Growth Performance and Meat Quality of Growing Pigs Fed with Black Soldier Fly (Hermetia illucens) Larvae as Alternative Protein Source
Abstract
:1. Introduction
2. Materials and Methods
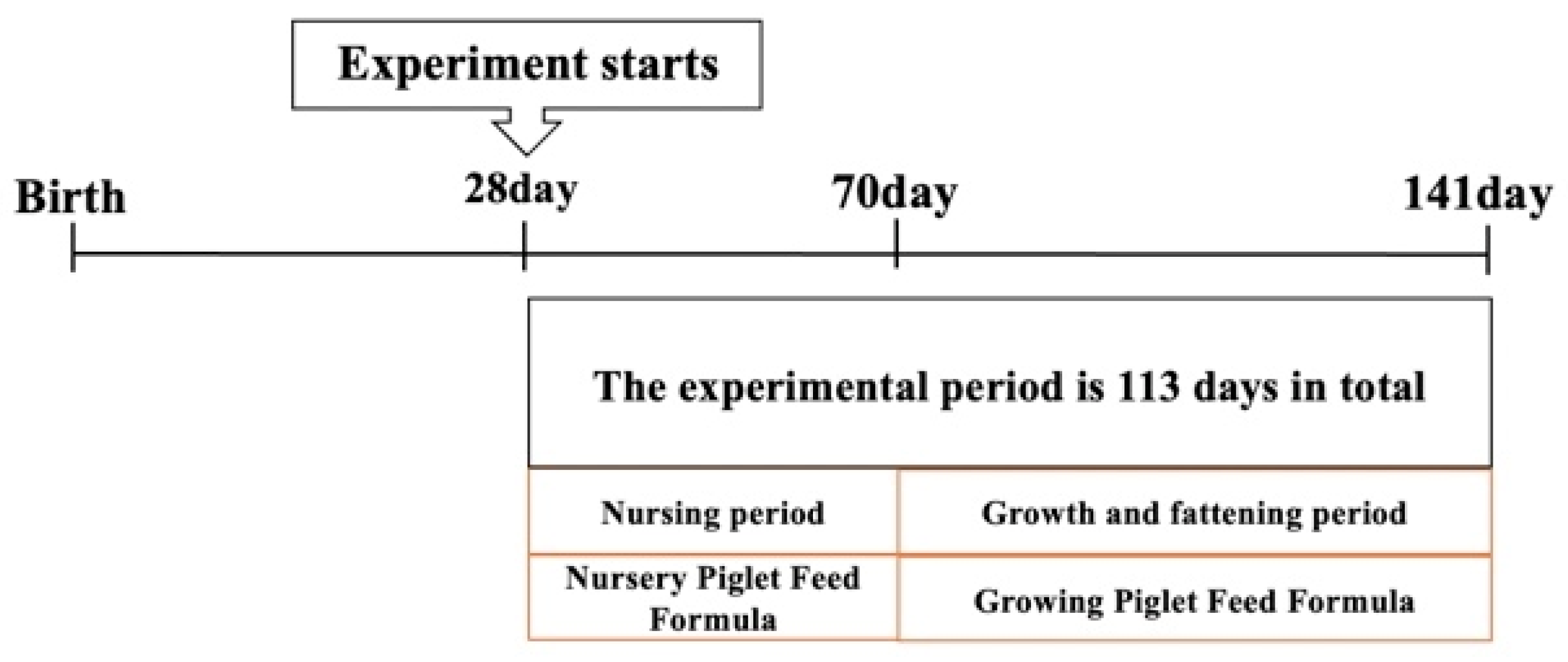
2.1. Animal, Experiment Design and Diets
2.2. Sample Collection
2.3. Measurement of Meat Quality
2.4. Tissue Histology
2.5. Meat Metabolomics
2.6. RNA Extraction and Quantitative Real-Time PCR
2.7. Statistical Analysis
3. Results
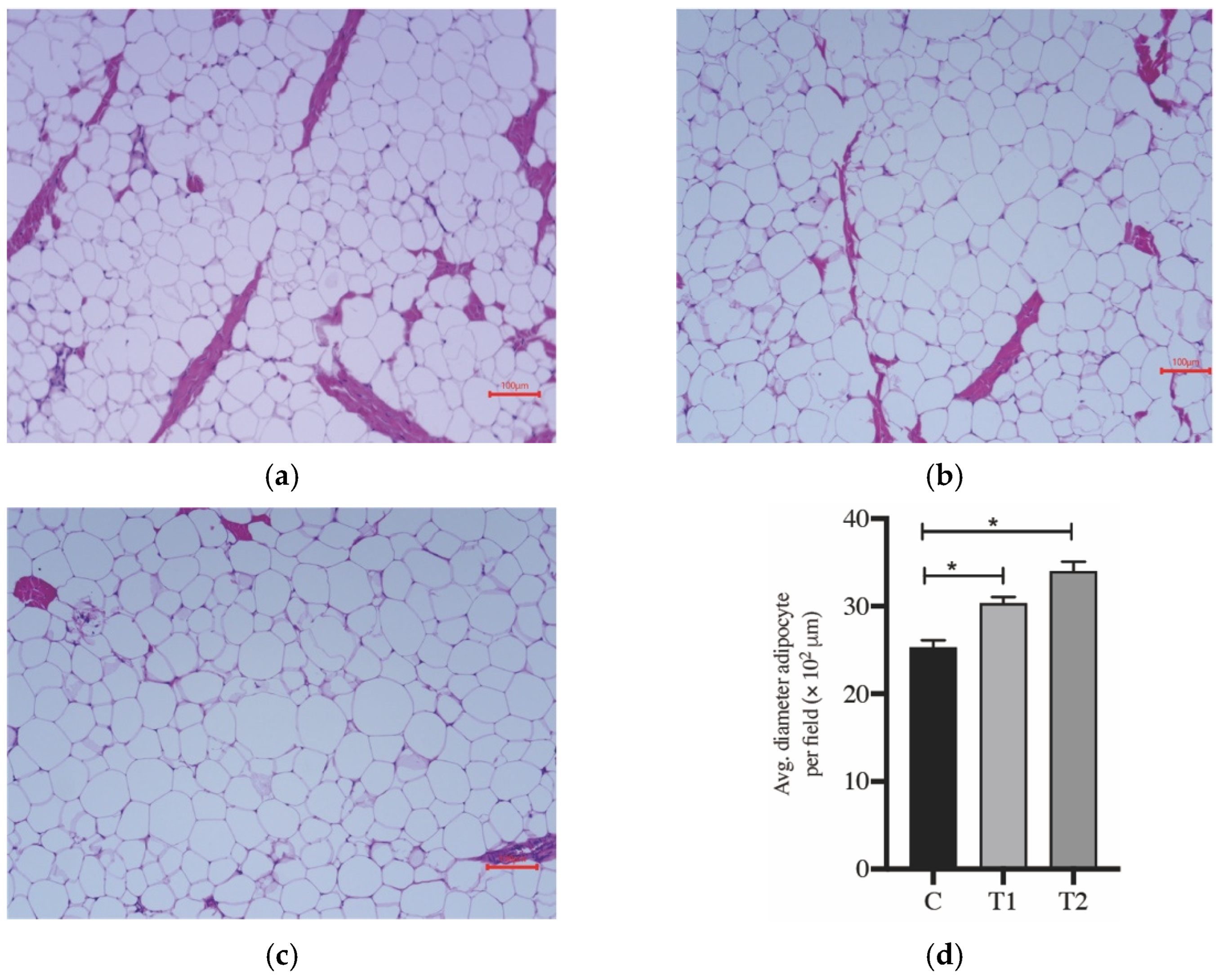
3.1. Growth Performance, Carcass Traits and Analysis of Tissue Histology
3.2. Meat Quality
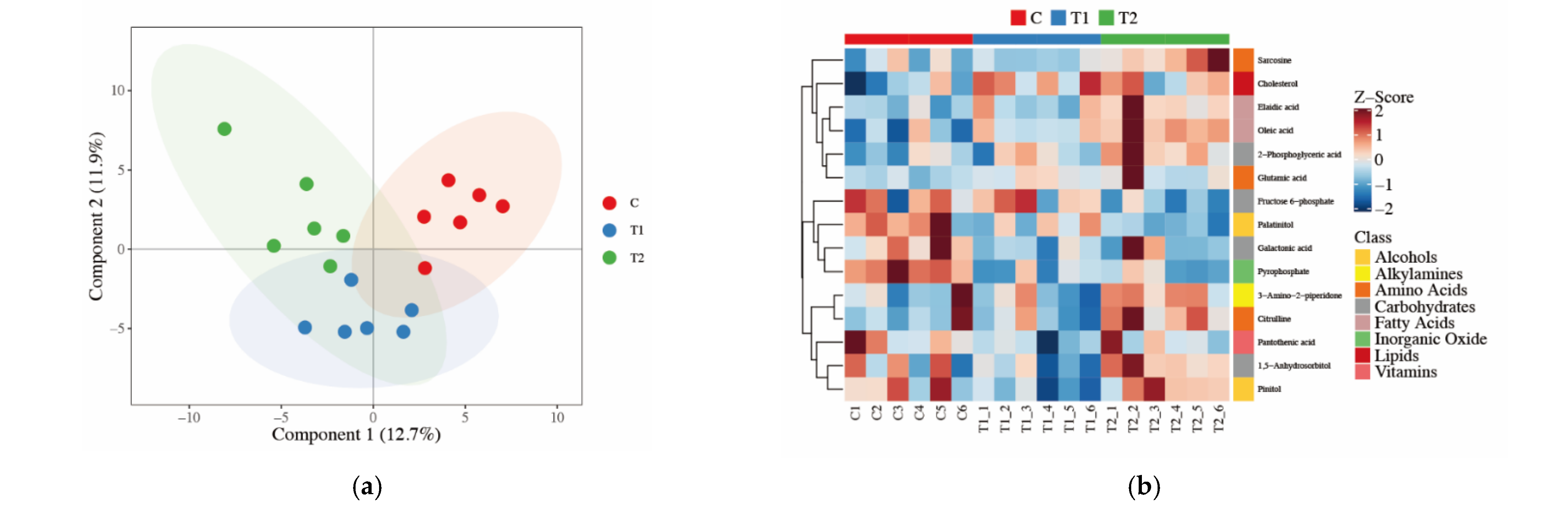
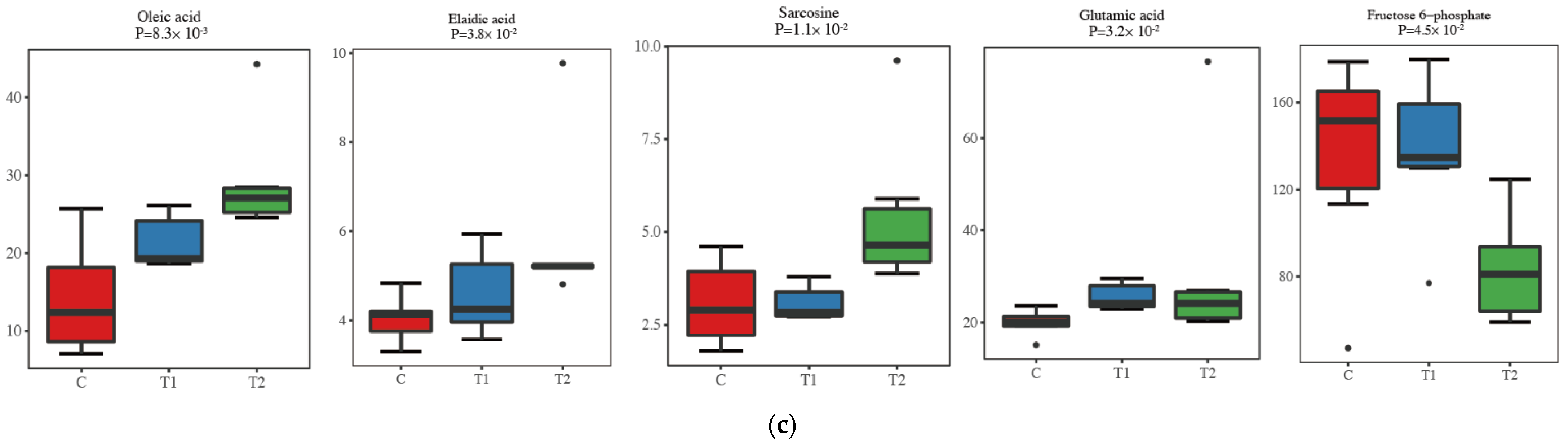
3.3. Detection of Loin Muscle Metabolomics
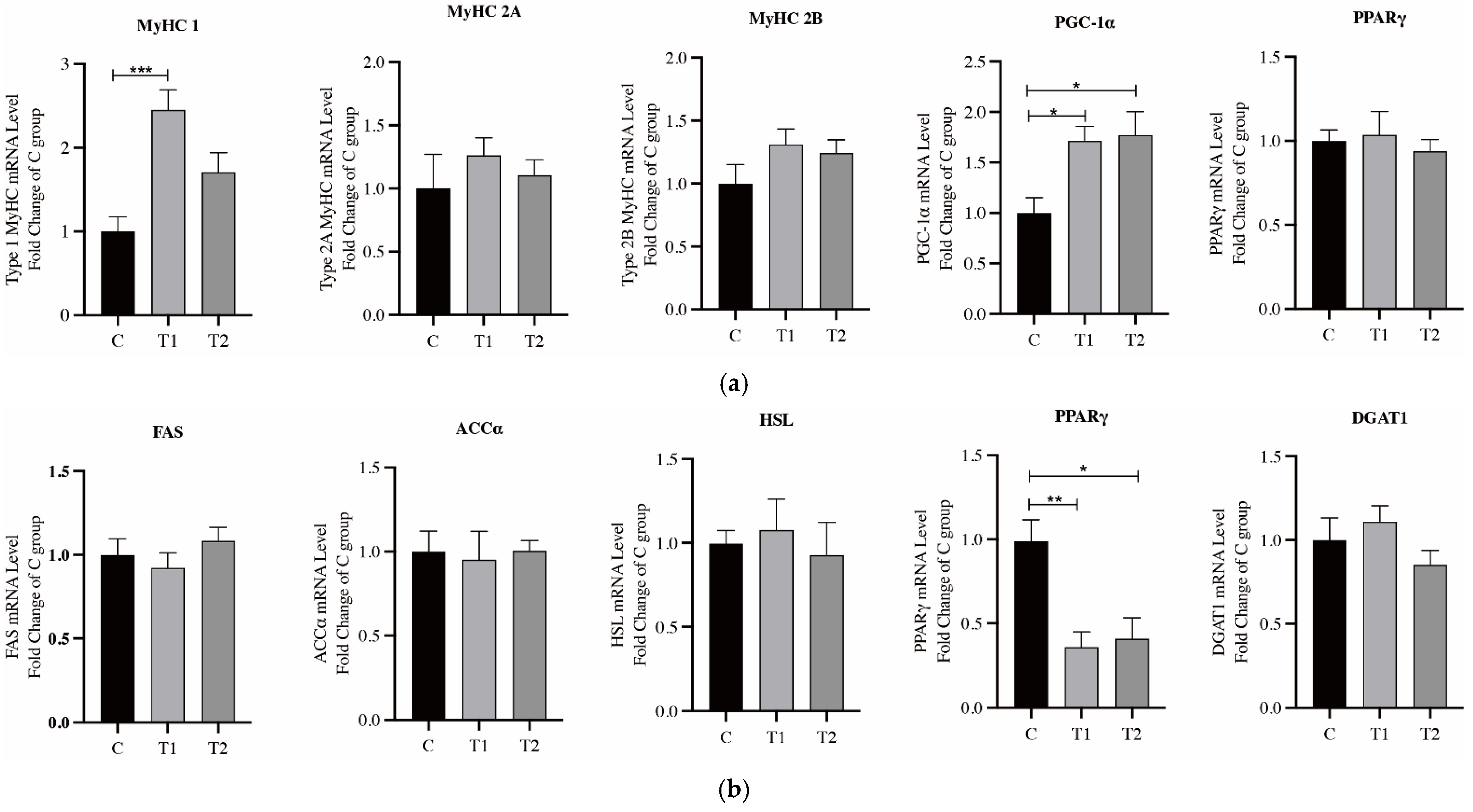
3.4. Expression of Genes Related to Fatty Acid Metabolism and Muscle Fiber Type
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Raamsdonk, L.W.D.; van der Fels-Klerx, H.J.; de Jong, J. New feed ingredients: The insect opportunity. Food. Addit. Contam. Part A 2017, 34, 1384–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bbosa, T.; Tamale Ndagire, C.; Muzira Mukisa, I.; Fiaboe, K.K.M.; Nakimbugwe, D. Nutritional Characteristics of Selected Insects in Uganda for Use as Alternative Protein Sources in Food and Feed. J. Insect. Sci. 2019, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Heuel, M.; Sandrock, C.; Leiber, F.; Mathys, A.; Gold, M.; Zurbrügg, C.; Gangnat, I.D.M.; Kreuzer, M.; Terranova, M. Black soldier fly larvae meal and fat can completely replace soybean cake and oil in diets for laying hens. Poult. Sci. 2021, 100, 101034. [Google Scholar] [CrossRef] [PubMed]
- Çabuk, B.; Yılmaz, B. Fortification of traditional egg pasta (erişte) with edible insects: Nutritional quality, cooking properties and sensory characteristics evaluation. J. Food. Sci. Technol. 2020, 57, 2750–2757. [Google Scholar] [CrossRef] [PubMed]
- Moretta, A.; Salvia, R.; Scieuzo, C.; Di Somma, A.; Vogel, H.; Pucci, P.; Sgambato, A.; Wolff, M.; Falabella, P. A bioinformatic study of antimicrobial peptides identified in the Black Soldier Fly (BSF) Hermetia illucens (Diptera: Stratiomyidae). Sci. Rep. 2020, 10, 16875. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Bang, H.T.; Kim, K.H.; Kim, M.J.; Jeong, J.Y.; Chun, J.L.; Ji, S.Y. Evaluation of black soldier fly larvae oil as a dietary fat source in broiler chicken diets. J. Anim. Sci. Technol. 2020, 62, 187–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dabbou, S.; Gai, F.; Biasato, I.; Capucchio, M.T.; Biasibetti, E.; Dezzutto, D.; Meneguz, M.; Plachà, I.; Gasco, L.; Schiavone, A. Black soldier fly defatted meal as a dietary protein source for broiler chickens: Effects on growth performance, blood traits, gut morphology and histological features. J. Anim. Sci. Biotechnol. 2018, 9, 49. [Google Scholar] [CrossRef]
- Biasato, I.; Renna, M.; Gai, F.; Dabbou, S.; Meneguz, M.; Perona, G.; Martinez, S.; Lajusticia, A.C.B.; Bergagna, S.; Sardi, L.; et al. Partially defatted black soldier fly larva meal inclusion in piglet diets: Effects on the growth performance, nutrient digestibility, blood profile, gut morphology and histological features. J. Anim. Sci. Biotechnol. 2019, 10, 12. [Google Scholar] [CrossRef]
- Ruhnke, I.; Normant, C.; Campbell, D.L.M.; Iqbal, Z.; Lee, C.; Hinch, G.N.; Roberts, J. Impact of on-range choice feeding with black soldier fly larvae (Hermetia illucens) on flock performance, egg quality, and range use of free-range laying hens. Anim. Nutr. 2018, 4, 452–460. [Google Scholar] [CrossRef]
- Pieterse, E.; Erasmus, S.W.; Uushona, T.; Hoffman, L.C. Black soldier fly (Hermetia illucens) pre-pupae meal as a dietary protein source for broiler production ensures a tasty chicken with standard meat quality for every pot. J. Sci. Food. Agric. 2019, 99, 893–903. [Google Scholar] [CrossRef]
- Dalle Zotte, A.; Singh, Y.; Michiels, J.; Cullere, M. Black Soldier Fly (Hermetia Illucens) as Dietary Source for Laying Quails: Live Performance, and Egg Physico-Chemical Quality, Sensory Profile and Storage Stability. Animals 2019, 9, 115. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Li, Z.; Chen, W.; Wang, G.; Rong, T.; Liu, Z.; Wang, F.; Ma, X. Hermetia illucens larvae as a Fishmeal replacement alters intestinal specific bacterial populations and immune homeostasis in weanling piglets. J. Anim. Sci. 2020, 98, skz395. [Google Scholar] [CrossRef]
- Biasato, I.; Ferrocino, I.; Colombino, E.; Gai, F.; Schiavone, A.; Cocolin, L.; Vincenti, V.; Capucchio, M.T.; Gasco, L. Effects of dietary Hermetia illucens meal inclusion on cecal microbiota and small intestinal mucin dynamics and infiltration with immune cells of weaned piglets. J. Anim. Sci. Biotechnol. 2020, 11, 64. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Joo, S.T. Fatty Acid Profiles, Meat Quality, and Sensory Palatability of Grain-fed and Grass-fed Beef from Hanwoo, American, and Australian Crossbred Cattle. Korean J. Food. Sci. Anim. Resour. 2017, 37, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Li, Z.; Chen, W.; Rong, T.; Wang, G.; Li, J.; Ma, X. Use of Hermetia illucens larvae as a dietary protein source: Effects on growth performance, carcass traits, and meat quality in finishing pigs. Meat Sci. 2019, 158, 107837. [Google Scholar] [CrossRef]
- Schiaffino, S. Muscle fiber type diversity revealed by anti-myosin heavy chain antibodies. FEBS J. 2018, 285, 3688–3694. [Google Scholar] [CrossRef]
- Ingjer, F. Effects of endurance training on muscle fibre ATP-ase activity, capillary supply and mitochondrial content in man. J. Physiol. 1979, 294, 419–432. [Google Scholar] [CrossRef]
- Ismail, I.; Joo, S.T. Poultry Meat Quality in Relation to Muscle Growth and Muscle Fiber Characteristics. Korean J. Food. Sci. Anim. Resour. 2017, 37, 873–883. [Google Scholar] [CrossRef]
- Zhang, C.; Luo, J.Q.; Zheng, P.; Yu, B.; Huang, Z.Q.; Mao, X.B.; He, J.; Yu, J.; Chen, J.L.; Chen, D.W. Differential expression of lipid metabolism-related genes and myosin heavy chain isoform genes in pig muscle tissue leading to different meat quality. Animal 2015, 9, 1073–1080. [Google Scholar] [CrossRef] [Green Version]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, B.; Zhang, J.; Zhang, X.; Yuan, J.; Shi, Y.; Qiao, L. Inhibition of lipogenesis and induction of apoptosis by valproic acid in prostate cancer cells via the C/EBPα/SREBP-1 pathway. Acta. Biochim. Biophys. Sin. 2021, 53, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ma, X.; Lin, Y.; Xiong, Y.; Zheng, C.; Hu, Y.; Yu, D.; Jiang, Z. Dietary supplementation with a high dose of daidzein enhances the antioxidant capacity in swine muscle but experts pro-oxidant function in liver and fat tissues. J. Anim. Sci. Biotechnol. 2016, 7, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldberg, R.A. National Pork Producers Council. 1997. Available online: https://nppc.org/ (accessed on 28 July 2022).
- Li, Y.H.; Li, F.N.; Duan, Y.H.; Guo, Q.P.; Wen, C.Y.; Wang, W.L.; Huang, X.G.; Yin, Y.L. Low-protein diet improves meat quality of growing and finishing pigs through changing lipid metabolism, fiber characteristics, and free amino acid profile of the muscle. J. Anim. Sci. 2018, 96, 3221–3232. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zheng, C.; Hu, Y.; Wang, L.; Yang, X.; Jiang, Z. Dietary L-arginine supplementation affects the skeletal longissimus muscle proteome in finishing pigs. PLoS ONE 2015, 10, e0117294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.; Honek, J.; Xue, Y.; Seki, T.; Cao, Z.; Andersson, P.; Yang, X.; Hosaka, K.; Cao, Y. Cold-induced activation of brown adipose tissue and adipose angiogenesis in mice. Nat. Protoc. 2012, 7, 606–615. [Google Scholar] [CrossRef]
- Rawski, M.; Mazurkiewicz, J.; Kierończyk, B.; Józefiak, D. Black Soldier Fly Full-Fat Larvae Meal as an Alternative to Fish Meal and Fish Oil in Siberian Sturgeon Nutrition: The Effects on Physical Properties of the Feed, Animal Growth Performance, and Feed Acceptance and Utilization. Animals 2020, 10, 2119. [Google Scholar] [CrossRef]
- Bruni, L.; Belghit, I.; Lock, E.J.; Secci, G.; Taiti, C.; Parisi, G. Total replacement of dietary fish meal with black soldier fly (Hermetia illucens) larvae does not impair physical, chemical or volatile composition of farmed Atlantic salmon (Salmo salar L.). J. Sci. Food. Agric. 2020, 100, 1038–1047. [Google Scholar] [CrossRef]
- Onsongo, V.O.; Osuga, I.M.; Gachuiri, C.K.; Wachira, A.M.; Miano, D.M.; Tanga, C.M.; Ekesi, S.; Nakimbugwe, D.; Fiaboe, K.K.M. Insects for Income Generation Through Animal Feed: Effect of Dietary Replacement of Soybean and Fish Meal With Black Soldier Fly Meal on Broiler Growth and Economic Performance. J. Econ. Entomol. 2018, 111, 1966–1973. [Google Scholar] [CrossRef] [Green Version]
- Ipema, A.F.; Bokkers, E.A.M.; Gerrits, W.J.J.; Kemp, B.; Bolhuis, J.E. Providing live black soldier fly larvae (Hermetia illucens) improves welfare while maintaining performance of piglets post-weaning. Sci. Rep. 2021, 11, 7371. [Google Scholar] [CrossRef]
- Heuel, M.; Kreuzer, M.; Sandrock, C.; Leiber, F.; Mathys, A.; Gold, M.; Zurbrügg, C.; Gangnat, I.D.M.; Terranova, M. Transfer of Lauric and Myristic Acid from Black Soldier Fly Larval Lipids to Egg Yolk Lipids of Hens Is Low. Lipids 2021, 56, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Soetemans, L.; Uyttebroek, M.; Bastiaens, L. Characteristics of chitin extracted from black soldier fly in different life stages. Int. J. Biol. Macromol. 2020, 165, 3206–3214. [Google Scholar] [CrossRef] [PubMed]
- Allee, G.L.; Baker, D.H.; Leveille, G.A. Influence of level of dietary fat on adipose tissue lipogenesis and enzymatic activity in the pig. J. Anim. Sci. 1971, 33, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Bakhsh, A.; Lee, J.G.; Joo, S.T. Differences in Muscle Fiber Characteristics and Meat Quality by Muscle Type and Age of Korean Native Black Goat. Food Sci. Anim. Resour. 2019, 39, 988–999. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Kim, J.M.; Ryu, Y.C.; Ko, K.S. Effects of Morphological Characteristics of Muscle Fibers on Porcine Growth Performance and Pork Quality. Korean J. Food. Sci. Anim. Resour. 2016, 36, 583–593. [Google Scholar] [CrossRef] [Green Version]
- Hua, N.; Takahashi, H.; Yee, G.M.; Kitajima, Y.; Katagiri, S.; Kojima, M.; Anzai, K.; Eguchi, Y.; Hamilton, J.A. Influence of muscle fiber type composition on early fat accumulation under high-fat diet challenge. PLoS ONE 2017, 12, e0182430. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; Kong, X.; Hu, C.; Zhou, B.; Wang, C.; Shen, Q.W. Fatty Acid Content, Flavor Compounds, and Sensory Quality of Pork Loin as Affected by Dietary Supplementation with l-arginine and Glutamic Acid. J. Food. Sci. 2019, 84, 3445–3453. [Google Scholar] [CrossRef]
- Kaewkot, A.; Boonkaewwan, C.; Noosud, J.; Kayan, A. Expression level of the cytochrome P450c21 (CYP21) protein correlating to drip loss in pigs. Anim. Sci. J. 2017, 88, 1855–1859. [Google Scholar] [CrossRef]
- Zierath, J.R.; Hawley, J.A. Skeletal muscle fiber type: Influence on contractile and metabolic properties. PLoS Biol. 2004, 2, e348. [Google Scholar] [CrossRef]
- Fry, A.C. The role of resistance exercise intensity on muscle fibre adaptations. Sports Med. 2004, 34, 663–679. [Google Scholar] [CrossRef]
- Pereyra, A.S.; Lin, C.T.; Sanchez, D.M.; Laskin, J.; Spangenburg, E.E.; Neufer, P.D.; Fisher-Wellman, K.; Ellis, J.M. Skeletal muscle undergoes fiber type metabolic switch without myosin heavy chain switch in response to defective fatty acid oxidation. Mol. Metab. 2022, 59, 101456. [Google Scholar] [CrossRef] [PubMed]
- Pette, D.; Staron, R.S. Myosin isoforms, muscle fiber types, and transitions. Microsc. Res. Tech. 2000, 50, 500–509. [Google Scholar] [CrossRef]
- Wang, L.; Luo, L.; Zhao, W.; Yang, K.; Shu, G.; Wang, S.; Gao, P.; Zhu, X.; Xi, Q.; Zhang, Y.; et al. Lauric Acid Accelerates Glycolytic Muscle Fiber Formation through TLR4 Signaling. J. Agric. Food. Chem. 2018, 66, 6308–6316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, Y.; Wu, W.; Hou, L.; Chen, H.; Zuo, B.; Xiong, Y.; Yang, J. Skeletal Muscle-Specific Overexpression of PGC-1α Induces Fiber-Type Conversion through Enhanced Mitochondrial Respiration and Fatty Acid Oxidation in Mice and Pigs. Int. J. Biol. Sci. 2017, 13, 1152–1162. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.; Lee, S.Y.; Park, J.Y.; Jung, S.; Jo, C.; Nam, K.C. Comparison of Functional Compounds and Micronutrients of Chicken Breast Meat by Breeds. Food. Sci. Anim. Resour. 2019, 39, 632–642. [Google Scholar] [CrossRef]
| Items | Weaned Piglet Feed Formula | Growing Piglet Feed Formula | ||||
|---|---|---|---|---|---|---|
| C | T1 | T2 | C | T1 | T2 | |
| Ingredient (%) | ||||||
| Corn | 52.98 | 51.53 | 50.08 | 57.02 | 57.16 | 57.29 |
| Soybean meal | 9.00 | 9.00 | 9.00 | 28.00 | 24.95 | 21.91 |
| Puffed soybeans | 9.00 | 9.34 | 9.68 | — | — | — |
| Fish meal | 4.00 | 2.00 | 0.00 | — | — | — |
| Soy protein concentrate | 6.00 | 6.00 | 6.00 | — | — | — |
| Whey powder | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Sucrose | 2.00 | 2.00 | 2.00 | — | — | — |
| Black soldier fly | 0.00 | 4.00 | 8.00 | 0.00 | 4.00 | 8.00 |
| Soybean oil | 2.00 | 1.18 | 0.36 | 2.00 | 1.18 | 0.35 |
| DL-Methionine | 0.36 | 0.38 | 0.40 | 0.08 | 0.09 | 0.10 |
| Lys-HCl (78%) | 0.80 | 0.82 | 0.84 | 0.18 | 0.18 | 0.19 |
| Threonine (98%) | 0.38 | 0.38 | 0.38 | 0.03 | 0.03 | 0.03 |
| Tryptophan (99%) | 0.08 | 0.08 | 0.08 | 0.00 | 0.01 | 0.02 |
| Limestone | 0.65 | 0.44 | 0.22 | 0.78 | 0.54 | 0.30 |
| Dicalcium phosphate | 1.10 | 1.20 | 1.30 | 0.53 | 0.48 | 0.43 |
| Choline chloride | 0.20 | 0.20 | 0.20 | 0.08 | 0.08 | 0.08 |
| Salt | 0.45 | 0.45 | 0.45 | 0.30 | 0.30 | 0.30 |
| Premix 1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Total | 100.00 | 100.00 | 100.00 | 100 | 100 | 100 |
| Calculated content | ||||||
| Crude protein (%) | 19.15 | 19.15 | 19.15 | 18.09 | 18.09 | 18.09 |
| Digestible energy (MJ/kg) | 14.59 | 14.59 | 14.59 | 14.36 | 14.36 | 14.36 |
| Ether extract | 6.65 | 7.07 | 7.55 | 4.54 | 5.39 | 6.15 |
| Ca | 0.80 | 0.80 | 0.80 | 0.60 | 0.60 | 0.60 |
| P | 0.65 | 0.65 | 0.65 | 0.52 | 0.52 | 0.52 |
| AP | 0.44 | 0.41 | 0.39 | 0.25 | 0.23 | 0.21 |
| Lysine | 1.35 | 1.35 | 1.35 | 0.99 | 0.99 | 0.99 |
| Methionine + cysteine | 0.76 | 0.75 | 0.73 | 0.58 | 0.58 | 0.58 |
| Threonine | 0.80 | 0.81 | 0.80 | 0.62 | 0.62 | 0.62 |
| Tryptophan | 0.22 | 0.22 | 0.22 | 0.19 | 0.19 | 0.19 |
| Gene | Primer Sequences (5′–3′) | Size (bp) | NCBI Gene ID |
|---|---|---|---|
| MyHC 1 | F: GAGGAAGCGGAGGAACAATCCA | 105 | NM_001104951.2 |
| R: GACCTGGGACTCAGCAATGTCA | |||
| MyHC 2A | F: GATGGAGATCGACGACCTTGCT | 127 | XM_021066217.1 |
| R: CTGCTGCTCTTCCTCCTTGGAT | |||
| MyHC 2B | F: CGCCAAGCTACTGAGGCAATAA | 127 | XM_021066036.1 |
| R: GTTCCACCATGGCCAGTTGTTC | |||
| PGC-1α | F: CGCAAGCTTCTCTGAGCTTCTTT | 188 | XM_021100444.1 |
| R: GGATACACTTTGCGCAGGTCGAA | |||
| PPAR-γ | F: CCAGCATTTCCACTCCACACTA | 124 | XM_005669788.3 |
| R: GACACAGGCTCCACTTTGATG | |||
| FAS | F: AGCCTAACTCCTCGCTGCAAT | 196 | NM_001099930.1 |
| R: TCCTTGGAACCGTCTGTGTTC | |||
| ACCα | F: AGCAAGGTCGAGACCGAAAG | 169 | XM_021066238.1 |
| R: TAAGACCACCGGCGGATAGA | |||
| HSL | F: GCAGCATCTTCTTCCGCACA | 195 | NM_214315.3 |
| R: AGCCCTTGCGTAGAGTGACA | |||
| DAGT1 | F: GACAAGGACGGACACGACGATG | 118 | XM_005655311.3 |
| R: AATTCAGGATGCCACGGTAGTTGC |
| Items | C | T1 | T2 | p-Value |
|---|---|---|---|---|
| Initial body weight (kg) | 7.82 ± 0.23 | 7.43 ± 0.37 | 7.62 ± 0.29 | 0.597 |
| Final body weight (kg) | 63.98 ± 2.53 | 60.94 ± 2.70 | 66.38 ± 3.51 | 0.427 |
| Average daily gain (kg/d) | 0.47 ± 0.01 b | 0.46 ± 0.02 b | 0.56 ± 0.03 a | 0.029 |
| First intercostal backfat, (cm) | 16.13 ± 1.48 b | 17.79 ± 2.05 b | 24.04 ± 1.82 a | 0.024 |
| Last intercostal backfat (cm) | 12.76 ± 0.51 | 11.87 ± 0.65 | 13.97 ± 0.72 | 0.318 |
| Lumbar backfat, (cm) | 8.83 ± 0.48 | 8.23 ± 0.43 | 10.82 ± 0.99 | 0.364 |
| Loin eye area, (cm) | 52.85 ± 2.62 a | 43.30 ± 0.95 b | 45.69 ± 1.59 b | 0.012 |
| Items | C | T1 | T2 | p-Value |
|---|---|---|---|---|
| IMF% | 1.49 ± 0.01 b | 1.74 ± 0.05 a | 1.65 ± 0.03 a | 0.013 |
| pH45 min | 6.62 ± 0.06 | 6.52 ± 0.07 | 6.52 ± 0.11 | 0.961 |
| pH24 h | 5.57 ± 0.02 | 5.56 ± 0.02 | 5.53 ± 0.02 | 0.834 |
| pH48 h | 5.52 ± 0.03 | 5.47 ± 0.04 | 5.52 ± 0.03 | 0.748 |
| L*45 min | 39.74 ± 1.23 | 39.24 ± 0.79 | 39.60 ± 1.00 | 0.931 |
| a*45 min | 5.59 ± 0.63 | 6.02 ± 0.87 | 5.62 ± 0.82 | 0.747 |
| b*45 min | 3.65 ± 0.39 | 3.16 ± 0.31 | 3.43 ± 0.37 | 0.712 |
| Marbling scores | 2.43 ± 0.22 | 2.75 ± 0.16 | 2.60 ± 0.40 | 0.234 |
| Shear force, (N) | 70.27 ± 4.35 | 68.20 ± 4.26 | 73.03 ± 12.46 | 0.546 |
| Cooking loss, % | 0.30 ± 0.01 | 0.33 ± 0.01 | 0.34 ± 0.01 | 0.638 |
| Drip loss, % | 0.17 ± 0.01 a | 0.12 ± 0.01 b | 0.13 ± 0.02 b | 0.026 |
| Amino Acids | Taste Attribute | C | T1 | T2 | p-Value |
|---|---|---|---|---|---|
| Sarcosine | Swt (+) | 3.08 ± 0.47 b | 3.07 ± 0.19 b | 5.46 ± 0.88 a | 0.011 |
| Glutamic acid | Uma (+) | 19.90 ± 1.18 b | 25.50 ± 1.21 a | 32.06 ± 8.98 a | 0.032 |
| Asparagine | Uma (+) | 8.38 ± 0.52 | 8.31 ± 0.53 | 9.56 ± 0.48 | 0.186 |
| Threonine | Swt (+) | 30.6 ± 3.17 | 26.5 ± 3.10 | 31.39 ± 4.42 | 0.631 |
| Serine | Swt (+) | 38.43 ± 4.20 | 39.76 ± 3.17 | 48.27 ± 5.62 | 0.331 |
| Glycine | Swt (+) | 305.99 ± 24.88 | 301.98 ± 27.98 | 297.93 ± 22.44 | 0.688 |
| Alanine | Swt (+) | 804.37 ± 45.79 | 791.35 ± 35.87 | 860.86 ± 40.10 | 0.455 |
| Citrulline | Swt (+)/Bit (−) | 15.93 ± 1.67 b | 14.41 ± 1.25 b | 19.64 ± 1.25 a | 0.028 |
| Cysteine | Bit (−) | 2.10 ± 0.40 | 1.41 ± 0.13 | 2.28 ± 0.49 | 0.347 |
| Valine | Bit (−) | 96.09 ± 13.12 | 87.79 ± 12.97 | 108.62 ± 13.81 | 0.550 |
| Isoleucine | Bit (−) | 40.69 ± 9.14 | 29.73 ± 5.57 | 40.27 ± 8.37 | 0.547 |
| Leucine | Bit (−) | 91.73 ± 7.83 | 94.39 ± 15.39 | 106.66 ± 8.81 | 0.385 |
| Tyrosine | Bit (−) | 62.45 ± 3.36 | 55.32 ± 5.81 | 57.18 ± 5.65 | 0.590 |
| Phenylalanine | Bit (−) | 32.47 ± 1.43 | 29.56 ± 2.71 | 35.01 ± 2.30 | 0.250 |
| Lysine | Bit (−) | 41.67 ± 11.15 | 32.20 ± 6.47 | 44.72 ± 8.70 | 0.599 |
| Histidine | Bit (−) | 16.04 ± 2.20 | 16.22 ± 2.12 | 19.34 ± 1.65 | 0.446 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, M.; Liu, M.; Yuan, B.; Jin, X.; Zhang, X.; Xie, G.; Wang, Z.; Lv, Y.; Wang, W.; Huang, Y. Growth Performance and Meat Quality of Growing Pigs Fed with Black Soldier Fly (Hermetia illucens) Larvae as Alternative Protein Source. Processes 2022, 10, 1498. https://doi.org/10.3390/pr10081498
Zhu M, Liu M, Yuan B, Jin X, Zhang X, Xie G, Wang Z, Lv Y, Wang W, Huang Y. Growth Performance and Meat Quality of Growing Pigs Fed with Black Soldier Fly (Hermetia illucens) Larvae as Alternative Protein Source. Processes. 2022; 10(8):1498. https://doi.org/10.3390/pr10081498
Chicago/Turabian StyleZhu, Mingqiang, Mingming Liu, Boyu Yuan, Xinxin Jin, Xue Zhang, Gaijie Xie, Zifan Wang, Yantao Lv, Wei Wang, and Yanhua Huang. 2022. "Growth Performance and Meat Quality of Growing Pigs Fed with Black Soldier Fly (Hermetia illucens) Larvae as Alternative Protein Source" Processes 10, no. 8: 1498. https://doi.org/10.3390/pr10081498
APA StyleZhu, M., Liu, M., Yuan, B., Jin, X., Zhang, X., Xie, G., Wang, Z., Lv, Y., Wang, W., & Huang, Y. (2022). Growth Performance and Meat Quality of Growing Pigs Fed with Black Soldier Fly (Hermetia illucens) Larvae as Alternative Protein Source. Processes, 10(8), 1498. https://doi.org/10.3390/pr10081498






