Abstract
The imbalance in the expression of matrix metalloproteinases (MMPs) and lysyl oxidases (LOXs) in synovial fibroblasts (SFs) caused by mechanical injury and inflammatory response prevents injured anterior cruciate ligaments (ACLs) from self-healing. However, research on the effect of growth factors on SFs on regulating the microenvironment is limited. In this study, mechanical injury and exogenous transform growth factor-β1 (TGF-β1) were employed to mimic a joint-cavity microenvironment with ACL trauma. The function of the NF-κB transcription factor was further studied. The study found that the gene expression of LOXs (except LOXL-1), MMP-1, -2, and -3 in SFs was promoted by the combination of injurious mechanical stretching and TGF-β1 and that the upregulation of MMPs was higher than that of LOXs. In addition, MMP-2 activity induced by the combination of injurious stretch and TGF-β1 was inhibited by NF-κB inhibitors such as Bay11-7082 and Bay11-7085. The findings concluded that the synovium was an important regulator of the knee joint-cavity microenvironment after ACL injury and that the NF-κB pathway mediated the regulation of MMP-2 in SFs via mechanical factors and TGF-β1.
1. Introduction
The anterior cruciate ligament (ACL) and the medial collateral ligament (MCL) are major ligaments contributing to the stability and normal functioning of the knee joint. Isolated ACL injuries are the most common knee ligament injury (48% of the total number), but isolated MCL injuries are also common (29%) [1]. Unfortunately, a damaged ACL cannot self-heal well, whereas a damaged MCL can self-heal well and restore function without any intervention [2]. ACL injury affects the stability of the joint and further causes osteoarthritis (OA) [3]. Thus far, the best treatment for ACL injury is reconstruction, including autotransplantation, allotransplantation, and artificial ligaments. However, the biomechanics of the joint is difficult to reproduce satisfactorily after surgery [4]. Thus, the poor healing of the ACL starting from the healing mechanism of the ligament should be investigated.
There is strong evidence suggesting that the cellular intrinsic properties of the ACL and MCL (such as proliferation, migration, extracellular matrix (ECM) synthesis, and especially ECM remodeling) may be important contributing factors to the dissimilar healing potential of these ligaments [5,6,7]. ECM remodeling is a dynamic process in which some old extracellular-matrix proteins are decomposed by proteolytic enzymes and some new extracellular-matrix proteins form fibers through aggregation and crosslinking [8]. Once the balance is disrupted, the tissue does not heal. Previous in vitro studies revealed that the inability of injured ACLs to heal is associated with an imbalance in extracellular-matrix synthesis and degradation adjusted and controlled by matrix metalloproteinases (MMPs) and lysyl oxidases (LOXs), which are expressed by ACL fibroblasts [9,10].
MMPs are zinc-dependent endopeptidases that can cleave various extracellular-matrix components, such as collagen, elastin, gelatin, etc. The degradation of these protein components plays an important role not only in normal physiological processes (such as wound healing, embryonic development, and morphogenesis) but also in pathological processes (such as tumor progression) [11,12]. LOXs, which are important for the mechanical properties of the ECM and the susceptibility of ECM proteins to degradation by proteinases (including MMPs), are a family of copper-dependent amine oxidases that catalyze lysine-derived crosslinking [13]. The presence of 0.1 Schiff-base crosslinks per collagen molecule results in twofold–threefold resistance to human collagenase compared with non-crosslinked controls or samples [14]. The balance between the degradation and the synthesis of new matrix in tissue remodeling processes is maintained by MMPs and LOXs.
In addition, the intrinsic differences between ACLs and MCLs, e.g., some environmental factors (such as blood supply, nutrition delivery, and especially the microenvironment of joint cavity), are also important in judging ligament healing [5]. An in vivo rat ACL-injury model proved that knee joint tissues released many more MMPs after ACL rotating injury, because the knee joint cavity is a relatively isolated fluid-containing microenvironment surrounded by the synovium, which facilitates the accumulation of MMPs in the synovial-fluid microenvironment [15]. An excessive amount of MMPs accumulated in the synovial-fluid microenvironment disrupts the delicate balance of removing damaged matrix components with the deposition of newly synthesized materials, leading to failure in the healing of cruciate ligaments.
Researchers found that among rat articular tissues (such as synovium, PCL, cartilage, and meniscus), the synovium had the greatest capacity for elevating the level of 72 kDa MMP-2 and 62 kDa active-MMP-2 based on a model of ACL injury in vivo. Hence, the synovium may be a major participant in the regulation of the knee joint-cavity microenvironment after knee tissue injury [15]. Researchers also showed that the regulation of the synovium in the microenvironment of the knee joint cavity was influenced by mechanical stimulation based on a cell-injury model in vitro. The mechanical compression of SFs increased the expression and the activity of MMP-2 [16]. Our previous in vitro study used the equibiaxial stretch chamber, revealed the increase in MMP expression and the decrease in LOX expression induced by mechanical injury, and further verified that SFs are involved in the regulation of the articular-cavity microenvironment after ACL injury [17].
Bigoni et al. found elevated levels of IL-1β, TNF-α, and IL-6 in human synovial-fluid samples after ACL injury [18]. Wang et al. [16] showed that TNF-α and IL-1α promoted the expression of MMP-2 in SFs and indicated that the regulation of the synovium in the articular-cavity microenvironment was also influenced by inflammatory factors. However, it was not reported whether the regulation of the synovium in the articular-cavity microenvironment was also affected by growth factors (such as transform growth factor-beta1 (TGF-β)).
TGF-β1 plays the most important role during connective-tissue repair. It can accelerate cell proliferation, induce cell migration, improve the synthesis of the extracellular matrix [19,20], and regulate the expression levels of LOXs and MMPs in many cells [21,22,23]. Previous studies showed that the difference in the expression of LOXs in TGF-β1-stimulated ACL and MCL fibroblasts resulted in a certain difference in the healing abilities of injured ACLs and MCLs [9]. From the above results, we speculated that the expression levels of LOXs and MMPs in SFs may also be regulated by TGF-β1, but this conjecture is yet to be confirmed. Therefore, an equibiaxial stretch chamber was used to study the in vitro effects of TGF-β1 on the expression levels of LOXs and MMPs in SFs under mechanical stimulation and verify the above-mentioned hypothesis. Previous studies showed that NF-κB-pathway inhibitors Bay11-7082 and Bay11-7085 could suppress MMP-2 activity in ACLs and had a therapeutic effect on injured ACLs. We also studied the inhibiting effects of Bay11-7082 and Bay11-7085 on the expression and activity of MMP-2 in SFs under injurious stretch and TGF-β1 treatment.
2. Materials and Methods
2.1. Cell Culture
The human synovium used for this study was obtained according to ethical principles.
Normal human SFs were obtained from the donor tissues of 2 males and 2 females (age = 30–60 years) who underwent limb amputation at First Affiliated Hospital of Chongqing Medical University, Chongqing, China. The synovium of donors who had long-term knee joint pathological changes or metabolic syndromes were excluded from the present study. The donor ligament tissue was obtained from patients after surgery. The SFs used for this study were harvested according to a previous procedure [18].
2.2. TGF-β1 Treatment
SFs (from passage 3 to passage 5) at the density of 5 × 105 cells per 25 cm2 flasks (Corning Inc., Corning, NY, USA) were seeded and equilibrated for 48 h. Then, SFs were starved for 16 h with 2% FBS high-glucose DMEM before adding TGF-β1 (PeproTech, Rocky Hill, NJ, USA). The medium was replaced with fresh 1% FBS high-glucose DMEM containing TGF-β1 (1, 5, 10, and 20 ng/mL) incubated for 3 h, and RT-PCR was performed on the collected samples. Then, 5 ng/mL TGF-β1 was chosen for further time-process experiments. Cell lysis samples from SFs were collected at 0, 1, 2, 3, and 6 h for detecting the levels of LOXs and MMPs using RT-PCR.
2.3. Mechanical Injury
SFs at the density of 5 × 105 cells per chamber were seeded on a silicone membrane in an equibiaxial stretch chamber and equilibrated for 48 h. Then, SFs were starved for 16 h with 2% FBS high-glucose DMEM. Immediately before stretching, the culture medium was replaced with fresh 1% FBS high-glucose DMEM. SFs were subjected to injurious (12%) and control (0%) stretch conditions. Then, 1000 μL of cell lysis samples was collected for gene expression at 0, 1, 2, 3, and 6 h. Samples of 600 μL of culture medium were collected for zymography analyses at 24, 48, and 72 h.
2.4. Signaling-Pathway-Inhibitor Treatment
SFs at the density of 5 × 105 cells per chamber were seeded in stretch chambers and equilibrated for 48 h. Then, SFs were starved for 16 h with 2% FBS high-glucose DMEM. The medium was replaced with fresh 1% FBS high-glucose DMEM containing 5 ng/mL TGF-β1 (PeproTech, Rocky Hill, NJ, USA) and NF-κB inhibitors (5 μM Bay11-7082 and 5 μM Bay11-7085; Calbiochem, San Diego, CA, USA) before mechanical injury. Samples of 600 μL of culture medium were collected for zymography analyses at 24, 48, and 72 h.
2.5. Real-Time Quantitative PCR
RNA samples were isolated from SFs using Rneasy Plus Mini Kit (Qiagen, Hilden, NRW, Germany). Isolated RNA was quantified through measuring the absorbance at 260 nm with a spectrophotometer (Bio-Rad Laboratories Inc., Hercules, CA, USA), and a volume of 20 μL of cDNA was prepared from 1 μg of RNA in accordance with the instructions of the reverse-transcription kit (Mbi). Real-time PCR was performed using a Quanti-Tect SYBR Green PCR kit (Qiagen, Hilden, NRW, German) and iCycler (Bio-Rad Laboratories Inc., Hercules, CA, USA). Selected sequences of primers are shown in Table 1.

Table 1.
Primers used in real-time RT-PCR.
2.6. Zymography
MMP-2 activity was determined using 0.05% gelatin zymography. Cell-culture-medium samples of 600 μL in volume were collected at 24, 48, and 72 h hours and put into centrifuge tubes; then, they were centrifuged at 12,000 rpm at 4 °C for 10 min to remove dead cells and other impurities. Equal amounts of total protein from different samples were separated in 10% SDS-PAGE gel copolymerized with 0.05% gelatin. After electrophoresis, gels were washed thrice for 1.5 h in 2.5% Triton X-100 at room temperature to regain enzyme activity. Washed gels were immersed in proteolysis buffer and incubated at 37 °C for 15 h. Then, gels were rinsed in a 2.5% Triton X-100 solution and stained at room temperature with Coomassie blue for 1 h on a rotator. Gels were destained until white bands clearly appeared.
2.7. Statistical Analysis
Data were expressed as means ± SDs. Statistical analyses were performed using one-way analyses of variance (ANOVAs). The Fisher LSD was used for post hoc analyses. The critical significance level was set at p < 0.05.
3. Results
3.1. Effects of Different Concentrations of TGF-β1 on Gene Levels of LOX and MMP Families in Normal SFs
The control group did not receive TGF-β1. TGF-β1 at all concentrations (1, 5, 10, and 20 ng/mL) upregulated the LOX gene levels. The expression levels of LOX mRNA and LOXL-1 mRNA in SFs treated with 10 ng/mL TGF-β1 reached maximum values (3.05- and 1.43-fold). Under the influence of 5 ng/mL TGF-β1, the expression of LOXL-2 reached the maximum value (1.53-fold) and the expression level of LOXL-3 decreased in a concentration-dependent manner, with a 1.98-fold reduction at the highest concentration (20 ng/mL). By contrast, the gene level of LOXL-4 increased in a concentration-dependent manner, with a 2.02-fold enhancement at the highest TGF-β1 concentration (20 ng/mL; Figure 1a).
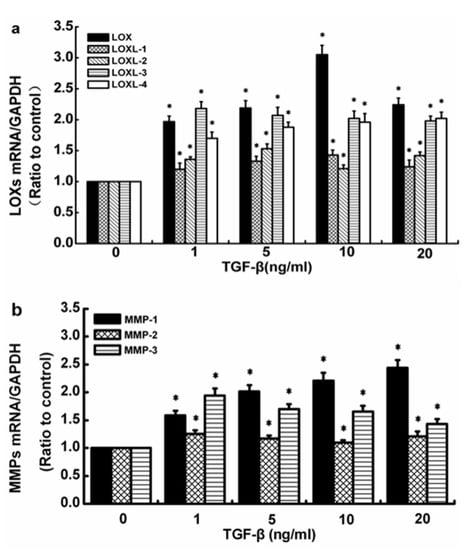
Figure 1.
Effects of TGF-β1 at different concentrations on the gene levels of the LOX and the MMP families in normal human SFs. (a) LOX-family expression levels. (b) MMP-family expression levels. The data are the means of three different experiments (n = 3). Statistical analyses were performed using ANOVAs. * Significant difference with respect to the control (p < 0.05).
TGF-β1 at all concentrations (1, 5, 10, and 20 ng/mL) also promoted the gene levels of MMP-1, -2, and -3. The gene expression of MMP-1 increased in a concentration-dependent manner, with a 2.44-fold enhancement at the highest TGF-β1 concentration (20 ng/mL). The expression level of MMP-2 showed a downward trend with the increase in TGF-β1 concentration from 1 ng/mL to 10 ng/mL but remained superior to the control values and was 1.25, 1.17, and 1.1 times, respectively, that of the untreated control cells; then, it continued to increase to 1.21 times that of the control at the highest TGF-β1 concentration (20 ng/mL). Although the gene level of MMP-3 in SFs showed a downward trend in response to the increase in TGF-β1 concentration, it still exceeded the level of the control group (Figure 1b).
3.2. Time Process of TGF-β1-Induced Expression Levels of LOXs, MMP-1, -2, and -3 in Normal SFs
The control group referred to 0 h. TGF-β1 at 5 ng/mL upregulated the LOX expression levels in normal SFs. The expression of LOX mRNA, LOXL-1 mRNA, and LOXL-4 mRNA showed maximum levels (3.05-, 1.43-, and 1.96-fold, respectively) at 3 h. The expression levels of LOXL-2 mRNA and LOXL-3 mRNA in SFs induced by 5 ng/mL TGF-β1 increased in a time-dependent manner (i.e., 1.45- and 2.29-fold) at 6 h (Figure 2a). Similar to LOXs, the gene levels of MMP-1, -2, and -3 were upregulated by 5 ng/mL TGF-β1. The gene level of MMP-1 induced by 5 ng/mL TGF-β1 was upregulated in a time-dependent manner and reached the highest level at 3 h (2.21-fold); then, it progressively decreased but remained superior to the control values. The gene level of MMP-2 was inhibited by 5 ng/mL TGF-β1 at 1 h; however, it was increased at 2, 3, and 6 h in a time-dependent manner, reaching the highest level at 6 h (1.37-fold). Compared with the control group, the gene level of MMP-3 was increased at each selected time point in SFs treated with 5 ng/mL TGF-β1 in a time-dependent manner, reaching a maximum at 6 h (2.24-fold) (Figure 2b).
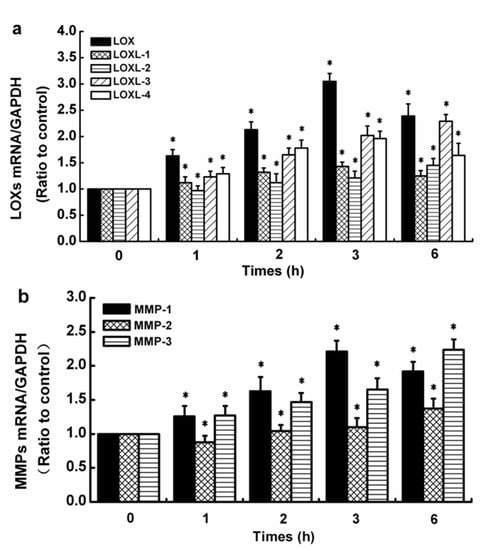
Figure 2.
Effects of TGF-β1 on gene levels of LOX and MMP families in normal human SFs. (a) LOX-family expression levels. (b) MMP-family expression levels. The data are the means of three different experiments (n = 3). Statistical analyses were performed using ANOVAs. * Significant difference with respect to the control (p < 0.05).
3.3. Time Course of TGF-β1-Induced Expression Levels of LOXs, MMP-1, -2, and -3 in Injured SFs
Injurious stretch (12%) was exerted on SFs using an equibiaxial stretch chamber and 5 ng/mL TGF-β1 to simulate the real microenvironment of the synovial cavity after ACL injury. The effects of TGF-β1 on the levels of mRNAs for LOXs, MMP-1, -2, and -3 in damaged human SFs were observed. The control group referred to 0 h. Compared with the control, injurious stretch downregulated the gene levels of LOXs except LOXL-2 in SFs. In the presence of TGF-β1, the injurious-stretch-induced upregulation of LOXs except LOXL-1 at 1, 2, and 3 h was significantly inhibited and further decreased after 6 h but remained superior to the control values (Figure 3). The expression levels of MMP-1, -2, and -3 mRNAs were significantly upregulated after injurious stretch. When injurious stretch was exerted on SFs together with TGF-β1, a promoting effect on the gene levels of MMP-1, -2, and -3 at all time points remained, compared with control values (Figure 4).
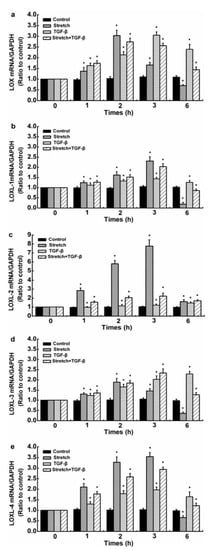
Figure 3.
Time processes of TGF-β1-induced LOX expression levels in injured human SFs. (a) LOX, (b) LOXL-1, (c) LOXL-2, (d) LOXL-3, and (e) LOXL-4 expression levels. The data are the means of three different experiments (n = 3). Statistical analyses were performed using ANOVAs. * Significant difference with respect to the control (p < 0.05).
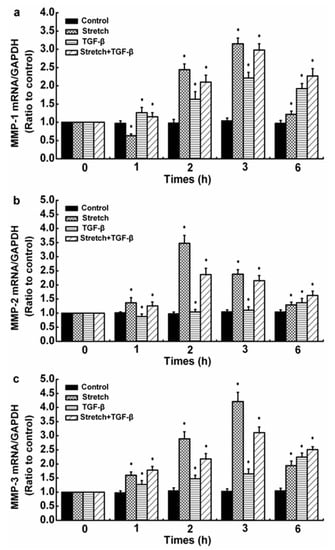
Figure 4.
Time processes of TGF-β1-induced MMP expressions in injured human SFs. (a) MMP-1, (b) MMP-2, and (c) MMP-3 expression levels. The data are the means of three different experiments (n = 3). Statistical analyses were performed using ANOVAs. * Significant difference with respect to the control (p < 0.05).
3.4. Effects of NF-κB-Pathway Inhibitors on Injurious Stretch and TGF-1-Induced MMP-2 Expression and Activity in SFs
In this experiment, NF-κB-pathway inhibitors Bay11-7082 and Bay11-7085 were added to the joint-cavity microenvironment simulated in vitro (injurious stretch and TGF-β1). Injurious stretch and TGF-1-induced MMP-2 activity were inhibited in SFs. The data showed that NF-κB was involved in the regulation of MMP-2 in SFs using mechanical factors and TGF-β1 (Figure 5). Based on this, more signaling-pathway inhibitors could regulate MMP-2 activity, which would provide a theoretical basis for the clinical treatment of cruciate ligament healing, should be found.
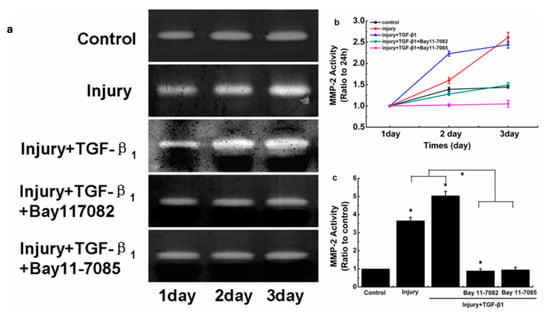
Figure 5.
Effects of NF-κB-signaling-pathway inhibitors on MMP-2 activity induced by injurious stretch and TGF-β1 in SFs. (a) Zymographic analyses of conditioned medium collected from SFs after different treatments for 24, 48, and 72 h. (b) MMP-2 quantification performed using Quantity One 4.6.3 software. (c) Indicated quantitative data referring to 72 h time points. The data are the means of three different experiments (n = 3). Statistical analyses were performed using ANOVAs. * Significant difference with respect to the control (p < 0.05).
4. Discussion
Previous research studies point out that during the process of ACL healing, synovial-joint-microenvironment regulation is affected by mechanical and inflammatory factors. However, whether the regulation of the microenvironment of the joint by the synovium is also affected by growth factors has not yet been studied. Data showed that the average concentration of TGF-β1 ranged from 0.75 to 4.95 ng/mL [24,25] in the joint fluid of patients with OA. Therefore, 5 ng/mL TGF-β1 was selected in this experiment to study the effects of injurious stretch and TGF-β1 on the gene levels of LOXs and MMPs, and MMP-2 activity in SFs and simulate the joint-cavity environment after cruciate-ligament injury.
Tissue repair is a complex and strictly regulated process and includes three overlapping phases, that is, inflammatory response, fibroblast proliferation, and extracellular-matrix reconstruction, and many types of cytokines are involved in this process. TGF-β1 is the most closely related to tissue repair and has a regulating effect on the progress of each stage in the repair process [26,27]. After tissue damage, TGF-β1 produced by platelet α particles promotes the migration of macrophages and neutrophils to the injury site. These inflammatory cells release many cytokines (including TGF-β1) and enter the stage of inflammatory response. During the cell-proliferation phase, exogenous fibroblasts migrate into the wound under the stimulation of TGF-β1, simultaneously proliferate, and synthesize various extracellular matrices, including collagen, proteoglycans, fibronectin, and tendin. During the remodeling process, TGF-β1 inhibits the degradation of the extracellular matrix by reducing protease synthesis and upregulates the level of protease inhibitors, thereby promoting the localization of the extracellular matrix. The regulatory role of TGF-β1 in various stages of the repair process remarkably promotes wound healing. Thus, some people call TGF-β1 a “trauma hormone” [28]. Many studies showed that TGF-β1 could promote wound healing in various animal trauma models (such as rabbits, pigs, and mice) [29,30,31].
However, TGF-β1 promotes and inhibits tissue repair. Many studies showed that the promotion or the inhibition of TGF-β1 in the tissue repair process depended on the dose of TGF-β1. For example, research reported that low-dose TGF-β1 and PDGF promoted the proliferation of canine ACL fibroblasts in a positive, synergistic manner. However, the proliferation-promoting effect of PDGF on fibroblasts was inhibited by high-dose TGF-β1 [32]. In addition, high levels of TGF-β1 also inhibited the proliferation of MCL fibroblasts, and bFGF- and VEGF-induced angiogenesis [33,34].
Our experimental results found that the gene level of MMP-1 induced by 5 ng/mL TGF-β1 increased in a time-dependent manner and reached a maximum level at 3 h; then, it progressively decreased at 6 h but remained superior to the control values. The reason why TGF-β1 induced a decrease in the MMP-1 gene level in human SFs is still under study. Current studies on other cells showed that there are three possible reasons: (1) The promoter regions of many MMP family members contain TGF-β1 inhibitory element (TIE), which acts as a cis-acting element for TGF-β1 to regulate the gene expression of MMPs. The TIE sequence is also included in the promoter region of the human MMP-1 gene. Nuclear proteins from TGF-β1-treated rabbit fibroblasts, including c-Fos, were shown to bind to this TIE sequence from the MMP-1 promoter region and mediated the repression effect of TGF-β1 on the MMP-1 level. (2) In human epidermal fibroblasts, TGF-β1 represses MMP-1 expression through Smad3 and Smad4. (3) TGF-β1 inhibits MMP-1 gene expression by activating the ERK 1 and 2 pathway in A-5 and UT SCC-7 [35,36]. We found that TGF-β1 also promoted the expression of MMP-2 and -3 at all time points (except MMP-2 at 1 h) in a time-dependent manner in SFs. When injurious stretch and TGF-β1 acted on SFs, the expression levels of MMP-1, -2, and -3 in SFs were further upregulated. Gelatin zymography indicated that a combination of injurious stretch and TGF-β1 also exhibited promotion effects on the induction of MMP-2 activity. The knee synovial cavity is a relatively isolated space that facilitates the accumulation of MMPs and TGF-β1 in synovial fluid following ACL injury. The increase in MMP expression and activity destroys the balance between the synthesis of new tissue and the degradation of necrotic tissue, and this phenomenon is not conducive to cruciate-ligament healing.
Similarly, TGF-β1 upregulates the gene levels of LOXs in SFs. TGF-β1 promotes LOXs in other cell types, such as lung fibroblasts [37], gingival cells [38], granulosa–lutein cells [23], and liver cancer cells [39]. The study observed that the expression of LOXs (except LOXL-2) in SFs was downregulated after 6 h of injurious stretch. A previous study showed that LOXL2 expression caused an upregulation of protective genes and reduced the levels of MMP-1, -3, and -13 mRNAs in the pathophysiology of arthritis in human TMJ, and hip and knee joints and evaluated its potential to play a protective feedback role in damaged cartilage in the pathophysiology of arthritis [40]. In view of this, we hypothesized that the highest upregulation of LOXL2 after injurious stretch was a stress response of cells and that this emergency response was protective. As to why stretch only upregulated LOXL2 but not the other members, there is no explanation at present. When TGF-β1 was added, the gene levels of LOXs except LOXL-1 were upregulated. This behavior of TGF-β1 proved its role in promoting wound healing. Increased LOXs initiated the formation of covalent cross-linkages in the ECM (collagen elastin), which enhanced the mechanical properties of the ECM and thereby reduced the degradation susceptibility of the ECM induced by MMPs. However, the increased levels of LOXs were lower than those of MMPs; this phenomenon causes the imbalance in extracellular-matrix synthesis and degradation during remodeling and eventually leads to failure in cruciate-ligament healing. Therefore, inhibiting the levels or activity of MMPs in SFs or promoting the expression of LOXs could be therapeutically targeted to improve the healing ability of ACLs.
At present, many chemical inhibitors of MMPs (such as cilastaz, rimamstat, doxycycline, ilomastat, and doxycycline) are in use [41]. However, these inhibitors produce certain side effects, and the clinical effect is not optimistic. Therefore, understanding the mechanism of the MMP signaling pathway and finally finding the signaling-molecule inhibitors would provide a strong theoretical basis for clinical drug development.
NF-κB is a nuclear transcription factor consisting of heterodimers p65–p50, which are widely present in cells. NF-κB can regulate the transcription of more than 150 genes (such as MMPs, cytokines, adhesion molecules, transcription factors, and growth factors). NF-κB also regulates some physiological processes (including repair, proliferation, apoptosis, and development) [42,43,44]. However, the dysregulation of NF-κB induces asthma, cancer, rheumatoid arthritis, and neurological disorders [45]. Therefore, NF-κB has gradually become a therapeutic target for a variety of related diseases. Researchers designed a variety of NF-κB biological and biochemical inhibitors (such as flavonoids, turmeric, DHEA, DHMEQ, and Bay11-7082) that can block the signaling pathway that activates NF-κB or inhibit the binding of NF-κB to gene sequences [46,47,48,49].
Tang et al. [50] found that NF-κB signaling molecules mediated the effects of damaging mechanical factors on the level and activity of MMP-2 in ACL fibroblasts. Based on this finding, Wang et al. [51] stimulated ACL fibroblasts by adding Bay11-7082, a NF-κB-signaling inhibitor, under simulated conditions of the joint-cavity microenvironment in vitro and found that MMP-2 activity in the cells was reduced. The data suggest that NF-κB is a major pathway that regulates the production of MMP-2 in ACL fibroblasts. However, whether NF-κB signaling is associated with the expression of MMP-2 in SFs under the conditions of the articular-cavity microenvironment simulated in vitro is unclear. The experimental results showed that under the simulated conditions of the joint-cavity microenvironment in vitro, the inhibitors of NF-κB signaling pathways, i.e., Bay11-7082 and Bay11-7085, significantly reduced MMP-2 activity. These results indicated that NF-κB mediated the regulation of MMP-2 production and activity in SFs subjected to stretch loading and TGF-β1 and provide a theoretical reference for improving the repair of ACLs.
The experiment does not represent the actual physiological environment after cruciate-ligament injury. Among the microenvironmental factors, as well as inflammatory factors and growth factors, hypoxia, which is another microenvironmental factor, also needs to be considered after ACL injury and has important significance for the study of ligament healing. The synovium located in the inner layer of the joint capsule is a connective-tissue membrane that contains highly abundant blood vessels. The main blood supply and tissue as well as joint homeostasis nourishment are maintained by the synovium. The synovium surrounds the ACL, so the injury of cruciate ligaments is bound to be accompanied by the damage of the synovium, which leads to a hypoxic microenvironment [52,53]. Previous studies reported that hypoxia promoted the secretion and activity of MMP-2 in ACL fibroblasts [53]. Therefore, as a microenvironmental factor, hypoxia may also regulate the expression of LOXs and MMPs, and MMP-2 activity in SFs.
5. Conclusions
In summary, the findings of this paper are that the changes in the expression of LOXs and MMPs or the changes in the microenvironment of the knee joint space are important causes of ACL healing failure and that SFs participate in the healing process of the ACL as microenvironment regulators. Therefore, improving the microenvironment of the synovial cavity after ACL injury through regulating LOXs and MMPs in SFs could have important significance for ACL repair. In addition, NF-κB signaling molecules mediate the regulation of MMP-2 in SFs via mechanical growth factors. This study can provide a strong theoretical foundation for the clinical treatment of the ACL in the future.
Author Contributions
Conceptualization, Y.Z. and L.Y.; methodology, Y.Z. and C.W.; data curation, Y.Z., C.W. and X.L.; writing—original draft preparation, Y.Z.; writing—review and editing, Y.Z. and X.L.; supervision, Y.Z.; funding acquisition, Y.Z. and L.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research study was funded by National Natural Science Foundation of China (grant No. 11702093) and Innovation and Attracting Talents Program for Colleges and Universities, China (grant No. B06023).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Reasearch Ethics Committee of the First Affiliated Hospital of Chongqing Medical University.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available in the article (table and figures).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Miyasaka, K.C.; Daniel, D.M.; Stone, M.L.; Hirshman, P. The incidence of knee ligament injuries in the general population. Am. J. Knee Surg. 1991, 4, 3–8. [Google Scholar]
- Dale, K.M.; Bailey, J.R.; Moorman, C.T. Surgical management and treatment of the anterior cruciate ligament/medial collateral ligament injured knee. Clin. Sport. Med. 2017, 36, 87–103. [Google Scholar] [CrossRef]
- Benedikt, L.P.; Jakob, T.S.; Martha, M.M.; Matthew, R.A.; Kaitlyn, E.C.; Gabriel, S.P.; Tarpit, K.P.; Braden, C.F. Extracellular matrix-blood composite injection reduces post-traumatic osteoarthritis after anterior cruciate ligament injury in the rat. J. Orthop. Res. 2016, 34, 995–1003. [Google Scholar]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, allograft, and aone graft substitutes: Clinical evidence and indications for use in the setting of orthopaedic trauma surgery. J. Orthop. Trauma 2019, 33, 203–213. [Google Scholar] [CrossRef]
- Nagineni, C.N.; Amiel, D.; Green, M.H.; Berchuck, M.; Akeson, W.H. Characterization of the intrinsic properties of the anterior cruciate and medial collateral ligament cells: An in vitro cell culture study. J. Orthop Res. 1992, 10, 465–475. [Google Scholar] [CrossRef]
- Wiig, M.E.; Amiel, D.; Ivarsson, M. Type I procollagen gene expression in normal and early healing of the medial collateral and anterior cruciate ligaments in rabbits: An in situ hybridization study. J. Orthop Res. 1991, 9, 374–382. [Google Scholar] [CrossRef]
- Zhou, D.; Lee, H.S.; Villarreal, F.; Teng, A.; Lu, E.; Reynolds, S.; Qin, C.; Smith, J.; Sung, K.L. Differential MMP-2 activity of ligaments cells under mechanical stretch injury: An in vitro study on human ACL and MCL fibroblasts. J. Orthop Res. 2005, 23, 949–957. [Google Scholar] [CrossRef]
- Rothman, S. How is the balance between protein synthesis and degradation achieved? Theor. Biol. Med. Model. 2010, 7, 25. [Google Scholar] [CrossRef]
- Xie, J.; Jiang, J.; Zhang, Y.; Xu, C.; Yin, L.; Wang, C.; Chen, P.C.Y.; Sung, K.L.P. Up-regulation expressions of lysyl oxidase family in anterior cruciate ligament and medial collateral ligament fibroblasts induced by transforming growth factor-beta1. Int. Orthop. 2012, 36, 207–213. [Google Scholar] [CrossRef][Green Version]
- Xie, J.; Jiang, J.; Huang, W.; Zhang, Y.; Xu, C.; Wang, C.; Yin, L.; Chen, P.C.Y.; Sung, K.L.P. TNF-α induced down-regulation of lysyl oxidase family in anterior cruciate ligament and medial collateral ligament fibroblasts. Knee 2014, 21, 47–53. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, Y.; Linawati, S.; Yang, L.; Chen, R.; Sung, K. Bay11-7082 facilitates wound healing by antagonizing mechanical injury- and TNF-α-induced expression of MMPs in posterior cruciate ligament. Connect. Tissue. Res. 2019, 60, 311–322. [Google Scholar] [CrossRef]
- Umezawa, K.; Lin, Y. Inhibition of matrix metalloproteinase expression and cellular invasion by NF-κB inhibitors of microbial origin. BBA-Proteins Proteom. 2020, 1868, 140412–140418. [Google Scholar] [CrossRef]
- Vater, C.A.; Harris, E.D.; Siegel, R.C. Native cross-links in collagen fbrils induce resistance to human synovial collagenase. Biochem. J. 1979, 181, 639–645. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, J.; Xie, J.; Xu, C.; Wang, C.; Yin, L.; Yang, L.; Sung, K.L. Combined effects of tumor necrosis factor-α and interleukin-1β on lysyl oxidase and matrix metalloproteinase expression in human knee synovial fibroblasts in vitro. Exp. Ther. Med. 2017, 14, 5258–5266. [Google Scholar] [CrossRef]
- Tang, Z.; Yang, L.; Wang, Y.; Xue, R.; Zhang, J.; Huang, W.; Chen, P.C.; Sung, K.L.P. Contributions of different intraarticular tissues to the acute phase elevation of synovial fluid MMP-2 following rat ACL rupture. J. Orthop. Res. 2010, 27, 243–248. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, L.; Zhang, J.; Xue, R.; Sung, K.L.P. Differential MMP-2 activity induced by mechanical compression and inflammatory factors in human synoviocytes. Mol. Cell. Biomech. 2010, 7, 105–114. [Google Scholar]
- Zhang, Y.; Huang, W.; Jiang, J.; Xie, J.; Xu, C.; Wang, C.; Yin, L.; Yang, L.; Zhou, K.; Chen, P.; et al. Influence of TNF-α and biomechanical stress on matrix metalloproteinases and lysyl oxidases expressions in human knee synovial fibroblasts. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 1997–2006. [Google Scholar] [CrossRef]
- Bigoni, M.; Sacerdote, P.; Turati, M.; Franchi, S.; Torsello, A. Acute and late changes in intraarticular cytokine levels following anterior cruciate ligament injury. J. Orthop. Res. 2013, 31, 315–321. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, Q.; Chen, Z.; Peng, R.; Chen, R.; Ma, Z.; Wan, X.; Liu, J.; Meng, M.; Peng, Z. MicroRNA-663 inhibits the proliferation, migration and invasion of glioblastoma cells via targeting TGF-β1. Oncol. Rep. 2016, 35, 1125–1134. [Google Scholar] [CrossRef]
- Wang, S.; Sun, Z.; Yang, S.; Chen, B.; Shi, J. CTRP6 inhibits cell proliferation and ECM expression in rat mesangial cells cultured under TGF-β1. Biomed. Pharmacother. 2018, 97, 280–285. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, Z.; Xue, R.; Gurinder, K.S.; Lv, Y.; Shi, K.; Cai, K.; Deng, L.; Yang, L. TGF-β1 promoted MMP-2 mediated wound healing of anterior cruciate ligament fibroblasts through NF-κB. Connect. Tissue Res. 2011, 52, 218–225. [Google Scholar] [CrossRef]
- Kim, H.S.; Shang, T.; Chen, Z.; Pflugfelder, S.C.; Li, D.Q. TGF-beta1 stimulates production of gelatinase (MMP-9), collagenases (MMP-1, -13) and stromelysins (MMP-3, -10, -11) by human corneal epithelial cells. Exp. Eye Res. 2004, 79, 263–274. [Google Scholar] [CrossRef]
- Fang, Y.; Chang, H.M.; Cheng, J.C.; Klausen, C.; Leung, P.C.K.; Yang, X. TGF-β1 increases lysyl oxidase by reducing miR-29a in human granulosa-lutein cells. Reproduction 2016, 152, 205–213. [Google Scholar] [CrossRef]
- Anitua, E.; Sánchez, M.; Maria, D.L.F.; Azofra, J.; Zalduendo, M.; Aguirre, J.J.; Andia, I. Relationship between investigative biomarkers and radiographic grading in patients with knee osteoarthritis. Int. J. Rheumatol. 2009, 2009, 747432. [Google Scholar] [CrossRef][Green Version]
- Schlaak, J.F.; Pfers, I.; Büschenfelde, K.H.M.Z.; Märker-Hermann, E. Different cytokine profiles in the synovial fluid of patients with osteoarthritis, rheumatoid arthritis and seronegative spondylarthropathies. Clin. Exp. Rheumatol. 1996, 14, 155–162. [Google Scholar]
- Monaco, J.A.L.; Lawrence, W.T. Acute wound healing-An overview. Clin. Plast. Surg. 2003, 30, 1–12. [Google Scholar] [CrossRef]
- Ding, J.; Kwan, P.; Ma, Z.; Iwashina, T.; Wang, J.; Shankowsky, H.A.; Tredget, E.E. Synergistic effect of vitamin D and low concentration of transforming growth factor beta 1, a potential role in dermal wound healing. Burns 2016, 42, 1277–1286. [Google Scholar] [CrossRef]
- Hunziker, E. Repair of partial-thickness defects in articular cartilage: Cell recruitment from the synovial membrane. J. Bone Joint. Surg. Am. 1996, 78, 721–733. [Google Scholar] [CrossRef]
- Davidson, J.M. Transforming growth factor-beta stimulates wound healing and modulates extracellular matrix gene expression in pig skin: Incisional wound model. Lab. Investig. 1990, 63, 307–319. [Google Scholar]
- Coşkun, Ş.; Peker, E.G.G.; Balabanli, B.; Ahiska, S.; Acartürk, F. Effect of transforming growth factor beta 1 (TGF-beta 1) on nitric oxide production and lipid peroxidation in oral mucosal wound healing. Med. Chem. Res. 2011, 20, 23–28. [Google Scholar] [CrossRef]
- Zhang, C.; Tan, C.K.; McFarlane, C.; Sharma, M.; Tan, N.S.; Kambadur, R. Myostatin-null mice exhibit delayed skin wound healing through the blockade of transforming growth factor-βsignaling by decorin. Am. J. Physiol. Cell Physiol. 2012, 302, 1213–1225. [Google Scholar] [CrossRef]
- Desrosiers, E.A.; Yahia, L.; Rivard, C.H. Proliferative and matrix synthesis response of canine anterior cruciate ligament fibroblasts submitted to combined growth factors. J. Orthop. Res. 1996, 14, 200–208. [Google Scholar] [CrossRef]
- Amiel, D.; Nagineni, C.N.; Choi, S.H.; Lee, J. Intrinsic properties of ACL and MCL cells and their responses to growth factors. Med. Sci. Sport Exerc. 1995, 27, 844–851. [Google Scholar] [CrossRef]
- Pepper, M.S.; Vassalli, J.D.; Orci, L.; Montesano, R. Biphasic effect of transforming growth factor-β1 on in vitro angiogenesis. Exp. Cell. Res. 1993, 204, 356–363. [Google Scholar] [CrossRef]
- Hall, M.C. The comparative role of activator protein 1 and Smad factors in the regulation of timp-1 and mmp-1 gene expression by transforming growth factor-β1. J. Biol. Chem. 2003, 278, 10304–10313. [Google Scholar] [CrossRef]
- Yuan, W.H.; Varga, J. Transforming growth factor-β repression of matrix metalloproteinase-1 in dermal fibroblasts involves smad3. J. Biol. Chem. 2016, 276, 38502–38510. [Google Scholar] [CrossRef]
- Roy, R.; Polgar, P.; Wang, Y.Y.; Goldstein, R.H.; And, L.T.; Kagan, H.M. Regulation of lysyl oxidase and cyclooxygenase expression in human lung fibroblasts: Interactions among TGF-beta, IL-1 beta, and prostaglandin E. J. Cell. Biochem. 2015, 62, 411–417. [Google Scholar] [CrossRef]
- Hong, H.H.; Uzel, M.I.; Duan, C.N.; Sheff, M.C.; Trackman, P.C. Regulation of lysyl oxidase, collagen, and connective tissue growth factor by TGF-beta1 and detection in human gingiva. Lab. Investig. 1999, 79, 1655–1667. [Google Scholar]
- Ezzoukhry, Z.; Henriet, E.; Piquet, L.; Boyé, K.; Bioulac-Sage, P.; Balabaud, C.; Couchy, G.; Zucman-Rossi, J.; Moreau, V.; Saltel, F. TGF-β1 promotes linear invadosome formation in hepatocellular carcinoma cells, through DDR1 up-regulation and collagen I cross-linking. Eur. J. Cell Biol. 2016, 95, 503–512. [Google Scholar] [CrossRef]
- Bais, M.; Mehra, P. Lysyl oxidase like-2: Potential anabolic agent expressed in hip, knee and temporomandibular joints arthritis. Int. J. Oral. Max. Surg. 2019, 48, 172–173. [Google Scholar] [CrossRef]
- Hajdú, I. Matrix metalloproteinase inhibitors: A critical appraisal of design principles and proposed therapeutic utility. Drugs 2010, 70, 949–964. [Google Scholar]
- Zhan, D.; Guo, L.; Zheng, L. Inhibition of the receptor for advanced glycation promotes proliferation and repair of human periodontal ligament fibroblasts in response to high glucose via the NF-kappa B signaling pathway. Arch. Oral. Biol. 2018, 87, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, Y.; Qu, X.; Wang, Z.; Yang, T. Effect of hyperoside on the proliferation and apoptosis of human gastric cancer cells by inhibiting the NF-κB pathway. Acta. Med. Mediterr. 2020, 36, 471–475. [Google Scholar]
- Lei, R.; Li, J.; Liu, F.; Li, W.; Zhang, S.; Wang, Y.; Chu, X.; Xu, J. HIF-1α promotes the keloid development through the activation of TGF-β/Smad and TLR4/MyD88/NF-κB pathways. Cell. Cycle 2019, 18, 3239–3250. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Shabnam, B.; Girisa, S.; Harsha, C.; Aggarwal, B.B. Inflammation, NF-κB, and chronic diseases: How are they linked? Crit. Rev. Immunol. 2020, 40, 1–39. [Google Scholar] [CrossRef]
- Choy, K.W.; Murugan, D.; Leong, X.F.; Abas, R.; Mustafa, M.R. Flavonoids as natural anti-inflammatory agents targeting nuclear factor-kappa B (NFκB) signaling in cardiovascular diseases: A mini review. Front. Pharmacol. 2019, 10, 1295. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Cao, J.; Yu, L.; Ma, H. Dehydroepiandrosterone alleviates E. Coli O157:H7-induced inflammation by preventing the activation of p38 MAPK and NF-κB pathways in mice peritoneal macrophages-sciencedirect. Mol. Immunol. 2019, 114, 114–122. [Google Scholar] [CrossRef]
- Horie, K.; Ma, J.; Umezawa, K. Inhibition of canonical NF-κB nuclear localization by (-)-DHMEQ via impairment of DNA binding. Oncol. Res. 2015, 22, 105–115. [Google Scholar] [CrossRef]
- Zhang, Q.; Mao, Z.; Sun, J. NF-κB inhibitor, BAY11-7082, suppresses M2 tumor-associated macrophage induced EMT potential via miR-30a/NF-κB/snail signaling in bladder cancer cells. Gene 2019, 710, 91–97. [Google Scholar] [CrossRef]
- Tang, Z.; Yang, L.; Xue, R.; Zhang, J.; Wang, Y.; Chen, P.C.; Sung, K.L.P. Differential expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in anterior cruciate ligament and medial collateral ligament fibroblasts after a mechanical injury: Involvement of the p65 subunit of NF-κB. Wound. Repair. Regen. 2009, 17, 709–716. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, Z.; Xue, R.; Singh, G.K.; Shi, K.; Lv, Y.; Yang, L. Combined effects of TNF-α, IL-1β, and HIF-1α on MMP-2 production in ACL fibroblasts under mechanical stretch: An in vitro study. J. Orthop. Res. 2011, 29, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Eisenbud, D.E. Oxygen in wound healing:nutrient, antibiotic, signaling molecule, and therapeutic agent. Clin. Plast. Surg. 2012, 39, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Tandara, A.A.; Mustoe, T.A. Oxygen in wound healing—More than a nutrient. World J. Surg. 2004, 28, 294–300. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).