Abstract
Microbial lipases are the biocatalyst of choice for the present and future because of their characteristics, including their ability to remain active as an enzyme throughout a broad pH, temperature, and substrate range. The goal of the current investigation was to find novel sources of substrates and isolates from soil contaminated by oil for the synthesis of lipase. On tributyrin media, 10 lipolytic bacterial strains that were isolated from oil-contaminated soil were grown. Using the zone of clearance, it was possible to identify the isolates with the highest activity. Following phylogenetic tree analysis, molecular characterization of the 16S rRNA sequence of the bacterial isolates revealed that it was Bacillus halotolerans (VSH 09). The enzyme was purified to near homogeneity. The enzyme activity was found to be optimum at a pH of 7.0 and a temperature of 35 °C. While Ni2+ and Cu2+ had no effect, the presence of Mg2+ and Ca2+ exhibited the highest levels of enzyme activity. At 1%, tributyrin as a substrate exhibited its highest level of activity. The molecular weight, as determined by SDS-PAGE, was found to be 38 kDa. The kinetics of the enzyme were found to be 41.66 and 9.37 mg/mL for Vmax and Km, respectively. The high yield of lipase produced by this method suggests that it holds potential for production on a large scale and could be used for various biotechnological applications.
1. Introduction
A significant group of biologically derived proteins, known as enzymes, function as biological catalysts. One of the crucial extracellular microbial enzymes is lipases (EC 3.1.1.3). Triglycerides are hydrolyzed by lipases into glycerol and free fatty acids. They hydrolyze insoluble substrates into more polar lipolytic products and are soluble in water. [1]. When compared to chemical catalysts, microbial lipases consume less energy [1]. Many researchers have been attracted to microbial-based lipases mainly because of the higher enzyme activity at neutral and alkaline pH and ease of isolation and production procedures [2]. Daiha et al. 2015, made an effort to review the literature on “whether lipases are still important Biocatalysts?” The main objective of this review was to determine the scientific publications and patents for technological forecasting of lipases. This article discusses the significance of the technological advancement in four significant industries where lipase is used, production of detergent formulation, kinetic resolution-focused organic synthesis, biodiesel manufacturing, and the production of food and feed products. Technology lifecycle concept S-curves were created, and the results led researchers to the conclusion that using lipases as a biocatalyst is still an important topic for the industrial sector [3]. For each application, a particular lipase was chosen based on its substrate specificity, position and stereospecificity, temperature, and pH, as well as other factors [4]. The optimum pH for the production of lipase has been reported to be higher in bacteria than in fungi [5].
Studies have also revealed that thermo tolerant strains belonging to different strains like Bacillus licheniformis MTCC 10498, B. flexus, B. pseudonymous, Pseudomonas MS017, and P. aeruginosa are known to produce lipases with proven commercial applications.
The objective of the present study is to investigate the prevalence of naturally occurring environmental strains of lipase-producing bacteria, which could be a potential source of lipases with good commercial significance.
The present study assumes significance in view of mining novel microbial resources and their molecular identification with their varied genetic diversity and potential for lipase production. Further, the lipase production by B. halotolerans strains is known to be a potent bio transformer of waste cooking oil to biodiesel conversion.
2. Materials and Methods
2.1. Chemicals
Peptone, olive, groundnut, sunflower, palm, and Gingelly oils, as well as sodium hydroxide, tryptone, urea, yeast extract, nutrient broth, phenolphthalein indicator, gram stain kit, agar, distilled water, ammonium sulfate, Tris-HCl buffer, NaCl, KCl, Na2HPO4, KH2PO4, and Coomassie brilliant blue (CBB), were purchased from Merck India Pvt Ltd. and all media components from Hi Media India Pvt Ltd.
2.2. Bacterial Strain Isolation and Characterization
B. halotolerans VSH 09 was isolated from oil-contaminated soil in the vicinity of Hubballi-Dharwad in Karnataka, India, situated at 786 m above mean sea level and geographic coordinates 14°25′42.71′′ N, in latitude and 74°25′8.04′′ E, ft. in longitude. The oil-contaminated soil samples were collected aseptically from the packaging area of an edible oil mill and taken to the laboratory for further analysis. Further, they were diluted serially in physiological saline (0.85% NaCl) and screened for production of lipase by Tributyrin Agar Plate assay using tributyrin as substrate. The isolates screened for lipase production were subjected to morphological (Gram staining and endospore staining) and biochemical tests (citrate consumption, indole generation, carbohydrate utilization, oxidase test, and nitrate reduction test) [6,7,8,9].
2.3. Lipase-Producing Bacterial Strain Screening
Using the Tributyrin Agar Plate Assay technique, isolated bacterial strains were tested for their lipolytic activity. The bacterial isolates were screened for lipolytic activity on agar plates containing tributyrin (1%, w/v), agar (2%, w/v), peptone (0.5 g); beef extract (0.3 g), sterilized at 121 °C for 15 min, and supplemented with 1% (v/v) olive oil. To visualize the halo zone, isolated strains were streaked on a tributyrin agar plate and cultured for 24 h at 48 °C [8,10].
2.4. Enzyme Production Media
The bacterial strains were found to be positive upon screening and grown on a lipase-producing medium [11]. Microbial cultures were added to 250 mL Erlenmeyer flasks with 100 mL of liquid medium and incubated at 37 °C for 24 h. On a rotary shaker (150 rpm), the sample was centrifuged at 10,000 rpm for 10 min at 40 °C to obtain the extracellular enzyme source from the cell-free culture supernatant fluid. The titrimetric approach was used to measure the lipase activity [12,13].
2.5. Optimization of Lipase Producing Media
Optimization of lipase-producing media was supplemented with various carbon and nitrogen sources, such as sucrose, glucose, lactose, peptone, yeast extract, and ammonium sulfate, at varying concentrations (1–5%). One Factor at a Time (OFAT) was studied to observe the effect of various factors that influenced the production of lipase in the fermentation process. Different cultural conditions considered were incubation period (12 h to 120 h), inoculums size (102 to 109 spores per mL), the effect of incubation temperature (25 °C to 50 °C), and effect of media pH (pH 3 to 10). The nutritional conditions considered included peptone concentration (0.4 to 2.8 g/100 mL), groundnut oil concentration (0.5 to 6.0 mL), KH2PO4 concentration (0.2 to 2.6 g/100 mL), agitation speed, the effect of different nitrogen sources, substrates (oils), and minerals were optimized for enhanced production of lipase by VSH 09 strain [14,15,16].
2.6. Extraction and Partial Purification of Lipase
The entire downstream purification process was carried out at 4 °C. The lipase was purified using the cell-free supernatant. A suitable volume of the supernatant combined with a saturated concentration of 70% w/v glacial ammonium sulfate solution based on the saturation level required to precipitate the enzyme (1:1) was maintained in an ice bath for 20 min. The precipitate was then removed by centrifuging at 3000 rpm for 15 min at 4 °C, dissolved in phosphate buffer (0.1 M, pH 7), and dialyzed overnight against a large volume of phosphate buffer (0.01 M). The purification was performed using a Sephadex G-100 column and gel filtration chromatography, and 10 mL of the dialysate was placed onto the column before it was pre-equilibrated with Tris-HCl buffer, and the same was used to elute the sample until it was divided into 20 fractions of 2 mL each. The lipase activity was performed using a protein assay by collecting the fractions every 10 min [17,18].
2.7. Molecular Identification
The DNA isolated was quantified with a spectrophotometer. The DNA was diluted 50 times by mixing 1 μL of stock with 49-μL sterile distilled water. The A260/A280 ratio was recorded to check the purity of DNA preparation. PCR amplification of the ribosomal 16S region of genomic DNA was performed using 16S Forward Primer (FP) and 16S Reverse Primer (RP). PCR intensification of 16S locale was conducted in 20 μL of response blend containing PCR support, 1X (Kappa, SA); 3 mM; MgCl2, 0.25 mM; Taq DNA polymerase, dNTP blend 0.05 U; preliminary, 1 picomol and layout DNA, 50 ng. Sterile nuclease-free water was utilized as a negative control. PCR items were gel cleaned and investigated in a DNA gel (3730 DNA Analyzer, Applied Biosystems, Waltham, MA, USA). Succession information was altered utilizing Chromas Pro form 2.1.8 [19,20].
Based on 16S rRNA sequences and homology analysis, the bacterial isolate B. halotolerans VSH 09 was identified molecularly. The isolate was tested for molecular identification using homology analysis and 16S rRNA sequencing. The 16S rRNA gene’s nucleotide sequences on both strands were amplified and determined. Using the BLAST search program, the obtained sequences were compared to the sequences present in the NCBI public database [19]. The sequences of the isolate and related strains from BLAST search results were retrieved from the Gen Bank database and aligned using the Clustal W tool. The phylogenetic tree was constructed using MEGA6 software, and the molecular evolutionary genetic relationship was constructed with the neighbor-joining (NJ) method [21].
2.8. Estimate of Protein and Lipase Activity
Tributyrin was used as a substrate in the production of lipase, and dilute NaOH was used for the titration method to measure lipase activity. To remove the cells, the fermentation broth was centrifuged at 5000 rpm at 4 °C for 20 min. A titration against 0.05 N NaOH using a phenolphthalein indicator was performed using 1 mL of the crude enzyme-containing supernatant, 9.9 mL of distilled water, and 0.1 mL of oil. This method was carried out at room temperature for 15 min. Additionally, using bovine serum albumin (BSA) as the standard, a calibration curve was drawn to measure the protein content using Lowry’s method [21,22].
2.9. Characterization of Enzyme
2.9.1. Study of pH and Stability on Enzyme Activity
Different physico-chemical parameters were used to characterize the partially purified lipase. The pH was maintained between 3 and 12 by adding the following buffers: 50 mM glycine-HCl (3), 50 mM sodium acetate (4–6), 50 mM Tris-HCl (7–9), and 50 mM glycine NaOH (10–12) and other factors were kept constant. The substrate was supplemented with 1% oil and incubated at 37 °C. The stability and residual activity of the enzyme were measured every 30 min over a period of 4 h of fermentation with a pH range of 3–12.
2.9.2. Effect of Temperature on Lipase Activity
The effect of temperature on lipase activity was determined by incubating media at different temperatures ranging between 25 °C and 65 °C; 1% oil was used as the substrate. The number of fatty acid units produced was estimated using the titration method. By evaluating the residual activity after incubating the enzyme at temperatures ranging from 25 °C to 65 °C up to 4 h.
2.9.3. Effect of Reaction Time on Lipase Activity
The effect of reaction time on enzyme activity was determined by varying reaction times between 10 and 60 min incubating at 37 °C, with oil (1%) as substrate, while other parameters were kept constant.
2.9.4. Effect of Metal Ions on Lipase Activity
The mixture of 0.5 mL of lipase substrate (1% concentration), 0.1 mL crude enzyme, and 0.1 mL of different metal ions (Mg2+, Cu2+, Mn2+, Ca2+, Fe2+, Co2+, Zn2+, Ni2+, K+, Hg+, Pb2+, EDTA) was incubated at 37 °C for 30 min and the enzyme activity was determined [23,24].
2.9.5. Effect of Substrate Concentration on Lipase Activity
The effect of substrate concentration (ranging from 0.1–1.5%) on enzyme activity was determined by keeping all other parameters constant. Kinematic parameters Vmax and Km were determined by plotting Lineweaver–Burk plots [25,26].
2.10. Application of Lipase Enzyme in Biodiesel Production
The transesterification reaction was conducted by using waste cooking oil as substrate, and lipase was used as biocatalyst. The waste cooking oil and methanol were mixed in a ratio of 1:4 (6.472 mL of oil and 43.36 mL of methanol) in a 250 mL beaker which was provided with a magnetic stirrer. A total of 15% (6.5 g) of immobilized enzymes were added under mixing conditions and kept at room temperature for 24 h under constant mixing with the help of a magnetic stirrer [27].
3. Results
3.1. Bacterial Isolate for Lipase Production
Ten strains were isolated from an oil-contaminated soil sample using the serial dilution method on the basis of colony morphology and appearance on nutrient agar plates [13]. The isolate VSH09, isolated near oil refineries, Dharwad, showed 2.3 cm (highest), and VSH 06 isolated from railway workshop, Hubballi showed 0.7 cm (lowest) halo zone on Tributyrin Agar Media (Figure 1). Hence, the VSH 09 strain was used for further experimentation. The zone of clearance was a result of hydrolysis of tributyrin due to lipase activity.
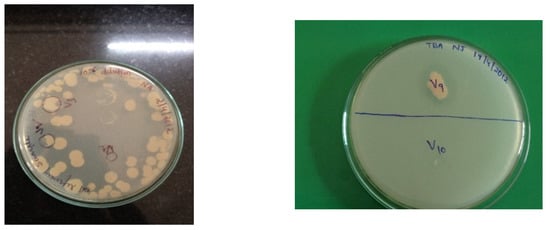
Figure 1.
Isolation and screening of lipase-producing bacteria on tributyrin agar plate for VSH 09.
The isolated 10 bacterial strains were designated as VSH01, VSH02, VSH03, VSH04, VSH05, VSH06, VSH07, VSH08, VSH09, and VSH10. Similar studies on screening and isolation were carried out by Beller et al. 1996 [28].
The isolated strain VSH 09, which showed the maximum zone of clearance on tributyrin agar, was characterized and identified according to Bergey’s manual. The isolate showed a purple/violet color, indicating it is Gram-positive, chain type, and short rod-shaped (Figure 2). The isolate showed positive for the catalase test, indicating it is an aerobic bacterium. Starch and casein hydrolysis gave a positive result. Hence, from these results, one could confirm that the VSH 09 isolate was a Bacillus sp. (Table 1) [29].
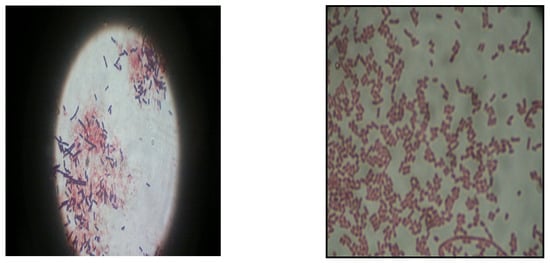
Figure 2.
Gram stain of bacterial isolate VSH 09 showing the crystal violet color and bacilli shape.

Table 1.
Morphological and biochemical characterization of bacterial isolates.
3.2. Phylogenetic Tree Construction by 16S rDNA Gen Sequence
Using a multiple alignment software, representative sequences of similar neighbors in the BLAST analysis were found and aligned (Figure 3). Using MAFFT (Multiple Alignment utilizing Fast Fourier Transform), a neighbor-joining tree was created using the multiple alignment file. The isolated pure bacterial culture VSH 09 was molecularly characterized by 16S rDNA sequencing followed by BLASTn and phylogenetic analysis.
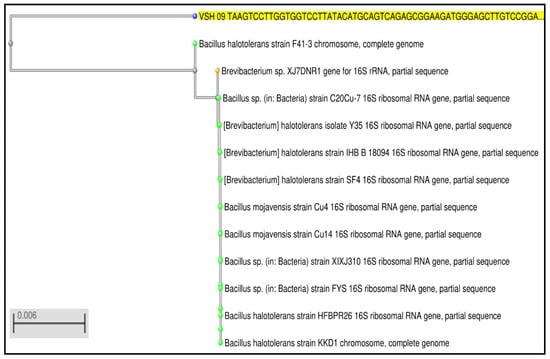
Figure 3.
Phylogenetic analysis of 16S rDNA gene for VSH 09.
Polymerase chain response enhancement of the fractional 16S rRNA came about in the anticipated 1500−bp amplicon in the isolated VSH 09. The amplicon groupings were contrasted with 16S rRNA successions in the NCBI database, and the arrangements displayed 92% character with Bacillus halotolerans. The incomplete 16S rRNA of the segregate VSH 09 was stored in Gen Bank (Accession Number: MN372454.1). The results are as per a few investigations revealing the lipase-creating Bacillus sp. under SmF [30,31,32].
After running nucleotide BLAST, the top 15 organisms from the blast results were selected for further analysis. The nucleotide sequence of the entire top 15 organisms was considered for performing multiple sequence alignment and phylogenetic analysis using Clustal Omega (https//www.ebi.ac.uk/tools/msa/clustalo/ (accessed on 10 June 2022). On the basis of nucleotide sequence homology and phylogenetic analysis, the sequence of primer showed maximum identity and shared phylogenetic relationship with Bacillus halotolerans. (KU821697.1—Gen bank accession number). Hence, the isolated strain VSH 09 was named Bacillus halotolerans. (Gen Bank of NCBI under the accession number: MN372454) [30].
3.3. Determination of Molecular Weight
The molecular weight of partially purified lipase was determined by SDS−PAGE, which indicated a solitary band (Figure 4), confirming that there was a chemical protein band with a molecular weight of around 36 kDa.

Figure 4.
SDS−PAGE, zymogram of purified Lipase produced by Bacillus halotolerans. VSH 09.
3.4. Batch Submerged Fermentation Studies on Lipase Production
Submerged fermentation (SmF) was carried out in order to study the growth pattern and also to analyze the kinetic parameters. SmF was carried out under the optimized conditions which were identified in OFAT studies (Figure 5). Microbial growth, substrate concentration, product concentration, and enzyme activity were analyzed in SmF. The growth and enzyme activity of bacteria was less for the initial 24 h of incubation and increased after 48 h. The growth was linear during the log phase and reached a maximum level at 56 h in the stationary phase. The maximum activity was 28.76 IU/mL at 56 h, and biomass concentration was 20.84 g/lit at 72 h of incubation [33].
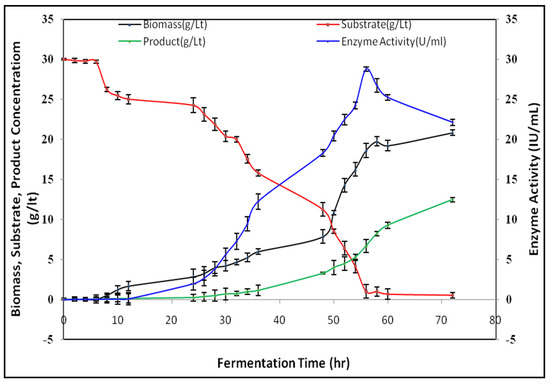
Figure 5.
Batch fermentation.
3.5. Optimum Temperature
The effect of temperature on lipase activity was considered in a range from 25 °C to 65 °C. Maximum lipase activity was found to be 27.28 ± 0.44 IU/mL at 35 °C, as shown in Figure 6. Lipase activity increased with an increase in temperature from 25 °C to 35 °C; a further increase in temperature decreased the enzyme activity up to 65 °C. Most bacterial lipases had an optimum temperature range of 30 °C to 60 °C [34,35].
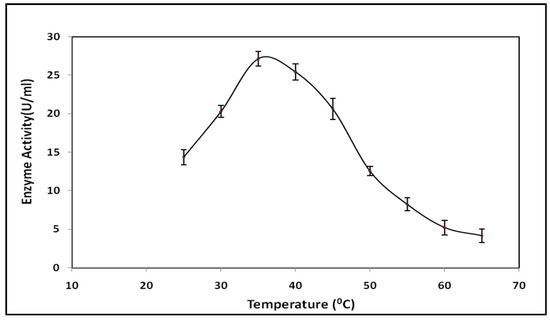
Figure 6.
Effect of temperature on lipase activity.
3.6. Optimum pH
Bacterial lipase showed a wide range of pH stability, over the range from pH 7 to pH 10. As we increased pH from 3 to 7, there was an increase in the enzyme activity, and a further increase in pH decreased the enzyme activity [36,37]. The optimum lipase activity of 29.68 ± 0.18 IU/mL was observed at pH 7 (Figure 7). Many authors have reported that the optimum pH for lipase production from Bacillus sp. is in the range of 8 to 9. Lipase enzymes retained 100% residual activity at pH 7 and 8 after 2 h of incubation. The enzyme showed more stability after 4 h of incubation at pH 7, 8, 9, and 10 with residual activity of 101.28, 90.32, 80.42, and 60.48, respectively. The stability of the lipase enzyme was only 50% after 4 h of incubation, which was maintained at pH 6 [38,39].
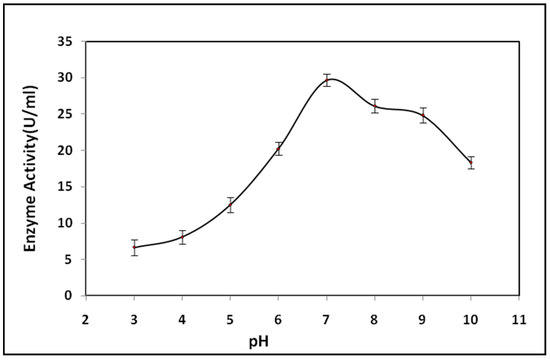
Figure 7.
Effect of pH on lipase activity.
3.7. Biodiesel Production
Lipase as a biocatalyst mediated the transesterification process of waste cooking oil to biodiesel. Glycerol, a byproduct of transesterification, was analyzed qualitatively by the glycerol assay method. A total of 1 mol of tri-glycerol and 3 moles of methanol were stoichiometrically required to produce 1 mol of glycerol and 3 moles of fatty acid methyl esters (biodiesel). The 3,5−diacetyl−1,4−dihydrolutidine compound was produced when formaldehyde and acetylacetone reacted with each other [40]. The greatest absorbance peak of this yellow substance was visible at 410 nm. Both pure glycerol and biodiesel samples had a yellow tint, which was an indication of glycerol formation. (Figure 8) [27,29].
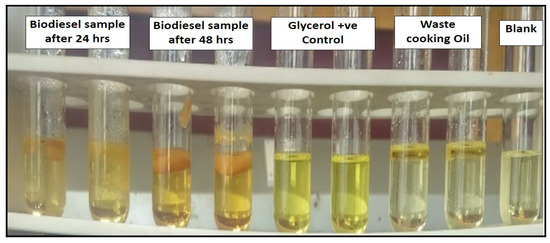
Figure 8.
Qualitative analysis of biodiesel by glycerol assay.
4. Discussion
Due to its characteristics, such as enzyme activity over a broad range of pH, temperature, and substrate range, microbial lipases are the biocatalyst of choice for the present and future. Bacterial strains are typically favored because they have neutral pH optima, higher activity than yeast, and are typically thermostable. The SmF system was selected with the bacterial strain B. halotolerans VSH 09. Different carbon sources, including glucose, fructose, and molasses, were used in submerged fermentation to produce lipase. Lactose, starch, and sucrose were investigated to ascertain their impacts on lipase activity by SmF. The OFAT method was used to investigate the impact of fermentation process variables such as incubation time, inoculum size, temperature, and pH on enzyme activity. Following OFAT analysis, the Plackett Burman design was used to screen media elements and process parameters. To identify important media components influencing the development of lipase, several sources of carbon, nitrogen, oils, and minerals were examined. Similar to this, process variables including pH, agitation rate, and temperature were tested to determine which of these variables had the greatest impact on the result. It was found that the kinetic parameters Km and Vmax were 9.37 mg/mL and 41.66 IU/mL, respectively. A single band displaying the purified protein with a molecular weight of 36 KDa was produced after the purified lipase was subjected to SDS PAGE. The lipase was characterized, and it was determined that the optimum pH of the free enzyme was pH 7. There was a drastic change in enzyme activity between pH 7 and 8. As a result, the optimum pH was found to be 8. The findings from the present study are in line with the findings of Tripathi et al. 2014, who reported on the isolation, purification, and characterization of lipase from Microbacterium sp. and its application in biodiesel production [40]. Similarly, Borkar et al. 2009, reported on the purification and characterization of extracellular lipase from a new strain, Pseudomonas aeruginosa SRT 9, and its suitability as a biotechnological tool with a variety of applications, including organo−synthetic reactions and preparation of enantiomerically pure pharmaceuticals [41].
Application studies performed for the production of biodiesel by transesterification have shown many industrial advantages over chemical catalysts and free enzymes. Biodiesel can be a renewable and environmentally beneficial alternative energy source. For biodiesel manufacturing, enzymes are better catalysts, as enzymes are more stable, and their production is more convenient [40]. Thus, the present study reveals that the bacterial strain B. halotolerans VSH 09 has high potential and is useful for lipase production. In summary, the media components are mainly industrial wastes, and optimization of cultural conditions and media components using statistical tools for higher lipase activity by B. halotolerans VSH 09 shows the potential for industrial application, especially for biodiesel production.
5. Conclusions
Due to its many industrial and biotechnology applications, the demand for enzymes produced from biological sources for industrial use is increasing globally. This study focused on the optimization of process and media parameters for enhanced lipase production from Bacillus sp. The kinetic parameters Km and Vmax were found to be 9.37 mg/mL and 41.66 IU/mL, respectively, and the purified lipase has a molecular weight of 36 KDa. The enzyme activity was found to be maximum at pH 7 and a temperature of 37 °C.
Author Contributions
Conceptualization, V.S.H. and U.M.M.; Data curation, S.V.D.; Formal analysis, U.M.M.; Funding acquisition, M.H.M., M.E.-S., M.M.G., M.M.A. and A.A.A.A.; Investigation, V.S.H., I.A.S., S.A., O.A.J. and B.A.M.; Methodology, M.H.M., V.S.H., S.V.D., A.A.A. and A.A.K.; Project administration, U.M.M., M.M.G., M.A. and A.A.K.; Resources, M.A. and A.A.A.; Software, I.A.S.; Supervision, S.V.D.; Validation, I.A.S. and M.E.-S.; Writing—original draft, V.S.H. and U.M.M.; Writing—review and editing, M.H.M., I.A.S., S.A., M.E.-S., M.M.G., O.A.J., M.M.A., M.A., A.A.A., B.A.M., A.A.A.A. and A.A.K. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful to the Deanship of Scientific Research, Najran University, Najran, Saudi Arabia, for funding this research through grant research code NU/NRP/MRC/11/2. This research was supported by AlMaarefa University researchers supporting program (grant number: MA−006), AlMaarefa University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data has been included in the results section of this article.
Acknowledgments
The authors are thankful to Department of Biotechnology, KLE Technological University, BVB Campus, Hubballi, India, for supporting this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ghosh, P.K.; Saxena, R.K.; Gupta, R.; Yadav, R.P.; Davidson, S. Microbial Lipases: Production and Applications. Sci. Prog. 1996, 79 Pt 2, 119–157. [Google Scholar] [PubMed]
- Schmidt-Dannert, C.; Rúa, M.L.; Schmid, R.D. Two Novel Lipases from Thermophile Bacillus thermocatenulatus: Screening, Purification, Cloning, Overexpression, and Properties. Methods Enzymol. 1997, 284, 194–220. [Google Scholar] [CrossRef]
- Chandra, P.; Enespa; Singh, R.; Arora, P.K. Microbial Lipases and Their Industrial Applications: A Comprehensive Review. Microb. Cell Fact. 2020, 19, 169. [Google Scholar] [CrossRef]
- de Godoy Daiha, K.; Angeli, R.; de Oliveira, S.D.; Almeida, R.V. Are Lipases Still Important Biocatalysts? A Study of Scientific Publications and Patents for Technological Forecasting. PLoS ONE 2015, 10, e0131624. [Google Scholar] [CrossRef]
- Bajpai, P. Application of Enzymes in the Pulp and Paper Industry. Biotechnol. Prog. 1999, 15, 147–157. [Google Scholar] [CrossRef]
- Pandey, A.; Benjamin, S.; Soccol, C.R.; Nigam, P.; Krieger, N.; Soccol, V.T. The Realm of Microbial Lipases in Biotechnology. Biotechnol. Appl. Biochem. 1999, 29, 119–131. [Google Scholar] [PubMed]
- Ertuğrul, S.; Dönmez, G.; Takaç, S. Isolation of Lipase Producing Bacillus sp. from Olive Mill Wastewater and Improving Its Enzyme Activity. J. Hazard. Mater. 2007, 149, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Bhavani, M.; Chowdary, G.V.; David, M.; Archana, G. Screening, Isolation and Biochemical Characterization of Novel Lipase Producing Bacteria from Soil Samples. Int. J. Biol. Eng. 2012, 2, 18–22. [Google Scholar] [CrossRef]
- Ilesanmi, O.I.; Adekunle, A.E.; Omolaiye, J.A.; Olorode, E.M.; Ogunkanmi, A.L. Isolation, Optimization and Molecular Characterization of Lipase Producing Bacteria from Contaminated Soil. Sci. Afr. 2020, 8, e00279. [Google Scholar] [CrossRef]
- Kiran, G.S.; Shanmughapriya, S.; Jayalakshmi, J.; Selvin, J.; Gandhimathi, R.; Sivaramakrishnan, S.; Arunkumar, M.; Thangavelu, T.; Natarajaseenivasan, K. Optimization of Extracellular Psychrophilic Alkaline Lipase Produced by Marine Pseudomonas sp. (MSI057). Bioprocess Biosyst. Eng. 2008, 31, 483–492. [Google Scholar] [CrossRef]
- Kumar, A.; Singh Parihar, S.; Batra, N.K. Enrichment, Isolation and Optimization of Lipase-Producing Staphylococcus sp. from Oil Mill Waste (Oil Cake). J. Exp. Sci. 2012, 3, 26–30. [Google Scholar]
- Alqahtani, Y.S.; More, S.S.; Shaikh, I.A.; KJ, A.; More, V.S.; Niyonzima, F.N.; Muddapur, U.M.; Khan, A.A. Production and Purification of Pectinase from Bacillus subtilis 15A-B92 and Its Biotechnological Applications. Molecules 2022, 27, 4195. [Google Scholar] [CrossRef]
- Mobarak-Qamsari, E.; Kasra-Kermanshahi, R.; Moosavi-Nejad, Z. Isolation and Identification of a Novel, Lipase-Producing Bacterium, Pseudomnas aeruginosa KM110. Iran. J. Microbiol. 2011, 3, 92–98. [Google Scholar]
- Dutta, S.; Ray, L. Production and Characterization of an Alkaline Thermostable Crude Lipase from an Isolated Strain of Bacillus cereus C(7). Appl. Biochem. Biotechnol. 2009, 159, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Demirkan, E.; Aybey Çetinkaya, A.; Abdou, M. Lipase from New Isolate Bacillus Cereus ATA179: Optimization of Production Conditions, Partial Purification, Characterization and Its Potential in the Detergent Industry. Turk. J. Biol. 2021, 45, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Isiaka Adetunji, A.; Olufolahan Olaniran, A. Optimization of Culture Conditions for Enhanced Lipase Production by an Indigenous Bacillus Aryabhattai SE3-PB Using Response Surface Methodology. Biotechnol. Biotechnol. Equip. 2018, 32, 1514–1526. [Google Scholar] [CrossRef]
- Nawani, N.; Kaur, J. Studies on Lipolytic Isoenzymes from a Thermophilic Bacillus sp.: Production, Purification and Biochemical Characterization. Enzym. Microb. Technol. 2007, 40, 881–887. [Google Scholar] [CrossRef]
- Rahman, R.N.Z.R.A.; Baharum, S.N.; Basri, M.; Salleh, A.B. High-Yield Purification of an Organic Solvent-Tolerant Lipase from Pseudomonas sp. Strain S5. Anal. Biochem. 2005, 341, 267–274. [Google Scholar] [CrossRef]
- Sonkar, K.; Singh, D.P. Biochemical Characterization and Thermodynamic Study of Lipase from Psychrotolerant Pseudomonas punonensis. Biocatal. Agric. Biotechnol. 2020, 28, 101686. [Google Scholar] [CrossRef]
- Sugihara, A.; Tani, T.; Tominaga, Y. Purification and Characterization of a Novel Thermostable Lipase from Bacillus sp. J. Biochem. 1991, 109, 211–216. [Google Scholar]
- Ko, W.H.; Wang, I.T.; Ann, P.J. A Simple Method for Detection of Lipolytic Microorganisms in Soils. Soil Biol. Biochem. 2005, 37, 597–599. [Google Scholar] [CrossRef]
- Bharathi, D.; Rajalakshmi, G.; Komathi, S. Optimization and Production of Lipase Enzyme from Bacterial Strains Isolated from Petrol Spilled Soil. J. King Saud Univ. Sci. 2019, 31, 898–901. [Google Scholar] [CrossRef]
- Peng, R.; Lin, J.; Wei, D. Purification and Characterization of an Organic Solvent-Tolerant Lipase from Pseudomonas Aeruginosa CS-2. Appl. Biochem. Biotechnol. 2010, 162, 733–743. [Google Scholar] [CrossRef]
- Hatzinikolaou, D.G.; Macris, J.B.; Christakopoulos, P.; Kekos, D.; Kolisis, F.N.; Fountoukidis, G. Production and Partial Characterisation of Extracellular Lipase from Aspergillus niger. Biotechnol. Lett. 1996, 18, 547–552. [Google Scholar] [CrossRef]
- Xie, W.; Khosasih, V.; Suwanto, A.; Kim, H.K. Characterization of Lipases from Staphylococcus aureus and Staphylococcus epidermidis Isolated from Human Facial Sebaceous Skin. J. Microbiol. Biotechnol. 2012, 22, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Hasan, F.; Shah, A.A.; Hameed, A. Purification and Characterization of a Mesophilic Lipase from Bacillus subtilis FH5 Stable at High Temperature and PH. Acta Biol. Hung. 2007, 58, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.-T.; Yeh, K.-L.; Chen, C.-L.; Chang, J.-S. Enzymatic Transesterification of Microalgal Oil from Chlorella Vulgaris ESP-31 for Biodiesel Synthesis Using Immobilized Burkholderia Lipase. Bioresour. Technol. 2012, 108, 119–127. [Google Scholar] [CrossRef]
- Beller, H.R.; Spormann, A.M.; Sharma, P.K.; Cole, J.R.; Reinhard, M. Isolation and Characterization of a Novel Toluene-Degrading, Sulfate-Reducing Bacterium. Appl. Environ. Microbiol. 1996, 62, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Gupta, N.; Rathi, P. Bacterial Lipases: An Overview of Production, Purification and Biochemical Properties. Appl. Microbiol. Biotechnol. 2004, 64, 763–781. [Google Scholar] [CrossRef]
- Jin, Z.; Zhang, K.; Zhang, L.; Zheng, S.-P.; Han, S.-Y.; Lin, Y. Quantification Analysis of Yeast-Displayed Lipase. Anal. Biochem. 2014, 450, 46–48. [Google Scholar] [CrossRef]
- Hu, J.; Cai, W.; Wang, C.; Du, X.; Lin, J.; Cai, J. Purification and Characterization of Alkaline Lipase Production by Pseudomonas aeruginosa HFE733 and Application for Biodegradation in Food Wastewater Treatment. Biotechnol. Biotechnol. Equip. 2018, 32, 583–590. [Google Scholar] [CrossRef]
- Iftikhar, T.; Niaz, M.; Zia, M.A.; Haq, I.U. Production of Extracellular Lipases by Rhizopus oligosporus in a Stirred Fermentor. Braz. J. Microbiol. 2010, 41, 1124–1132. [Google Scholar] [CrossRef]
- Dharmsthiti, S.; Pratuangdejkul, J.; Theeragool, G.; Luchai, S. Lipase Activity and Gene Cloning of Acinetobacter Calcoacetlcus LP009. J. Gen. Appl. Microbiol. 1998, 44, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Igibah, E.C.; Agashua, L.O.; Sadiq, A.A. Hydro-Geochemical Features and Groundwater Attribute Evaluation in North-Central Abuja, Nigeria. Sci. Afr. 2020, 8, e00324. [Google Scholar] [CrossRef]
- Ghori, M.I.; Iqbal, M.J.; Hameed, A. Characterization of a Novel Lipase from Bacillus sp. Isolated from Tannery Wastes. Braz. J. Microbiol. 2011, 42, 22–29. [Google Scholar] [CrossRef]
- Lee, D.-W.; Koh, Y.-S.; Kim, K.-J.; Kim, B.-C.; Choi, H.-J.; Kim, D.-S.; Suhartono, M.T.; Pyun, Y.-R. Isolation and Characterization of a Thermophilic Lipase from Bacillus thermoleovorans ID-1. FEMS Microbiol. Lett. 1999, 179, 393–400. [Google Scholar] [CrossRef]
- Lee, S.Y.; Rhee, J.S. Production and Partial Purification of a Lipase from Pseudomonas putida 3SK. Enzym. Microb. Technol. 1993, 15, 617–623. [Google Scholar] [CrossRef]
- Tran, D.-T.; Chen, C.-L.; Chang, J.-S. Effect of Solvents and Oil Content on Direct Transesterification of Wet Oil-Bearing Microalgal Biomass of Chlorella vulgaris ESP-31 for Biodiesel Synthesis Using Immobilized Lipase as the Biocatalyst. Bioresour. Technol. 2013, 135, 213–221. [Google Scholar] [CrossRef]
- Yagar, H.; Balkan, U. Entrapment of Laurel Lipase in Chitosan Hydrogel Beads. Artif. Cells Nanomed. Biotechnol. 2017, 45, 864–870. [Google Scholar] [CrossRef]
- Tripathi, R.; Singh, J.; Bharti, R.K.; Thakur, I.S. Isolation, Purification and Characterization of Lipase from Microbacterium sp. and Its Application in Biodiesel Production. Energy Procedia 2014, 54, 518–529. [Google Scholar] [CrossRef]
- Borkar, P.S.; Bodade, R.G.; Rao, S.R.; Khobragade, C.N. Purification and Characterization of Extracellular Lipase from a New Strain: Pseudomonas aeruginosa SRT 9. Braz. J. Microbiol. 2009, 40, 358–366. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).