Abstract
Wheat straw is a suitable source material for bioethanol production. Removing lignin and hemicellulose in wheat straw to improve enzymatic hydrolysis efficiency is essential because of its complex structure. Deep eutectic solvents (DESs) have become substitutes for ionic liquids (ILs), with the characteristics of good biocompatibility, simple synthesis procedure and low cost. However, the process of removing lignin and hemicellulose using present DESs requires a high operation temperature or long operation time. Therefore, we studied a novel method under mild conditions for screening a series of novel DESs based on an inorganic base to remove lignin and hemicellulose in wheat straw. In this work, the effect of DES type, the pH of the DESs, the operation temperature and operation time for enhancing enzymatic hydrolysis, and the crystal structure and the chemical structure and surface morphology of wheat straw were investigated. In particular, Na:EG exhibited the most excellent solubility for wheat straw under mild conditions, removing 80.6% lignin and 78.5% hemicellulose, while reserving 87.4% cellulose at 90 °C for 5 h, resulting in 81.6% reducing sugar produced during hydrolysis for 72 h. Furthermore, XRD, FT-IR and SEM analysis verified the lignin and hemicellulose removal. Hence, DESs based on an inorganic base used for removing lignin and hemicellulose will enhance enzymatic hydrolysis, and thus promote the industrial application of wheat straw to produce bioethanol.
1. Introduction
Lignocellulose for second generation bioethanol production, such as agriculture wastes and wood residues, has attracted extensive attention from researchers. Wheat straw as agriculture waste has been considered as a good candidate for the raw material of bioethanol because of the advantages of renewability, low cost, abundance, and wide distribution [1,2]. However, the complex structure of lignocellulose, which consists of lignin, hemicellulose, and cellulose [3], limits efficient enzymatic hydrolysis and thus causes low bioethanol production. Lignin acts as a glue for binding hemicellulose and cellulose; hemicellulose acts as a martrix for the cellulose and a bridge between cellulose and lignin; thus, lignin and hemicellulose hinder the contact between cellulose and enzyme [4]. Therefore, the enzymatic hydrolysis of wheat straw should be enhanced to improve reducing sugar yield [5].
Chemical methods such as acids, bases, organic solvents, and ionic liquids (ILs) [6,7,8,9] are usually used to improve enzymatic hydrolysis efficiency by removing lignin and hemicellulose. Although ILs, which show many advantages such as strong dissolution ability, low vapor pressure, and high thermal stability, have the better performance on removing lignin and hemicellulose than other solvents [10], there still exist several drawbacks, such as the complex synthesis process, high cost and toxicity to enzymes and yeast [11]. These drawbacks cause a big controversy in the greenness of ILs [12].
Deep eutectic solvents (DESs), which are new-type IL analogues, have become more and more popular in the removal of lignin and hemicellulose because they do not have the drawbacks of ILs. DESs mainly consist of a hydrogen bond donor (HBD) and hydrogen bond acceptor (HBA) [13,14,15], and possess similar characteristics to ILs, for example, low vapor pressure and strong dissolution ability. However, there are some advantages of DESs that ILs do not have such as good biocompatibility, simple synthesis procedure, and low-cost raw material [16]. The advantages enhance the greenness of DESs [17]. In previous studies, DESs have exhibited excellent performance on removing lignin and hemicellulose in lignocellulose. Zulkefli et al. [18] used ethylammonium chloride: ethylene glycol (EAC:EG) to enhance enzymatic hydrolysis of palm efficiently by removing 42% of lignin and 83% of hemicellulose at 100 °C for 48 h, resulting in a maximum glucose yield of 74% for hydrolysis for 24 h. Li et al. [19] reported that choline chloride: urea (C:U) can remove 20% of lignin and 84.4% of hemicellulose in poplar wood at 115 °C for 15 h. Ji et al. [20] found choline chloride: lactic acid (C:L) is a good candidate for enhancing enzymatic hydrolysis. For example, 7.07% of cellulose conversion and 17.95% of hemicellulose conversion were achieved when sugarcane bagasse was hydrolyzed by enzymes for 24 h, which was attributed to C:L removing 80.13% of lignin and 78.08% of hemicellulose under ultrasound at 120 °C for 3 h. Morán-Aguilar et al. [21] reported choline chloride: glycerol (C:G) and C:U have good dissolution ability on sugar cane bagasse at 140 °C and 160 °C for 15 h. The glucan conversion was 56.29% and 68.57% after the residue was hydrolyzed by enzymes for 72 h, respectively. However, the current DES method for enhanced enzymatic hydrolysis reported in the literatures still poses several problems, such as requiring high operation temperature and long operation time, which leads to increased energy consumption and cost. Thus, it is necessary to find a new class of DES to remove lignin and hemicellulose under mild conditions for improving enzymatic hydrolysis efficiency.
In this work, three DESs based on an inorganic base (i.e., choline chloride: potassium hydroxide, potassium carbonate: glycerin and sodium hydroxide: ethylene glycol) and two DESs based on polyalcohol (i.e., choline chloride: ethylene glycol and choline chloride: propylene glycol) and an organic base (i.e., choline chloride: monoethanolamine) were studied to remove lignin and hemicellulose for enhancing the enzymatic hydrolysis of wheat straw. The influence of the operation temperature (i.e., 50 °C, 70 °C, 90 °C, 110 °C, 130 °C, 150 °C) and time (i.e., 1 h, 3 h, 5 h, 7 h, 9 h, 11 h) and the pH value of DESs on removing lignin and hemicellulose were also investigated. In addition, the crystallinity, chemical structure and surface morphology of wheat straw and wheat straw residues were investigated for revealing the mechanism of removing lignin and hemicellulose by DESs based on an inorganic base.
2. Materials and Methods
2.1. Materials
Wheat straw (50–80 mesh; Shijiazhuang farm); cellulase (C805042, enzymatic activity 10,000 U·g−1); monoethanolamine, propylene glycol, glycerol and potassium carbonate (purity: 99%), potassium hydroxide (purity: 95%), ethylene glycol and choline chloride (purity 98%). All above-mentioned chemical reagents were purchased from Shanghai Maclean Biochemical Technology Co., Ltd. In order to prepare DESs, the HBD and HBA were mixed at the certain molar ratio listed in Table 1, and then magnetically stirred at 90°C until colorless and transparent liquids were formed [22,23,24,25,26].

Table 1.
The composition of DESs.
2.2. Component Analysis
2.2.1. Determination of Cellulose and Hemicellulose Content
Firstly, 5 mL KOH solution (4 mol·L−1), which contained NaBH4 (1 mg·mL−1), was used to treat 0.1 g wheat straw samples at 25 °C for 1 h. Afterwards, the volume of suspension was brought to volume reaching 20 mL by adding an equal volume of KOH solution (4 mol·L−1) and 10 mL deionized water. The supernatant was taken to measure the absorbance of glucose and xylose at 620 nm and 660 nm, respectively. The glucose and xylose concentrations were calculated according to the absorbance and the predetermined standard curves of glucose (y = 102.75x + 0.0075, R2 = 0.9992) and xylose (y = 300.97x − 0.016, R2 = 0.9994) [27].
Then, alkali-treated residue continued to be treated with 4 mL sulfuric acid (72%, w/w) at 25 °C for 1 h. Afterwards, the deionized water was added into the treatment fluid until reaching 30 mL. The supernatant was taken to measure the absorbance of glucose and xylose at 620 nm and 660 nm, respectively. The glucose and xylose concentrations were calculated according to the absorbance and the predetermined standard curves of glucose (y = 102.75x + 0.0075, R2 = 0.9992) and xylose (y = 300.97x − 0.016, R2 = 0.9994) [27]. The cellulose content was calculated from the glucose concentration obtained by acid treatment with dehydration correction coefficient of 0.9 for glucose. The hemicellulose content was calculated according to the concentrations of glucose and xylose obtained by alkali treatment and the concentration of xylose obtained by acid treatment with dehydration coefficient of 0.9 for glucose and 0.88 for xylose. The hemicellulose removal rate and cellulose preservation rate were calculated according to Equations (1) and (2).
Cellulose preservation rate = (residue recovery rate × cellulose content of residue)/cellulose content of untreated wheat straw
Hemicellulose removal rate = 1 − (residue recovery rate × hemicellulose content of residue)/(hemicellulose content of untreated wheat straw)
2.2.2. Determination of Lignin Content
A 0.3 g sample of pretreatment residue was treated with 10 mL sulfuric acid (72%, w/w) to hydrolyze at 30 °C for 1.5 h. Subsequently, the hydrolysis residues and filtrate were separated by filtering the hydrolysate. The hydrolysis residues, which contain lignin and ash, were used to determine acid-insoluble lignin content by weighing. The filtrate was used to determine acid-soluble lignin by measuring the absorbance at 205 nm [28]. The total lignin content was calculated by the sum of acid-insoluble lignin content and acid-soluble lignin content. The lignin removal rate was calculated according to equation (3).
Lignin removal rate = 1 − (residue recovery rate × lignin content of residue)/(lignin content of untreated wheat straw)
2.3. Process of Enhancing Enzymatic Hydrolysis by DESs
Wheat straw and DESs were mixed with mass ratio of 1:20, and then magnetically stirred for 1–11 h at 50–130 °C. Afterwards, the deionized water was added into pretreatment suspension and then the diluted suspension was centrifuged at 3000 r/s to separate solid and liquid. Subsequently, the solid, i.e., residue, was washed by using deionized water and centrifuging suspension until the supernatant was neutral. Finally, the washed residues were freeze-dried for 48 h, and then stored at room temperature.
The severity factor (R0) of treatment was calculated by Equation (4) [29]:
t-operation time (min), T-operation temperature (°C).
2.4. Enzymatic Hydrolysis
The mixture of 0.4 g wheat straw, 52.5 mL dipotassium hydrogen phosphate-citrate buffer solution (pH = 4.8) and 7.5 mL cellulase solution (10,000 U·g−1) was heated at 45 °C in water bath oscillator at 120 r·min−1 for 72 h. The hydrolysate was taken 0.6 mL at certain time intervals and then heated in a boiling water bath [30]. After cooling to room temperature, the sample was used to determine reducing sugar yield by DNS method with the predetermined reducing sugar standard curve (y = 1.68x − 0.075, R2 = 0.9998).
2.5. X-ray Diffraction Analysis
The crystallinity of untreated wheat straw and residues was analyzed by using Bruker D8 Advance diffractometer. The scanning range was 5°–50° with the rate of 5°/min. The crystallinity of samples was calculated from the spectrum peak intensity according to Equation (5) [29].
CrI—crystallinity index, I002—intensity of crystalline cellulose peak at 2θ = 22.5°, Iam—intensity of lignin, hemicellulose and amorphous cellulose peak at 2θ = 18°
2.6. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis
A Vertex 80 V spectrometer was used for analyzing chemical structure of untreated wheat straw and residues [29]. Samples and potassium bromide were pressed into tablets in a mass ratio of 1:100. Each tablet was scanned for 32 s with the wavenumber range of 4000–400 cm−1 and the resolution of 2 cm−1.
2.7. Scanning Electron Microscope Analysis
The samples were evenly dispersed on discs coated with conductive adhesive and then were sprayed with gold. Then, the surface appearances of untreated wheat straw and residues were observed with JSM-IT100 (A) scanning electron microscope (SEM) [23].
3. Results and Discussion
3.1. Enhancement of Enzymatic Hydrolysis of Wheat Straw with Different DESs
Figure 1 shows the influence of DES type on the enhancement of the enzymatic hydrolysis of wheat straw. In Figure 1, the residue recovery rates of C:K and Na:EG-treated residues were obviously lower than the other DESs, which demonstrates that C:K and Na:EG can remove more of the three components than the other DESs. More than 80% of the cellulose was retained from residues obtained from the enhancement of enzymatic hydrolysis by six DESs. This indicates that six kinds of DES almost cannot dissolve cellulose; the more the cellulose is reserved, the more sugar may be produced. The reservation of cellulose is a benefit of enzymatic hydrolysis. C:M, C:K, Na:EG and K:G have a better capacity to dissolve lignin and hemicellulose than C:PG and C:EG (lignin and hemicellulose removal rate: 49.8–85.5% and 53.3–85.8% vs. 5.2–10.6% and 11.1–16.1%). This can be explained by the pH value of DESs. We measured the pH value of 0.1 mol·L−1 DES aqueous solutions listed in Table 2. The pH values of C:M, C:K, NaEG and K:G are all above 11, which indicates that these four DESs are basic solvents, while, C:PG and C:EG are neutral solvents. Therefore, basic solvents promote the removal of lignin and hemicellulose, but remove less cellulose.
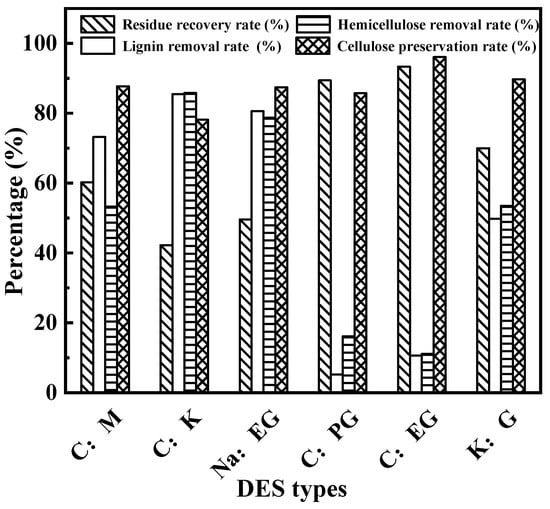
Figure 1.
Enhancement of enzymatic hydrolysis of wheat straw with different DESs.

Table 2.
pH value of DESs.
Among the four basic DESs, C:K and Na:EG exhibit excellent performance for removing lignin and hemicellulose. After C:K treatment, the lignin and hemicellulose removal rates were 85.5% and 85.8%, respectively. In total, 80.6% of lignin and 78.7% of hemicellulose were removed from wheat straw by Na:EG. This is also due to C:K and Na:EG having stronger basicity than the other DESs. Figure 2 shows the correlations between the pH value of DESs and the lignin or hemicellulose removal rate. It can be observed from Figure 2, the pH value of DESs is positively correlated with lignin and hemicellulose removal rate, which indicates that the stronger the basicity of the solvent, the more lignin and hemicellulose are removed. This similar conclusion was also summarized by our previous study [31].
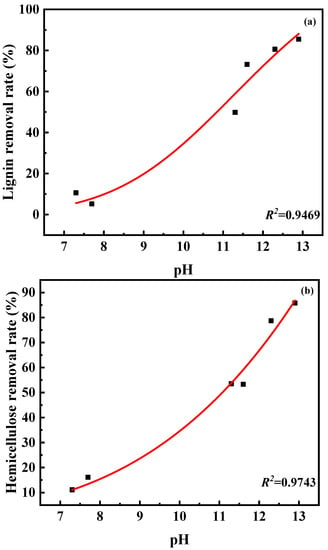
Figure 2.
Correlation between pH value of DESs and lignin removal rate (a) and hemicellulose removal rate (b).
Figure 3 represents the influence of DES type on enzymatic hydrolysis. It can be seen from Figure 3, with the prolongation of enzymatic hydrolysis time, the production of reducing sugar at first increases rapidly, and then there is little change. The initial saccharification rate and reducing sugar yield after hydrolysis for 72 h of C:PG- and C:EG-treated residues were similar to those of untreated wheat straw (1.6–2.7 mg·mL−1·h−1 and 12.4–14.0% vs. 1.4 mg·mL−1·h−1 and 9.1%, respectively). This indicates C:PG and C:EG have little effect on enhancing enzymatic hydrolysis. However, basic DESs, especially C:K and Na:EG, showed distinct accelerations on improving reducing sugar yield. For example, amounts of 16.2 mg·mL−1 and 9.7 mg·mL−1 of reducing sugar were produced, respectively when C:K and Na:EG-treated residue was hydrolyzed for 1 h; as much as 93.8% and 81.6% of reducing sugar was obtained after hydrolyzing for 72 h. The initial saccharification rate of C:K and Na:EG-treated residues were 11.6 times and 6.9 times as much as that of wheat straw. The reducing sugar yields obtained by hydrolysis for 72 h of C:K and Na:EG-treated residues were 10.3 times and 9 times that of wheat straw. Figure 4 illustrates the correlation between the reducing sugar yield and lignin removal rate or hemicellulose removal rate. As depicted in Figure 4, the reducing sugar yield is also positively correlated with the lignin and hemicellulose removal rate. The more the lignin or hemicellulose was removed, the higher the reducing sugar yield obtained.
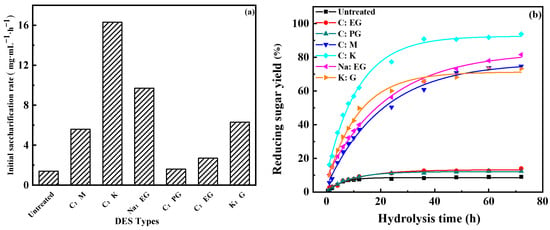
Figure 3.
Effect of DES type on enzyme hydrolysis: (a) initial saccharification rate, (b) reducing sugar yield.
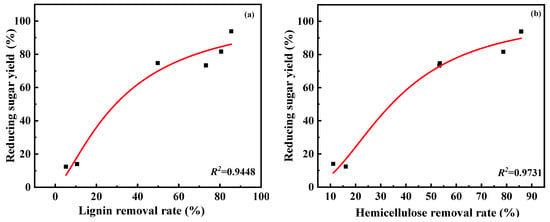
Figure 4.
Correlation between reducing sugar yield and lignin removal rate (a) and hemicellulose removal rate (b).
Although C:K has a preferable dissolving ability of lignin and hemicellulose, the high viscosity, which was 33.8 cP at 90 °C, and the release of a pungent odor during the preparation process, offset the advantage of the great facilitation of hydrolysis. Therefore, we investigated the operation temperature and operation time by using Na:EG.
3.2. Influence of Operation Temperature on Enhancing Enzymatic Hydrolysis of Wheat Straw
Operation temperature was investigated by using Na:EG for enhancing the enzymatic hydrolysis of wheat straw for 12 h, as depicted in Figure 5. In Figure 5, it can be seen that the recovery rates of residue gradually decreased with the increase in operation temperature, which shows that the rise of operation temperature contributes to the removal of the three components of the wheat straw. The lignin and hemicellulose removal rates first rapidly increased and then changed little with increasing operation temperature. The lignin removal rate–operation temperature and hemicellulose removal rate–operation temperature bar graphs show maximum values at 110 °C and 90 °C, respectively. At the two temperatures, the lignin and hemicellulose removal rates reach 90.5% and 78.7%, respectively. In addition, the increase in temperature also caused cellulose degradation. For example, the cellulose preservation rate dropped from 98.4% to 79.2%, when the operation temperature varied from 50 °C to 150 °C. As a consequence, the operation temperature need not be too high.
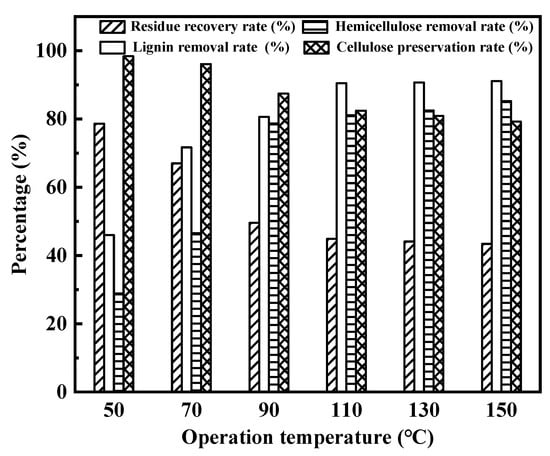
Figure 5.
Influence of operation temperature on enhancing enzymatic hydrolysis of wheat straw.
The initial saccharification rates and reducing sugar yields of pretreated residues at different temperatures are shown in Figure 6. As depicted in Figure 6, the production of reducing sugar first rapidly increased, then changed little, with prolonged hydrolysis time. The initial saccharification rates and reducing sugar yields of the residues at different temperatures significantly increased (1.4 mg·mL−1·h−1 vs. 5.4–11.0 mg·mL−1·h−1 and 9.1% vs. 59.3–84.3%), compared with untreated wheat straw. In addition, the initial saccharification rate and reducing sugar yield resulting from hydrolysis for 72 h increased with operation temperature. When the operation temperature was varied from 50 °C to 90 °C, the initial saccharification rises rose from 5.4 mg·mL−1·h−1 to 9.9 mg·mL−1·h−1 and the reducing sugar yield rose from 59.3% to 81.6%. This demonstrates that further enzymatic hydrolysis is favored by lignin and hemicellulose. When the operation temperature continued to increase to 150 °C, the initial saccharification rate and reducing sugar yield showed little change, which also indicates that moderate temperatures also can promote hydrolysis. Thus, 90 °C was chosen to continue investigating the operation time.
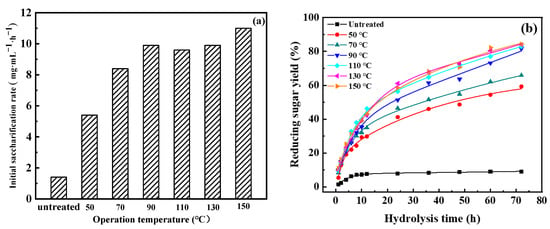
Figure 6.
Effect of operation temperature on enzymatic hydrolysis: (a) initial saccharification rate, and (b) reducing sugar yield.
3.3. Influence of Operation Time on Enhancing Enzymatic Hydrolysis of Wheat Straw
Operation time was investigated by using Na:EG for wheat straw pretreatment at 90 °C, as depicted in Figure 7. In Figure 7, with the prolongation of operation time, the recovery rates of residue decreased. It can be seen from this phenomenon that the longer the operation time, the more the three components of wheat straw were removed. In addition, with the increase in operation time, the lignin and hemicellulose removal rate sharply increased from 53.0% to 80.6% and from 46.8% to 84.2%, respectively; however, the removal rates barely changed until the time was prolonged to 5 h and 7 h, respectively. Moreover, when the operation time went longer, the cellulose degradation became more and more serious. Therefore, the operation time should be chosen by considering the balance between cellulose loss and lignin and hemicellulose removal.
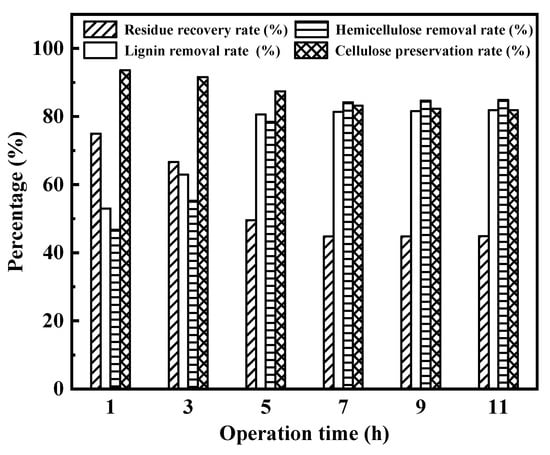
Figure 7.
Influence of operation time on enhancing enzymatic hydrolysis of wheat straw.
Figure 8 shows the enzymatic hydrolysis results of Na:EG-treated residues for different operation times at 90 °C. In Figure 8, the production of reducing sugar first rapidly increased, then changed little, with prolonged hydrolysis time. The initial saccharification rates and reducing sugar yields after hydrolysis for 72 h of residues were obviously higher than untreated wheat straw (1.4 mg·mL−1·h−1 vs. 8.0~10.8 mg·mL−1·h−1 and 9.1% vs. 58.9%~84.0%). In addition, the initial saccharification rate and reducing sugar yield after 72 h of hydrolysis increased with prolonged operation time, which was due to the removal of lignin and hemicellulose. Similar hydrolysis results were obtained when the pretreatment time varied from 5 h to 11 h, e.g., the initial saccharification rate only changed from 10.4 mg·mL−1·h−1 to 10.8 mg·mL−1·h−1, and the reducing sugar yield only changed from 81.6% to 84.0%. This also indicates an appropriate operation time that is enough to obtain satisfying hydrolysis results and small cellulose loss. Thus, 5 h can be chosen as the optimal operation time.
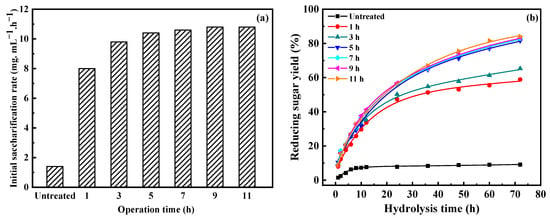
Figure 8.
Influence of operation time on enzymatic hydrolysis: (a) initial saccharification rate, and (b) reducing sugar yield.
3.4. Severity Factor
The severity factor (R0) of all operation conditions (50–150 °C and 1–11 h) was calculated to investigate the effect of operation conditions on lignin removal, hemicellulose removal, and reducing sugar yield, as shown in Figure 9. In Figure 9, it can be observed that the reducing sugar yield was only 60–65% when the severity factor was below 2.18. However, the lignin and hemicellulose removal rate, and the reducing sugar yield increased significantly to 80%. This demonstrates that the increase in reducing sugar yield was associated with an increase in the lignin and hemicellulose removal rate, which is consistent with the removal of lignin and hemicellulose and enzymaitc hydrolysis results.
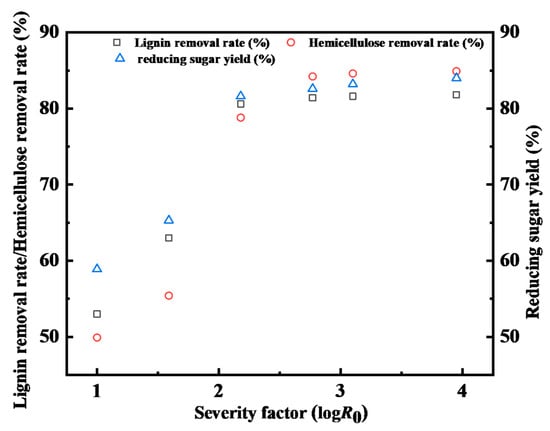
Figure 9.
Correlation of lignin removal, hemicellulose removal, and reducing sugar yields with severity factor (log R0).
3.5. Comparison of Different Chemical Methods for Enhancing Enzymatic Hydrolysis of Wheat Straw
Table 3 shows the comparison of different chemical methods for enhancing the enzymatic hydrolysis of wheat straw. As shown in Table 3, Na:EG is highly competitive compared with traditional solvents, ILs, acidic DESs, and DESs coupled with a base. For instance, traditional solvents used for removing lignin and hemicellulose need harsh operation conditions, such as high temperature (121–205 °C), while the operation temperature was only 90 °C when Na:EG was used to remove lignin and hemicellulose. In addition, there are several drawbacks, such as high operation temperature, long operation time and low sugar yield, when 1-Ethyl-3-methylimidazolium diethyl phosphate, Triethylbenzylammonium chloride: lactic acid or choline chloride: urea (1:2)—10 wt% H2O—1 wt% NaOH was used for removing lignin and hemicellulose in wheat straw, as listed in Table 3.

Table 3.
The comparison of different chemical methods.
3.6. Characterization Analysis
3.6.1. XRD Analysis
Figure 10 shows the change of the crystal structures of untreated wheat straw and residue. In Figure 10, the CrI value of Na:EG-treated residue is distinctly higher than that of untreated wheat straw (56.6 vs. 45.2), which suggests that Na:EG contributes to the obviously increased degree of crystalline of wheat straw. One reason can explain this phenomenon: basic Na:EG can remove much more lignin and hemicellulose than cellulose, thus the removal of lignin and hemicellulose, which are both disordered structures, results in an increase in the degree of order in the wheat straw structure [38,39]. The increase in the CrI value of the residue confirms the removal of lignin and hemicellulose by Na:EG.
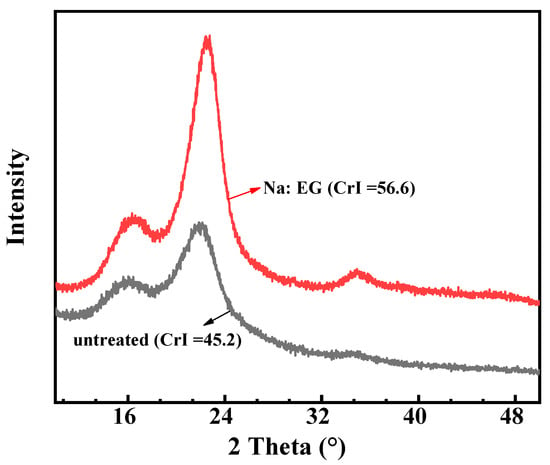
Figure 10.
XRD pattern of untreated wheat straw and Na:EG-treated residue.
3.6.2. FT-IR Analysis
Figure 11 depicted the chemical structure changes between untreated wheat straw and residue. In Figure 11, compared with untreated wheat straw, the stretching vibration peak at 1735 cm−1 in Na:EG-treated residue, which is the C=O stretching vibration peak of the carbonyl or ester group, almost disappeared [40], demonstrating that the ester bond between the lignin and hemicellulose was almost completely broken and the carbonyl group in the hemicellulose was almost all removed. The peaks at 1618 cm−1, 1512 cm−1, and 1242 cm−1 were significantly weakened, indicating that the aromatic ring skeleton in the lignin was seriously damaged [41]. It is consistent with the results in Figure 7. Thus, the changes in the chemical structure of the residues confirm that the lignin and hemicellulose can be removed by Na:EG.
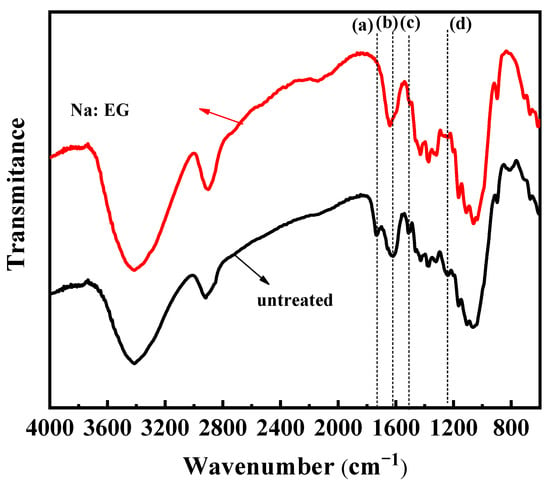
Figure 11.
FT-IR spectra of untreated wheat straw and Na:EG-treated residue: (a) 1735 cm−1, (b) 1618 cm−1, (c) 1512 cm−1, and (d) −1242 cm−1.
3.6.3. SEM Analysis
SEM characterization was performed to investigate the changes in the surface morphology of untreated wheat straw and Na:EG-treated residue, as shown in Figure 12. It can be observed in Figure 12 that untreated wheat straw presents dense fiber bundle structures with smooth and flat surface, which makes it difficult to achieve contact between the enzyme and the cellulose. In contrast, SEM images of the Na:EG-treated residue show that the surface of the residue is obviously uneven and rough, and the fiber bundle structures became loose and exposed in a disorderly manner. This is because Na:EG removes a large amount of lignin and hemicellulose (i.e., disordered structure), which greatly promotes the accessibility of the enzyme to the cellulose, thus increasing the reducing sugar yield, as shown in Figure 7 and Figure 8. Therefore, the changes in the surface morphology of the residue verify that Na:EG can remove the lignin and hemicellulose.
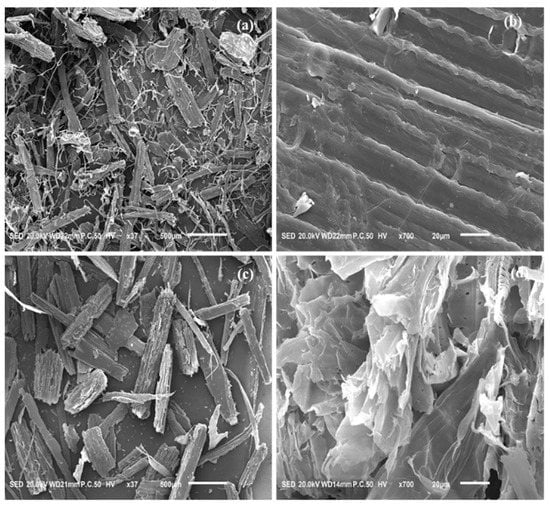
Figure 12.
Surface morphology of untreated wheat straw and Na:EG-treated residue: (a) untreated wheat straw × 37, (b) untreated wheat straw × 700, (c) Na:EG-treated wheat straw × 37, and (d) Na:EG-treated wheat straw × 700.
4. Conclusions
Six kinds of basic and neutral DESs were screened for enhancing the enzymatic hydrolysis of wheat straw. Basic DESs have a stronger capacity than neutral DESs to remove lignin and hemicellulose. Among these DESs, Na:EG, which is a DES based on an inorganic base, possesses the strongest removal capacity, with 80.6% lignin and 78.5% hemicellulose removal, and 87.4% cellulose reservation at 90 °C for 5 h. Subsequently, the residue reducing sugar yield reaches 81.6% when the residue is hydrolyzed by enzymes for 72 h. The pH value of the solvent has an impact on the removal of lignin and hemicellulose. The stronger basicity of the solvent, the more lignin and hemicellulose are removed. The removal of lignin and hemicellulose accelerates enzymatic hydrolysis. In addition, the XRD, FT-IR and SEM analysis of pretreated residue confirms the lignin and hemicellulose removal from wheat straw pretreated with Na:EG. Further, the removal of lignin and hemicellulose by DESs based on an inorganic base resulted in a high reducing sugar yield under mild conditions, indicating DESs based on an inorganic base used for enhancing enzymatic hydrolysis of wheat straw is a promising application in the industrial production of bioethanol.
Author Contributions
Conceptualization, X.Z. and Z.Z.; Formal analysis, X.Z.; Investigation, X.Z., X.M. and Z.Z.; Project administration, Z.Z.; Resources, L.H., X.S., and Z.Z.; Supervision, L.H., X.S., and Z.Z.; Writing—original draft, X.Z.; Writing—review and editing, Z.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Key Program of Hebei Province Department of Science and Technology, grant number 20373805D, and the Basic Scientific Research Project of Colleges and Universities of Hebei Province, grant number JQN2022011.
Data Availability Statement
The research data has been presented in the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, J.; Lu, X.; Liu, X.; Xu, J.; Zhou, Q.; Zhang, S. Rapid and productive extraction of high purity cellulose material via selective depolymerization of the lignin-carbohydrate complex at mild conditions. Green Chem. 2017, 19, 2234–2243. [Google Scholar] [CrossRef]
- Xia, Q.; Liu, Y.; Meng, J.; Cheng, W.; Chen, W.; Liu, S.; Liu, Y.; Li, J.; Yu, H. Multiple hydrogen bond coordination in three-constituent deep eutectic solvents enhances lignin fractionation from biomass. Green Chem. 2018, 20, 2711–2721. [Google Scholar] [CrossRef]
- Jung, S.J.; Kim, S.H.; Chung, I.M. Comparison of lignin, cellulose, and hemicellulose contents for biofuels utilization among 4 types of lignocellulosic crops. Biomass Bioenergy 2015, 83, 322–327. [Google Scholar] [CrossRef]
- Fu, D.; Mazza, G.; Tamaki, Y. Lignin extraction from straw by ionic liquids and enzymatic hydrolysis of the cellulosic residues. J. Agric. Food Chem. 2010, 58, 2915–2922. [Google Scholar] [CrossRef]
- Hou, X.D.; Feng, G.J.; Ye, M.; Huang, C.-M.; Zhang, Y. Significantly enhanced enzymatic hydrolysis of rice straw via a high-performance two-stage deep eutectic solvents synergistic pretreatment. Bioresour. Technol. 2017, 238, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Buruiană, C.-T.; Georgescu, L.; Isticioaia, S.-F.; Constantin, O.E.; Vizireanu, C.; Dinică, R.M.; Furdui, B. Insights on monosaccharides and bioethanol production from sweet sorghum stalks using dilute acid pretreatment. Processes 2020, 8, 1486. [Google Scholar] [CrossRef]
- Prasad, S.; Malav, M.K.; Kumar, S.; Singh, A.; Pant, D.; Radhakrishnan, S. Enhancement of bio-ethanol production potential of wheat straw by reducing furfural and 5-hydroxymethylfurfural (HMF). Bioresour. Technol. Rep. 2018, 4, 50–56. [Google Scholar] [CrossRef]
- Joy, S.P.; Krishnan, C. Modified organosolv pretreatment for improved cellulosic ethanol production from sorghum biomass. Ind. Crops Prod. 2022, 177, 114409. [Google Scholar] [CrossRef]
- Xu, J.; Xu, J.; Zhang, S.; Xia, J.; Liu, X.; Chu, X.; Duan, J.; Li, X. Synergistic effects of metal salt and ionic liquid on the pretreatment of sugarcane bagasse for enhanced enzymatic hydrolysis. Bioresour. Technol. 2018, 249, 1058–1061. [Google Scholar] [CrossRef]
- Ma, L.; Goldfarb, J.L.; Ma, Q. Enabling lower temperature pyrolysis with aqueous ionic liquid pretreatment as a sustainable ap-proach to rice husk conversion to biofuels. Renew. Energy 2022, 198, 712–722. [Google Scholar] [CrossRef]
- Wang, Z.K.; Li, H.; Lin, X.C.; Tang, L.; Chen, J.J.; Mo, J.W.; Yu, R.S.; Shen, X.J. Novel recyclable deep eutectic solvent boost biomass pretreatment for enzymatic hydrolysis. Bioresour. Technol. 2020, 307, 123237. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef]
- Chourasia, V.R.; Pandey, A.; Pant, K.K.; Henry, R.J. Improving enzymatic digestibility of sugarcane bagasse from different varieties of sugarcane using deep eutectic solvent pretreatment. Bioresour. Technol. 2021, 337, 125480. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Munro, H.L.; Rasheed, R.K.; Tambyrajah, V. Preparation of novel, moisture-stable, Lewis-acidic ionic liquids containing quaternary ammonium salts with functional side. Chem. Commun. 2001, 19, 2010–2011. [Google Scholar] [CrossRef] [PubMed]
- Hansen, F.A.; Santigosa-Murillo, E.; Ramos-Payán, M.; Muñoz, M.; Øiestad, E.L.; Pedersen-Bjergaard, S. Electromembrane extraction using deep eutectic solvents as the liquid membrane. Anal. Chim. Acta 2021, 1143, 109–116. [Google Scholar] [CrossRef]
- Guo, Z.; Ling, Z.; Wang, C.; Zhang, X.; Xu, F. Integration of facile deep eutectic solvents pretreatment for enhanced enzymatic hydrolysis and lignin valorization from industrial xylose residue. Bioresour. Technol. 2018, 265, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.K.U.; Hadinoto, K. Deep eutectic solvent as green solvent in extraction of biological macromolecules: A review. Int. J. Mol. Sci. 2022, 23, 3381. [Google Scholar] [CrossRef]
- Zulkefli, S.; Abdulmalek, E.; Rahman, M.B.A. Pretreatment of oil palm trunk in deep eutectic solvent and optimization of enzy-matic hydrolysis of pretreated oil palm trunk. Renew. Energy 2017, 107, 36–41. [Google Scholar] [CrossRef]
- Okur, M.; Koyuncu, D.D.E. Investigation of pretreatment parameters in the delignification of paddy husks with deep eutectic solvents. Biomass Bioenergy 2020, 142, 105811. [Google Scholar] [CrossRef]
- Ji, Q.; Yu, X.; Yagoub, A.E.-G.A.; Chen, L.; Fakayode, O.A.; Zhou, C. Synergism of sweeping frequency ultrasound and deep eutectic solvents pretreatment for fractionation of sugarcane bagasse and enhancing enzymatic hydrolysis. Ultrason. Sonochem. 2021, 73, 105470. [Google Scholar] [CrossRef]
- Morán-Aguilar, M.G.; Costa-Trigo, I.; Ramírez-Pérez, A.M.; de Blas, E.; Calderón-Santoyo, M.; Aguilar-Uscanga, M.G.; Domínguez, J.M. Development of Sustainable Biorefinery Processes Applying Deep Eutectic Solvents to Agrofood Wastes. Energies 2022, 15, 4101. [Google Scholar] [CrossRef]
- Adeyemi, I.; Abu-Zahra, M.R.M.; AlNashef, I.M. Physicochemical properties of alkanolamine-choline chloride deep eutectic solvents: Measurements, group contribution and artificial intelligence prediction techniques. J. Mol. Liq. 2018, 256, 581–590. [Google Scholar] [CrossRef]
- Mamilla, J.L.K.; Novak, U.; Grilc, M.; Likozar, B. Natural deep eutectic solvents (DES) for fractionation of waste lignocellulosic biomass and its cascade conversion to value-added bio-based chemicals. Biomass Bioenergy 2019, 120, 417–425. [Google Scholar] [CrossRef]
- Grudniewska, A.; Popłoński, J. Simple and green method for the extraction of xanthohumol from spent hops using deep eutectic solvents. Sep. Purif. Technol. 2020, 250, 117196. [Google Scholar] [CrossRef]
- Rasool, M.H.; Zamir, A.; Elraies, K.A.; Ayoub, M.; Abbas, M.A. Potassium carbonate based deep eutectic solvent (DES) as a potential drilling fluid additive in deep water drilling applications. Pet. Sci. Technol. 2021, 39, 612–631. [Google Scholar] [CrossRef]
- Chen, J.; Ali, M.C.; Liu, R.; Munyemana, J.C.; Li, Z.; Zhai, H.; Qiu, H. Basic deep eutectic solvents as reactant, template and solvents for ultra-fast preparation of transition metal oxide nanomaterials. Chin. Chem. Lett. 2020, 31, 1584–1587. [Google Scholar] [CrossRef]
- Fry, S.C. The Growing Plant Cell Wall: Chemical and Metabolic Analysis; The Blackburn Press: Caldwell, NJ, USA, 1988. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of structural carbohydrates and lignin in biomass. Lab. Anal. Proced. 2008, 1617, 1–16. [Google Scholar]
- Hossain, M.A.; Rahaman, M.S.; Yelle, D.; Shang, H.; Sun, Z.; Renneckar, S.; Dong, J.; Tulaphol, S.; Sathitsuksanoh, N. Effects of polyol-based deep eutectic solvents on the efficiency of rice straw enzymatic hydrolysis. Ind. Crops Prod. 2021, 167, 113480. [Google Scholar] [CrossRef]
- Li, Y.; Zhuo, J.; Liu, P.; Chen, P.; Hu, H.; Wang, Y.; Zhou, S.; Tu, Y.; Peng, L.; Wang, Y. Distinct wall polymer deconstruction for high biomass digestibility under chemical pretreatment in Miscanthus and rice. Carbohydr. Polym. 2018, 192, 273–281. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, X.; Ali, M.F.; Abdeltawab, A.A.; Yakout, S.M.; Yu, G. Pretreatment of wheat straw using basic ethanolamine-based deep eutectic solvents for im-proving enzymatic hydrolysis. Bioresour. Technol. 2018, 263, 325–333. [Google Scholar] [CrossRef]
- Sheng, Y.; Tan, X.; Gu, Y.; Zhou, X.; Tu, M.; Xu, Y. Effect of ascorbic acid assisted dilute acid pretreatment on lignin removal and enzyme digestibility of agricultural residues. Renew. Energy 2021, 163, 732–739. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhou, T.; Wang, Y.; Cao, X.; Wu, S.; Zhao, M.; Wang, H.; Xu, M.; Zheng, B.; Zheng, J.; et al. Pretreatment of wheat straw leads to structural changes and improved enzymatic hydrolysis. Sci. Rep. 2018, 8, 1321. [Google Scholar] [CrossRef] [PubMed]
- Huijgen, W.J.J.; Reith, J.H.; den Uil, H. Pretreatment and fractionation of wheat straw by an acetone-based organosolv process. Ind. Eng. Chem. Res. 2010, 49, 10132–10140. [Google Scholar] [CrossRef]
- Li, Q.; He, Y.C.; Xian, M.; Jun, G.; Xu, X.; Yang, J.M.; Li, L.Z. Improving enzymatic hydrolysis of wheat straw using ionic liquid 1-ethyl-3-methyl imidazolium diethyl phosphate pretreatment. Bioresour. Technol. 2009, 100, 3570–3575. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, J.; Xiao, J.; He, X.; Zhang, K.; Yuan, S.; Peng, Z.; Chen, Z.; Lin, X. Enhanced enzymatic hydrolysis and lignin extraction of wheat straw by triethylbenzyl ammonium chloride/lactic acid-based deep eutectic solvent pretreatment. ACS Omega 2019, 4, 19829–19839. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Wang, L.; Huang, B.; Yu, Y.; Liu, J.; Li, T. Synthesis of green deep eutectic solvents for pretreatment wheat straw: Enhance the solubility of typical lignocellulose. Sustainability 2022, 14, 657. [Google Scholar] [CrossRef]
- Pan, M.; Zhao, G.; Ding, C.; Wu, B.; Lian, Z.; Lian, H. Physicochemical transformation of rice straw after pretreatment with a deep eutectic solvent of choline chloride/urea. Carbohydr. Polym. 2017, 176, 307–314. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, Y.; Abdeltawab, A.; Yakout, S.; Chen, X.; Yu, G. Cholinium amino acids-glycerol mixtures: New class of solvents for pretreating wheat straw to facilitate enzymatic hydrolysis. Bioresour. Technol. 2017, 245, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Nor, N.A.M.; Mustapha, W.A.W.; Hassan, O. Deep eutectic solvent (DES) as a pretreatment for oil palm empty fruit bunch (OPEFB) in sugar production. Procedia Chem. 2016, 18, 147–154. [Google Scholar] [CrossRef]
- Han, Y.; Bai, Y.; Zhang, J.; Liu, D.; Zhao, X. A comparison of different oxidative pretreatments on polysaccharide hydrolyzability and cell wall structure for interpreting the greatly improved enzymatic digestibility of sugarcane bagasse by delignification. Bioresour. Bioprocess. 2020, 7, 24. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).