A Novel Method to Detoxify Steam-Exploded Biomass and Produce a Substrate for Biorefinery
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass Pre-Treatment
2.2. Detoxification by Water Washing
2.3. Detoxification by Drying
2.4. Detoxification with a Bench Scale Vibro-Fluidized Bed System
2.5. Detoxification with a Pilot Scale Vibro-Fluidized Bed System
2.6. Analytical Methods
2.7. Hydrolysis and Fermentation Tests
3. Results and Discussion
3.1. Substrates Characterization
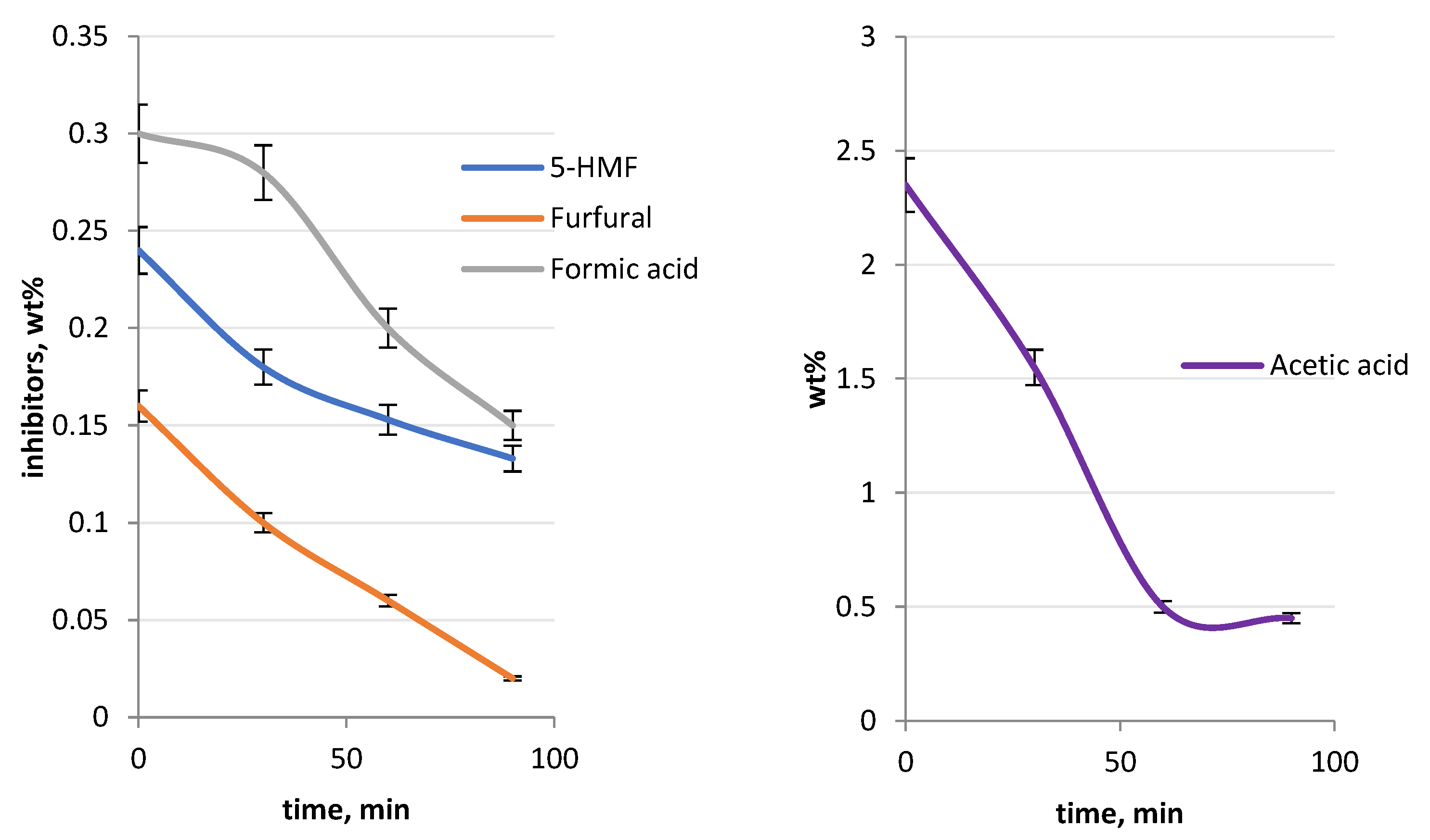
3.2. Detoxification with the Bench Scale Vibro-Fluidized Bed System
- To use steam-exploded biomass as a substrate for the fermentation process, the pulp needs to be detoxified;
- The detoxification with the vibro-fluidized system leads to higher yield of ethanol;
- When the substrate was detoxified by drying, a lower alcoholic production was observed. This could be related to a lower saccharification yield due to hornification;
- When the substrate is detoxified by washing, a lower alcoholic production was observed. This could be due to the loss of soluble carbohydrates.
3.3. Detoxification with the Pilot-Scale Vibro-Fluidized Bed System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chornet, E.; Overend, R.P. Phenomenological kinetics and reaction engineering aspects of steam/aqueous treatments. In Proceedings of the International Workshop on Steam Explosion Techniques, Milan, Italy, 20–21 October 1988; Focher, B., Marzetti, A., Crescenzi, V., Eds.; Gordon and Breach: Philadelphia, PA, USA, 1998. [Google Scholar]
- Olsson, L.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates for ethanol production. Enzym. Microb. Technol. 1996, 18, 312–331. [Google Scholar] [CrossRef]
- De Bari, I.; Viola, E.; Barisano, D.; Cardinale, M.; Nanna, F.; Zimbardi, F.; Cardinale, G.; Braccio, G. Ethanol production at flask and pilot scale from concentrated slurries of steam exploded aspen. Ind. Eng. Chem. Res. 2002, 41, 1745–1753. [Google Scholar] [CrossRef]
- Hongqiang, L.; Hongzhang, C. Detoxification of steam-exploded corn straw produced by an industrial-scale reactor. Process Biochem. 2008, 43, 1447–1451. [Google Scholar]
- Zimbardi, F.; Viggiano, D.; Demichele, M.; Cuna, D.; Cardinale, G. Steam explosion of straw in batch and continuous systems. Appl. Biochem. Biotechnol. 1999, 77–79, 117–132. [Google Scholar] [CrossRef]
- Viola, E.; Arcieri, G.; Zimbardi, F.; Valerio, V.; Cerone, N.; De Corato, U. Evaluation of a pilot-scaled paddle dryer for the production of ethanol from lignocellulose including inhibitor removal and high-solids enzymatic hydrolysis. Biotechnol. Rep. 2016, 9, 38–45. [Google Scholar] [CrossRef]
- Luo, X.; Zhu, J.Y. Effects of dryin-induced fiber hornification on enzymatic saccharification of lignocellulose. Enzym. Microb. Technol. 2011, 48, 92–99. [Google Scholar] [CrossRef]
- Bura, R.; Mansfield, S.D.; Saddler, J.N.; Bothast, R.J. SO2-catalyzed steam explosion of corn fiber for ethanol production. Appl. Biochem. Biotechnol. 2002, 98, 59–72. [Google Scholar] [CrossRef]
- Converti, A.; Domínguez, J.M.; Perego, P.; da Silva, S.S.; Zilli, M. Wood Hydrolysis and Hydrolyzate Detoxification for Subsequent Xylitol Production. Chem. Eng. Technol. 2000, 23, 1013–1020. [Google Scholar] [CrossRef]
- Marchal, R.; Ropars, M.; Vandecasteele, J.P. Conversion into acetone and butanol of lignocellulosic substrates pretreated by steam explosion. Biotechnol. Lett. 1986, 8, 365–370. [Google Scholar] [CrossRef]
- Cantarella, M.; Cantarella, L.; Gallifuoco, A.; Spera, A.; Alfani, F. Comparison of different detoxification methods for steam-exploded poplar wood as a substrate for the bioproduction of ethanol in SHF and SSF. Process Biochem. 2004, 39, 1533–1542. [Google Scholar] [CrossRef]
- Ge, J.-P.; Cai, B.-Y.; Liu, G.-M.; Ling, H.-Z.; Fang, B.-Z.; Song, G.; Yang, X.-F.; Ping, W.-X. Comparison of different detoxification methods for corn cob hemicelluose hydrolysate to improve ethanol production by Candida shehatae ACCC 20335. Afr. J. Microbiol. Res. 2011, 5, 1163–1168. [Google Scholar] [CrossRef][Green Version]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. I: Inhibition and detoxification. Bioresour. Technol. 2000, 74, 17–24. [Google Scholar] [CrossRef]
- Canilha, L.; Chandel, A.K.; Suzanne dos Santos Milessi, T.; Antunes, F.A.F.; Luiz da Costa Freitas, W.; das Graças Almeida Felipe, M.; da Silva, S.S. Bioconversion of Sugarcane Biomass into Ethanol: An Overview about Composition, Pretreatment Methods, Detoxification of Hydrolysates, Enzymatic Saccharification, and Ethanol Fermentation. J. Biomed. Res. 2012, 2012, 989572. [Google Scholar] [CrossRef] [PubMed]
- Devia, A.; Singha, A.; Bajarb, S.; Pantc, D.; Ud Dina, Z. Ethanol from lignocellulosic biomass: An in-depth analysis of pre-treatment methods, fermentation approaches and detoxification processes. J. Environ. Chem. Eng. 2021, 9, 105798. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.-O. Bioconversion of lignocellulose: Inhibitors and detoxification. Biotechnol. Biofuels 2013, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.D.; Ibarra, D.; Fernández, J.L.; Ballesteros, M. Different laccase detoxification strategies for ethanol production from lignocellulosic biomass by the thermotolerant yeast Kluyveromyces marxianus CECT 10875. Bioresour. Technol. 2012, 106, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Jurado, M.; Prieto, A.; Martínez-Alcalá, Á.; Martínez, Á.T.; Martínez, M.J. Laccase detoxification of steam-exploded wheat straw for second generation bioethanol. Bioresour. Technol. 2009, 100, 6378–6384. [Google Scholar] [CrossRef]
- Grzenia, D.L.; Schell, D.J.; Wickramsinghe, S.R. Detoxification of biomass hydrolysates by reactive membrane extraction. J. Membr. Sci. 2010, 348, 6–12. [Google Scholar] [CrossRef]
- Carter, B.; Squillace, P.; Gilcrease, P.C.; Menkhaus, T.J. Detoxification of a lignocellulosic biomass slurry by soluble polyelectrolyte adsorption for improved fermentation efficiency. Biotechnol. Bioeng. 2011, 108, 2053–2060. [Google Scholar] [CrossRef]
- de Albuquerque Wanderley, M.C.; Martín, C.; de Moraes Rocha, G.J.; Gouveia, E.R. Increase in ethanol production from sugarcane bagasse based on combined pretreatments and fed-batch enzymatic hydrolysis. Bioresour. Technol. 2013, 128, 448–453. [Google Scholar] [CrossRef]
- Roman, K.; Barwicki, J.; Rzodkiewicz, W.; Dawidowski, M. Evaluation of mechanical and energetic properties of the forest residues shredded chips during briquetting process. Energies 2021, 14, 3270. [Google Scholar] [CrossRef]
- Muntean, A.; Ivanova, T.; Hutla, P.; Havrland, B. Influence of raw material properties on the quality of solid biofuel and energy consumption in briquetting process. Agron. Res. 2017, 15, 1708–1715. [Google Scholar] [CrossRef]
- Arrieche, R.; Saloni, D.; van Dyk, H.; Lemaster, R.L. Evaluation of the energy balance for the production of briquettes from biomass. For. Prod. J. 2011, 61, 302–309. [Google Scholar] [CrossRef]



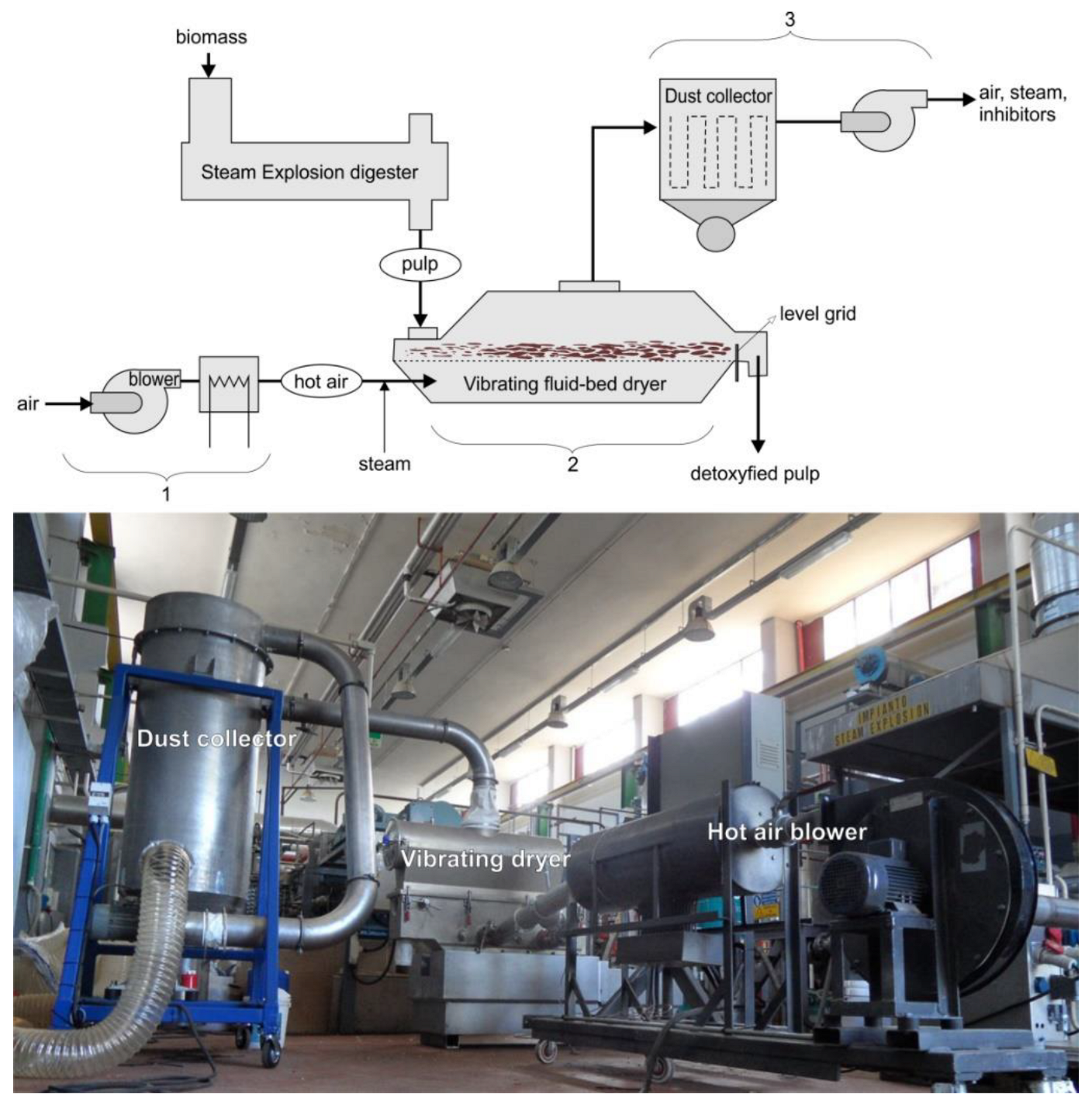



| Parameter | Setting |
|---|---|
| Oscillation amplitude | 2 cm |
| Oscillation frequency | 7 Hz |
| Airflow | 9 m3/h |
| Air pressure | 0.4 Barg |
| Steam flow 80 mL/h | 80 mL/h |
| Air–steam flow temperature | 60 °C |
| Factor | Name | Units | Minimum | Maximum | Coded Values | Mean | |
|---|---|---|---|---|---|---|---|
| A | Ur | % | 40 | 80 | −1.0 = 40 | 1.0 = 80 | 60 |
| B | t | min | 10 | 50 | −1.0 = 10 | 1.0 = 50 | 30 |
| Arundo Donax | Wheat Straw | |||||||
|---|---|---|---|---|---|---|---|---|
| Constituent, Wt% 1 | Raw | SE Pulp | WI Pulp | WS Pulp | Raw | SE Pulp | WI Pulp | WS Pulp |
| Glucan | 37.6 | 35.6 | 33.2 | 2.4 | 38.0 | 34.2 | 32.6 | 1.6 |
| Galactan | 0.7 | 0.4 | 0 | 0.4 | 0.7 | 0 | 0 | 0 |
| Xylan | 19.7 | 12.1 | 5.7 | 6.4 | 19.4 | 7.1 | 2.2 | 4.9 |
| Arabinan | 1.5 | 0.6 | 0.3 | 0.3 | 2.3 | 0.2 | 0 | 0.2 |
| Ashes | 4.9 | 4.9 | 2.2 | 2.5 | 6.9 | 6.9 | 2.9 | 4.0 |
| Lignin | 26.6 | 29.9 | 25.4 | 3.09 | 22.0 | 30.4 | 21.6 | 8.8 |
| Extractives 2 | 6.0 | 8 | ||||||
| Undetermined | 3.1 | 6.3 | 0.5 | 5.8 | 2.7 | 5.5 | 1.4 | 4.1 |
| Water | 61.3 | 144 | 164 | 995 | 13.6 | 101 | 250 | 665 |
| Inhibitors 3 | 2.75 | 2.75 | 2.41 | 2.41 | ||||
| DM balance | 100.0 | 89.8 | 67.2 | 22.6 | 100 | 84.3 | 60.7 | 23.6 |
| Compound, Wt% 1 | SE Arundo | SE Wheat Straw |
|---|---|---|
| Acetic acid | 2.35 | 2.44 |
| Formic acid | 0.3 | 0.01 |
| Furfural | 0.16 | 0.26 |
| 5-HMF | 0.24 | 0.14 |
| Catechol | traces | 0.01 |
| 4-hydroxybenzaldehyde | 0.01 | traces |
| Syringaldehyde | traces | traces |
| Exp. | Ur, % | t, min | DM, % | Inhibitors, % | Enz. Hydrolysis Yield, % |
|---|---|---|---|---|---|
| 1 | 80 | 50 | 83.5 | 0.136 | 80.6 |
| 2 | 60 | 10 | 81.5 | 0.428 | 88.2 |
| 3 | 60 | 50 | 84.1 | 0.206 | 83.6 |
| 4 | 40 | 30 | 94.7 | 0.198 | 81.1 |
| 5 | 80 | 30 | 87.5 | 0.096 | 85.7 |
| 6 | 80 | 10 | 70.8 | 0.381 | 85.7 |
| 7 | 40 | 10 | 92.6 | 0.416 | 85.6 |
| 8 | 60 | 30 | 93.2 | 0.171 | 88.8 |
| 9 | 40 | 50 | 94.9 | 0.375 | 86.2 |
| 10 | 60 | 30 | 95.5 | 0.172 | 84.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimbardi, F.; Viola, E.; Arcieri, G.; Valerio, V.; Carnevale, M. A Novel Method to Detoxify Steam-Exploded Biomass and Produce a Substrate for Biorefinery. Processes 2022, 10, 2611. https://doi.org/10.3390/pr10122611
Zimbardi F, Viola E, Arcieri G, Valerio V, Carnevale M. A Novel Method to Detoxify Steam-Exploded Biomass and Produce a Substrate for Biorefinery. Processes. 2022; 10(12):2611. https://doi.org/10.3390/pr10122611
Chicago/Turabian StyleZimbardi, Francesco, Egidio Viola, Giuseppe Arcieri, Vito Valerio, and Massimo Carnevale. 2022. "A Novel Method to Detoxify Steam-Exploded Biomass and Produce a Substrate for Biorefinery" Processes 10, no. 12: 2611. https://doi.org/10.3390/pr10122611
APA StyleZimbardi, F., Viola, E., Arcieri, G., Valerio, V., & Carnevale, M. (2022). A Novel Method to Detoxify Steam-Exploded Biomass and Produce a Substrate for Biorefinery. Processes, 10(12), 2611. https://doi.org/10.3390/pr10122611









