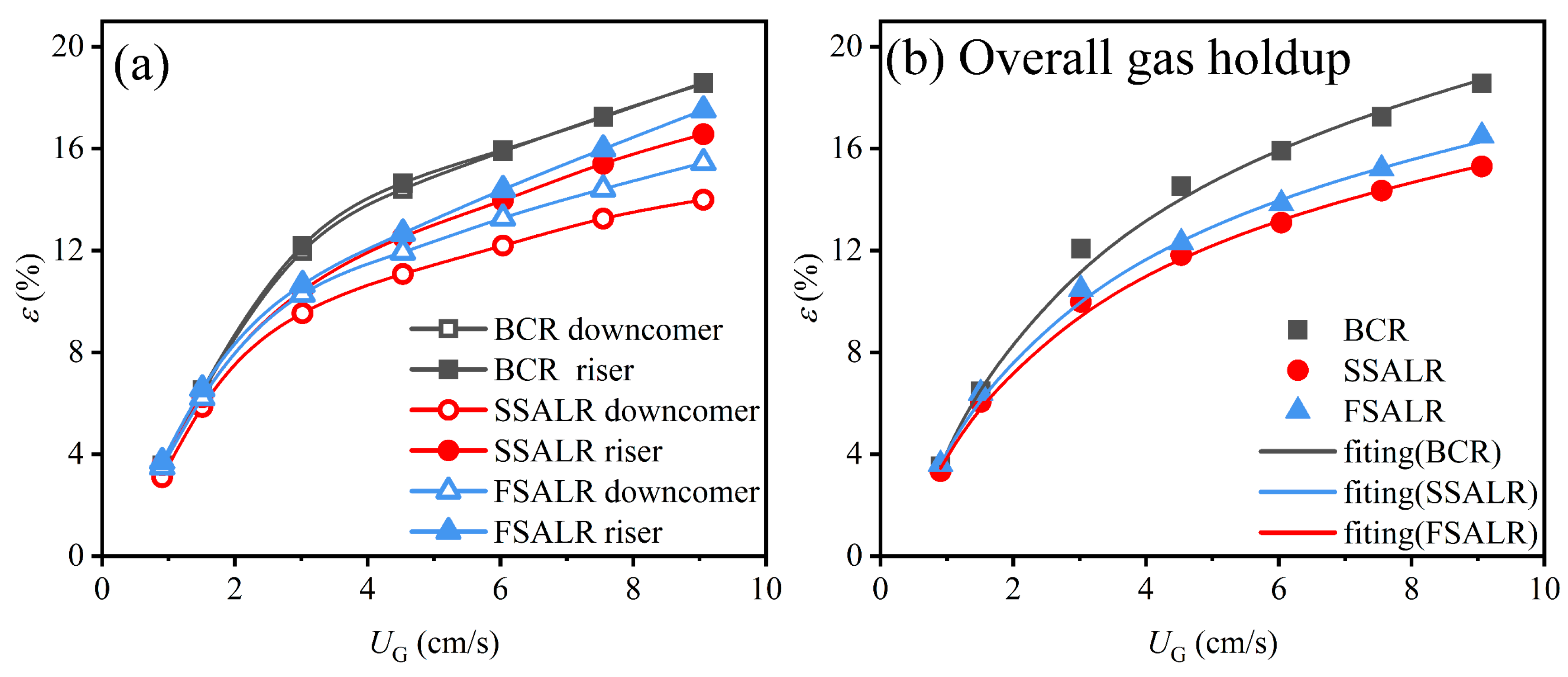
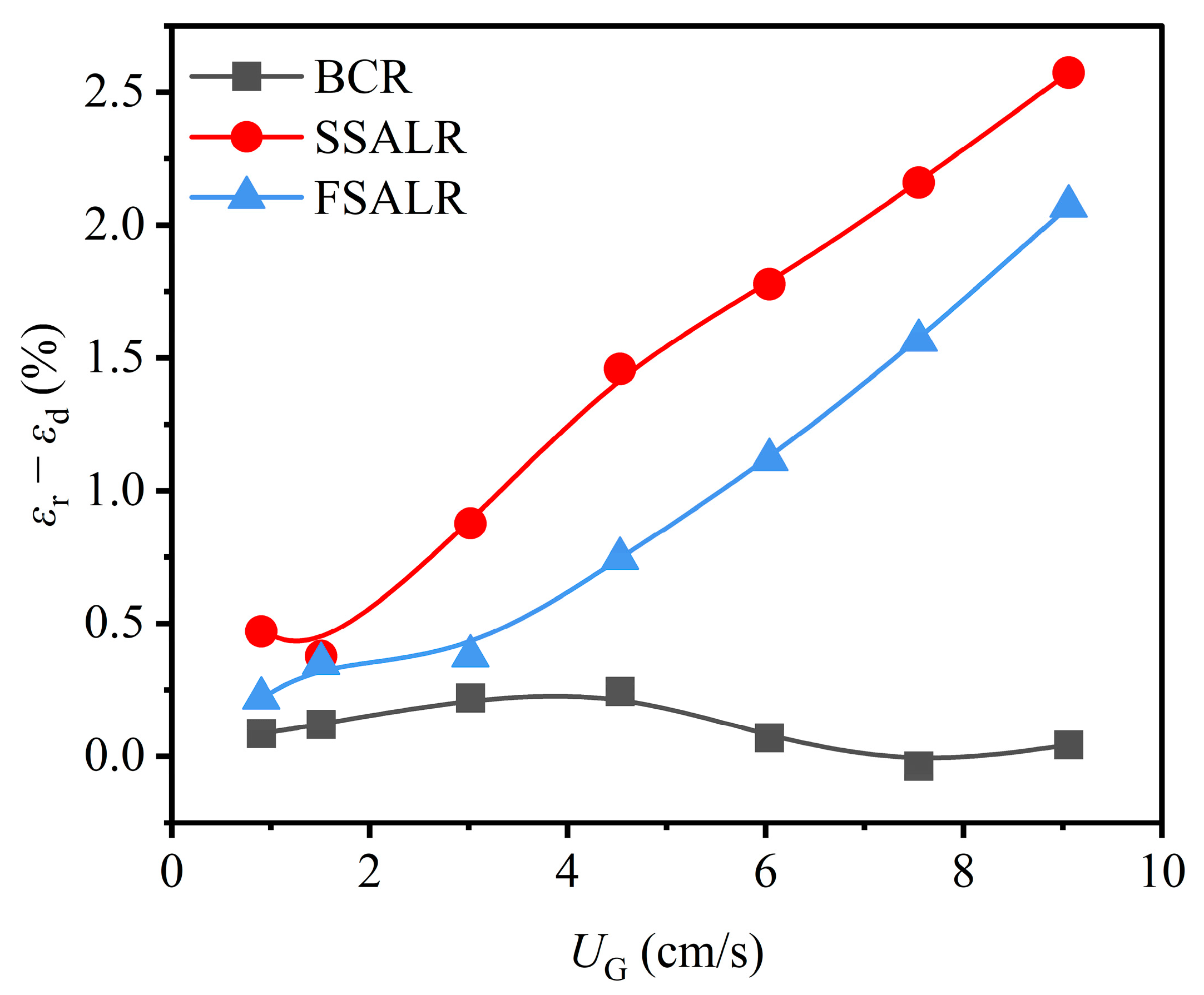
3.1. Gas Holdup
3.2. Liquid Circulating Velocity
3.3. Volumetric Mass Transfer Coefficient
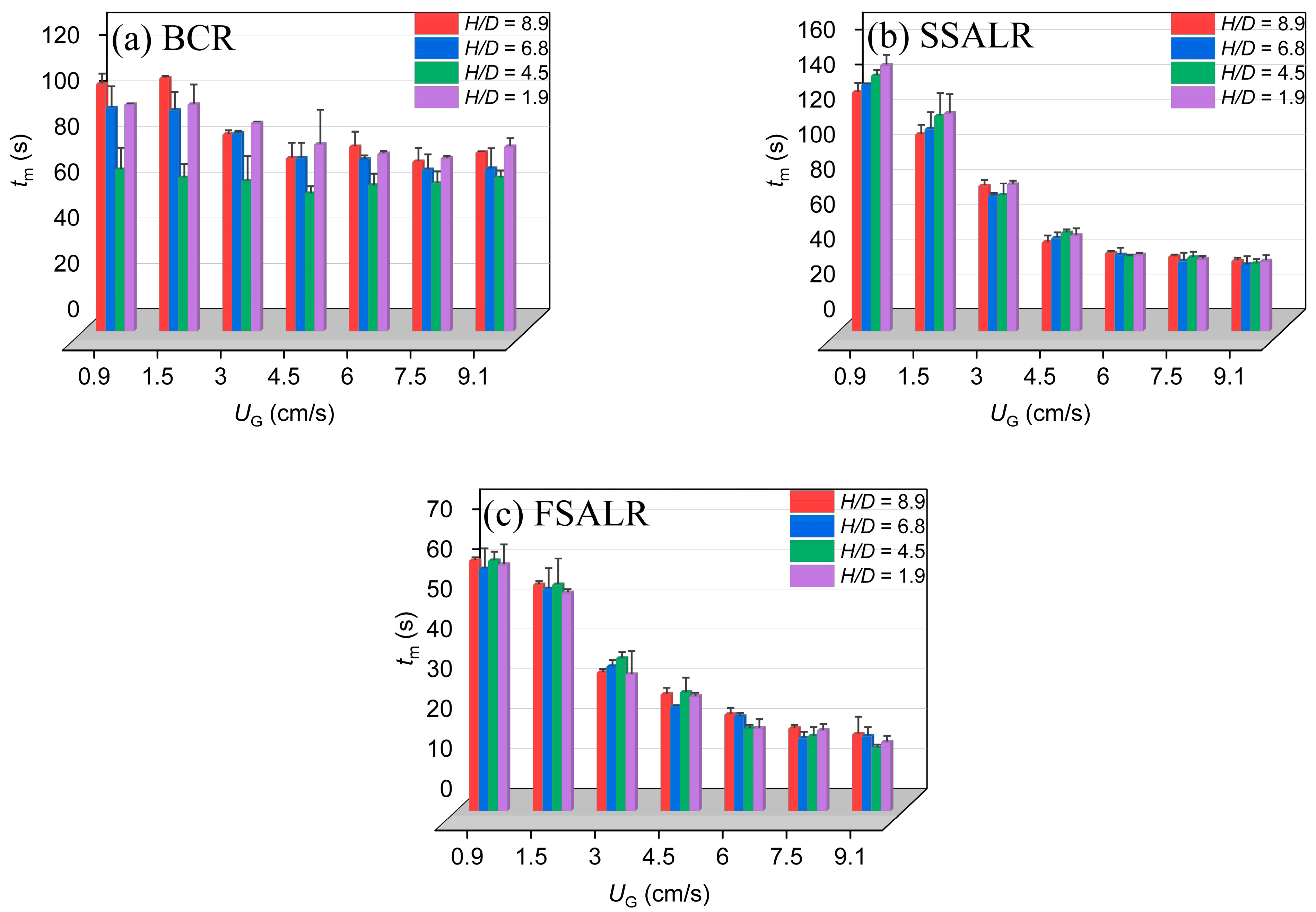
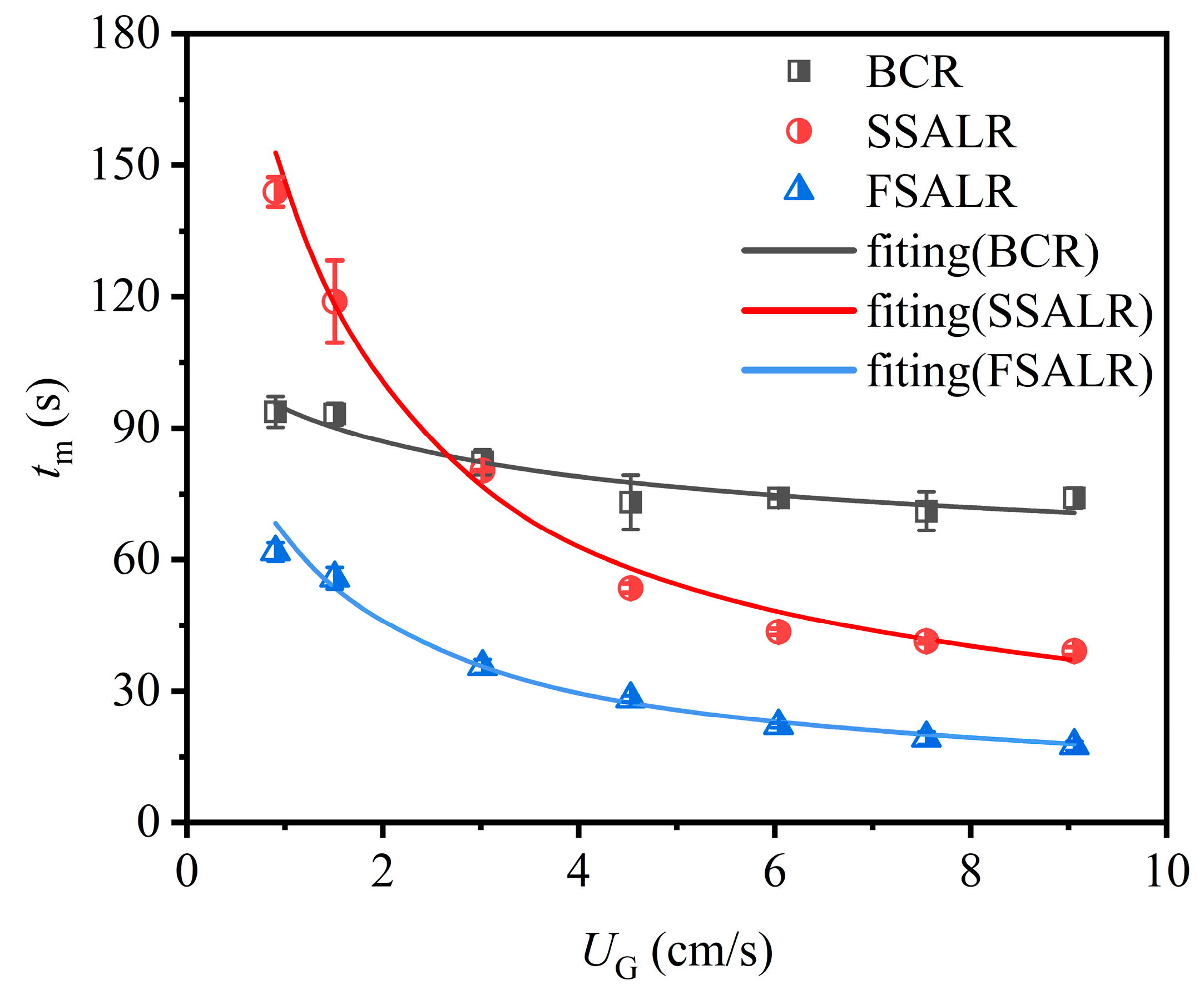
3.4. Mixing Time
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| a | Mass transfer area: m2∙m−3 |
| D | Inner diameter of column, m |
| g | Gravitational acceleration, m∙s−2 |
| H | Height, m |
| kL | Liquid side mass transfer coefficient, m∙s−1 |
| P | Pressure, Pa |
| r/R | Dimensionless radial position, dimensionless |
| t | Time, s |
| tm | Mixing time, s |
| Ucir | Circulating liquid velocity, m∙s−1 |
| UG | Superficial gas velocity, cm∙s−1 |
| Greek letters | |
| ε | Gas holdup, dimensionless |
| η | Degree of macro-mixing, dimensionless |
| ρ | Density, kg∙m−3 |
| Subscripts | |
| G | Gas |
| d | Downcomer |
| L | Liquid |
| r | Riser |
References
- Behin, J.; Amiri, P. A review of recent advances in airlift reactors technology with emphasis on environmental remediation. J. Environ. Manag. 2023, 335, 117560–117583. [Google Scholar] [CrossRef]
- Yang, H.; Chen, A.; Geng, S.; Cheng, J.; Gao, F.; Huang, Q.; Yang, C. Influences of fluid physical properties, solid particles, and operating conditions on the hydrodynamics in slurry reactors. Chin. J. Chem. Eng. 2022, 44, 51–71. [Google Scholar] [CrossRef]
- Li, L.; Xu, X.; Wang, W.; Lau, R.; Wang, C.H. Hydrodynamics and mass transfer of concentric-tube internal loop airlift reactors: A review. Bioresour. Technol. 2022, 359, 127451–127469. [Google Scholar] [CrossRef] [PubMed]
- Bergault, I.; Rajashekharam, M.V.; Chaudhari, R.V.; Schweich, D.; Delmas, H. Modeling and comparison of acetophenone hydrogenation in trickle-bed and slurry airlift reactors. Chem. Eng. Sci. 1997, 52, 4033–4043. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, Q.; Geng, S.; Chen, A.; Liu, Y.; Zhang, H. Impacts of solid physical properties on the performances of a slurry external airlift loop reactor integrating mixing and separation. Chin. J. Chem. Eng. 2023, 55, 1–12. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, W.; Yang, C. Modeling transport phenomena and reactions in a pilot slurry airlift loop reactor for direct coal liquefaction. Chem. Eng. Sci. 2015, 135, 441–451. [Google Scholar] [CrossRef]
- Li, G.; Chen, K.; Wei, Y.; Zeng, J.; Yang, Y.; He, F.; Li, H.; Ouyang, P. Mass transfer, gas holdup, and kinetic models of batch and continuous fermentation in a novel rectangular dynamic membrane airlift bioreactor. Engineering 2022, 13, 153–163. [Google Scholar] [CrossRef]
- Lohwacharin, J.; Annachhatre, A.P. Biological sulfide oxidation in an airlift bioreactor. Bioresour. Technol. 2010, 101, 2114–2120. [Google Scholar] [CrossRef]
- Chen, Z.; Min, H.; Hu, D.; Wang, H.; Zhao, Y.; Cui, Y.; Zou, X.; Wu, P.; Ge, H.; Luo, K.; et al. Performance of a novel multiple draft tubes airlift loop membrane bioreactor to treat ampicillin pharmaceutical wastewater under different temperatures. Chem. Eng. J. 2020, 380, 122521–122534. [Google Scholar] [CrossRef]
- Xu, P.; Wei, Y.; Ma, C.; Li, S.; Guo, T.; Wang, X.; Li, W. Multi-factorial analysis of the removal of dichloromethane and toluene in an airlift packing bioreactor. J. Environ. Manag. 2020, 261, 109665–109672. [Google Scholar] [CrossRef]
- Tao, J.; Huang, J.; Geng, S.; Gao, F.; He, T.; Huang, Q. Experimental investigation of hydrodynamics and mass transfer in a slurry multistage internal airlift loop reactor. Chem. Eng. J. 2020, 386, 122769–122778. [Google Scholar] [CrossRef]
- Li, D.; Guo, K.; Li, J.; Huang, Y.; Zhou, J.; Liu, H.; Liu, C. Hydrodynamics and bubble behaviour in a three-phase two-stage internal loop airlift reactor. Chin. J. Chem. Eng. 2018, 26, 1359–1369. [Google Scholar] [CrossRef]
- Ramonet, F.; Haddadi, B.; Harasek, M. Optimal Design of Double Stage Internal Loop Air-Lift Bioreactor. Energies 2023, 16, 3267. [Google Scholar] [CrossRef]
- Ramonet, F.; Haddadi, B.; Jordan, C.; Harasek, M. Modelling and Design of Optimal Internal Loop Air-Lift Reactor Configurations Through Computational Fluid Dynamics. Chem. Eng. Trans. 2022, 94, 817–822. [Google Scholar]
- Shi, J.; Guo, K.; Wang, Z.; Zheng, L.; Liu, H.; Xiang, W.; Liu, C.; Li, X. Computational fluid dynamics simulation of hydrodynamics in a two-stage internal loop airlift reactor with contraction-expansion guide vane. ACS Omega 2021, 6, 6981–6995. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, K.; Qi, W.; Zhang, T.; Liu, C. Gas holdup, bubble behaviour, and mass transfer characteristics in a two-stage internal loop airlift reactor with different screens. Can. J. Chem. Eng. 2017, 95, 1202–1212. [Google Scholar] [CrossRef]
- Li, S.; Qi, T. Hydrodynamics and flow regimes of a multi-stage internal airlift loop reactor. Mater. Focus 2014, 3, 205–210. [Google Scholar] [CrossRef]
- Li, S.; Qi, T.; Zhang, Y.; Liu, C. Hydrodynamics of a Multi-Stage Internal Loop Airlift Reactor. Chem. Eng. Technol. 2009, 32, 80–85. [Google Scholar] [CrossRef]
- Zuber, N.; Findlay, J.A. Average volumetric concentration in two-phase flow systems. J. Heat Transfer. 1965, 87, 453–468. [Google Scholar] [CrossRef]
- Behin, J. Modeling of modified airlift loop reactor with a concentric double-draft tube. Chem. Eng. Res. Des. 2010, 88, 919–927. [Google Scholar] [CrossRef]
- Yu, W.; Wang, T.; Liu, M.; Wang, Z. Liquid Backmixing and Particle Distribution in a Novel Multistage Internal-Loop Airlift Slurry Reactor. Ind. Eng. Chem. Res. 2008, 47, 3974–3982. [Google Scholar] [CrossRef]
- Yu, W.; Wang, T.; Liu, M.; Wang, Z. Bubble circulation regimes in a multi-stage internal-loop airlift reactor. Chem. Eng. J. 2008, 142, 301–308. [Google Scholar] [CrossRef]
- Yu, W.; Wang, T.; Song, F.; Wang, Z. Investigation of the Gas Layer Height in a Multistage Internal-Loop Airlift Reactor. Ind. Eng. Chem. Res. 2009, 48, 9278–9285. [Google Scholar] [CrossRef]
- Yu, W.; Wang, T.; Liu, M.; Song, F. Investigation of Operation Regimes in a Multistage Internal-Loop Airlift Reactor. Ind. Eng. Chem. Res. 2010, 49, 11752–11759. [Google Scholar] [CrossRef]
- Mohanty, K.; Das, D.; Biswas, M.N. Hydrodynamics of a novel multi-stage external loop airlift reactor. Chem. Eng. Sci. 2006, 61, 4617–4624. [Google Scholar] [CrossRef]
- Mohanty, K.; Das, D.; Biswas, M.N. Mass transfer characteristics of a novel multi-stage external loop airlift reactor. Chem. Eng. J. 2007, 133, 257–264. [Google Scholar] [CrossRef]
- Sarkar, S.; Mohanty, K.; Meikap, B. Hydrodynamic modeling of a novel multi-stage gas–liquid external loop airlift reactor. Chem. Eng. J. 2008, 145, 69–77. [Google Scholar] [CrossRef]
- Liu, Y.-Q.; Tay, J.-H. Cultivation of aerobic granules in a bubble column and an airlift reactor with divided draft tubes at low aeration rate. Biochem. Eng. J. 2007, 34, 1–7. [Google Scholar] [CrossRef]
- Kumar, N.; Gupta, R.; Bansal, A. Effect of Surface Tension on Hydrodynamics and Mass Transfer Coefficient in Airlift Reactors. Chem. Eng. Technol. 2020, 43, 995–1004. [Google Scholar] [CrossRef]
- Huang, Z.-B.; Cheng, Z.-M. Liquid circulation hydrodynamics in an external loop airlift reactor. Can. J. Chem. Eng. 2013, 91, 223–230. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, H.; Zhou, T.; Cheng, Z.; Huang, Z. Influence of sparger type on mass transfer in a pilot-scale internal loop airlift reactor. Processes 2022, 10, 429. [Google Scholar] [CrossRef]
- Vasconcelos, J.M.T.; Rodrigues, J.M.L.; Orvalho, S.C.P.; Alves, S.S.; Mendes, R.L.; Reis, A. Effect of contaminants on mass transfer coefficients in bubble column and airlift contactors. Chem. Eng. Sci. 2003, 58, 1431–1440. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Haryono, M.H.; Tomida, T.; Hatano, H.; Oaki, H. Performance of a hollow-fiber spiral disk for effective gas dispersion toward high mass transfer rate. Ind. Eng. Chem. Res. 1996, 35, 613–620. [Google Scholar] [CrossRef]
- Merchuk, J.C.; Ladwa, N.; Cameron, A.; Bulmer, M.; Pickett, A. Concentric-tube airlift reactors: Effects of geometrical design on performance. AIChE J. 1994, 40, 1105–1117. [Google Scholar] [CrossRef]
- Zhang, W.; Yong, Y.; Zhang, G.; Yang, C.; Mao, Z. Mixing characteristics and bubble behavior in an airlift internal loop reactor with low aspect ratio. Chin. J. Chem. Eng. 2014, 22, 611–621. [Google Scholar] [CrossRef]
- Gourich, B.; Vial, C.; Essadki, A.H.; Allam, F.; Belhaj Soulami, M.; Ziyad, M. Identification of flow regimes and transition points in a bubble column through analysis of differential pressure signal—Influence of the coalescence behavior of the liquid phase. Chem. Eng. Process. Process Intensif. 2006, 45, 214–223. [Google Scholar] [CrossRef]
- Blažej, M.; Kiša, M.; Markoš, J. Scale influence on the hydrodynamics of an internal loop airlift reactor. Chem. Eng. Process. Process Intensif. 2004, 43, 1519–1527. [Google Scholar] [CrossRef]
- Lee, J.S.; Jin, H.R.; Lim, H.; Lim, D.H.; Kang, Y.; Kim, S.D.; Jun, K.W. Interfacial area and liquid-side and overall mass transfer coefficients in a three-phase circulating fluidized bed. Chem. Eng. Sci. 2013, 100, 203–211. [Google Scholar] [CrossRef]
- van Baten, J.M.; Krishna, R. Comparison of hydrodynamics and mass transfer in airlift and bubble column reactors using CFD. Chem. Eng. Technol. 2003, 26, 1074–1079. [Google Scholar] [CrossRef]
- McClure, D.D.; Aboudha, N.; Kavanagh, J.M.; Fletcher, D.F.; Barton, G.W. Mixing in bubble column reactors: Experimental study and CFD modeling. Chem. Eng. J. 2015, 264, 291–301. [Google Scholar] [CrossRef]
- Sánchez Mirón, A.; Cerón García, M.C.; García Camacho, F.; Molina Grima, E.; Chisti, Y. Mixing in bubble column and airlift reactors. Chem. Eng. Res. Des. 2004, 82, 1367–1374. [Google Scholar] [CrossRef]
- Pandit, A.B.; Joshi, J.B. Mixing in mechanically agitated gas-liquid contactors, bubble columns and modified bubble columns. Chem. Eng. Sci. 1983, 38, 1189–1215. [Google Scholar] [CrossRef]





| Reactor type | Correlation | R2 |
|---|---|---|
| BCR | 0.996 | |
| SSALR | 0.999 | |
| FSALR | 0.999 |
| UG (cm/s) | FSALR Compared with SSALR (%) | FSALR Compared with BCR (%) |
|---|---|---|
| 0.9 | 17.1 | 113.9 |
| 1.5 | 29.4 | 86.2 |
| 3.0 | 17.9 | 129.7 |
| 4.5 | 15.2 | 143.6 |
| 6.0 | 12.8 | 151.4 |
| 7.5 | 10.5 | 152.7 |
| 9.1 | 8.1 | 161.2 |
| Reactor Type | Correlation | R2 |
|---|---|---|
| BCR | 0.994 | |
| SSALR | 0.997 | |
| FSALR | 0.991 |
| Reactor Type | Correlation | R2 |
|---|---|---|
| BCR | kLa = 0.0262lnUG + 0.0165 | 0.994 |
| SSALRs | kLa = 0.0243lnUG + 0.0129 | 0.995 |
| FSALR | kLa = 0.0244lnUG + 0.0159 | 0.995 |
| Reactor Type | Correlation | R2 |
|---|---|---|
| BCR | tm = 94.227UG−0.130 | 0.91 |
| SSALR | tm = 143.880UG−0.615 | 0.98 |
| FSALR | tm = 64.574UG−0.581 | 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Cheng, Z.; Yuan, P.; Huang, Z. The Influence of Draft Tubes on the Mass Transfer and Mixing Performance of a Pilot-Scale Internal-Loop Airlift Reactor. Processes 2023, 11, 2866. https://doi.org/10.3390/pr11102866
Wang Z, Cheng Z, Yuan P, Huang Z. The Influence of Draft Tubes on the Mass Transfer and Mixing Performance of a Pilot-Scale Internal-Loop Airlift Reactor. Processes. 2023; 11(10):2866. https://doi.org/10.3390/pr11102866
Chicago/Turabian StyleWang, Zongliang, Zhenmin Cheng, Peiqing Yuan, and Zibin Huang. 2023. "The Influence of Draft Tubes on the Mass Transfer and Mixing Performance of a Pilot-Scale Internal-Loop Airlift Reactor" Processes 11, no. 10: 2866. https://doi.org/10.3390/pr11102866
APA StyleWang, Z., Cheng, Z., Yuan, P., & Huang, Z. (2023). The Influence of Draft Tubes on the Mass Transfer and Mixing Performance of a Pilot-Scale Internal-Loop Airlift Reactor. Processes, 11(10), 2866. https://doi.org/10.3390/pr11102866







