A Systematic Review on Waste as Sustainable Feedstock for Bioactive Molecules—Extraction as Isolation Technology
Abstract
:1. Introduction
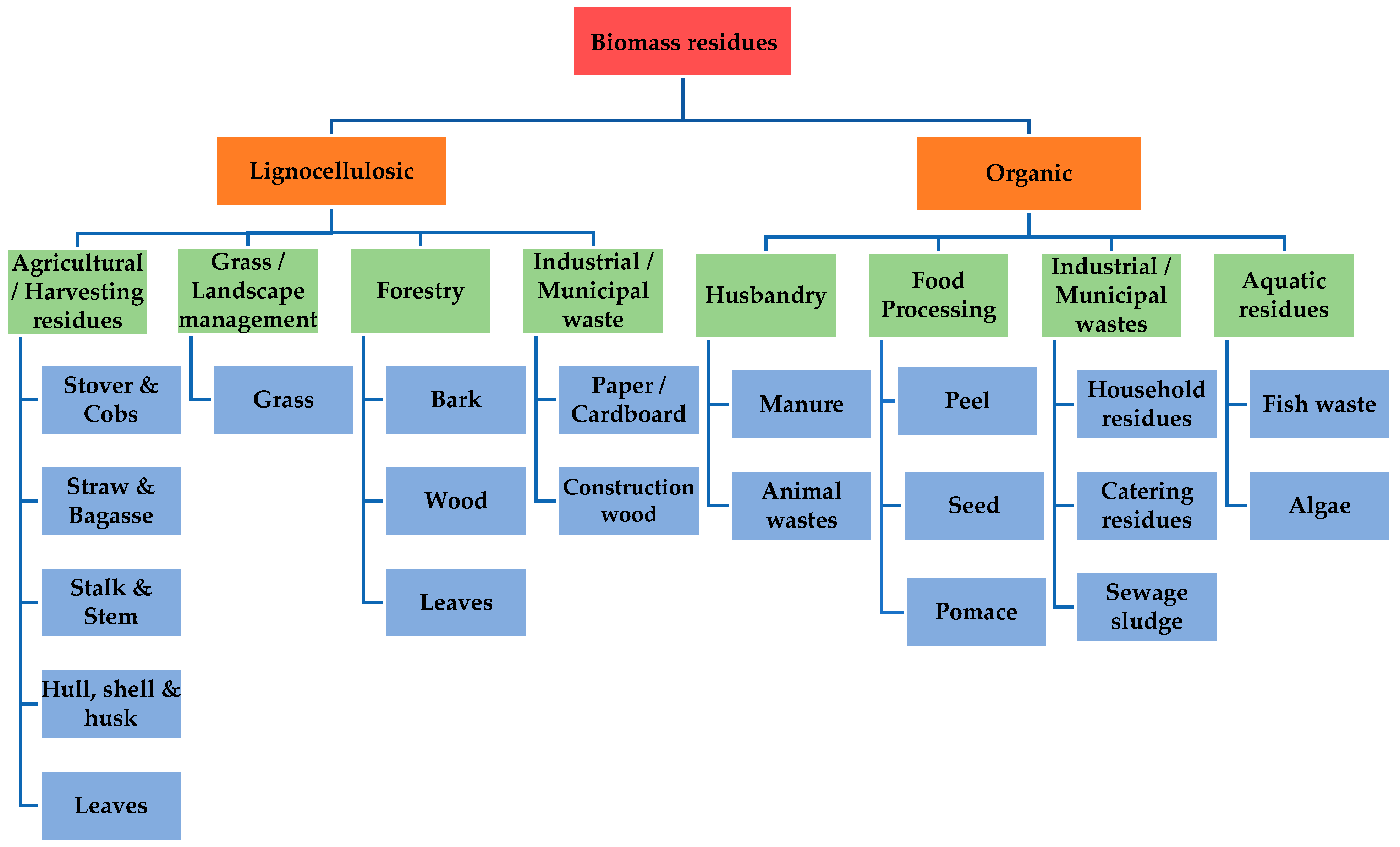
2. Feedstock Classification
- Agricultural cultivation;
- Residues from nature and landscape management;
- Forest-based management;
- Industrial/municipal residues.
- Animal waste and manure;
- Processed food waste;
- Industrial/municipal residues;
- Aquatic residues.
2.1. Lignocellulosic Residues
- Lignocellulosic wastes as agricultural residues. Leftovers of harvesting, as well as secondary residues, which are produced as byproducts from downstream processing of, e.g., maize, sugar cane, wheat, sunflowers, rice, olives, and others such as straw, olive pits, nutshells, leaves, peels, seeds, pomace, or cobs, which also count in this group [14,15,16,17,18].
- Table 1 summarizes the biomass composition of the lignocellulosic biomasses and residues thereof [20,21,22,23,24]. About 44% of the globally produced primary residues can be assigned to wheat and rice residues, which are left on fields. The practice of harvesting primary crop residues could contribute to the reduction of greenhouse gas emissions by hindering the natural degradation of the crops on the field. Nevertheless, these emissions are not counted towards the GHG emissions, because they are kept within the soil–plant–atmosphere continuum. To avoid soil degradation, keeping the nutrient balance as well as the protective behavior of the residues on the soil surface, only a defined percentage of the leftovers can be harvested for further processing [23]. The major problem for producers is the seasonal availability of agricultural residues.
- Residues from nature and landscape management. Worldwide, grasslands account for the largest ecosystems on land, with an area of about 52.5 million km2 [25]. Data for the amount of grass from landscape management are hardly available. During the “Forschungsforum” in Austria, innovative technologies for green biorefineries were discussed, and the annual availability of grass from grassland in the very small country of Austria was evaluated to be 750,000 tons of dry matter. This number shows the high potential of grass as feedstock for future biorefineries [26].
- Industrial residues. Sawdust, bark, and spent or black liquor are byproducts of wood processing. Around 53% of the primary biomass from forestry industries consists of residues such as roots, stumps, and bark. Only 47% is stem, which is the main raw material for wood processing. Data from 2020 expel wood residues of about 227 mio m3. According to technical, environmental, and economic restrictions, not all of the residues can be utilized for further production. In the EU, it is estimated that around 79% of the primary residues, including bark, can be used for the production of bioenergy and biochemicals. This percentage should be transferable to any other wooden-based economy in the world [27]. Sawmill byproducts, bark, and black liquor are mostly burned for power generation. The potential of sawmill byproducts and bark for the isolation of bioactive molecules and the recovery of carbohydrates or organic acid is high [28].
- Lignocellulosic industrial and municipal residues. Mostly paper, cardboard, and wood waste from packaging, construction, and demolition wood is meant here [27]. The paper and cardboard residues are mostly recycled in paper mills, the European Union reports a recycling rate of 73.9% [29]. When the fibers are no longer valid for recycling, the paper is burned to produce energy in the mill. For the valorization of bioactive molecules, this type of lignocellulosic material does not play any role.
2.2. Organic Waste
- Fruits and vegetables;
- Starchy foodstuff;
- Meat, fish and byproducts;
- Others, like dairy products, sweets and nonedible products;
- To improve the waste management also the discarding causes of MFWs has to be noted [39];
- Nonedible food: out of date, not consumed full meals, excessive portion sizes, or not processed feedstock;
- Overproduction of food: in restaurants and other services;
- Waste, based on hygienic, quality, and storage standards;
- Aquatic biomass residues consist of seaweed, algae, and fish residues. Fish residues such as skin, heads, frames, and viscera account for 60% of the global fish production, which was 175 million tons in 2017 [40]. Fish waste is a source of many bioactive molecules and peptides, collagen, gelatin, oil, and pigments [41]. The lower amount of nutrient requirements of algae compared to plants, and the fact that it has not to be cultivated on farmland is beneficial for its footprint. Nevertheless, around 70% of algal biomass remains as waste after the extraction of lipids for food and biodiesel production [42,43].
3. Bioactive Compounds
4. Isolation
- Unsteady chemical composition of biomass across the seasons;
- Varying supply masses, and;
- Low concentration of targeted compounds.
4.1. Pre-Treatment
4.1.1. Electric Discharge Extraction
4.1.2. Hydrolysis & Fermentation
4.2. Extraction
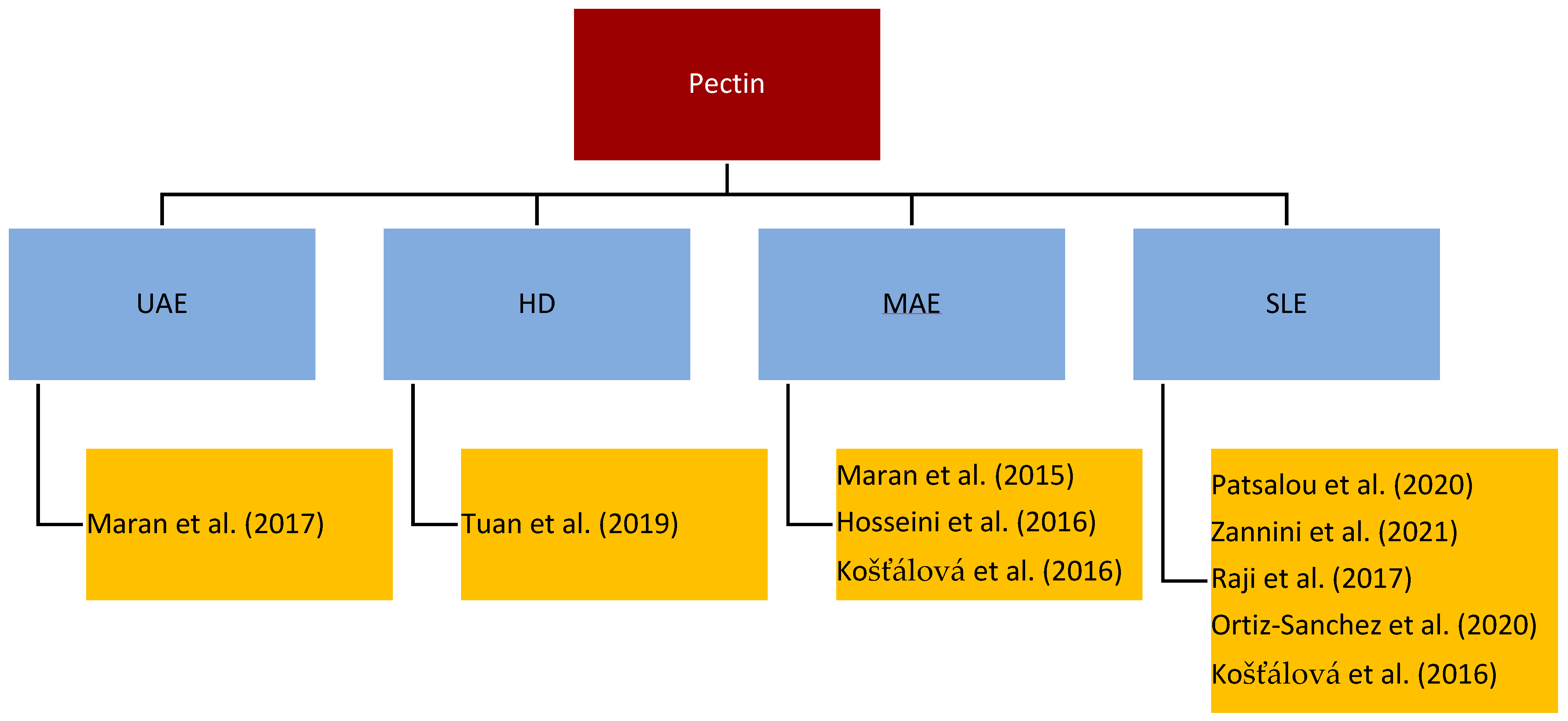
4.3. Pectin Extraction
4.4. Pyrolysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Process | Literature | ||
|---|---|---|---|
| ATPE | Chong et al. [80] | Dordevic et al. [81] | Ran et al. [68] |
| HVED | Xi et al. [89] | Yan et al. [53] | Zhang et al. [54] |
| MAE | Cvetanovic et al. [94] Cvjetko et al. [82] Dahmoune et al. [123] | Drevelegka et al. [32] Hiranvarachat et al. [93] Ho et al. [69] | Klavins et al. [90,124] Routray et al. [71] Wang et al. [74] |
| OHM | Loypimai et al. [100] | ||
| SE | Klavins et al. [90] | ||
| SFE | Benito–Roman et al. [101] Garcia–Mendoza et al. [99] | Kitryte et al. [64] Squillace et al. [125] | |
| SLE | Allison et al. [54] Amyrgialaki et al. [76] Cvjetko et al. [82] Dahmoune et al. [124] Espinosa–Pardo et al. [98] | Goldsmith et al. [79] Guthrie et al. [97] Kehili et al. [78] Klavins et al. [90] Masci et al. [87] | Osojnik et al. [72] Ozturk et al. [70] Routray et al. [71] |
| UAE | Bosiljkov et al. [83] Chanioti et al. [84] Cvetanovic et al. [94] Cvjetko et al. [82] Dahmoune et al. [124] Dordevic et al. [81] Drevelegka et al. [32] | Fernandez et al. [85] Gassara et al. [63] Huang et al. [86] Klavins et al. [90,123] Nipornram et al. [92] Rahimi et al. [77] Rajha et al. [91] | Ran et al. [68] Routray et al. [71] Wang et al. [74] Zhang et al. [54] Zivkovic et al. [75] |
| PEF | Martin–Garcia et al. [56] Pataro et al. [51] | Redondo et al. [55] Zhang et al. [54] | |
| PLE | Cvetanovic et al. [94] Garcia–Mendoza et al. [99] Grunovaite et al. [33] | Guthrie et al. [97] Kheirkhah et al. [96] Kim et al. [126] | Kitryte et al. [64] Loarce et al. [95] |
| Extraction Feedstock | Plant Part | Extraction Process | Extraction Solvent | Extraction Condition | Main Target Compounds | Max. Extraction Yields Content | Possible Utilization | Ref. |
|---|---|---|---|---|---|---|---|---|
| Apple | peel | PEF | Water | 480–1200 V/cm, 0–2 s, 23–25 °C, 1:1–10 (s/l) | Phenolic compounds | ~180 mg/100 g dw | [127,128] | |
| pomace | SSF– UAEUAE | 80% Acetone, P. chrysoporium | 40 °C, 30 min, 1:2 s/l (w/v) | 720 mg GAE/l | [63] | |||
| 80% Ethanol | 639 mg GAE/l | |||||||
| Aronia | stem | MAE | Water | 580 W, 30 min, 1:25 (w/v) | Phenolic compounds flavonoids | 16.65 mg CAE/g 12.12 RE/g | Antioxidant | [94] |
| PLE | 40 bar, 140 °C, 3 Hz stirring, 30 min | 48.62 mg CAE/g 39.19 RE/g | ||||||
| UAE | 1:26 (w/v) | 5.22 mg CAE/g 3.94 RE/g | ||||||
| Banana | peel | UAE | Water, citric acid | pH 1–5, 200–500 W, 5–45 min, 1:10–20 g/mL | Pectin | 9.02% | [129] | |
| Berry | press pomace | UAE | 99.5% ethanol (96%), 0.5% Trifluoroacetic acid | 1:30–100 (w/v), 100 W, 30 °C; 24 h without ultrasound | Phenolic compounds, anthocyanins | 1.68 g/100 g dw 147 mg/100 g dw | [90,123] | |
| SLE | 1:30–100 (w/v), 100 W, 30 °C; 24 h | 1.12 g/100 g dw 98 mg/100 g dw | ||||||
| MAE | 10 min heat up, 600 W, 80 °C, 20 min | 1.09 g/100 g dw 54 mg/100 g dw | ||||||
| SE | 80 °C, 12 h, 25 cycles | 1.21 g/100 g dw 65 mg/100 g dw | ||||||
| Black chokeberry | pomace | SE | Hexane, acetone, ethanol | 1:40 (w:v) | Phenolic compounds anthocyanins | 25.92 g/100 g dw | [33] | |
| PLE | Hexane, methanol, water, acetone; 80:20 (v/v) acetone/water and methanol/water | 40 and 130 °C, 10.3 MPa, 45 min | 48.13 g/100 g dw | |||||
| SFE | CO2 | 149 min, 40 MPa, 40 °C, 2 l/min | 7.08 g/100 g dw | |||||
| Blackberry | pomace | EAE | Citrate buffer, water | Viscozyme L, 50 °C, 250 rpm, 360 min, pH 4.8 | Phenolic compoundsLipophilic fraction | 2.28 mg GAE/g dw 7.83 g/100 g dw | [64] | |
| Citrate buffer, water | 50 °C, 250 rpm, 360 min, pH 4.8 | 0.84 mg GAE/g dw 5.78 g/100 g dw | ||||||
| PLE | Ethanol | 50–90 °C, 10.3 MPa, 3 cycles á 5–15–45 min, after SFE | 29.14 mg GAE/g dw 26.34 g/100 g dw | |||||
| Water | 7.81 mg GAE/g dw 5.09 g/100 g dw | |||||||
| SFE | CO2 | 25–55 MPa, 50–80 °C, 60–180 min | 2.91 mg GAE/g dw 9.93 g/100 g dw | |||||
| SLE | 70% Water, 30% ethanol (v/v) | 800 rpm, 20 °C, 360 min, after SFE | 23.34 mg GAE/g dw 19.88 g/100 g dw | |||||
| SE | Hexane | 6 h | 3.41 mg GAE/g dw 9.53 g/100 g dw | |||||
| Blueberry | leaves | MAE | 15–30 % Ethanol, 1.5 M citric acid | 10–20 % (710.5 W), 4–16 min | Phenolic compounds Anthocyanin Chlorogenic acid | 92.719–128.76 mg GAE/g dw 2.419–2.636 mg M 3–G equiv./g dw 49.542–53.270 mg/g dw | [71] | |
| SLE | 30% Ethanol, 1.5 M citric acid, 80 mL, 97:3 (v/v) | 24 h, 1:16 (w/v) | 89.164 mg GAE/g dw 2.196 mg M 3–G equiv./g dw 47.271 mg/g dw | |||||
| UAE | 30% Ethanol, 1.5 M citric acid, 80 mL, 97:3 (v/v) | 1 h, 1:16 (w/v), 40 kHz; 24 h without ultrasound | 97.77 mg GAE/g 2.46 mg M 3–G equiv./g dw 48.838 mg/g dw | |||||
| Brewers | spend grain | PEF | Ethanol water (4:1 v/v) | 0.5–2.5 kV/cm, 50–150 Hz, 5–15 s | Tricin | 97.936–46.125 µg/g dw | [56] | |
| Ethanol water (4:1 v/v) | 0.5–2.5 kV/cm, 50–150 Hz, 5–15 s | Sinapoyl hexose | 21.08– 36.08 µg/g dw | |||||
| Buckwheat | hull | PLE | Water | 80–120 °C, 5 MPa, 1–3 mL/min | Rutin | 91%, 24.2 mg/g | Medical use | [126] |
| UAE | NADES | 20 kHz, 200 W, 40 °C | 9.5 mg/g | [86] | ||||
| Carrot | peel | MAE | 50 % Hexane, 25 % ethanol, 25 % acetone (v/v) | 180 and 300 W | Carotenoids | 289.2 mg/100 g (d.b.) | [93] | |
| β–carotene | 132.7 mg/100 g (d.b.) | |||||||
| Citrus | peel | HD | Water | 116 °C, 10 min, 5% (w/v) CPW and 0.5% (v/v) H2 SO4 | Essential oils Pectin | 0.43% 30.53% | [66] | |
| SSF | A. succinogenes Z130 | Succinic acid | 0.73 g/g | |||||
| pomace | SLE | Acetic acid 3% (v/v), | 20 g, 200 mL, pH 2.6, 90–100 °C, 6 h | Pectin, flavonoids, polyphenol | 23.70% | Bio–composite | [108] | |
| Cupressus lusitanica Mill. Cistus ladanifer L. | leaves | SD | Water | 1. 30 °C, 30 min ethanol, s:l 1:20, 320 W, 35 kHz 2. 1. + 30 °C, 30 min acetone | Phenolic compounds | 140 mg GAE/g extract 210 mg GAE/g extract | [73] | |
| Flavonoid | 1.3 mg QE/g extract 11.5 mg QE/g extract | |||||||
| Tannins | 86.8 mg GAE/g extract 133.3 mg GAE/g extract | |||||||
| UAE | Ethanol, 70% acetone | Phenolic compounds | 251.3 mg GAE/g extract 275.6 mg GAE/gextract | |||||
| Flavonoid | 6.3 mg QE/g extract 15.2 mg QE/g extract | |||||||
| Tannins | 82.2 mg GAE/g extract 116.6 mg GAE/g extract | |||||||
| Fig | leaves | MAE | NADES | 1:20 g/mL, 10 min, 55 °C, 250 W | Phenolic compounds Furanocoumarins | 45.724 mg/g | [74] | |
| UAE | NADES | 1:20 g/mL, 60 min, 60 °C, 700 W | 45.724 mg/g | |||||
| Grape | pomace | MAE | Water ethanol | 100–600 W, 8–24 mL/g (l/s) | Phenolic compounds | 30.66 mg GAE/g dry pomace 45.35 GAE/g dry pomace | [32] | |
| PLE | Water NADES | 2 g pomace, 1 g diatomaceous earth, 2*10 min, 10.34 MPa, 40–120 °C, | 135.24 mg/g | [95] | ||||
| UAE | Water, 0–100 % ethanol (v/v) | 20–60% (20 kHz), 20–60 °C, 8–24 mL/g (l/s) | 48.76 mg GAE/g dry pomace | Food industry | [32] | |||
| seed | UAE–ATPE | Ionic liquids Ethyl acetate Isopropanol Ethanol Water Methanol 70% ethanol 70% methanol | 1:5 (s:l), 40 °C, 20 min | Procyanidin B2 catechin epicatechin | 0.14 mg/g dry weight 1.21 mg/g dry weight 1.22 mg/g dry weight | Natural antioxidants | [68] | |
| peel | MAE | NADES | 50–90 °C, 15–90 min, 100 W | Anthocyanins | 26 mg/g dw | [82] | ||
| SLE | 12 h, RT, 1:11 (s/l) | 18 mg/g dw | ||||||
| UAE | 50–90 °C, 15–90 min, 35 kHz | 30 mg/g dw | ||||||
| stalk | SSF | LiP, MnP, MnIP, and Lacc iP MnP MnIP, Lacc, CMCase, xylanase, and avicelase (CMCase xylanase and avicelase) | Phenolic compounds | 1.5 mg GAE/l control 0.45 mg GAE/l pre–treated | [58] | |||
| Haskap | leaves | ATPE | Salt/ethanol | (NH4)2 SO4, NaH2 PO4 25 °C, 5–120 min | Chlorogenic acid flavonoids Phenolic compounds | 89.31%–97.82% 36.43 µg/mg | [80] | |
| SLE | 80% Methanol (v/v) | 75 mg, 150 mL, 24 h | 76.28 µg/mg leaves 0.23 mg/mg leaves 0.14 mg/mg leaves | |||||
| ATPE | Sugar/propanol | glucose and maltose, 25 °C, 5–120 min, 0.1–1 wt% sample loading | 69.52%–82.13% | |||||
| Juçara | residues | PLE | Ethanol, water, acidified water | 10 MPa, 40–80 °C, 1.5 mL/min, 90 kg solvent/kg dry residue | Phenolic compoundsanthocyanins | 51.4 mgGAE/g dw | [99] | |
| 1.76 mg/g dw | ||||||||
| SFE | CO2, ethanol water 50% (v/v) | 46 min, 60 °C, 20 MPa, 10% (w/w) co–solvent | 30 mg GAE/g dw | |||||
| 6.2 mg/g dw | ||||||||
| SE | Ethanol | 180 mL, 3 g, 6 h | 21.9 mg GAE/g dw | |||||
| 10.5 mg C3 RE/g dw | ||||||||
| UAE | Ethanol, water 50% (v/v) | 800 W, 19 kHz, 45 min | 23.3 mg GAE/g dw | |||||
| 8.7 mg C3 RE/g dw | ||||||||
| ABE | 2.5 g, pH 2.0, 45 min, 60 °C | 22 mg GAE/g dw | ||||||
| 7.9 mg C3 RE/g dw | ||||||||
| Kiwifruit | Peel | PLE | Water | 500 mL, 30 bar, 30 min, 120–160 °C, pH 2–5.5, 2–6% (s/l) | Phenolic compounds | 51.2 mg GAE/g DW | Medical use | [97] |
| Flavonoids | 22.5 mg CE/g DW | |||||||
| SLE | Ethanol 50% | 2–24 h, pH 2–7 | Phenolic compounds | 26.15 mg GAE/g DW | ||||
| Flavonoids | 18.93 mg CE/g DW | |||||||
| pomace | PLE | Water | 50 bar, 170–225 °C, 10–180 min, 1:100 g/mL (M/S) | Phenolic compounds | 86.26 mg CaE/g DW | [96] | ||
| Flavonoids | 24.18–34.59 mg QE/g DW | |||||||
| SLE | Ethanol, methanol (80%), acetone (70%) | 2 h, 1 h/70 rpm, 1 h/200 rpm | Phenolic compounds | 8.1 CaE/g DW | ||||
| Flavonoids | ||||||||
| Mandarin | peel | SLE | Acetone 80% | 1:20 g/mL (s/l), 30–50 °C, 20–40 min | Phenolic compounds | 12,519.73 mg GAE/100 g DW | [92] | |
| Hesperidin | 6153.22 mg/100 g DW | |||||||
| UAE | 1:20 g/mL (s/l), 38.5 kHz, 30.34–59.36 W, 30–50 °C, 20–40 min | Phenolic compounds | 15,263.32 mg GAE/100 g DW | |||||
| Hesperidin | 6435.53 mg/100 g DW | |||||||
| Mango | peel | MAE | Water | 413 W, 2450 MHz, pH 2.7, 134 s, 1:18 g/mL (s/l) | Pectin | 28.86% | [105] | |
| Melon | peel | SLE | Water, tartaric acid, citric acid, hydrochloric acid, acetic acid, lactic acid, nitric acid, Phosphoric acid, and sulfuric acid | 35–95 °C, 40–200 min, pH (1–3) 10–50:1 v/w (l/s) | Pectin | 29.48% with citric acid | [107] | |
| Ethanol/0.1 M nitric acid | 1:25 w/v (s/l), 1 h, boiling point | 19.3% (fresh); 14.2% (dried) | [130] | |||||
| Microalgae pomace | PEF | Ethanol 95% (v/v) | 24 h, 1:20 w/w (s/l), 150 rpm | Carbohydrates | 37.50% | [54] | ||
| Proteins | 10.10% | |||||||
| Chlorophyll α | 9 mg/g dw | |||||||
| HVED | Carbohydrates | 19% | ||||||
| Proteins | 5.5% | |||||||
| Chlorophyll α | 10.5 mg/g dw | |||||||
| UAE | Carbohydrates | 31% | ||||||
| Proteins | 4% | |||||||
| Chlorophyll α | 25 mg/g dw | |||||||
| SLE | Carbohydrates | 10% | ||||||
| Proteins | 3% | |||||||
| Chlorophyll α | 4.5 mg/g dw | |||||||
| Olive | cake | UAE | Lactic acid and glucose, 5:1; citric acid and glucose, 1:1; fructose and citric acid, 1:1, water 0–15% | 40–80 °C, buffer 0–0.1%, 24 h | Secoiridoids, simple phenol, flavonoids, hydroxycinnamic and hydroxybenzoic acids | 669.433 µg/g dry by–product | Pharmaceutical, cosmetic, agricultural and food industries | [85] |
| pomace | SLE | Hexane | 62.05 µM TYE/g | [79] | ||||
| UAE | NADES | 2:25 g/mL (s/l), 40–60 °C, 30 min, 60 kHz, 280 W | 20.14 GA/g dw | [84] | ||||
| PLE | NADES | 300–600 MPa, 5–10 min | Phenolic compounds | 5.31 mg/g dw | ||||
| Onion | pomace | SLE | Water or ethanol 70% (v/v) | 1:10 w/v (s/l), 90 min, 25 °C, 175 rpm | Quercetin | 400 mg/kg dw | [72] | |
| seed | UAE | Lactic acid and glucose, 5:1; citric acid and glucose, 1:1; fructose and citric acid, 1:1, water 0–15% | 15–60 min ultrasound, 15–75:1 mg/mL (s/l), 40 °C, 20 kHz, 200 W | Secoiridoids, simple phenol, flavonoids, hydroxycinnamic and hydroxybenzoic acids | 344.551 µg/g dry by–product | Pharmaceutical, cosmetic, agricultural and food industries | [85] | |
| Orange | peel | SSF | Aspergillus fumigatus, water | 1:5 (s/l), 60 °C, 30 min | Ellagic acid | 18.68 mg/g | [65] | |
| EAE | Viscozyme L | 1:8 (s/l), 50 °C, 180 min, pH 4.5 | Sugar | 48% | [52] | |||
| Phenolic compounds | 0.85% | |||||||
| MAE | Water | 1:10 g/mL (s/l), 240 min, 100 °C, 300–1000 W | Essential oils | 1.80% | [131] | |||
| Water, citric acid | 15:1 v/w (l/s), pH 1.5–3, 300–700 W, 1–3 min | Pectin | 29.10% | [106] | ||||
| SLE | Water, 5.7 mM citric acid, 0.7% sodium hexametaphosphate | 348.15 K, pH 2, 90 min, 200 rpm | Pectin | 15.85% 0.15±0.77 g/g | D–galacturonic acid as a platform chemical, mucic acid | [110] | ||
| Hexane, CPME, EL, IPA, PEG 300, IAc, DMC, MEK, 2–MeTHF, EAc | 1:10 (s/l), 120 min, 30 °C, 900 rpm | Limonene | 0.81% | Limonene | [70] | |||
| pomace | Water | 1:8 w/w (s/l), 50 °C, 160 rpm | Phenolic compounds | 0.4–0.6 g/100 g dw | [52] | |||
| Ethanol | 1/15 g/mL (w/v), 6 h | 51–71 mg GAE/g extract | [98] | |||||
| HVED | Water, Viscozyme® L | 200 W, 1:8 w/w (s/l), 50 °C, 40 kV, 10 kA, 0.5 Hz, 160 J/pulse, 180 min, pH 4.5, 44–448 kJ/kg | 700 mg/100 g DM | [52] | ||||
| EAE–SFE | CO2, ethanol/water 9:1 (v/v), Paecilomyces variotii | 124 ± 2 kg solvent/kg pomace, 15–35 MPa, 95 min, 40–60 °C, 2.67 × 10 4 kg/s | 18–47 mg GAE/g dry extract | [98] | ||||
| Peach | pomace | PEF | Water, 0–80% methanol | 10–50 pulses, 3 µs, 0–5 kV/cm, 0.61–9.98 kJ/kg, 15–35 °C | Phenolic compounds | 6.4–83.3 mg GEA/100 g | [55] | |
| Flavonoids | 0.6–54.3 CE/100 g | |||||||
| Peanut | shell | HVED | Water, 0–30% ethanol | 1000 Hz, 2 µs, 12–16 kV, 10 kA, 20:1–60:1 mL/g (l/s), 20–100 mL/min | Flavonoids | 0.117–0.948% | [53] | |
| SLE | Water, 80% ethanol | 30 mL/g (l/s), 60°C, 120 min | 0.866% | |||||
| Pear | pomace | UAE | NADES | 15–60 min ultrasound, 15–75:1 mg/mL (s/l), 40 °C, 20 kHz, 200 W | Secoiridoids, simple phenol, flavonoids, hydroxycinnamic and hydroxybenzoic acids | 32.808 µg/g dry by–product | [85] | |
| Pistachio green | hull | SLE | Citric acid | pH 0.5–2.5, 50–90 °C, 30–150 min, 10–50:1 v/w (l/s) | Pectin | 22.10% | Health and cosmetic applications | [103] |
| Pistacia lentiscus | leaves | MAE | 20–100% Ethanol (v/v) | 30–210 s, 300–900 W, 10–40:1 mL/g (l/s) | Phenolic compounds Flavonoid Tannin | 185.69 mg GAE/g dw 5.16 mg QE/g dw 40.21 mg/g dw | [88] | |
| SLE | 60% Ethanol (v/v) | 60 °C, 2 h, 110 rpm, 50:1 mL/g (l/s) | 178.00 mg GAE/g dw 4.79 mg QE/g dw 31.15 mg/g dw | Antioxidant | ||||
| UAE | 40% Ethanol (v/v) | 20 kHz, 15 min, 27 °C, 0.01 W/mL, 1:250 g/mL (s/l) | 142.76 mg GAE/g dw 4.61 mg QE/g dw 35.94 mg/g dw | |||||
| Pomelo | peels | HD | Water, 0–0.55% (w/w) citric acid | pH 2.1–7.3, 2.5 h | Pectin | 11–24% (0–0.55% citric acid) | [109] | |
| Essential oils (limonene, β–pinene, α–phellandrene) | 4.6%/g dw (89.87%, 2.83%, 1.38%) | |||||||
| Pomegranate | peel | HVED | Ethanol, methanol, water, acetic acid, ethyl acetate | 8–14 mL/min, 18–45 kV/cm, 20–50 mL/g (l/s), 30 min | Phenolic compounds | 196.7 mg/g | [89] | |
| SLE | 70 °C, 35 mL/g (l/s), 60 min | Phenolic compounds, Flavonoids, anthocyanins | 158.9 mg/g | |||||
| Ethanol, water, 1 g/l citric acid, 1 N NaOH | 1:10 w/v (s/l), 400 rpm, 22 °C | Phenolic compounds | 324.9 mg GAE/g dry weight | [76] | ||||
| Ethanol, methanol, Ethanol/water 10% acetic acid (1:1, 3:1), ethyl acetate | 1:4 g/mL (s/l), RT, 24 h | Phenolic compounds, Flavonoids, anthocyanins | 26.6 mg GAE/g fw 1.9 mg/100 g fw | [87] | ||||
| seed peel | Water | 50 °C, 10:1 w/w (l/s), 200 min, 20 rpm | Phenolic compounds (ellagic acid, chlorogenic acid, gallic acid) | 38 mg GAE/g DM (peel) | [91] | |||
| 10.6 mg GAE/g DM (mix) | ||||||||
| UAE | 13.7 mg GAE/g DM (mix) | |||||||
| 51 mg GAE/g DM (peel) | ||||||||
| 10–90% Ethanol | 1:10–1:50 (s/l), 20–80 °C, 5–65 min | Phenolic compounds | 118.01–190.94 mg GAE/g dw | [75] | ||||
| Ellagic acid | 4.05–12.54 mg/g dw | |||||||
| Gallic acid | 1.13–3.58 mg/g dw | |||||||
| Punicalin | 28.38–65.67 mg/g dw | |||||||
| Punicalagin | 7.04–35.05 mg/g dw | |||||||
| Pumpkin | pomace | SLE | Water, HCl | 30:1–50:1 mL/g (l/s), pH 2.5, 85 °C, 1 h, 100 rpm | Pectin | 5.7–7.3% | [104] | |
| MAE | Water | 30:1–50:1 mL/g (l/s), pH 2.5, 1200 W, 80–120 °C, 2–10 min | 3.1–7.4% | |||||
| Rice | bran | OHM | Water: 95% ethanol, 1:1, 0.1 M HCl | 30–40% moist, RT, pH 2.5, 100 rpm, 90 min, 1:5 g/mL (s/l) | Tocols and γ–oryzanol | 53,077µg/g | [100] | |
| Anthocyanins | 10818.5 µg/g | |||||||
| SD | water | RT pH 2.5, 100 rpm, 90 min, 1:5 g/mL (s/l) | Tocols and γ–oryzanol | 28.54 µg/g | ||||
| Anthocyanins | 7806.9 µg/g | |||||||
| SFE | CO2, ethanol (modifier, 0–10%) | 40–60 °C, 30–40 MPa, 0.40 kg/h, 2 h, 100 g | Phenolic compounds | 1 mg GAE/g 1.61–3.42 GAE/g (EtOH modifier) | [101] | |||
| Flavonoids | 1.05 mg QE/g 4.47 mg QE/g (EtOH modifier) | |||||||
| γ–oryzanol | 13.19–20.63 mg/g | |||||||
| SLE | Hexane | Soxhlet, 5 g, 25 cycles | Phenolic compounds | 1.58 mg GAE/g | ||||
| Flavonoids | 1.01 mg QE/g | |||||||
| γ–oryzanol | 12.47 0.25 mg/g | |||||||
| Fatty acids | 784 mg/g of oil | |||||||
| Sesamin | 25.7 mg SES/100 g, | |||||||
| Sesaminol triglucoside | 537.5 mg SE/100 g | |||||||
| SSF | Bg352, Bw367 Bg406, H 4 Methanolic extraction solvent | Phenolic compounds | 8.81 mg GAE/g FW | [132] | ||||
| Flavonoids | 14.75 mg RE/g FW | |||||||
| Carotenoids | 1.65 mg Cy 3 glc/g FW | |||||||
| Tomato | peel | MAE | Hexane, ethyl acetate (1:0, 1.5:0.5, 1:1, 2:8, 1:9, 0:1 v/v) | 1:20–4:20 g/mL (s/l), 400–1600 W, 24–48 kJ | Lycopene | 13.592 mg/100 g | [69] | |
| PEF | Acetone, ethyl lactate | 10 Hz, 20 µs pulse width, 0.012–0.475 kJ/kg, 0–5 kV/cm, 10–833 pulses, 1:40 g/mL (s/l), 25 °C, 160 rpm, 0–1440 min | 17.532 g/kg Dw | [51] | ||||
| SLE | 1:40 g/mL (s/l), 25 °C, 160 rpm, 0–1440 min | 14.823 g/kg Dw | ||||||
| pomace | SFE | CO2 | 80 °C, 380 bar, 15 kg/h | Lycopene | 406 μg g−1 | [125] | ||
| SLE | 50% Hexane, 25% ethanol, 25% acetone | 1:100 g/mL (s/l) | Lycopene | 293–476 mg/g dry pomace | Antioxidant, health applications | [31] | ||
| Olive oil | 40–80 °C, 200–400 rpm, 2.5–5.5% w/v (s/l), 15–150 min | Lycopene | 1235.7 mg/kg dw | Lycopene | [78] | |||
| Hexane, hexane: methanol:acetone (2:1:1 v/v) | 1 g, 1 h, RT | Lycopene | 63.66 mg/100 g, 74.89 mg/100 g | [77] | ||||
| UAE | NADES | 15–60 min ultrasound, 15–75:1 mg/mL (s/l), 40 °C, 20 kHz, 200 W | Secoiridoids, simple phenol, flavonoids, hydroxycinnamic and hydroxybenzoic acids | 1,093.475 µg/g dry by–product | Pharmaceutical, cosmetic, agricultural and food industries | [85] | ||
| Sunflower oil | 3.18–36.82% w/v (s/l), 1.59–18.41 min, 30–70 W/m2 | Lycopene | 91.49 mg/100 g | [77] | ||||
| Wheat | chaff | SLE | 22.5% w/w Ethanol, water | 10 min, 25 °C, 3% w/w (s/l) | Phenolic compounds | 2 mg GAE/g (ethanol), 1.8 mg GAE/g (water) | [81] | |
| UAE | 22.5% w/w Ethanol/ammonium–sulfate | 10 min, 25 °C, 30 kHz, 500 W, 3% w/w (s/l) | 2.1 mg GAE/g (water), 2.5 mg GAE/g (ethanol) | |||||
| ATPE | 22.5–24.5% w/w Ethanol, 20–23.8% w/w salt, water | 10 min, 25 °C, 3% w/w (s/l), ammonium–sulfate | 2.1 mg GAE/g | |||||
| ATPE UAE | 10 min, 25 °C, 30 kHz, 500 W, 3% w/w (s/l), ammonium–sulfate, | 2.67 mg GAE/g | ||||||
| Wine | lees | UAE | Ethanol/water/formic acid, 50:48.5:1.5, v/v/v | 380 W, 37 kHz, 35°C, 1:10 g/mL (s/l), pH 2.7, 3 h | Anthocyanin | 4.35 mg/g dw | [83] | |
| NADES, 10–50% water | 190–380 W, 37 kHz, 35°C, 1:10 g/mL (s/l), pH 2.7, 15–45 min | 6.42 mg/g dw | ||||||
| Feedstock | Plant Part | Condition | Liquid Yield | Major Chemicals | Possible Utilization of Bioactive Molecules | Reference |
|---|---|---|---|---|---|---|
| Camellia oleifera | shell extraction residue | 800 °C | Acetic acid, (E)–Stilbene, 4,4′–(1–methylethylidene)bis–phenol, and 33 other constituents | Bio–oil | [133] | |
| Cocoa | shell | 114–514 °C | 33.58% at 214 °C | Acetic acid, cyclopropyl carbinol, 1,6–anhydrous–beta and 24 other constituents. | Liquid fuels, source of chemicals | [121] |
| Coconut | 400 °C | 51% | Phenol, 2–methoxy phenol, furfural, 29 other constituents. | A traumatic ulcer healing agent | [120] | |
| Corn | cob | 300–500 °C | 47.78% at 400 °C | Not analyzed | Pesticides, liquid smoke | [115] |
| Cotton | shell | 400–600 °C | 51% at 450 °C | Thiofanox sulfoxide, 2–hexane, 3–hexane, Linalool, Conyrine, and 260 other compounds listed | Medical, industrial, and agricultural products | [116] |
| Durian | peel | 340–380 °C | Acetic acid, phenol and small amounts of ketones, aldehydes, and carboxylic acids | Pesticide, natural preservative for mackerel | [118] | |
| Groundnut | press cake | 200–500 °C | 50% at 450 °C | Oleic acid amide, oleanitrile, p–cresol, palmitamide, n–methyloctadecanamide, indole, and 64 constituents | Bio–fuel | [134] |
| Hemp | herds | 275–350 °C | 38% | Methanol, acetic acid, furfural, formic acid, 1–hydroxybutan–2–one | [135] | |
| fiber | 350 °C | Propionic acid, acetic acid, methanol, formic acid, hydroxymethylfurfural, and furfural | Pesticide | [117] | ||
| Jatropha | husk shell branch | 550 °C | 42.7% | Benzene, toluene, xylenes, methyl indene, methylnaphthalene, phenols, alkylphenols, methoxy phenols, and eugenols | [122] | |
| Palm | fruit bunch | 425–550 °C | 72.4% at 500 °C | not analyzed | Bio–fuel | [136] |
| Rice | hull | 450–500 °C | 161 compounds characterized | [137] | ||
| Soybean | hulls | 300 °C | Levoglucosenone | Antimicrobial activity | [138] | |
| Sunflower | seed hull | 450 °C | 36% | Acetic acid, methoxy–phenolics, furfural | Insecticide | [114] |
| Switchgrass | 400–600 °C | 48% at 400 °C | 36% hydrocarbons and alkanes, 20.5% phenolic compounds, 14.1% aromatics and 29.4% furans, acids, ketones, alcohols, esters, and amides | Bio–fuel | [113] | |
| Tomato | leaves stems roots fruits | 300–500 °C | 37.8% at 500 °C | Neophytadiene, diene, phytol, and 404 minor constituents | Pesticide | [111] |
| Wood | bark | 350 °C | Liquid smoke Propionic acid, acetic acid, methanol, formic acid, hydroxymethylfurfural, and furfural | Wood preservative | [117,119] |
References
- Department of Economics and Social Affairs. How Certain Are the United Nations Global Population Projections? Popul. Facts 2019, No. 2019/6. Available online: https://www.un.org/en/development/desa/population/publications/factsheets/index.asp (accessed on 28 July 2022).
- Kaza, S.; Yao, L.; Bhada-Tata, P.; van Woerden, F.; Lonkova, K.; Morton, J.; Poveda, R.A.; Sarraf, M.; Malkawi, F.; Harinath, A.S.; et al. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank Group: Washington, DC, USA, 2018; ISBN 978-1-4648-1329-0. [Google Scholar]
- Terra Green. Global Waste—Solvable Problem as a Renewable Energy Resource. Available online: https://medium.com/@support_61820/global-waste-solvable-problem-as-a-renewable-energy-resource-5d8f05cc1a7d (accessed on 5 August 2020).
- Attard, T.M.; Clark, J.H.; McElroy, C.R. Recent developments in key biorefinery areas. Curr. Opin. Green Sustain. Chem. 2020, 21, 64–74. [Google Scholar] [CrossRef]
- Haigh, L.; de Wit, M.; Hoogzaad, J.; von Daniels, C.; Colloricchio, A.; Heidtmann, A. Circularity Gap Report 2021. Available online: https://www.circularity-gap.world/2021#downloads (accessed on 21 August 2021).
- De Wit, M.; Hoogzaad, J.; von Daniels, C. Circularity Gap Report 2020. Available online: https://www.circularity-gap.world/2020 (accessed on 21 February 2021).
- De Jong, E.; Stichnothe, H.; Bell, G.; Jørgensen, H. Bio-Based Chemicals: A 2020 Update; IEA: Wageningen, The Netherlands, 2020; ISBN 978-1-910154-69-4. [Google Scholar]
- Chen, T.-L.; Kim, H.; Pan, S.-Y.; Tseng, P.-C.; Lin, Y.-P.; Chiang, P.-C. Implementation of green chemistry principles in circular economy system towards sustainable development goals: Challenges and perspectives. Sci. Total Environ. 2020, 716, 136998. [Google Scholar] [CrossRef] [PubMed]
- Joelsson, E.; Wallberg, O.; Börjesson, P. Integration potential, resource efficiency and cost of forest-fuel-based biorefineries. Comput. Chem. Eng. 2015, 82, 240–258. [Google Scholar] [CrossRef]
- Budzianowski, W.M. High-value low-volume bioproducts coupled to bioenergies with potential to enhance business development of sustainable biorefineries. Renew. Sustain. Energy Rev. 2017, 70, 793–804. [Google Scholar] [CrossRef]
- Tomei, J.; Helliwell, R. Food versus fuel? Going beyond biofuels. Land Use Policy 2016, 56, 320–326. [Google Scholar] [CrossRef]
- Bharathiraja, B.; Chakravarthy, M.; Ranjith Kumar, R.; Yogendran, D.; Yuvaraj, D.; Jayamuthunagai, J.; Praveen Kumar, R.; Palani, S. Aquatic biomass (algae) as a future feed stock for bio-refineries: A review on cultivation, processing and products. Renew. Sustain. Energy Rev. 2015, 47, 634–653. [Google Scholar] [CrossRef]
- Cuevas-Castillo, G.A.; Navarro-Pineda, F.S.; Baz Rodríguez, S.A.; Sacramento Rivero, J.C. Advances on the processing of microalgal biomass for energy-driven biorefineries. Renew. Sustain. Energy Rev. 2020, 125, 109606. [Google Scholar] [CrossRef]
- Mohammed, N.I.; Kabbashi, N.; Alade, A. Significance of Agricultural Residues in Sustainable Biofuel Development. In Agricultural Waste and Residues; Aladjadjiyan, A., Ed.; InTechOpen: London, UK, 2018; ISBN 978-1-78923-572-2. [Google Scholar]
- Iqbal, Y.; Lewandowsk, I.; Weinreich, A.; Wippel, B.; Pforte, B.; Hadai, O.; Tryboi, O.; Spöttle, M.; Peters, D. Maximising the Yield of Biomass from Residues of Agricultural Crops and Biomass from Forestry: Final Report. Available online: https://ec.europa.eu/energy/sites/ener/files/documents/Ecofys%20-%20Final_%20report_%20EC_max%20yield%20biomass%20residues%2020151214.pdf (accessed on 26 June 2021).
- Jose, S.; Bhaskar, T. Biomass and Biofuels: Advanced Biorefineries for Sustainable Production and Distribution; CRC Press: Boca Raton, FL, USA, 2015; ISBN 9781466595323. [Google Scholar]
- Antizar-Ladislao, B.; Turrion-Gomez, J.L. Second-generation biofuels and local bioenergy systems. Biofuels Bioprod. Bioref. 2008, 2, 455–469. [Google Scholar] [CrossRef]
- RedCorn, R.; Fatemi, S.; Engelberth, A.S. Comparing End-Use Potential for Industrial Food-Waste Sources. J. Eng. 2018, 4, 371–380. [Google Scholar] [CrossRef]
- Dahmen, N.; Lewandowski, I.; Zibek, S.; Weidtmann, A. Integrated lignocellulosic value chains in a growing bioeconomy: Status quo and perspectives. GCB Bioenergy 2019, 11, 107–117. [Google Scholar] [CrossRef] [Green Version]
- Kumar, B.; Bhardwaj, N.; Agrawal, K.; Chaturvedi, V.; Verma, P. Current perspective on pretreatment technologies using lignocellulosic biomass: An emerging biorefinery concept. Fuel Process. Technol. 2020, 199, 106244. [Google Scholar] [CrossRef]
- Dahadha, S.; Amin, Z.; Bazyar Lakeh, A.A.; Elbeshbishy, E. Evaluation of Different Pretreatment Processes of Lignocellulosic Biomass for Enhanced Biomethane Production. Energy Fuels 2017, 31, 10335–10347. [Google Scholar] [CrossRef]
- Vu, H.P.; Nguyen, L.N.; Vu, M.T.; Johir, M.A.H.; McLaughlan, R.; Nghiem, L.D. A comprehensive review on the framework to valorise lignocellulosic biomass as biorefinery feedstocks. Sci. Total Environ. 2020, 743, 140630. [Google Scholar] [CrossRef] [PubMed]
- Cherubin, M.R.; Oliveira, D.M.d.S.; Feigl, B.J.; Pimentel, L.G.; Lisboa, I.P.; Gmach, M.R.; Varanda, L.L.; Morais, M.C.; Satiro, L.S.; Popin, G.V.; et al. Crop residue harvest for bioenergy production and its implications on soil functioning and plant growth: A review. Sci. Agric. 2018, 75, 255–272. [Google Scholar] [CrossRef] [Green Version]
- Mubofu, E.B.; Mgaya, J.E. Chemical Valorization of Cashew Nut Shell Waste. Top. Curr. Chem. 2018, 376, 8. [Google Scholar] [CrossRef]
- Han, P.; Zhao, X.; Dong, Z.; Yan, Y.; Niu, J.; Zhang, Q. A new approach for the classification of grassland utilization in Inner Mongolia—Based on ecological sites and state-and-transition models. Ecol. Indic. 2022, 137, 108733. [Google Scholar] [CrossRef]
- Thang, V.H.; Novalin, S. Green biorefinery: Separation of lactic acid from grass silage juice by chromatography using neutral polymeric resin. Bioresour. Technol. 2008, 99, 4368–4379. [Google Scholar] [CrossRef]
- PricewaterhouseCoopers EU Services EESV’s Consortium. Sustainable and Pptimal Use of Biomass for Energy in the EU beyond 2020: Annexes of the Final Report. Available online: https://energy.ec.europa.eu/sustainable-and-optimal-use-biomass-energy-eu-beyond-2020_en (accessed on 20 August 2020).
- E4tech, RE-CORD, WUR. From the Sugar Platform to Biofuels and Biochemicals: Final Report for the European Commission, Directorate-General Energy, Contract no. ENER/C2/423-2012/SI2.673791, 2015. Available online: https://ec.europa.eu/search/?queryText=SI2.673791&query_source=europa_default&filterSource=europa_default&swlang=de&more_options_language=en&more_options_f_formats=pdf&more_options_date=* (accessed on 26 February 2022).
- European Paper Recycling Council. Monitoring Report 2020: European Declaration on Paper Recycling 2016–2020. 2020. Available online: https://www.paperforrecycling.eu/publications/ (accessed on 17 August 2021).
- Cherubini, F.; Jungmeier, G.; Wellisch, M.; Willke, T.; Skiadas, I.; van Ree, R.; de Jong, E. Toward a common classification approach for biorefinery systems. Biofuels Bioprod. Bioref. 2009, 3, 534–546. [Google Scholar] [CrossRef]
- Allison, B.J.; Simmons, C.W. Valorization of tomato pomace by sequential lycopene extraction and anaerobic digestion. Biomass Bioenergy 2017, 105, 331–341. [Google Scholar] [CrossRef]
- Drevelegka, I.; Goula, A.M. Recovery of grape pomace phenolic compounds through optimized extraction and adsorption processes. Chem. Eng. Process.-Process Intensif. 2020, 149, 107845. [Google Scholar] [CrossRef]
- Grunovaitė, L.; Pukalskienė, M.; Pukalskas, A.; Venskutonis, P.R. Fractionation of black chokeberry pomace into functional ingredients using high pressure extraction methods and evaluation of their antioxidant capacity and chemical composition. J. Funct. Foods 2016, 24, 85–96. [Google Scholar] [CrossRef]
- Molinuevo-Salces, B.; Riaño, B.; Hijosa-Valsero, M.; González-García, I.; Paniagua-García, A.I.; Hernández, D.; Garita-Cambronero, J.; Díez-Antolínez, R.; García-González, M.C. Valorization of apple pomaces for biofuel production: A biorefinery approach. Biomass Bioenergy 2020, 142, 105785. [Google Scholar] [CrossRef]
- Loyon, L. Overview of Animal Manure Management for Beef, Pig, and Poultry Farms in France. Front. Sustain. Food Syst. 2018, 2, 35. [Google Scholar] [CrossRef]
- Zhang, H.; Schroder, J. Animal Manure Production and Utilization in the US. In Applied Manure and Nutrient Chemistry for Sustainable Agriculture and Environment; He, Z., Zhang, H., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 1–21. ISBN 978-94-017-8806-9. [Google Scholar]
- Battista, F.; Frison, N.; Pavan, P.; Cavinato, C.; Gottardo, M.; Fatone, F.; Eusebi, A.L.; Majone, M.; Zeppilli, M.; Valentino, F.; et al. Food wastes and sewage sludge as feedstock for an urban biorefinery producing biofuels and added-value bioproducts. J. Chem. Technol. Biotechnol. 2020, 95, 328–338. [Google Scholar] [CrossRef]
- Caldeira, C.; Vlysidis, A.; Fiore, G.; de Laurentiis, V.; Vignali, G.; Sala, S. Sustainability of food waste biorefinery: A review on valorisation pathways, techno-economic constraints, and environmental assessment. Bioresour. Technol. 2020, 312, 123575. [Google Scholar] [CrossRef]
- Carmona-Cabello, M.; García, I.L.; Sáez-Bastante, J.; Pinzi, S.; Koutinas, A.A.; Dorado, M.P. Food waste from restaurant sector—Characterization for biorefinery approach. Bioresour. Technol. 2020, 301, 122779. [Google Scholar] [CrossRef]
- Välimaa, A.-L.; Mäkinen, S.; Mattila, P.; Marnila, P.; Pihlanto, A.; Mäki, M.; Hiidenhovi, J. Fish and fish side streams are valuable sources of high-value components. Food Qual. Saf. 2019, 3, 209–226. [Google Scholar] [CrossRef] [Green Version]
- Maschmeyer, T.; Luque, R.; Selva, M. Upgrading of marine (fish and crustaceans) biowaste for high added-value molecules and bio(nano)-materials. Chem. Soc. Rev. 2020, 49, 4527–4563. [Google Scholar] [CrossRef]
- Fawzy, M.A.; Gomaa, M. Use of algal biorefinery waste and waste office paper in the development of xerogels: A low cost and eco-friendly biosorbent for the effective removal of congo red and Fe (II) from aqueous solutions. J. Environ. Manag. 2020, 262, 110380. [Google Scholar] [CrossRef]
- Khoo, C.G.; Dasan, Y.K.; Lam, M.K.; Lee, K.T. Algae biorefinery: Review on a broad spectrum of downstream processes and products. Bioresour. Technol. 2019, 292, 121964. [Google Scholar] [CrossRef]
- Shirahigue, L.D.; Ceccato-Antonini, S.R. Agro-industrial wastes as sources of bioactive compounds for food and fermentation industries. Cienc. Rural 2020, 50, 4. [Google Scholar] [CrossRef]
- Segneanu, A.-E.; Velciov, S.M.; Olariu, S.; Cziple, F.; Damian, D.; Grozescu, I. Bioactive Molecules Profile from Natural Compounds. In Amino Acid—New Insights and Roles in Plant and Animal; Asao, T., Asaduzzaman, M., Eds.; InTech: London, UK, 2017; ISBN 978-953-51-3241-7. [Google Scholar]
- Abreu-Naranjo, R.; Paredes-Moreta, J.G.; Granda-Albuja, G.; Iturralde, G.; González-Paramás, A.M.; Alvarez-Suarez, J.M. Bioactive compounds, phenolic profile, antioxidant capacity and effectiveness against lipid peroxidation of cell membranes of Mauritia flexuosa L. fruit extracts from three biomes in the Ecuadorian Amazon. Heliyon 2020, 6, e05211. [Google Scholar] [CrossRef]
- Fekadu Gemede, H. Antinutritional Factors in Plant Foods: Potential Health Benefits and Adverse Effects. IJNFS 2014, 3, 284. [Google Scholar] [CrossRef] [Green Version]
- De Andrade Lima, M.; Charalampopoulos, D.; Chatzifragkou, A. Optimisation and modelling of supercritical CO2 extraction process of carotenoids from carrot peels. J. Supercrit. Fluids 2018, 133, 94–102. [Google Scholar] [CrossRef]
- Arruda, H.S.; Pastore, G.M. Araticum (Annona crassiflora Mart.) as a source of nutrients and bioactive compounds for food and non-food purposes: A comprehensive review. Food Res. Int. 2019, 123, 450–480. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Pataro, G.; Carullo, D.; Falcone, M.; Ferrari, G. Recovery of lycopene from industrially derived tomato processing by-products by pulsed electric fields-assisted extraction. Innov. Food Sci. Emerg. Technol. 2020, 63, 102369. [Google Scholar] [CrossRef]
- El Kantar, S.; Boussetta, N.; Rajha, H.N.; Maroun, R.G.; Louka, N.; Vorobiev, E. High voltage electrical discharges combined with enzymatic hydrolysis for extraction of polyphenols and fermentable sugars from orange peels. Food Res. Int. 2018, 107, 755–762. [Google Scholar] [CrossRef]
- Yan, L.-G.; Deng, Y.; Ju, T.; Wu, K.; Xi, J. Continuous high voltage electrical discharge extraction of flavonoids from peanut shells based on “annular gap type” treatment chamber. Food Chem. 2018, 256, 350–357. [Google Scholar] [CrossRef]
- Zhang, R.; Lebovka, N.; Marchal, L.; Vorobiev, E.; Grimi, N. Pulsed electric energy and ultrasonication assisted green solvent extraction of bio-molecules from different microalgal species. Innov. Food Sci. Emerg. Technol. 2020, 62, 102358. [Google Scholar] [CrossRef]
- Redondo, D.; Venturini, M.E.; Luengo, E.; Raso, J.; Arias, E. Pulsed electric fields as a green technology for the extraction of bioactive compounds from thinned peach by-products. Innov. Food Sci. Emerg. Technol. 2018, 45, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Martín-García, B.; Tylewicz, U.; Verardo, V.; Pasini, F.; Gómez-Caravaca, A.M.; Caboni, M.F.; Dalla Rosa, M. Pulsed electric field (PEF) as pre-treatment to improve the phenolic compounds recovery from brewers’ spent grains. Innov. Food Sci. Emerg. Technol. 2020, 64, 102402. [Google Scholar] [CrossRef]
- Bilal, M.; Wang, Z.; Cui, J.; Ferreira, L.F.R.; Bharagava, R.N.; Iqbal, H.M.N. Environmental impact of lignocellulosic wastes and their effective exploitation as smart carriers—A drive towards greener and eco-friendlier biocatalytic systems. Sci. Total Environ. 2020, 722, 137903. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.M.C.; Fraga, I.; Sousa, R.M.O.F.; Rodrigues, M.A.M.; Sampaio, A.; Bezerra, R.M.F.; Dias, A.A. Pretreatment of Grape Stalks by Fungi: Effect on Bioactive Compounds, Fiber Composition, Saccharification Kinetics and Monosaccharides Ratio. Int. J. Environ. Res. Public Health 2020, 17, 5900. [Google Scholar] [CrossRef]
- Xu, J.; Dai, L.; Gui, Y.; Yuan, L.; Ma, J.; Zhang, C. Towards a waste-free biorefinery: A cascade valorization of bamboo for efficient fractionation, enzymatic hydrolysis and lithium-sulfur cathode. Ind. Crop. Prod. 2020, 149, 112364. [Google Scholar] [CrossRef]
- Cheng, F.; Brewer, C.E. Conversion of protein-rich lignocellulosic wastes to bio-energy: Review and recommendations for hydrolysis + fermentation and anaerobic digestion. Renew. Sustain. Energy Rev. 2021, 146, 111167. [Google Scholar] [CrossRef]
- Hammed, A.M.; Jaswir, I.; Amid, A.; Alam, Z.; Asiyanbi-H, T.T.; Ramli, N. Enzymatic Hydrolysis of Plants and Algae for Extraction of Bioactive Compounds. Food Rev. Int. 2013, 29, 352–370. [Google Scholar] [CrossRef]
- Cruz-Casas, D.E.; Aguilar, C.N.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R.; Chávez-González, M.L.; Flores-Gallegos, A.C. Enzymatic hydrolysis and microbial fermentation: The most favorable biotechnological methods for the release of bioactive peptides. Food Chem. 2021, 3, 100047. [Google Scholar] [CrossRef]
- Gassara, F.; Ajila, C.M.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Valero, J.R. Liquid state fermentation of apple pomace sludge for the production of ligninolytic enzymes and liberation of polyphenolic compounds. Process Biochem. 2012, 47, 999–1004. [Google Scholar] [CrossRef]
- Kitrytė, V.; Narkevičiūtė, A.; Tamkutė, L.; Syrpas, M.; Pukalskienė, M.; Venskutonis, P.R. Consecutive high-pressure and enzyme assisted fractionation of blackberry (Rubus fruticosus L.) pomace into functional ingredients: Process optimization and product characterization. Food Chem. 2020, 312, 126072. [Google Scholar] [CrossRef]
- Sepúlveda, L.; Laredo-Alcalá, E.; Buenrostro-Figueroa, J.J.; Ascacio-Valdés, J.A.; Genisheva, Z.; Aguilar, C.; Teixeira, J. Ellagic acid production using polyphenols from orange peel waste by submerged fermentation. Electron. J. Biotechnol. 2020, 43, 1–7. [Google Scholar] [CrossRef]
- Patsalou, M.; Chrysargyris, A.; Tzortzakis, N.; Koutinas, M. A biorefinery for conversion of citrus peel waste into essential oils, pectin, fertilizer and succinic acid via different fermentation strategies. Waste Manag. 2020, 113, 469–477. [Google Scholar] [CrossRef]
- European Parliament. Directive 2009/32/EC of the European Parliament and of the Council on the approximation of the laws of the Member States on extraction solvents used in the production of foodstuffs and food ingredients. Off. J. Eur. Union 2009, 141, 11. [Google Scholar]
- Ran, L.; Yang, C.; Xu, M.; Yi, Z.; Ren, D.; Yi, L. Enhanced aqueous two-phase extraction of proanthocyanidins from grape seeds by using ionic liquids as adjuvants. Sep. Purif. Technol. 2019, 226, 154–161. [Google Scholar] [CrossRef]
- Ho, K.; Ferruzzi, M.G.; Liceaga, A.M.; San Martín-González, M.F. Microwave-assisted extraction of lycopene in tomato peels: Effect of extraction conditions on all-trans and cis-isomer yields. LWT 2015, 62, 160–168. [Google Scholar] [CrossRef]
- Ozturk, B.; Winterburn, J.; Gonzalez-Miquel, M. Orange peel waste valorisation through limonene extraction using bio-based solvents. Biochem. Eng. J. 2019, 151, 107298. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. MAE of phenolic compounds from blueberry leaves and comparison with other extraction methods. Ind. Crop. Prod. 2014, 58, 36–45. [Google Scholar] [CrossRef]
- Osojnik Črnivec, I.G.; Skrt, M.; Šeremet, D.; Sterniša, M.; Farčnik, D.; Štrumbelj, E.; Poljanšek, A.; Cebin, N.; Pogačnik, L.; Smole Možina, S.; et al. Waste streams in onion production: Bioactive compounds, quercetin and use of antimicrobial and antioxidative properties. Waste Manag. 2021, 126, 476–486. [Google Scholar] [CrossRef]
- Tavares, C.S.; Martins, A.; Miguel, M.G.; Carvalheiro, F.; Duarte, L.C.; Gameiro, J.A.; Figueiredo, A.C.; Roseiro, L.B. Bioproducts from forest biomass II. Bioactive compounds from the steam-distillation by-products of Cupressus lusitanica Mill. and Cistus ladanifer L. wastes. Ind. Crop. Prod. 2020, 158, 112991. [Google Scholar] [CrossRef]
- Wang, T.; Jiao, J.; Gai, Q.-Y.; Wang, P.; Guo, N.; Niu, L.-L.; Fu, Y.-J. Enhanced and green extraction polyphenols and furanocoumarins from Fig (Ficus carica L.) leaves using deep eutectic solvents. J. Pharm. Biomed. Anal. 2017, 145, 339–345. [Google Scholar] [CrossRef]
- Živković, J.; Šavikin, K.; Janković, T.; Ćujić, N.; Menković, N. Optimization of ultrasound-assisted extraction of polyphenolic compounds from pomegranate peel using response surface methodology. Sep. Purif. Technol. 2018, 194, 40–47. [Google Scholar] [CrossRef]
- Amyrgialaki, E.; Makris, D.P.; Mauromoustakos, A.; Kefalas, P. Optimisation of the extraction of pomegranate (Punica granatum) husk phenolics using water/ethanol solvent systems and response surface methodology. Ind. Crop. Prod. 2014, 59, 216–222. [Google Scholar] [CrossRef]
- Rahimi, S.; Mikani, M. Lycopene green ultrasound-assisted extraction using edible oil accompany with response surface methodology (RSM) optimization performance: Application in tomato processing wastes. Microchem. J. 2019, 146, 1033–1042. [Google Scholar] [CrossRef]
- Kehili, M.; Sayadi, S.; Frikha, F.; Zammel, A.; Allouche, N. Optimization of lycopene extraction from tomato peels industrial by-product using maceration in refined olive oil. Food Bioprod. Process. 2019, 117, 321–328. [Google Scholar] [CrossRef]
- Goldsmith, C.D.; Vuong, Q.V.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. Ultrasound increases the aqueous extraction of phenolic compounds with high antioxidant activity from olive pomace. LWT 2018, 89, 284–290. [Google Scholar] [CrossRef] [Green Version]
- Chong, K.Y.; Stefanova, R.; Zhang, J.; Brooks, M.S.-L. Aqueous two-phase extraction of bioactive compounds from haskap leaves (Lonicera caerulea): Comparison of salt/ethanol and sugar/propanol systems. Sep. Purif. Technol. 2020, 252, 117399. [Google Scholar] [CrossRef]
- Dorđević, T.; Antov, M. Ultrasound assisted extraction in aqueous two-phase system for the integrated extraction and separation of antioxidants from wheat chaff. Sep. Purif. Technol. 2017, 182, 52–58. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Ćurko, N.; Tomašević, M.; Kovačević Ganić, K.; Radojčić Redovniković, I. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef]
- Bosiljkov, T.; Dujmić, F.; Cvjetko Bubalo, M.; Hribar, J.; Vidrih, R.; Brnčić, M.; Zlatic, E.; Radojčić Redovniković, I.; Jokić, S. Natural deep eutectic solvents and ultrasound-assisted extraction: Green approaches for extraction of wine lees anthocyanins. Food Bioprod. Process. 2017, 102, 195–203. [Google Scholar] [CrossRef]
- Chanioti, S.; Tzia, C. Extraction of phenolic compounds from olive pomace by using natural deep eutectic solvents and innovative extraction techniques. Innov. Food Sci. Emerg. Technol. 2018, 48, 228–239. [Google Scholar] [CrossRef]
- Fernández, M.d.L.Á.; Espino, M.; Gomez, F.J.V.; Silva, M.F. Novel approaches mediated by tailor-made green solvents for the extraction of phenolic compounds from agro-food industrial by-products. Food Chem. 2018, 239, 671–678. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, F.; Jiang, J.; Qiao, Y.; Wu, T.; Voglmeir, J.; Chen, Z.-G. Green and efficient extraction of rutin from tartary buckwheat hull by using natural deep eutectic solvents. Food Chem. 2017, 221, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Masci, A.; Coccia, A.; Lendaro, E.; Mosca, L.; Paolicelli, P.; Cesa, S. Evaluation of different extraction methods from pomegranate whole fruit or peels and the antioxidant and antiproliferative activity of the polyphenolic fraction. Food Chem. 2016, 202, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Dahmoune, F.; Spigno, G.; Moussi, K.; Remini, H.; Cherbal, A.; Madani, K. Pistacia lentiscus leaves as a source of phenolic compounds: Microwave-assisted extraction optimized and compared with ultrasound-assisted and conventional solvent extraction. Ind. Crop. Prod. 2014, 61, 31–40. [Google Scholar] [CrossRef]
- Xi, J.; He, L.; Yan, L.-G. Continuous extraction of phenolic compounds from pomegranate peel using high voltage electrical discharge. Food Chem. 2017, 230, 354–361. [Google Scholar] [CrossRef]
- Klavins, L.; Kviesis, J.; Klavins, M. Comparison of methods of extraction of phenolic compounds from american cranberry (Vaccinium macrocarpon L.). Agron. Res. 2017, 15, 1316–1329. [Google Scholar]
- Rajha, H.N.; Koubaa, M.; Boussetta, N.; Maroun, R.G.; Louka, N.; Lebovka, N.; Vorobiev, E. Selective ultrasound-assisted aqueous extraction of polyphenols from pomegranate peels and seeds. J. Food Process. Preserv. 2020, 44, e14545. [Google Scholar] [CrossRef]
- Nipornram, S.; Tochampa, W.; Rattanatraiwong, P.; Singanusong, R. Optimization of low power ultrasound-assisted extraction of phenolic compounds from mandarin (Citrus reticulata Blanco cv. Sainampueng) peel. Food Chem. 2018, 241, 338–345. [Google Scholar] [CrossRef]
- Hiranvarachat, B.; Devahastin, S. Enhancement of microwave-assisted extraction via intermittent radiation: Extraction of carotenoids from carrot peels. J. Food Eng. 2014, 126, 17–26. [Google Scholar] [CrossRef]
- Cvetanović, A.; Švarc-Gajić, J.; Zeković, Z.; Mašković, P.; Đurović, S.; Zengin, G.; Delerue-Matos, C.; Lozano-Sánchez, J.; Jakšić, A. Chemical and biological insights on aronia stems extracts obtained by different extraction techniques: From wastes to functional products. J. Supercrit. Fluids 2017, 128, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Loarce, L.; Oliver-Simancas, R.; Marchante, L.; Díaz-Maroto, M.C.; Alañón, M.E. Implementation of subcritical water extraction with natural deep eutectic solvents for sustainable extraction of phenolic compounds from winemaking by-products. Food Res. Int. 2020, 137, 109728. [Google Scholar] [CrossRef]
- Kheirkhah, H.; Baroutian, S.; Quek, S.Y. Evaluation of bioactive compounds extracted from Hayward kiwifruit pomace by subcritical water extraction. Food Bioprod. Process. 2019, 115, 143–153. [Google Scholar] [CrossRef]
- Guthrie, F.; Wang, Y.; Neeve, N.; Quek, S.Y.; Mohammadi, K.; Baroutian, S. Recovery of phenolic antioxidants from green kiwifruit peel using subcritical water extraction. Food Bioprod. Process. 2020, 122, 136–144. [Google Scholar] [CrossRef]
- Espinosa-Pardo, F.A.; Nakajima, V.M.; Macedo, G.A.; Macedo, J.A.; Martínez, J. Extraction of phenolic compounds from dry and fermented orange pomace using supercritical CO2 and cosolvents. Food Bioprod. Process. 2017, 101, 1–10. [Google Scholar] [CrossRef]
- Garcia-Mendoza, M.D.P.; Espinosa-Pardo, F.A.; Baseggio, A.M.; Barbero, G.F.; Maróstica Junior, M.R.; Rostagno, M.A.; Martínez, J. Extraction of phenolic compounds and anthocyanins from juçara (Euterpe edulis Mart.) residues using pressurized liquids and supercritical fluids. J. Supercrit. Fluids 2017, 119, 9–16. [Google Scholar] [CrossRef]
- Loypimai, P.; Moongngarm, A.; Chottanom, P.; Moontree, T. Ohmic heating-assisted extraction of anthocyanins from black rice bran to prepare a natural food colourant. Innov. Food Sci. Emerg. Technol. 2015, 27, 102–110. [Google Scholar] [CrossRef]
- Benito-Román, O.; Varona, S.; Sanz, M.T.; Beltrán, S. Valorization of rice bran: Modified supercritical CO2 extraction of bioactive compounds. J. Ind. Eng. Chem. 2019, 80, 273–282. [Google Scholar] [CrossRef]
- Xiao, C.; Anderson, C.T. Roles of pectin in biomass yield and processing for biofuels. Front. Plant Sci. 2013, 4, 67. [Google Scholar] [CrossRef] [Green Version]
- Chaharbaghi, E.; Khodaiyan, F.; Hosseini, S.S. Optimization of pectin extraction from pistachio green hull as a new source. Carbohydr. Polym. 2017, 173, 107–113. [Google Scholar] [CrossRef]
- Košťálová, Z.; Aguedo, M.; Hromádková, Z. Microwave-assisted extraction of pectin from unutilized pumpkin biomass. Chem. Eng. Process.-Process Intensif. 2016, 102, 9–15. [Google Scholar] [CrossRef]
- Maran, J.P.; Swathi, K.; Jeevitha, P.; Jayalakshmi, J.; Ashvini, G. Microwave-assisted extraction of pectic polysaccharide from waste mango peel. Carbohydr. Polym. 2015, 123, 67–71. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Khodaiyan, F.; Yarmand, M.S. Optimization of microwave assisted extraction of pectin from sour orange peel and its physicochemical properties. Carbohydr. Polym. 2016, 140, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Raji, Z.; Khodaiyan, F.; Rezaei, K.; Kiani, H.; Hosseini, S.S. Extraction optimization and physicochemical properties of pectin from melon peel. Int. J. Biol. Macromol. 2017, 98, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Zannini, D.; Dal Poggetto, G.; Malinconico, M.; Santagata, G.; Immirzi, B. Citrus Pomace Biomass as a Source of Pectin and Lignocellulose Fibers: From Waste to Upgraded Biocomposites for Mulching Applications. Polymers 2021, 13, 1280. [Google Scholar] [CrossRef] [PubMed]
- Tuan, N.T.; Le Dang, N.; Huong, B.T.C.; Danh, L.T. One step extraction of essential oils and pectin from pomelo (Citrus grandis) peels. Chem. Eng. Process.-Process. Intensif. 2019, 142, 107550. [Google Scholar] [CrossRef]
- Ortiz-Sanchez, M.; Solarte-Toro, J.-C.; González-Aguirre, J.-A.; Peltonen, K.E.; Richard, P.; Cardona Alzate, C.A. Pre-feasibility analysis of the production of mucic acid from orange peel waste under the biorefinery concept. Biochem. Eng. J. 2020, 161, 107680. [Google Scholar] [CrossRef]
- Cáceres, L.A.; McGarvey, B.D.; Briens, C.; Berruti, F.; Yeung, K.K.-C.; Scott, I.M. Insecticidal properties of pyrolysis bio-oil from greenhouse tomato residue biomass. J. Anal. Appl. Pyrolysis 2015, 112, 333–340. [Google Scholar] [CrossRef]
- Costa, F.F.; de Oliveira, D.T.; Brito, Y.P.; Rocha Filho, G.N.d.; Alvarado, C.G.; Balu, A.M.; Luque, R.; Nascimento, L.A.S.d. Lignocellulosics to biofuels: An overview of recent and relevant advances. Curr. Opin. Green Sustain. Chem. 2020, 24, 21–25. [Google Scholar] [CrossRef]
- Imam, T.; Capareda, S. Characterization of bio-oil, syn-gas and bio-char from switchgrass pyrolysis at various temperatures. J. Anal. Appl. Pyrolysis 2012, 93, 170–177. [Google Scholar] [CrossRef]
- Urrutia, R.I.; Yeguerman, C.; Jesser, E.; Gutierrez, V.S.; Volpe, M.A.; Werdin González, J.O. Sunflower seed hulls waste as a novel source of insecticidal product: Pyrolysis bio-oil bioactivity on insect pests of stored grains and products. J. Clean. Prod. 2021, 287, 125000. [Google Scholar] [CrossRef]
- Aladin, A.; Yani, S.; Modding, B.; Wiyani, L. Pyrolisis Of Corncob Waste To Produce Liquid Smoke. IOP Conf. Ser. Earth Environ. Sci. 2018, 175, 12020. [Google Scholar] [CrossRef]
- Madhu, P.; Periyanayagi, G. Identification of Bioactive compounds of pyrolysis oil obtained from cotton residues (Gossypium arboreum) by flash pyrolysis. Int. J. ChemTech Res. 2017, 10, 51–66. [Google Scholar]
- Barbero-López, A.; Chibily, S.; Tomppo, L.; Salami, A.; Ancin-Murguzur, F.J.; Venäläinen, M.; Lappalainen, R.; Haapala, A. Pyrolysis distillates from tree bark and fibre hemp inhibit the growth of wood-decaying fungi. Ind. Crop. Prod. 2019, 129, 604–610. [Google Scholar] [CrossRef]
- Faisal, M.; Gani, A.; Mulana, F. Preliminary assessment of the utilization of durian peel liquid smoke as a natural preservative for mackerel. F1000Research 2019, 8, 240. [Google Scholar] [CrossRef]
- Zhao, Q.; Mäkinen, M.; Haapala, A.; Jänis, J. Valorization of Bark from Short Rotation Trees by Temperature-Programmed Slow Pyrolysis. ACS Omega 2021, 6, 9771–9779. [Google Scholar] [CrossRef]
- Surboyo, M.D.C.; Arundina, I.; Rahayu, R.P.; Mansur, D.; Bramantoro, T. Potential of Distilled Liquid Smoke Derived from Coconut (Cocos nucifera L) Shell for Traumatic Ulcer Healing in Diabetic Rats. Eur. J. Dent. 2019, 13, 271–279. [Google Scholar] [CrossRef] [Green Version]
- Wijaya, M.; Wiharto, M. Synthesis and characterization of bioactive compound from Cocoa fruit shell by pyrolysis process. J. Phys. Conf. Ser. 2020, 1567, 22025. [Google Scholar] [CrossRef]
- Murata, K.; Liu, Y.; Inaba, M.; Takahara, I. Catalytic fast pyrolysis of jatropha wastes. J. Anal. Appl. Pyrolysis 2012, 94, 75–82. [Google Scholar] [CrossRef]
- Dahmoune, F.; Boulekbache, L.; Moussi, K.; Aoun, O.; Spigno, G.; Madani, K. Valorization of Citrus limon residues for the recovery of antioxidants: Evaluation and optimization of microwave and ultrasound application to solvent extraction. Ind. Crop. Prod. 2013, 50, 77–87. [Google Scholar] [CrossRef]
- Klavins, L.; Kviesis, J.; Nakurte, I.; Klavins, M. Berry press residues as a valuable source of polyphenolics: Extraction optimisation and analysis. LWT 2018, 93, 583–591. [Google Scholar] [CrossRef]
- Squillace, P.; Adani, F.; Scaglia, B. Supercritical CO2 extraction of tomato pomace: Evaluation of the solubility of lycopene in tomato oil as limiting factor of the process performance. Food Chem. 2020, 315, 126224. [Google Scholar] [CrossRef]
- Kim, D.-S.; Lim, S.-B. Subcritical water extraction of rutin from the aerial parts of common buckwheat. J. Supercrit. Fluids 2019, 152, 104561. [Google Scholar] [CrossRef]
- Wang, L.; Boussetta, N.; Lebovka, N.; Vorobiev, E. Cell disintegration of apple peels induced by pulsed electric field and efficiency of bio-compound extraction. Food Bioprod. Process. 2020, 122, 13–21. [Google Scholar] [CrossRef]
- Wang, L.; Boussetta, N.; Lebovka, N.; Vorobiev, E. Purification of polyphenols from apple skins by membrane electro-filtration. LWT 2021, 145, 111357. [Google Scholar] [CrossRef]
- Maran, J.P.; Priya, B.; Al-Dhabi, N.A.; Ponmurugan, K.; Moorthy, I.G.; Sivarajasekar, N. Ultrasound assisted citric acid mediated pectin extraction from industrial waste of Musa balbisiana. Ultrason. Sonochem. 2017, 35, 204–209. [Google Scholar] [CrossRef]
- Petkowicz, C.; Vriesmann, L.C.; Williams, P.A. Pectins from food waste: Extraction, characterization and properties of watermelon rind pectin. Food Hydrocoll. 2017, 65, 57–67. [Google Scholar] [CrossRef]
- Bustamante, J.; van Stempvoort, S.; García-Gallarreta, M.; Houghton, J.A.; Briers, H.K.; Budarin, V.L.; Matharu, A.S.; Clark, J.H. Microwave assisted hydro-distillation of essential oils from wet citrus peel waste. J. Clean. Prod. 2016, 137, 598–605. [Google Scholar] [CrossRef]
- Janarny, G.; Gunathilake, K. Changes in rice bran bioactives, their bioactivity, bioaccessibility and bioavailability with solid-state fermentation by Rhizopus oryzae. Biocatal. Agric. Biotechnol. 2020, 23, 101510. [Google Scholar] [CrossRef]
- Wang, Y.; Ke, L.; Yang, Q.; Peng, Y.; Hu, Y.; Dai, L.; Jiang, L.; Wu, Q.; Liu, Y.; Ruan, R.; et al. Biorefinery process for production of bioactive compounds and bio-oil from Camellia oleifera shell. Int. J. Agric. Biol. Eng. 2019, 12, 190–194. [Google Scholar] [CrossRef]
- Agrawalla, A.; Kumar, S.; Singh, R.K. Pyrolysis of groundnut de-oiled cake and characterization of the liquid product. Bioresour. Technol. 2011, 102, 10711–10716. [Google Scholar] [CrossRef]
- Salami, A.; Raninen, K.; Heikkinen, J.; Tomppo, L.; Vilppo, T.; Selenius, M.; Raatikainen, O.; Lappalainen, R.; Vepsäläinen, J. Complementary chemical characterization of distillates obtained from industrial hemp hurds by thermal processing. Ind. Crop. Prod. 2020, 155, 112760. [Google Scholar] [CrossRef]
- Abdullah, N.; Gerhauser, H. Bio-oil derived from empty fruit bunches. Fuel 2008, 87, 2606–2613. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.P.; Yang, J.Y.; Kang, M.Y.; Park, J.C.; Nam, S.H.; Friedman, M. Composition of liquid rice hull smoke and anti-inflammatory effects in mice. J. Agric. Food Chem. 2011, 59, 4570–4581. [Google Scholar] [CrossRef]
- Giri, G.F.; Viarengo, G.; Furlán, R.L.; Suárez, A.G.; Garcia Véscovi, E.; Spanevello, R.A. Soybean hulls, an alternative source of bioactive compounds: Combining pyrolysis with bioguided fractionation. Ind. Crop. Prod. 2017, 105, 113–123. [Google Scholar] [CrossRef]
| Species | Cellulose % | Hemicellulose % | Lignin % | Residue Production [106 t/a] | Ref. |
|---|---|---|---|---|---|
| Corn stover | 37.5 | 22.4 | 17.6 | 1016.7 | [20,21,22,23] |
| Corn cobs | 45.0 | 35.0 | 15.0 | ||
| Cotton seed hair | 80–95 | 5–20 | 0.0 | 109.5 | [21,23] |
| Wheat straw | 38.2, 29–35 | 21.2 | 23.4 | 1069.7 | [21,23] |
| Bagasse | 38.2 | 27.1 | 20.2 | 92.0 | [20,21] |
| Sugar cane | 25.0 | 17.0 | 12.0 | 563.1 | [21,22,23] |
| Rice straw | 32.0 | 24.0 | 13–18 | 1118.5 | [20,21,22,23] |
| Sunflower stalk | 31.0 | 15.6 | 29.2 | 44.7 | [21,23] |
| (Cashew) nut shells | 25–30 | 25–30 | 30–40 | 4.44 | [21,24] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drescher, A.; Kienberger, M. A Systematic Review on Waste as Sustainable Feedstock for Bioactive Molecules—Extraction as Isolation Technology. Processes 2022, 10, 1668. https://doi.org/10.3390/pr10081668
Drescher A, Kienberger M. A Systematic Review on Waste as Sustainable Feedstock for Bioactive Molecules—Extraction as Isolation Technology. Processes. 2022; 10(8):1668. https://doi.org/10.3390/pr10081668
Chicago/Turabian StyleDrescher, Adrian, and Marlene Kienberger. 2022. "A Systematic Review on Waste as Sustainable Feedstock for Bioactive Molecules—Extraction as Isolation Technology" Processes 10, no. 8: 1668. https://doi.org/10.3390/pr10081668
APA StyleDrescher, A., & Kienberger, M. (2022). A Systematic Review on Waste as Sustainable Feedstock for Bioactive Molecules—Extraction as Isolation Technology. Processes, 10(8), 1668. https://doi.org/10.3390/pr10081668






