Two-Stage Pretreatment of Jerusalem Artichoke Stalks with Wastewater Recycling and Lignin Recovery for the Biorefinery of Lignocellulosic Biomass
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
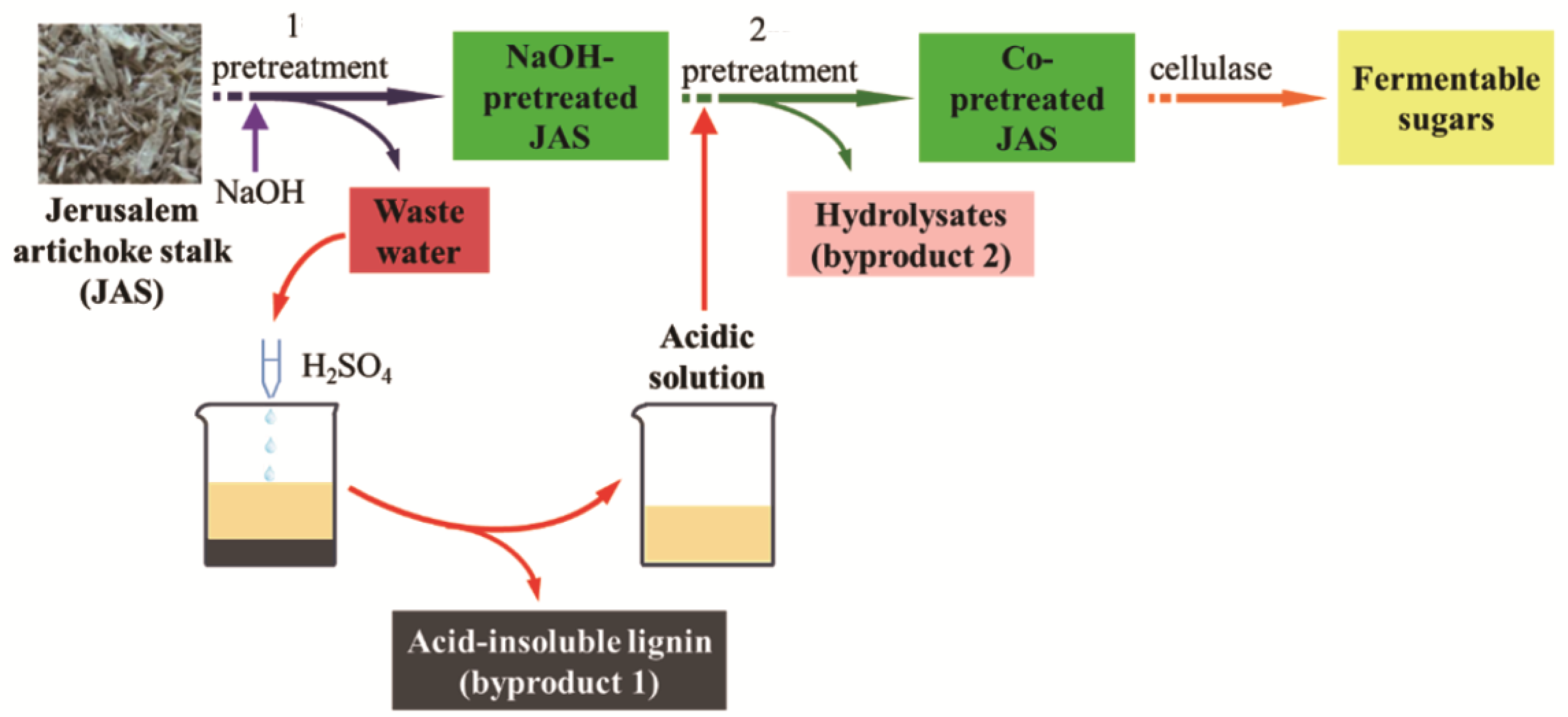
2.2. Pretreatment Process Design
2.3. Enzymatic Assays
2.4. Enzymatic Saccharification
2.5. Analysis Methods
2.6. Raw and Pretreated Material Characterization
2.6.1. Scanning Electron Microscopy (SEM) Analysis
2.6.2. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.6.3. Analysis of Cellulose Crystallinity
3. Results and Discussion
3.1. Chemical Composition of Raw and Pretreated Jerusalem Artichoke Stalk
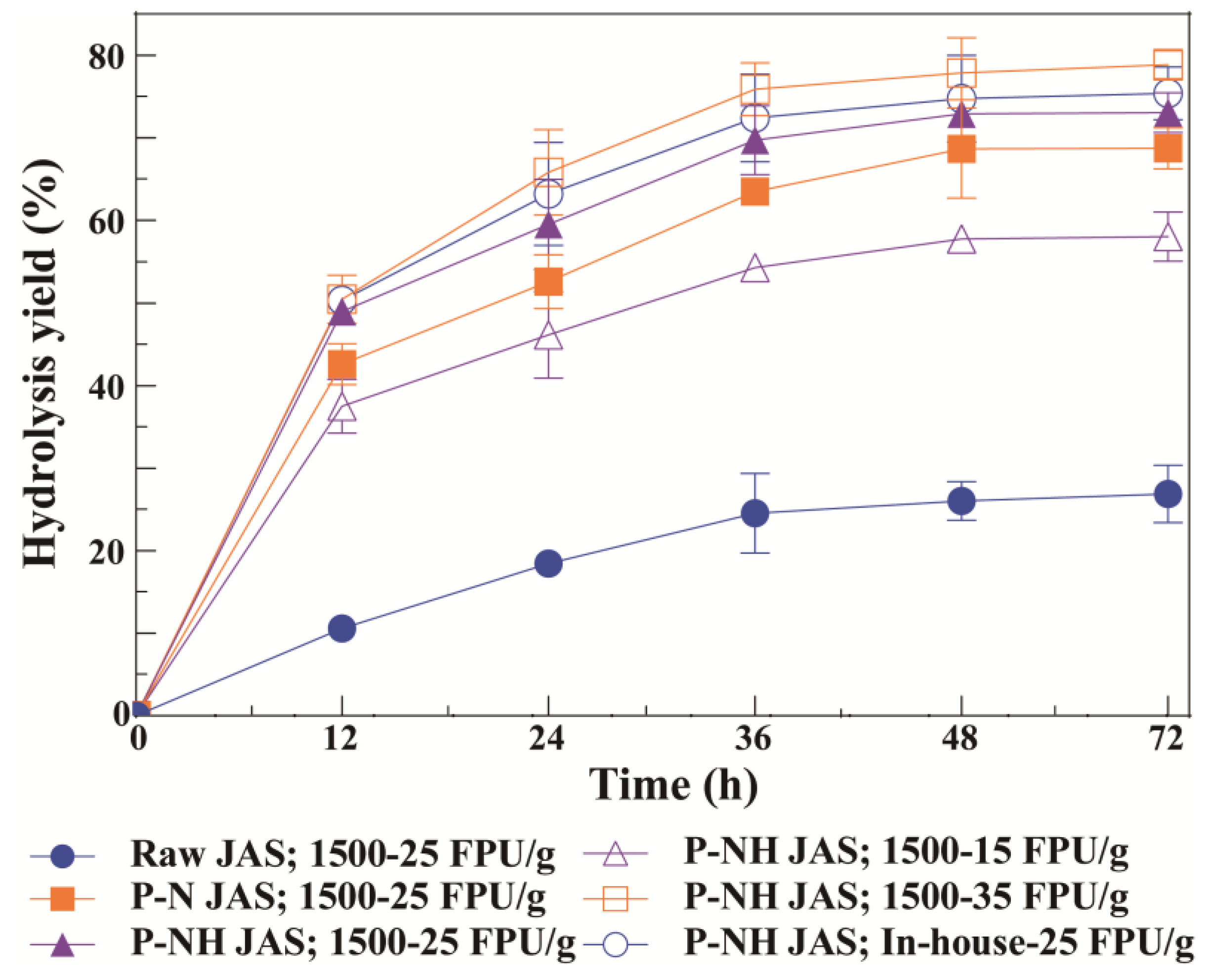
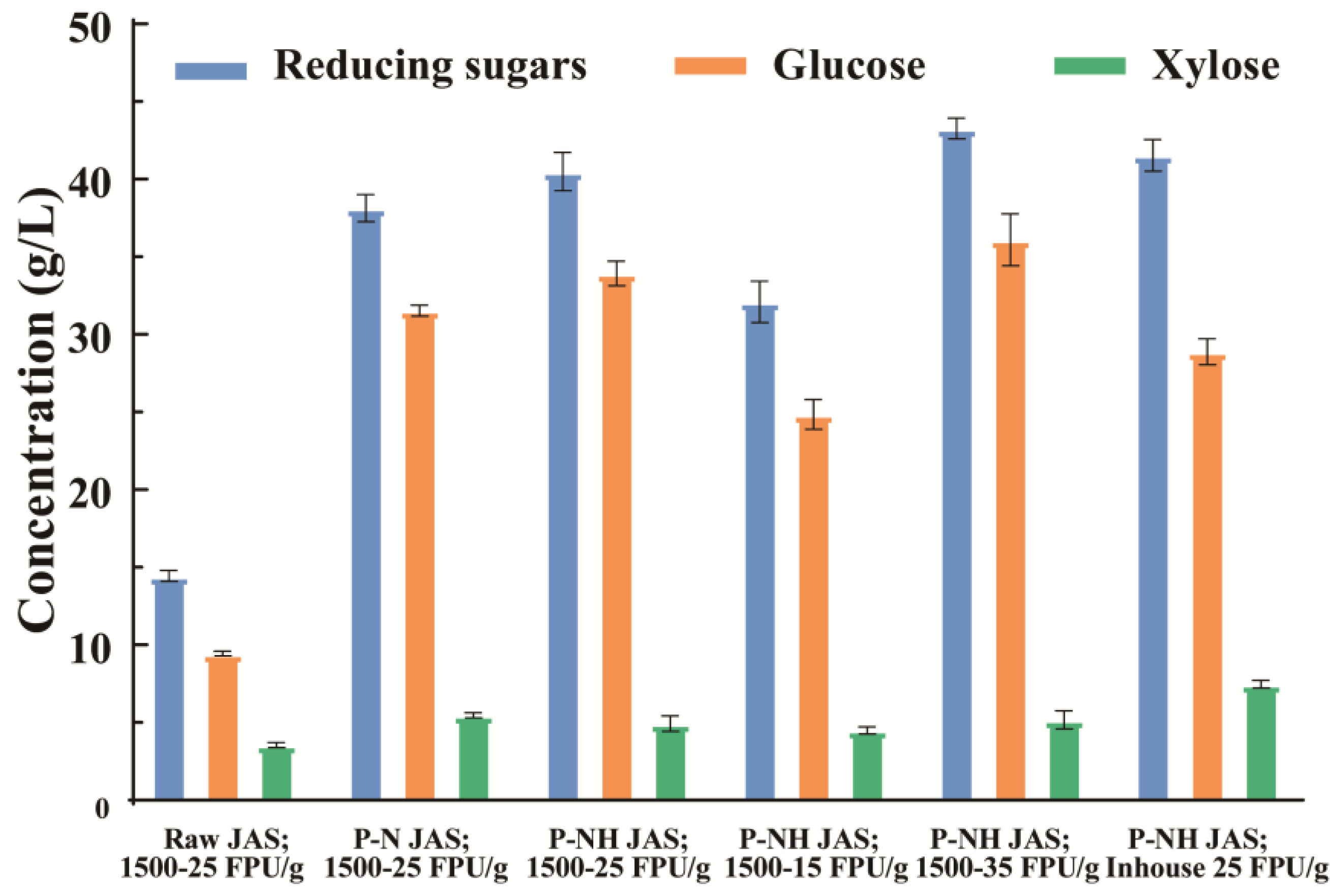
3.2. Enzymatic Hydrolysis of Raw and Pretreated JAS
3.3. Byproducts Obtained during the Two-Stage Pretreatment Process
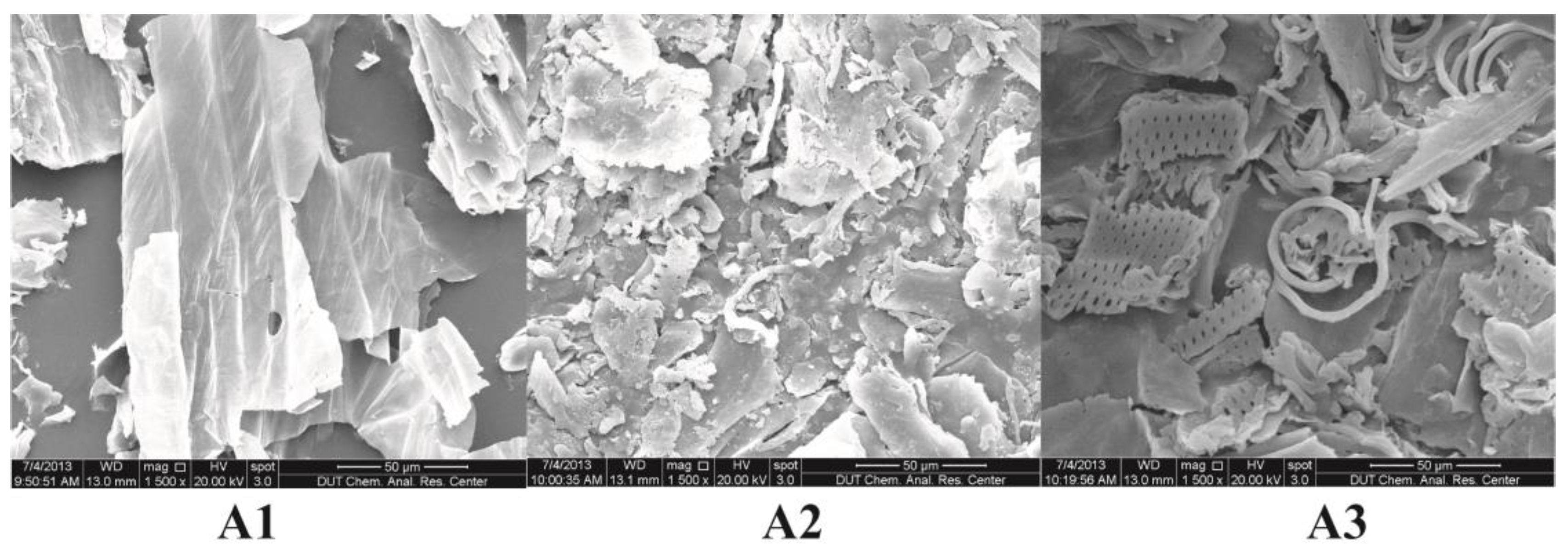
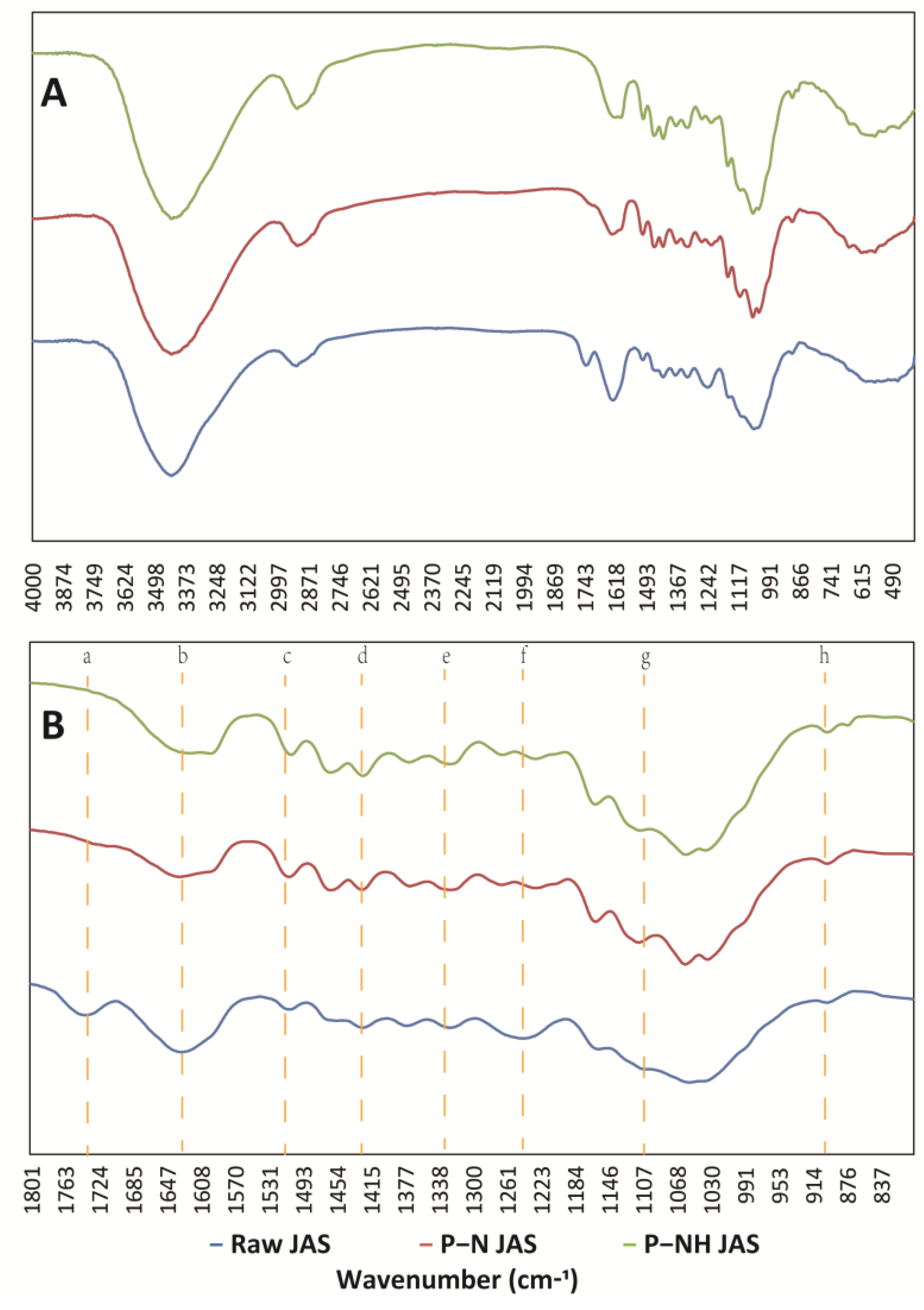
3.4. Structure of the Regenerated JAS Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| JA | Jerusalem artichoke |
| JAS | Jerusalem artichoke stalk |
| JAT | Jerusalem artichoke tuber |
| P-N JAS | NaOH-pretreated Jerusalem artichoke stalk |
| P-NH JAS | Combined alkali-acid pretreated Jerusalem artichoke stalk |
| NREL | National Renewable Energy Laboratory |
| DNS | Dinitrosalicylic acid |
| HMF | Hydroxymenthylfurfural |
| HPLC | High-Performance Liquid Chromatography |
| SEM | Scanning Electron Microscopy |
| FTIR | Fourier Transform infrared spectroscopy |
| XRD | X-ray diffraction |
| KBr | potassium bromide |
| CrI | Crystallinity index |
| CBHI | Cellobiohydrolase I |
| FPU | Filter Paper Unit |
| T. reesei | Trichoderma reesei |
References
- Fan, B.; Ni, J.; Li, Q.; He, Y.; Ma, C. Enhanced enzymatic saccharification of tomato stalk by combination pretreatment with NaOH and ChCl:Urea-Thioure in one-pot manner. Processes 2022, 10, 1905. [Google Scholar] [CrossRef]
- Jimenez-Gutierrez, J.M.; Verlinden, R.A.J.; Van der Meer, P.C.; Van der wielen, L.A.M.; Straathof, A.J.J. Liquid Hot Water Pretreatment of Lignocellulosic Biomass at Lab and Pilot Scale. Processes 2021, 9, 1518. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Patel, A.K.; Pant, D.; Banu, J.R.; Rao, C.V.; Kim, Y.G.; Yang, Y.H. Recent developments in pretreatment technologies on lignocellulosic biomass: Effect of key parameters, technological improvements, and challenges. Bioresour. Technol. 2019, 300, 122724. [Google Scholar] [CrossRef] [PubMed]
- Karungi, A.; Pogrebnoi, A.; Kivevele, T. Optimization of microwave-assisted alkali pretreatment followed by acid hydrolysis of sugarcane straw for production of acetone-butanol-ethanol. Energy Source Part A 2020, 1–17. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Q.; Chen, Y.D.; Peng, N.; Liu, W.S.; Wang, X.M.; Li, Y.H. Induction of cellulase production in Trichoderma reesei by a glucose–sophorose mixture as an inducer prepared using stevioside. RSC Adv. 2022, 12, 17392. [Google Scholar] [CrossRef]
- Byun, J.; Cha, Y.L.; Park, S.M.; Kim, K.S.; Lee, J.E.; Kang, Y.G. Lignocellulose pretreatment combining continuous alkaline single-screw extrusion and ultrasonication to enhance biosugar production. Energies 2020, 13, 5636. [Google Scholar] [CrossRef]
- Wu, J.; Dong, L.; Liu, B.; Xing, D.; Zhou, C.; Wang, Q.; Wu, X.; Feng, L.; Cao, G. A novel integrated process to convert cellulose and hemicellulose in rice straw to biobutanol. Environ. Res. 2020, 186, 109580. [Google Scholar] [CrossRef]
- Triwahyuni, E.; Hendarsyah, H.; Abimanyu, H. Reuse black liquor of alkali pretreatment in bioethanol production. Energy Procedia 2015, 68, 236–243. [Google Scholar] [CrossRef]
- Prajapati, B.P.; Kango, N. Evaluation of alkali black liquor recycling for rice straw delignification and its effect on enzymatic saccharification. Ind. Crops Prod. 2022, 180, 114709. [Google Scholar] [CrossRef]
- Han, M.; Moon, S.K.; Choi, G.W. Pretreatment solution recycling and high-concentration output for economical production of bioethanol. Bioproc. Biosyst. Eng. 2014, 37, 2205–2213. [Google Scholar] [CrossRef]
- Tofani, G.; Cornet, I.; Tavernier, S. Separation and recovery of lignin and hydrocarbon derivatives from cardboard. Biomass Conv. Bioref. 2022, 12, 3409–3424. [Google Scholar] [CrossRef]
- Mousavioun, P.; Doherty, W. Chemical and thermal properties of fractionated bagasse soda lignin. Ind. Crops Prod. 2010, 31, 52–58. [Google Scholar] [CrossRef]
- Liu, S.; Yu, Y.; Xu, Z.; Chen, S.; Shen, G.; Yuan, X.; Deng, Q.; Shen, W.; Yang, S.; Zhang, C.; et al. Efficient corncob biorefinery for ethanol initiated by a novel pretreatment of densifying lignocellulosic biomass with sulfuric acid. Fermentation 2022, 8, 661. [Google Scholar] [CrossRef]
- Zanellati, A.; Spina, F.; Bonaterra, M.; Dinuccio, E.; Varese, G.C.; Scarpeci, T.E. Screening and evaluation of phenols and furans degrading fungi for the biological pretreatment of lignocellulosic biomass. Int. Biodeter. Biodegr. 2021, 161, 105246. [Google Scholar] [CrossRef]
- Kim, S. Xylitol production from byproducts generated during sequential acid-/alkali-pretreatment of empty palm fruit bunch fiber by an adapted candida tropicalis. Front. Energy Res. 2019, 7, 72. [Google Scholar] [CrossRef]
- Dziekońska-Kubczak, U.; Berłowska, J.; Dziugan, P.; Patelski, P.; Balcerek, M.; Pielech-Przybylska, K.; Robak, K. Two-stage pretreatment to improve saccharification of oat straw and Jerusalem artichoke biomass. Energies 2019, 12, 1715. [Google Scholar] [CrossRef]
- Vaz, F.L.; da Rocha Lins, J.; Alencar, B.R.A.; de Abreu, Í.B.S.; Vidal, E.E.; Ribeiro, E.; Sampaio, E.V.D.S.B.; Menezes, R.S.C.; Dutra, E.D. Chemical pretreatment of sugarcane bagasse with liquid fraction recycling. Renew. Energy 2021, 174, 666–673. [Google Scholar] [CrossRef]
- Kashcheyeva, E.I.; Skiba, E.A.; Zolotukhin, V.N.; Budaeva, V.V. Recycling of nitric acid solution in chemical pretreatment of oat hulls for biorefining. Bioresources 2020, 15, 1575–1586. [Google Scholar] [CrossRef]
- Zou, H.X.; Zhao, D.; Wen, H.; Li, N.; Qian, W.; Yan, X.F. Salt stress induced differential metabolic responses in the sprouting tubers of Jerusalem artichoke (Helianthus tuberosus L.). PLoS ONE 2020, 15, e0235415. [Google Scholar] [CrossRef]
- Bedzo, O.; Mandegari, M.; Johann, F.; Görgens, J.F. Techno-economic analysis of inulooligosaccharides, protein, and biofuel co-production from Jerusalem artichoke tubers: A biorefinery approach. Biofuels Bioprod. Biorefin. 2020, 14, 766–793. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, D.; Yang, H.; Ren, H. Production of ethanol from Jerusalem artichoke by mycelial pellets. Sci. Rep.-UK 2019, 9, 18510. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ren, L.; Zhao, J.; Xia, Y.; Zhang, Z.; Guan, X.; Huang, S.; Wang, Q.; Wu, J.; Yu, Z.; et al. Ergosterol production at elevated temperatures by Upc2-overexpressing Kluyveromyces marxianus using Jerusalem artichoke tubers as feedstock. Bioresour. Technol. 2022, 362, 127878. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.; Kim, D.; Kim, J.; Kim, J.; Yoon, S.; Rhie, S.; Ha, S.; Ryu, Y. Optimization of alkaline pretreatment on corn stover for enhanced production of 1.3-propanediol and 2,3-butanediol by Klebsiella pneumoniae AJ4. Biomass Bioenergy 2015, 77, 177–185. [Google Scholar] [CrossRef]
- Guo, Z.W.; Ni, Z.F.; Zong, M.H. Modular metabolic engineering of Bacillus licheniformis for efficient 2,3-butanediol production by consolidated bioprocessing of Jerusalem Artichoke tubers. ACS Sustain. Chem. Eng. 2022, 10, 9624–9634. [Google Scholar] [CrossRef]
- Khatun, M.M.; Liu, C.G.; Zhao, X.Q.; Yuan, W.J.; Bai, F.W. Consolidated ethanol production from Jerusalem Artichoke tubers at elevated temperature by saccharomyces cerevisiae engineered with inulinase expression through cell surface display. J. Ind. Microbiol. Biotechnol. 2017, 44, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Matías, J.; Encinar, J.M.; González, J.; González, J.F. Optimisation of ethanol fermentation of Jerusalem artichoke tuber juice using simple technology for a decentralised and sustainable ethanol production. Energy Sustain. Dev. 2015, 25, 34–39. [Google Scholar] [CrossRef]
- Yuan, W.J.; Li, N.N.; Zhao, X.Q.; Chen, L.J.; Kong, L.; Bai, F.W. Engineering an industrial Saccharomyces cerevisiae strain with the inulinase gene for more efficient ethanol production from Jerusalem artichoke tubers. Eng. Life Sci. 2014, 13, 472–478. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, X.; Shi, X.; Hou, Y.; Yi, Y. Extraction and purification of inulin from Jerusalem Artichoke with response surface method and ion exchange resins. ACS Omega 2022, 7, 12048–12055. [Google Scholar] [CrossRef]
- Khatun, M.M.; Li, Y.H.; Liu, C.G.; Zhao, X.Q.; Bai, F.W. Fed-batch saccharification and ethanol fermentation of Jerusalem artichoke stalks by an inulinase producing Saccharomyces cerevisiae MK01. RSC Adv. 2015, 5, 107112–107118. [Google Scholar] [CrossRef]
- McIntosh, S.; Vancov, T. Enhanced enzyme saccharification of Sorghum bicolor straw using dilute alkali pretreatment. Bioresour. Technol. 2010, 101, 6718–6727. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, J.; Xia, L. Comparison of four different chemical pretreatments of corn stover for enhancing enzymatic digestibility. Biomass Bioenergy 2009, 33, 1381–1385. [Google Scholar] [CrossRef]
- Ghose, T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Kim, S.B.; Lee, S.J.; Lee, J.H.; Jung, Y.R.; Thapa, L.P.; Kim, J.S.; Um, Y.; Park, C.; Kim, S.W. Pretreatment of rice straw with combined process using dilute sulfuric acid and aqueous ammonia. Biotechnol. Biofuels 2013, 6, 109. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, M.; Wang, D. A comprehensive investigation on the effects of biomass particle size in cellulosic biofuel production. J. Energy Resour. Technol. 2018, 140, 041804. [Google Scholar] [CrossRef]
- Liu, K.; Lin, X.; Yue, J.; Li, X.; Fang, X.; Zhu, M.; Lin, J.; Qu, Y.; Xiao, L. High concentration ethanol production from corncob residues by fed-batch strategy. Bioresour. Technol. 2010, 101, 4952–4958. [Google Scholar] [CrossRef]
- Dziekońska-Kubczak, U.; Berłowska, J.; Dziugan, P.; Patelski, P.; Pielech-Przybylska, K.; Balcerek, M. Nitric acid pretreatment of Jerusalem Artichoke stalks for enzymatic saccharification and bioethanol production. Energies 2018, 11, 2153. [Google Scholar] [CrossRef]
- Li, K.; Qin, J.C.; Liu, C.G.; Bai, F.W. Optimization of pretreatment, enzymatic hydrolysis and fermentation for more efficient ethanol production by Jerusalem artichoke stalk. Bioresour. Technol. 2016, 221, 188–194. [Google Scholar] [CrossRef]
- Bischof, R.H.; Ramoni, J.; Seiboth, B. Cellulases and beyond: The first 70 years of the enzyme producer Trichoderma reesei. Microb. Cell Fact. 2016, 15, 1–13. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, P.; Zhu, D.; Yao, B.; Hasunuma, T.; Kondo, A.; Zhao, X. Efficient preparation of soluble inducer for cellulase production and saccharification of corn stover using in-house generated crude enzymes. Biochem. Eng. J. 2022, 178, 108296. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Gao, Y.; Mo, Y.; Long, T.; Yao, B.; Li, Y. Engineering Trichoderma reesei for the hyperproduction of cellulose induced protein 1 (Cip1) on a sophorose-containing inducer to efficiently saccharify alkali-pretreated corn stover. Prep. Biochem. Biotechnol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Long, T.; Zhang, P.; Yu, J.; Gao, Y.; Ran, X.; Li, Y. Regulation of β-disaccharide accumulation by β-glucosidase inhibitors to enhance cellulase production in Trichoderma reesei. Fermentation 2022, 8, 232. [Google Scholar] [CrossRef]
- Li, Y.; Yu, J.; Zhang, P.; Long, T.; Mo, Y.; Li, J.; Li, Q. Comparative transcriptome analysis of Trichoderma reesei reveals different gene regulatory networks induced by synthetic mixtures of glucose and β-disaccharide. Bioresour. Bioprocess. 2021, 8, 57. [Google Scholar] [CrossRef]
- Jacob, O.; van Lill, G.R.; den Haan, R. CRISPR-based multi-gene integration strategies to create Saccharomyces cerevisiae strains for consolidated bioprocessing. Appl. Sci. 2022, 12, 12317. [Google Scholar] [CrossRef]
- Arnthong, J.; Bussadee, P.; Phienluphon, A.; Deenarn, P.; Tulsook, K.; Plupjeen, S.-n.; Siamphan, C.; Tachaapaikoon, C.; Champreda, V.; Suwannarangsee, S. Overexpression of LAS21 in cellulase-displaying Saccharomyces cerevisiae for high-yield ethanol production from pretreated Sugarcane Bagasse. Fermentation 2022, 8, 652. [Google Scholar] [CrossRef]
- Klinke, H.B.; Thomsen, A.; Ahring, B.K. Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl. Microbiol. Biotechnol. 2004, 66, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Aslam, S.; Hussain, N.; Bilal, M.; Iqbal, H. Transforming lignin biomass to value: Interplay between ligninolytic enzymes and lignocellulose depolymerization. Bioenergy Res. 2022. [Google Scholar] [CrossRef]
- Stewart, D. Lignin as a base material for materials applications: Chemistry, application and economics. Ind. Crops Prod. 2008, 27, 202–207. [Google Scholar] [CrossRef]
- Asada, C.; Sasaki, C.; Takamatsu, T.; Nakamura, Y. Conversion of steam-exploded cedar into ethanol using simultaneous saccharification, fermentation and detoxification process. Bioresour. Technol. 2015, 176, 203–209. [Google Scholar] [CrossRef]
- Sanz, M.T. Subcritical water as pretreatment technique for bioethanol production from brewer’s spent grain within a biorefinery concept. Polymers 2022, 14, 5218. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, M.; Wu, Z. Pretreatment of sugarcane bagasse with NH4OH-H2O2 and ionic liquid for efficient hydrolysis and bioethanol production. Bioresour. Technol. 2012, 119, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Shi, Y.C.; Wang, D. X-ray scattering studies of lignocellulosic biomass: A review. Carbohydr. Polym. 2013, 94, 904–917. [Google Scholar] [CrossRef] [PubMed]
- Awoyale, A.A.; Lokhat, D. Experimental determination of the effects of pretreatment on selected nigerian lignocellulosic biomass in bioethanol production. Sci. Rep. 2021, 11, 557. [Google Scholar] [CrossRef] [PubMed]

| Enzymes | Specific Activity (U/mg Protein) | |
|---|---|---|
| Accellerase® 1500 | In-House Cellulase | |
| Protein (mg mL−1) | 27.16 ± 2.63 | 3.71 ± 0.58 |
| Cellulase | 4.40 ± 0.09 | 2.85 ± 0.21 |
| β-Glucosidase | 15.31 ± 1.33 | 1.00 ± 0.05 |
| Xylanase | 21.10 ± 1.25 | 204.80 ± 6.39 |
| Samples | Chemical Composition (%) | ||
|---|---|---|---|
| Cellulose | Hemicellulose | Lignin | |
| Raw JAS | 35.58 ± 0.11 | 21.73 ± 0.31 | 18.39 ± 0.13 |
| NaOH-pretreated JAS | 63.50 ± 0.32 | 25.68 ± 0.13 | 7.78 ± 0.05 |
| CO-pretreated JAS | 70.07 ± 0.59 | 17.63 ± 0.50 | 9.06 ± 0.01 |
| Pretreated Liquid | Inhibitor Composition (g/L) | ||
|---|---|---|---|
| Acetic Acid | Furfural | 5-HMF | |
| Raw JAS | 0.18 ± 0.09 | ND | ND |
| NaOH-pretreated JAS | 0.19 ± 0.01 | ND | ND |
| CO-pretreated JAS | 0.43 ± 0.10 | 0.06 ± 0.002 | ND |
| Sugar and Inhibitor Composition (g/L) | Pretreated Liquid | ||
|---|---|---|---|
| NaOH-Pretreated Liquid | Recycling Solution | Hydrolysates | |
| Reducing sugar | 7.93 ± 0.17 | 1.86 ± 0.02 | 9.85 ± 0.05 |
| Glucose | 3.37 ± 0.13 | 0.41 ± 0.04 | 1.07 ± 0.21 |
| Xylose | 3.29 ± 0.12 | 0.37 ± 0.05 | 5.82 ± 0.07 |
| Acetic acid | 1.75 ± 0.07 | 0.89 ± 0.04 | 1.42 ± 0.11 |
| Furfural | 0.25 ± 0.00 | 0.01 ± 0.00 | 0.16 ± 0.00 |
| 5-HMF | ND | ND | 0.08 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Peng, N.; Gao, Y.; Li, Q.; Wang, Z.; Yao, B.; Li, Y. Two-Stage Pretreatment of Jerusalem Artichoke Stalks with Wastewater Recycling and Lignin Recovery for the Biorefinery of Lignocellulosic Biomass. Processes 2023, 11, 127. https://doi.org/10.3390/pr11010127
Chen Y, Peng N, Gao Y, Li Q, Wang Z, Yao B, Li Y. Two-Stage Pretreatment of Jerusalem Artichoke Stalks with Wastewater Recycling and Lignin Recovery for the Biorefinery of Lignocellulosic Biomass. Processes. 2023; 11(1):127. https://doi.org/10.3390/pr11010127
Chicago/Turabian StyleChen, Yudian, Nian Peng, Yushan Gao, Qian Li, Zancheng Wang, Bo Yao, and Yonghao Li. 2023. "Two-Stage Pretreatment of Jerusalem Artichoke Stalks with Wastewater Recycling and Lignin Recovery for the Biorefinery of Lignocellulosic Biomass" Processes 11, no. 1: 127. https://doi.org/10.3390/pr11010127
APA StyleChen, Y., Peng, N., Gao, Y., Li, Q., Wang, Z., Yao, B., & Li, Y. (2023). Two-Stage Pretreatment of Jerusalem Artichoke Stalks with Wastewater Recycling and Lignin Recovery for the Biorefinery of Lignocellulosic Biomass. Processes, 11(1), 127. https://doi.org/10.3390/pr11010127







