Recent Criterion on Stability Enhancement of Perovskite Solar Cells
Abstract
:1. Introduction
2. Stability Problems in Perovskite Solar Cells
2.1. Chemical Instability
2.1.1. Instability Due to UV Light Exposure
2.1.2. Instability Due to Moisture and O2
2.1.3. Influence of Ion Migration on Stability
2.2. Thermal Instability
2.2.1. Thermal Degeneracy of Perovskite or Crystal Structure
2.2.2. Thermal Degeneracy of HTM Layers
2.2.3. Thermal Degeneracy of ETM Layers
2.3. Hysteresis Problem of Perovskite
3. Improvement Strategies for the Instability Problems
3.1. Enhancing Stability of Perovskite Sensitizer
3.2. Enhancing Intrinsic (Device) Stabilities
3.2.1. Improvement and Modification of Electron Transport Layers (ETLs)
Single-Layered Electron Transport Materials (ETMs)
Bi-Layered ETMs
Doping of ETLs
Interfacial Passivation between ETL and the Perovskite Layer
Structural Alteration of ETLs
3.2.2. Improvement and Modification of Hole Transport Layers (HTLs)
Using Non-Hygroscopic Additives
Alternative HTLs
HTL-Free Devices
3.2.3. Improvement of Electrode Materials
3.3. Strategies for Mitigating Hysteresis Problem
3.3.1. Interfacial Passivation
3.3.2. Doping of Perovskite Layer
3.3.3. Reduction in Unbalanced Charged Transportation
3.3.4. Structural Development
3.4. Sealing/Encapsulation
4. Future Recommendations
- More intense research is needed to understand the degradation mechanisms and varying conditions for all types of perovskites such as CH3NH3PbCl3, CH3NH3SnI3, and CH3NH3PbBr3.
- Research is required on the degradation mechanisms for all types of HTM layer and ETM layer under high thermal conditions.
- Investigation is needed to find the proper alternative to toxic Pb in perovskite to reduce environmental pollution. The alternative metal must be non-toxic and enhance the stability of PSC with high efficiency.
- Various organic modifiers must be applied to passivate the direct contact between the metal oxide films (ETLs) and the respective perovskite sensitizer to reduce the undesirable photocatalytic phenomena of the perovskite layer.
- Various potential bi-layer interfacial structures must be used instead of the single metal oxide-based ETLs to significantly reduce the metal ion diffusion and the charge recombination at the perovskite/ETL interfaces.
- Mixed-metal-based ETMs (such as Zn2SnO4 and La-doped BaSnO3) must be developed with a focus on improving PCE in such devices.
- Developing alternative polymeric or inorganic p-type (i.e., hole-conductive) materials instead of the conventional spiro-OMeTAD that exhibits well-matched energy level alignment with the conventional perovskite materials, good hole mobility, conductivity, as well as better stability against moisture, oxygen, and thermal stress.
- Developing various non-hygroscopic dopants or additives that may help diminish the decomposition rate of conventional HTLs (i.e., spiro-OMeTAD).
- Enhancing PCE of HTL-free devices. For this purpose, gradient doping in carbon-based PSCs can be an effective solution for the future. More research should be provided in such devices to improve their PCE balancing and enhance stability.
- Focusing on the newer molecular doping strategies in the perovskite layer of PSCs to reduce the device hysteresis.
- Enhancing the PCE of inverted planar structured PSCs will make them more promising in the future as they amicably reduce device hysteresis only by their identical configurations.
- Focusing on the improvement of PCE in mesoscopic structured PSCs—such devices will be more promising than the planar structured devices in the future for commercialization if their PCE can be somehow improved.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, J.; Saunders, B.R. Third-generation solar cells: A review and comparison of polymer: Fullerene, hybrid polymer and perovskite solar cells. RSC Adv. 2014, 4, 43286–43314. [Google Scholar] [CrossRef]
- Gholipour, S.; Saliba, M. From exceptional properties to stability challenges of perovskite solar cells. Small 2018, 14, 1802385. [Google Scholar] [CrossRef] [PubMed]
- Mitzi, D.B.; Chondroudis, K.; Kagan, C.R. Organic-inOrg. Electron. IBM J. Res. Dev. 2001, 45, 29–45. [Google Scholar] [CrossRef]
- Mitzi, D.; Wang, S.; Feild, C.; Chess, C.; Guloy, A. Conducting layered organic-inorganic halides containing <110>-oriented perovskite sheets. Science 1995, 267, 1473–1476. [Google Scholar] [CrossRef] [PubMed]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Cui, Y.; Zhu, J.; Meng, F.; Zoras, S.; McKechnie, J.; Chu, J. Energy assessment and economic sensitivity analysis of a grid-connected photovoltaic system. Renew. Energy 2020, 150, 101–115. [Google Scholar] [CrossRef]
- Guo, P.; Ye, Q.; Liu, C.; Cao, F.; Yang, X.; Ye, L.; Zhao, W.; Wang, H.; Li, L.; Wang, H. Double barriers for moisture degradation: Assembly of hydrolysable hydrophobic molecules for stable perovskite solar cells with high open-circuit voltage. Adv. Funct. Mater. 2020, 30, 2002639. [Google Scholar] [CrossRef]
- Zhao, J.; Cai, B.; Luo, Z.; Dong, Y.; Zhang, Y.; Xu, H.; Hong, B.; Yang, Y.; Li, L.; Zhang, W. Investigation of the hydrolysis of perovskite organometallic halide CH3NH3PbI3 in humidity environment. Sci. Rep. 2016, 6, 21976. [Google Scholar] [CrossRef] [Green Version]
- Eames, C.; Frost, J.M.; Barnes, P.R.; O’regan, B.C.; Walsh, A.; Islam, M.S. Ionic transport in hybrid lead iodide perovskite solar cells. Nat. Commun. 2015, 6, 7497. [Google Scholar] [CrossRef] [Green Version]
- Mosconi, E.; Meggiolaro, D.; Snaith, H.J.; Stranks, S.D.; De Angelis, F. Light-induced annihilation of Frenkel defects in organo-lead halide perovskites. Energy Environ. Sci. 2016, 9, 3180–3187. [Google Scholar] [CrossRef]
- Meloni, S.; Moehl, T.; Tress, W.; Franckevičius, M.; Saliba, M.; Lee, Y.H.; Gao, P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Rothlisberger, U.J.N. Ionic polarization-induced current–voltage hysteresis in CH3NH3PbX3 perovskite solar cells. Nat. Commun. 2016, 7, 10334. [Google Scholar] [CrossRef]
- Wojciechowski, K.; Leijtens, T.; Siprova, S.; Schlueter, C.; Hörantner, M.T.; Wang, J.T.-W.; Li, C.-Z.; Jen, A.K.Y.; Lee, T.-L.; Snaith, H.J. C60 as an efficient n-type compact layer in perovskite solar cells. J. Phys. Chem. Lett. 2015, 6, 2399–2405. [Google Scholar] [CrossRef]
- Sandhu, S.; Saharan, C.; Buruga, S.K.; Kumar, S.A.; Rana, P.S.; Nagajyoti, P.; Mane, S.D. Micro structurally engineered hysteresis-free high efficiency perovskite solar cell using Zr-doped TiO2 electron transport layer. Ceram. Int. 2021, 47, 14665–14672. [Google Scholar] [CrossRef]
- Gaur, D.; Sharma, S.; Ghoshal, S. Modified structures, optical and photovoltaic characteristics of low energy ions beam irradiated TiO2/TiO2-Graphene thin films as electron transport layer in perovskite solar cell. Mater. Today Proc. 2021, 43, 3826–3832. [Google Scholar] [CrossRef]
- You, Y.; Tian, W.; Min, L.; Cao, F.; Deng, K.; Li, L. TiO2/WO3 bilayer as electron transport layer for efficient planar perovskite solar cell with efficiency exceeding 20%. Adv. Mater. Interfaces 2020, 7, 1901406. [Google Scholar] [CrossRef]
- Cheng, L.; Liu, Z.; Li, S.; Zhai, Y.; Wang, X.; Qiao, Z.; Xu, Q.; Meng, K.; Zhu, Z.; Chen, G. Highly thermostable and efficient formamidinium-based low-dimensional perovskite solar cells. Angew. Chem. Int. Ed. 2021, 60, 856–864. [Google Scholar] [CrossRef]
- Keshavarzi, R.; Molabahrami, N.; Afzali, N.; Omrani, M. Improving Efficiency and Stability of Carbon-Based Perovskite Solar Cells by a Multifunctional Triple-Layer System: Antireflective, UV-Protective, Superhydrophobic, and Self-Cleaning. Sol. RRL 2020, 4, 2000491. [Google Scholar] [CrossRef]
- Zaky, A.A.; Christopoulos, E.; Gkini, K.; Arfanis, M.K.; Sygellou, L.; Kaltzoglou, A.; Stergiou, A.; Tagmatarchis, N.; Balis, N.; Falaras, P. Enhancing efficiency and decreasing photocatalytic degradation of perovskite solar cells using a hydrophobic copper-modified titania electron transport layer. Appl. Catal. B 2021, 284, 119714. [Google Scholar] [CrossRef]
- Mohammed, M.K.; Shekargoftar, M. Surface treatment of ZnO films with carbon nanotubes for efficient and stable perovskite solar cells. Sustain. Energy Fuels 2021, 5, 540–548. [Google Scholar] [CrossRef]
- Aleksandrova, M.; Ivanova, T.; Strijkova, V.; Tsanev, T.; Singh, A.K.; Singh, J.; Gesheva, K. Ga-Doped ZnO Coating—A Suitable Tool for Tuning the Electrode Properties in the Solar Cells with CdS/ZnS Core-Shell Quantum Dots. Crystals 2021, 11, 137. [Google Scholar] [CrossRef]
- Sun, J.; Li, N.; Dong, L.; Niu, X.; Zhao, M.; Xu, Z.; Zhou, H.; Shan, C.; Pan, C. Interfacial-engineering enhanced performance and stability of ZnO nanowire-based perovskite solar cells. Nanotechnology 2021, 32, 475204. [Google Scholar] [CrossRef]
- Wang, Q.; Peng, C.; Du, L.; Li, H.; Zhang, W.; Xie, J.; Qi, H.; Li, Y.; Tian, L.; Huang, Y. Enhanced Performance of Perovskite Solar Cells via Low-Temperature-Processed Mesoporous SnO2. Adv. Mater. Interfaces 2020, 7, 1901866. [Google Scholar] [CrossRef]
- Mattiello, S.; Lucarelli, G.; Calascibetta, A.; Polastri, L.; Ghiglietti, E.; Podapangi, S.K.; Brown, T.M.; Sassi, M.; Beverina, L. Sustainable, Efficient, and Scalable Preparation of Pure and Performing Spiro-OMeTAD for Perovskite Solar Cells. ACS Sustain. Chem. Eng. 2022, 10, 4750–4757. [Google Scholar]
- Abdellah, I.M.; Chowdhury, T.H.; Lee, J.-J.; Islam, A.; Nazeeruddin, M.K.; Gräetzel, M.; El-Shafei, A. Facile and low-cost synthesis of a novel dopant-free hole transporting material that rivals Spiro-OMeTAD for high efficiency perovskite solar cells. Sustain. Energy Fuels 2021, 5, 199–211. [Google Scholar] [CrossRef]
- Guo, W.; Ren, G.; Han, W.; Deng, Y.; Wu, W.; Li, Z.; Guo, J.; Bao, H.; Liu, C. Strategies of modifying spiro-OMeTAD materials for perovskite solar cells: A review. J. Mater. Chem. A 2021, 9, 4589–4625. [Google Scholar]
- Lin, L.; Lian, C.; Jones, T.W.; Bennett, R.; Mihaylov, B.; Yang, T.C.-J.; Wang, J.T.-W.; Chi, B.; Duffy, N.; Li, J. Tunable transition metal complexes as hole transport materials for stable perovskite solar cells. ChemComm 2021, 57, 2093–2096. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.-C.; Raga, S.R.; Ono, L.K.; Qi, Y. Substantial improvement of perovskite solar cells stability by pinhole-free hole transport layer with doping engineering. Sci. Rep. 2015, 5, 9863. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Hua, Y.; Xu, B.; Yang, L.; Liu, P.; Johansson, M.B.; Vlachopoulos, N.; Kloo, L.; Boschloo, G.; Johansson, E.M. The Role of 3D Molecular Structural Control in New Hole Transport Materials Outperforming Spiro-OMeTAD in Perovskite Solar Cells. Adv. Energy Mater. 2016, 6, 1601062. [Google Scholar] [CrossRef]
- Kato, Y.; Ono, L.K.; Lee, M.V.; Wang, S.; Raga, S.R.; Qi, Y. Silver iodide formation in methyl ammonium lead iodide perovskite solar cells with silver top electrodes. Adv. Mater. Interfaces 2015, 2, 1500195. [Google Scholar] [CrossRef]
- Ren, Y.; Ren, M.; Xie, X.; Wang, J.; Cai, Y.; Yuan, Y.; Zhang, J.; Wang, P. A spiro-OMeTAD based semiconductor composite with over 100 °C glass transition temperature for durable perovskite solar cells. Nano Energy 2021, 81, 105655. [Google Scholar] [CrossRef]
- Wu, F.; Pathak, R.; Qiao, Q. Origin and Alleviation of JV Hysteresis in Perovskite Solar Cells: A Short Review. Catal. Today 2021, 374, 86–101. [Google Scholar] [CrossRef]
- Wu, F.; Pathak, R.; Chen, C.; Tong, Y.; Xu, H.; Zhang, T.; Jian, R.; Li, X.; Qiao, Q. Reduced hysteresis in perovskite solar cells using metal oxide/organic hybrid hole transport layer with generated interfacial dipoles. Electrochim. Acta 2020, 354, 136660. [Google Scholar] [CrossRef]
- Zuo, X.; He, Y.; Ji, H.; Li, Y.; Yang, X.; Yu, B.; Wang, T.; Liu, Z.; Huang, W.; Gou, J.; et al. In-situ photoisomerization of azobenzene to inhibit ion-migration for stable high-efficiency perovskite solar cells. J. Energy Chem. 2022. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, Y.; Li, H.; Li, G.; Pan, J.; Xu, D.; Zhao, Q.; Yu, D. Hysteresis analysis based on the ferroelectric effect in hybrid perovskite solar cells. J. Phys. Chem. Lett. 2014, 5, 3937–3945. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, S.; Zhang, X.; Wang, A.; Wu, C.; Hao, F. Ion Migration in Organic–Inorganic Hybrid Perovskite Solar Cells: Current Understanding and Perspectives. Small 2022, 18, 2105783. [Google Scholar] [CrossRef]
- Dualeh, A.; Moehl, T.; Tétreault, N.; Teuscher, J.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Impedance spectroscopic analysis of lead iodide perovskite-sensitized solid-state solar cells. ACS Nano 2014, 8, 362–373. [Google Scholar] [CrossRef]
- Li, C.; Tscheuschner, S.; Paulus, F.; Hopkinson, P.E.; Kießling, J.; Köhler, A.; Vaynzof, Y.; Huettner, S. Iodine migration and its effect on hysteresis in perovskite solar cells. Adv. Mater. 2016, 28, 2446–2454. [Google Scholar] [CrossRef]
- Kruszyńska, J.; Ostapko, J.; Ozkaya, V.; Surucu, B.; Szawcow, O.; Nikiforow, K.; Hołdyński, M.; Tavakoli, M.M.; Yadav, P.; Kot, M.; et al. Atomic Layer Engineering of Aluminum-Doped Zinc Oxide Films for Efficient and Stable Perovskite Solar Cells. Adv. Mater. Interfaces 2022, 9, 2200575. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, Y.; He, B.; Zhang, W.; Cui, L.; Wang, S.; Chen, H.; Duan, Y.; Tang, Q. Efficient interface engineering of N, N′-Dicyclohexylcarbodiimide for stable HTMs-free CsPbBr3 perovskite solar cells with 10.16%-efficiency. Chem. Eng. J. 2022, 428, 131950. [Google Scholar] [CrossRef]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Johnston, M.B.; Snaith, H.J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 2013, 501, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yang, Z.; Cui, X.; Zeng, P.; Li, F.; Liu, X.; Feng, G.; Liu, M. Construction of Charge Transport Channels at the NiOx/Perovskite Interface through Moderate Dipoles toward Highly Efficient Inverted Solar Cells. ACS Appl. Mater. Interfaces 2022, 14, 13431–13439. [Google Scholar] [CrossRef]
- Kim, H.; Lee, K.S.; Paik, M.J.; Lee, D.Y.; Lee, S.U.; Choi, E.; Yun, J.S.; Seok, S.I. Polymethyl Methacrylate as an Interlayer Between the Halide Perovskite and Copper Phthalocyanine Layers for Stable and Efficient Perovskite Solar Cells. Adv. Funct. Mater. 2022, 32, 2110473. [Google Scholar] [CrossRef]
- Ye, Z.; Wei, Q.; Cheng, Y.; Zhang, X.; Ji, P.; Ren, X.; Zan, L.; Fu, F. Highly-efficient perovskite solar cells based on controllable oxygen defects in tin oxide electron transport layers. Ceram. Int. 2022. [Google Scholar] [CrossRef]
- Pan, W.; Lin, J.; Wu, J.; Rong, B.; Zhang, X.; Chen, Q.; Zhang, M.; Wang, S.; Sun, W.; Wang, X.; et al. Interface modification by formamidine acetate for efficient perovskite solar cells. Solar Energy 2022, 232, 304–311. [Google Scholar] [CrossRef]
- Pandey, M.; Kapil, G.; Sakamoto, K.; Hirotani, D.; Kamrudin, M.A.; Wang, Z.; Hamada, K.; Nomura, D.; Kang, H.-G.; Nagayoshi, H. Efficient, hysteresis free, inverted planar flexible perovskite solar cells via perovskite engineering and stability in cylindrical encapsulation. Sustain. Energy Fuels 2019, 3, 1739–1748. [Google Scholar] [CrossRef]
- Asghar, M.; Zhang, J.; Wang, H.; Lund, P. Device stability of perovskite solar cells—A review. Renew. Sustain. Energ. Rev. 2017, 77, 131–146. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Xia, J.; Yang, H.; Chen, L.; Wan, Z.; Han, F.; Malik, H.A.; Zhu, X.; Jia, C. Toward high-efficiency, hysteresis-less, stable perovskite solar cells: Unusual doping of a hole-transporting material using a fluorine-containing hydrophobic Lewis acid. Energy Environ. Sci. 2018, 11, 2035–2045. [Google Scholar] [CrossRef]
- Ava, T.T.; Al Mamun, A.; Marsillac, S.; Namkoong, G. A review: Thermal stability of methylammonium lead halide based perovskite solar cells. Appl. Sci. 2019, 9, 188. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Guan, Y.; Sheng, Y.; Hu, Y.; Rong, Y.; Mei, A.; Han, H. A review on additives for halide perovskite solar cells. Adv. Energy Mater. 2020, 10, 1902492. [Google Scholar] [CrossRef]
- Burschka, J.; Pellet, N.; Moon, S.-J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Leijtens, T.; Eperon, G.E.; Pathak, S.; Abate, A.; Lee, M.M.; Snaith, H.J. Overcoming ultraviolet light instability of sensitized TiO2 with meso-superstructured organometal tri-halide perovskite solar cells. Nat. Commun. 2013, 4, 2885. [Google Scholar] [CrossRef] [PubMed]
- Aristidou, N.; Sanchez-Molina, I.; Chotchuangchutchaval, T.; Brown, M.; Martinez, L.; Rath, T.; Haque, S.A. The role of oxygen in the degradation of methylammonium lead trihalide perovskite photoactive layers. Angew. Chem. 2015, 127, 8326–8330. [Google Scholar] [CrossRef] [Green Version]
- Bryant, D.; Aristidou, N.; Pont, S.; Sanchez-Molina, I.; Chotchunangatchaval, T.; Wheeler, S.; Durrant, J.R.; Haque, S.A. Light and oxygen induced degradation limits the operational stability of methylammonium lead triiodide perovskite solar cells. Energy Environ. Sci. 2016, 9, 1655–1660. [Google Scholar] [CrossRef] [Green Version]
- Nickel, N.H.; Lang, F.; Brus, V.V.; Shargaieva, O.; Rappich, J. Unraveling the Light-Induced Degradation Mechanisms of CH3NH3PbI3 Perovskite Films. Adv. Electron. Mater. 2017, 3, 1700158. [Google Scholar] [CrossRef]
- Salhi, B.; Wudil, Y.; Hossain, M.; Al-Ahmed, A.; Al-Sulaiman, F. Review of recent developments and persistent challenges in stability of perovskite solar cells. Renew. Sustain. Energ. Rev. 2018, 90, 210–222. [Google Scholar] [CrossRef]
- Niu, G.; Guo, X.; Wang, L. Review of recent progress in chemical stability of perovskite solar cells. J. Mater. Chem. A 2015, 3, 8970–8980. [Google Scholar] [CrossRef]
- Tai, Q.; You, P.; Sang, H.; Liu, Z.; Hu, C.; Chan, H.L.W.; Yan, F. Efficient and stable perovskite solar cells prepared in ambient air irrespective of the humidity. Nat. Commun. 2016, 7, 11105. [Google Scholar] [CrossRef]
- Frost, J.M.; Butler, K.T.; Brivio, F.; Hendon, C.H.; Van Schilfgaarde, M.; Walsh, A. Atomistic origins of high-performance in hybrid halide perovskite solar cells. Nano Lett. 2014, 14, 2584–2590. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Siempelkamp, B.D.; Liu, D.; Kelly, T.L. Investigation of CH3NH3PbI3 degradation rates and mechanisms in controlled humidity environments using in situ techniques. ACS Nano 2015, 9, 1955–1963. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Y.; Qin, C.; Yang, X.; Yasuda, T.; Islam, A.; Zhang, K.; Peng, W.; Chen, W.; Han, L. A dopant-free hole-transporting material for efficient and stable perovskite solar cells. Energy Environ. Sci. 2014, 7, 2963–2967. [Google Scholar] [CrossRef]
- Wali, Q.; Iftikhar, F.J.; Khan, M.E.; Ullah, A.; Iqbal, Y.; Jose, R. Advances in stability of perovskite solar cells. Org. Electron. 2020, 78, 105590. [Google Scholar] [CrossRef]
- Ghadiri, M.; Kang, A.K.; Gorji, N.E. XRD characterization of graphene-contacted perovskite solar cells: Moisture degradation and dark-resting recovery. Superlattice Microst. 2020, 146, 106677. [Google Scholar] [CrossRef]
- Lan, D. The physics of ion migration in perovskite solar cells: Insights into hysteresis, device performance, and characterization. Prog. Photovolt. Res. Appl. 2020, 28, 533–537. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, W.; Han, Z.; Yu, D.; Zhao, Q. Effects of ion migration and improvement strategies for the operational stability of perovskite solar cells. Phys. Chem. Chem. Phys. 2021, 23, 94–106. [Google Scholar] [CrossRef]
- Swartz, C.H.; Khakurel, N.; Najar, S.R.; Hossain, M.I.; Zakhidov, A. Temperature and Bias Dependent Degradation and Regeneration of Perovskite Solar Cells with Organic and Inorganic Hole Transport Layers. Phys. Status Solidi 2021, 218, 2000721. [Google Scholar] [CrossRef]
- Gu, C.; Zhou, Z.; Xu, S.; Sun, L. Light illumination and temperature induced current-voltage hysteresis in single crystal perovskite photodiodes. CrystEngComm 2021, 23, 1663–1670. [Google Scholar] [CrossRef]
- Goodenough, J.B. Electronic and ionic transport properties and other physical aspects of perovskites. Rep. Prog. Phys. 2004, 67, 1915. [Google Scholar] [CrossRef]
- Bischoff, C.; Schuller, K.; Beckman, S.P.; Martin, S.W. Non-Arrhenius ionic conductivities in glasses due to a distribution of activation energies. Phys. Rev. Lett. 2012, 109, 075901. [Google Scholar] [CrossRef] [Green Version]
- Xing, J.; Wang, Q.; Dong, Q.; Yuan, Y.; Fang, Y.; Huang, J. Ultrafast ion migration in hybrid perovskite polycrystalline thin films under light and suppression in single crystals. Phys. Chem. Chem. Phys. 2016, 18, 30484–30490. [Google Scholar] [CrossRef]
- Shao, Y.; Fang, Y.; Li, T.; Wang, Q.; Dong, Q.; Deng, Y.; Yuan, Y.; Wei, H.; Wang, M.; Gruverman, A. Grain boundary dominated ion migration in polycrystalline organic–inorganic halide perovskite films. Energy Environ. Sci. 2016, 9, 1752–1759. [Google Scholar] [CrossRef]
- Kang, D.-H.; Kim, S.-Y.; Lee, J.-W.; Park, N.-G. Efficient surface passivation of perovskite films by a post-treatment method with a minimal dose. J. Mater. Chem. A 2021, 9, 3441–3450. [Google Scholar] [CrossRef]
- Farrington, G.C.; Briant, J.L. Fast ionic transport in solids. Science 1979, 204, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, R.; Haltzi, E.; Gouda, L.; Tirosh, S.; Bouhadana, Y.; Zaban, A.; Mosconi, E.; De Angelis, F. Extremely slow photoconductivity response of CH3NH3PbI3 perovskites suggesting structural changes under working conditions. J. Phys. Chem. Lett. 2014, 5, 2662–2669. [Google Scholar] [CrossRef] [Green Version]
- Yun, J.S.; Seidel, J.; Kim, J.; Soufiani, A.M.; Huang, S.; Lau, J.; Jeon, N.J.; Seok, S.I.; Green, M.A.; Ho-Baillie, A. Critical role of grain boundaries for ion migration in formamidinium and methylammonium lead halide perovskite solar cells. Adv. Energy Mater. 2016, 6, 1600330. [Google Scholar] [CrossRef]
- Yun, J.S.; Kim, J.; Young, T.; Patterson, R.J.; Kim, D.; Seidel, J.; Lim, S.; Green, M.A.; Huang, S.; Ho-Baillie, A. Humidity-induced degradation via grain boundaries of HC (NH2)2PbI3 planar perovskite solar cells. Adv. Funct. Mater. 2018, 28, 1705363. [Google Scholar] [CrossRef]
- Lin, D.; Shi, T.; Xie, H.; Wan, F.; Ren, X.; Liu, K.; Zhao, Y.; Ke, L.; Lin, Y.; Gao, Y. Ion Migration Accelerated Reaction between Oxygen and Metal Halide Perovskites in Light and Its Suppression by Cesium Incorporation. Adv. Energy Mater. 2021, 11, 2002552. [Google Scholar] [CrossRef]
- Di Girolamo, D.; Phung, N.; Kosasih, F.U.; Di Giacomo, F.; Matteocci, F.; Smith, J.A.; Flatken, M.A.; Köbler, H.; Turren Cruz, S.H.; Mattoni, A. Ion Migration-Induced Amorphization and Phase Segregation as a Degradation Mechanism in Planar Perovskite Solar Cells. Adv. Energy Mater. 2020, 10, 2000310. [Google Scholar] [CrossRef]
- Tress, W.; Marinova, N.; Moehl, T.; Zakeeruddin, S.M.; Nazeeruddin, M.K.; Grätzel, M. Understanding the rate-dependent J–V hysteresis, slow time component, and aging in CH3NH3PbI3 perovskite solar cells: The role of a compensated electric field. Energy Environ. Sci. 2015, 8, 995–1004. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.; Williams, S.T.; Xiong, D.; Zhang, W.; Chueh, C.C.; Chen, W.; Jen, A.K.Y. SrCl2 Derived Perovskite Facilitating a High Efficiency of 16% in Hole-Conductor-Free Fully Printable Mesoscopic Perovskite Solar Cells. Adv. Mater. 2017, 29, 1606608. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, Q.; Shao, Y.; Lu, H.; Li, T.; Gruverman, A.; Huang, J. Electric-field-driven reversible conversion between Methylammonium lead triiodide perovskites and lead iodide at elevated temperatures. Adv. Energy Mater. 2016, 6, 1501803. [Google Scholar] [CrossRef]
- Guerrero, A.; You, J.; Aranda, C.; Kang, Y.S.; Garcia-Belmonte, G.; Zhou, H.; Bisquert, J.; Yang, Y. Interfacial degradation of planar lead halide perovskite solar cells. ACS Nano 2016, 10, 218–224. [Google Scholar] [CrossRef]
- Yang, D.; Ming, W.; Shi, H.; Zhang, L.; Du, M.-H. Fast diffusion of native defects and impurities in perovskite solar cell material CH3NH3PbI3. Chem. Mater. 2016, 28, 4349–4357. [Google Scholar] [CrossRef]
- Yu, Z.; Hu, P.; Xu, Y.; Bao, Q.; Ni, D.; Wei, C.; Shi, J. Efficient Gene Therapy of Pancreatic Cancer via a Peptide Nucleic Acid (PNA)-Loaded Layered Double Hydroxides (LDH) Nanoplatform. Small 2020, 16, 1907233. [Google Scholar] [CrossRef]
- Zhou, Y.; Yin, Y.; Zuo, X.; Wang, L.; Li, T.-D.; Zhou, Y.; Padture, N.P.; Yang, Z.; Guo, Y.; Xue, Y. Enhancing Chemical Stability and Suppressing Ion Migration in CH3NH3PbI3 Perovskite Solar Cells via Direct Backbone Attachment of Polyesters on Grain Boundaries. Chem. Mater. 2020, 32, 5104–5117. [Google Scholar] [CrossRef]
- Choi, H.; Choi, K.; Choi, Y.; Kim, T.; Lim, S.; Park, T. A Review on Reducing Grain Boundaries and Morphological Improvement of Perovskite Solar Cells from Methodology and Material-Based Perspectives. Small Methods 2020, 4, 1900569. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, Y.; Chen, S.; Wang, S.; Ni, Z.; Brackle, C.; Zhao, J.; Yu, Z.; Dai, X.; Wang, Q. Revealing defective nanostructured surfaces and their impact on intrinsic stability of hybrid perovskites. Energy Environ. Sci. 2021, 14, 1563–1572. [Google Scholar] [CrossRef]
- Rybin, N.; Ghosh, D.; Tisdale, J.; Shrestha, S.; Yoho, M.; Vo, D.; Even, J.; Katan, C.; Nie, W.; Neukirch, A.J. Effects of chlorine mixing on optoelectronics, Ion Migration, and Gamma-Ray detection in bromide Perovskites. Chem. Mater. 2020, 32, 1854–1863. [Google Scholar] [CrossRef]
- Kim, M.R.; Choi, H.W.; Bark, C.W. Low-Temperature Thermally Evaporated SnO2 Based Electron Transporting Layer for Perovskite Solar Cells with Annealing Process. J. Nanosci. Nanotechnol. 2020, 20, 5491–5497. [Google Scholar] [CrossRef]
- Lin, Y.; Bai, Y.; Fang, Y.; Chen, Z.; Yang, S.; Zheng, X.; Tang, S.; Liu, Y.; Zhao, J.; Huang, J. Enhanced Thermal Stability in Perovskite Solar Cells by Assembling 2D/3D Stacking Structures. J. Phys. Chem. Lett. 2018, 9, 654–658. [Google Scholar] [CrossRef]
- Zhang, J.; Mao, W.; Duan, J.; Huang, S.; Zhang, Z.; Ou-Yang, W.; Zhang, X.; Sun, Z.; Chen, X. Enhanced Efficiency and Thermal Stability of Perovskite Solar Cells Using Poly (9-Vinylcarbazole) Modified Perovskite/PCBM Interface. Electrochim. Acta 2019, 318, 384–391. [Google Scholar] [CrossRef]
- Rong, Y.; Liu, L.; Mei, A.; Li, X.; Han, H. Beyond Efficiency: The Challenge of Stability in Mesoscopic Perovskite Solar Cells. Adv. Energy Mater. 2015, 5, 1501066. [Google Scholar] [CrossRef]
- Divitini, G.; Cacovich, S.; Matteocci, F.; Cinà, L.; Di Carlo, A.; Ducati, C. In Situ Observation of Heat-Induced Degradation of Perovskite Solar Cells. Nat. Energy 2016, 1, 15012. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Mujahid, M.; Duan, Y.; Wang, Z.-K.; Xue, J.; Yang, Y. A Review of Perovskites Solar Cell Stability. Adv. Funct. Mater. 2019, 29, 1808843. [Google Scholar] [CrossRef]
- Li, D.; Cheng, H.-C.; Wang, Y.; Zhao, Z.; Wang, G.; Wu, H.; He, Q.; Huang, Y.; Duan, X. The Effect of Thermal Annealing on Charge Transport in Organolead Halide Perovskite Microplate Field-Effect Transistors. Adv. Mater. 2017, 29, 1601959. [Google Scholar] [CrossRef] [PubMed]
- Juarez-Perez, E.J.; Hawash, Z.; Raga, S.R.; Ono, L.K.; Qi, Y. Thermal Degradation of CH3 NH3PbI3 Perovskite into NH3 and CH3I Gases Observed by Coupled Thermogravimetry—Mass Spectrometry Analysis. Energy Environ. Sci. 2016, 9, 3406–3410. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.-K.; Min, Y.H.; Noh, S.; Cho, E.; Jeong, G.; Joo, M.; Ahn, S.-W.; Lee, J.S.; Kim, S.; Ihm, K. Investigation of Thermally Induced Degradation in Perovskite Solar Cells Using In-Situ Synchrotron Radiation Analysis. Sci. Rep. 2017, 7, 4645. [Google Scholar] [CrossRef]
- Conings, B.; Drijkoningen, J.; Gauquelin, N.; Babayigit, A.; D’Haen, J.; D’Olieslaeger, L.; Ethirajan, A.; Verbeeck, J.; Manca, J.; Mosconi, E. Intrinsic Thermal Instability of Methylammonium Lead Trihalide Perovskite. Adv. Energy Mater. 2015, 5, 1500477. [Google Scholar] [CrossRef]
- Philippe, B.; Park, B.-W.; Lindblad, R.; Oscarsson, J.; Ahmadi, S.; Johansson, E.M.; Rensmo, H. Chemical and Electronic Structure Characterization of Lead Halide Perovskites and Stability Behavior under Different Exposures—A Photoelectron Spectroscopy Investigation. Chem. Mater. 2015, 27, 1720–1731. [Google Scholar] [CrossRef]
- Dualeh, A.; Gao, P.; Seok, S.I.; Nazeeruddin, M.K.; Grätzel, M. Thermal Behavior of Methylammonium Lead-Trihalide Perovskite Photovoltaic Light Harvesters. Chem. Mater. 2014, 26, 6160–6164. [Google Scholar] [CrossRef]
- Pisoni, A.; Jacimovic, J.; Barisic, O.S.; Spina, M.; Gaál, R.; Forró, L.; Horváth, E. Ultra-Low Thermal Conductivity in Organic–Inorganic Hybrid Perovskite CH3NH3PbI3. J. Phys. Chem. Lett. 2014, 5, 2488–2492. [Google Scholar] [CrossRef] [Green Version]
- Amat, A.; Mosconi, E.; Ronca, E.; Quarti, C.; Umari, P.; Nazeeruddin, M.K.; Gratzel, M.; De Angelis, F. Cation-Induced Band-Gap Tuning in Organohalide Perovskites: Interplay of Spin–Orbit Coupling and Octahedra Tilting. Nano Lett. 2014, 14, 3608–3616. [Google Scholar] [CrossRef] [PubMed]
- Mitzi, D.B. Synthesis, Structure, and Properties of Organic-Inorganic Perovskites and Related Materials. In Progress in Inorganic Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1999; pp. 1–121. [Google Scholar] [CrossRef]
- Krishna, A.; Grimsdale, A.C. Hole Transporting Materials for Mesoscopic Perovskite Solar Cells—Towards a Rational Design? J. Mater. Chem. A 2017, 5, 16446–16466. [Google Scholar] [CrossRef]
- Kim, G.-W.; Kang, G.; Malekshahi Byranvand, M.; Lee, G.-Y.; Park, T. Gradated Mixed Hole Transport Layer in a Perovskite Solar Cell: Improving Moisture Stability and Efficiency. ACS Appl. Mater. Interfaces 2017, 9, 27720–27726. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Sun, W.; Li, Y.; Yan, W.; Peng, H.; Bian, Z.; Liu, Z.; Huang, C. CuSCN-Based Inverted Planar Perovskite Solar Cell with an Average PCE of 15.6%. Nano Lett. 2015, 15, 3723–3728. [Google Scholar] [CrossRef]
- Labban, A.E.; Chen, H.; Kirkus, M.; Barbe, J.; Del Gobbo, S.; Neophytou, M.; McCulloch, I.; Eid, J. Improved Efficiency in Inverted Perovskite Solar Cells Employing a Novel Diarylamino-Substituted Molecule as PEDOT: PSS Replacement. Adv. Energy Mater. 2016, 6, 1502101. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.; Song, M.; Park, S.; Nam, S.; Seo, J.; Kim, H.; Kim, Y. Acidity-Controlled Conducting Polymer Films for Organic Thermoelectric Devices with Horizontal and Vertical Architectures. Sci. Rep. 2016, 6, 33795. [Google Scholar] [CrossRef] [Green Version]
- Vitoratos, E.; Sakkopoulos, S.; Dalas, E.; Paliatsas, N.; Karageorgopoulos, D.; Petraki, F.; Kennou, S.; Choulis, S.A. Thermal Degradation Mechanisms of PEDOT: PSS. Org. Electron. 2009, 10, 61–66. [Google Scholar] [CrossRef]
- Hu, L.; Li, M.; Yang, K.; Xiong, Z.; Yang, B.; Wang, M.; Tang, X.; Zang, Z.; Liu, X.; Li, B. PEDOT: PSS Monolayers to Enhance the Hole Extraction and Stability of Perovskite Solar Cells. J. Mater. Chem. A 2018, 6, 16583–16589. [Google Scholar] [CrossRef]
- You, J.; Meng, L.; Song, T.-B.; Guo, T.-F.; Yang, Y.M.; Chang, W.-H.; Hong, Z.; Chen, H.; Zhou, H.; Chen, Q. Improved Air Stability of Perovskite Solar Cells via Solution-Processed Metal Oxide Transport Layers. Nat. Nanotechnol. 2016, 11, 75–81. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, J.; Lin, F.; He, H.; Mao, J.; Wong, K.S.; Jen, A.K.-Y.; Choy, W.C. Pinhole-Free and Surface-Nanostructured NiO x Film by Room-Temperature Solution Process for High-Performance Flexible Perovskite Solar Cells with Good Stability and Reproducibility. ACS Nano 2016, 10, 1503–1511. [Google Scholar] [CrossRef]
- Zhao, X.; Kim, H.-S.; Seo, J.-Y.; Park, N.-G. Effect of Selective Contacts on the Thermal Stability of Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 7148–7153. [Google Scholar] [CrossRef] [PubMed]
- Habisreutinger, S.N.; Leijtens, T.; Eperon, G.E.; Stranks, S.D.; Nicholas, R.J.; Snaith, H.J. Carbon Nanotube/Polymer Composites as a Highly Stable Hole Collection Layer in Perovskite Solar Cells. Nano Lett. 2014, 14, 5561–5568. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Wu, Q.; Zhou, P.; Li, Y.; Chen, X.; Liu, Q.; Zhu, J.; Dai, S.; Lu, Y.; Yang, S. Efficiency Enhancement of Inverted Structure Perovskite Solar Cells via Oleamide Doping of PCBM Electron Transport Layer. ACS Appl. Mater. Interfaces 2015, 7, 13659–13665. [Google Scholar] [CrossRef]
- Bera, A.; Sheikh, A.D.; Haque, M.A.; Bose, R.; Alarousu, E.; Mohammed, O.F.; Wu, T. Fast Crystallization and Improved Stability of Perovskite Solar Cells with Zn2SnO4 Electron Transporting Layer: Interface Matters. ACS Appl. Mater. Interfaces 2015, 7, 28404–28411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lian, J.; Lu, B.; Niu, F.; Zeng, P.; Zhan, X. Electron-Transport Materials in Perovskite Solar Cells. Small Methods 2018, 2, 1800082. [Google Scholar] [CrossRef]
- Kim, H.-S.; Lee, J.-W.; Yantara, N.; Boix, P.P.; Kulkarni, S.A.; Mhaisalkar, S.; Gratzel, M.; Park, N.-G. High Efficiency Solid-State Sensitized Solar Cell-Based on Submicrometer Rutile TiO2 Nanorod and CH3NH3PbI3 Perovskite Sensitizer. Nano Lett. 2013, 13, 2412–2417. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Fang, G.; Liu, Q.; Xiong, L.; Qin, P.; Tao, H.; Wang, J.; Lei, H.; Li, B.; Wan, J. Low-Temperature Solution-Processed Tin Oxide as an Alternative Electron Transporting Layer for Efficient Perovskite Solar Cells. J. Am. Chem. Soc. 2015, 137, 6730–6733. [Google Scholar] [CrossRef]
- Wang, Q.; Chueh, C.-C.; Eslamian, M.; Jen, A.K.-Y. Modulation of PEDOT: PSS PH for Efficient Inverted Perovskite Solar Cells with Reduced Potential Loss and Enhanced Stability. ACS Appl. Mater. Interfaces 2016, 8, 32068–32076. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Zhang, X.W.; Yin, Z.G.; Jiang, Q.; Liu, X.; Meng, J.H.; Zhao, Y.J.; Wang, H.L. Highly Efficient and Stable Planar Heterojunction Perovskite Solar Cells via a Low Temperature Solution Process. J. Mater. Chem. A 2015, 3, 12133–12138. [Google Scholar] [CrossRef]
- Shao, Y.; Xiao, Z.; Bi, C.; Yuan, Y.; Huang, J. Origin and Elimination of Photocurrent Hysteresis by Fullerene Passivation in CH3NH3PbI3 Planar Heterojunction Solar Cells. Nat. Commun. 2014, 5, 5784. [Google Scholar] [CrossRef] [PubMed]
- Pathak, S.K.; Abate, A.; Ruckdeschel, P.; Roose, B.; Gödel, K.C.; Vaynzof, Y.; Santhala, A.; Watanabe, S.-I.; Hollman, D.J.; Noel, N. Performance and Stability Enhancement of Dye-Sensitized and Perovskite Solar Cells by Al Doping of TiO2. Adv. Funct. Mater. 2014, 24, 6046–6055. [Google Scholar] [CrossRef]
- Ahn, N.; Kwak, K.; Jang, M.S.; Yoon, H.; Lee, B.Y.; Lee, J.-K.; Pikhitsa, P.V.; Byun, J.; Choi, M. Trapped Charge-Driven Degradation of Perovskite Solar Cells. Nat. Commun. 2016, 7, 13422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojciechowski, K.; Stranks, S.D.; Abate, A.; Sadoughi, G.; Sadhanala, A.; Kopidakis, N.; Rumbles, G.; Li, C.-Z.; Friend, R.H.; Jen, A.K.-Y. Heterojunction Modification for Highly Efficient Organic–Inorganic Perovskite Solar Cells. ACS Nano 2014, 8, 12701–12709. [Google Scholar] [CrossRef] [PubMed]
- Snaith, H.J.; Abate, A.; Ball, J.M.; Eperon, G.E.; Leijtens, T.; Noel, N.K.; Stranks, S.D.; Wang, J.T.-W.; Wojciechowski, K.; Zhang, W. Anomalous hysteresis in perovskite solar cells. J. Phys. Chem. Lett. 2014, 5, 1511–1515. [Google Scholar] [CrossRef]
- Shah, S.A.A.; Sayyad, M.H.; Sun, J.; Guo, Z. Hysteresis Analysis of Hole-Transport-Material-Free Monolithic Perovskite Solar Cells with Carbon Counter Electrode by Current Density–Voltage and Impedance Spectra Measurements. Nanomaterials 2021, 11, 48. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, L.; Mu, X.; Chen, C.-C.; Wu, Y.; Cao, J.; Tang, Y. Intramolecular Electric Field Construction in Metal Phthalocyanine as Dopant-Free Hole Transporting Material for Stable Perovskite Solar Cells with>21% Efficiency. Angew. Chem. 2021, 60, 6294–6299. [Google Scholar] [CrossRef]
- Chen, B.; Yang, M.; Priya, S.; Zhu, K. Origin of J–V hysteresis in perovskite solar cells. J. Phys. Chem. Lett. 2016, 7, 905–917. [Google Scholar] [CrossRef]
- Song, D.H.; Jang, M.H.; Lee, M.H.; Heo, J.H.; Park, J.K.; Sung, S.-J.; Kim, D.-H.; Hong, K.-H.; Im, S.H. A discussion on the origin and solutions of hysteresis in perovskite hybrid solar cells. J. Phys. D Appl. Phys. 2016, 49, 473001. [Google Scholar] [CrossRef]
- Mohseni, H.; Dehghanipour, M.; Dehghan, N.; Tamaddon, F.; Ahmadi, M.; Sabet, M.; Behjat, A. Enhancement of the photovoltaic performance and the stability of perovskite solar cells via the modification of electron transport layers with reduced graphene oxide/polyaniline composite. Sol. Energy 2021, 213, 59–66. [Google Scholar] [CrossRef]
- Qasim, U.B.; Qasim, H.B.; Saeed, M.M.; Riaz, M.H.; Imran, H. Investigating physical origin of dominant hysteresis phenomenon in perovskite solar cell. J. Mater. Sci. Mater. Electron. 2021, 32, 5274–5285. [Google Scholar] [CrossRef]
- Unger, E.L.; Hoke, E.T.; Bailie, C.D.; Nguyen, W.H.; Bowring, A.R.; Heumüller, T.; Christoforo, M.G.; McGehee, M.D. Hysteresis and transient behavior in current–voltage measurements of hybrid-perovskite absorber solar cells. Energy Environ. Sci. 2014, 7, 3690–3698. [Google Scholar] [CrossRef]
- Chen, H.-W.; Sakai, N.; Ikegami, M.; Miyasaka, T. Emergence of hysteresis and transient ferroelectric response in organo-lead halide perovskite solar cells. J. Phys. Chem. Lett. 2015, 6, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Ku, Z.; Rong, Y.; Xu, M.; Liu, T.; Han, H. Full printable processed mesoscopic CH3NH3PbI3/TiO2 heterojunction solar cells with carbon counter electrode. Sci. Rep. 2013, 3, 3132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Liang, C.; Zhao, Y.; Sun, M.; Liu, H.; Liang, J.; Li, D.; Zhang, F.; He, Z. Dynamic interface charge governing the current–voltage hysteresis in perovskite solar cells. Phys. Chem. Chem. Phys. 2015, 17, 9613–9618. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.W.; Eperon, G.E.; Roe, E.T.; Warren, C.W.; Snaith, H.J.; Lonergan, M.C. Defect states in perovskite solar cells associated with hysteresis and performance. Appl. Phys. Lett. 2016, 109, 153902. [Google Scholar] [CrossRef]
- Son, D.-Y.; Kim, S.-G.; Seo, J.-Y.; Lee, S.-H.; Shin, H.; Lee, D.; Park, N.-G. Universal approach toward hysteresis-free perovskite solar cell via defect engineering. J. Am. Chem. Soc. 2018, 140, 1358–1364. [Google Scholar] [CrossRef]
- Deng, Y.; Xiao, Z.; Huang, J. Light-induced self-poling effect on organometal trihalide perovskite solar cells for increased device efficiency and stability. Adv. Energy Mater. 2015, 5, 1500721. [Google Scholar] [CrossRef]
- Juarez-Perez, E.J.; Sanchez, R.S.; Badia, L.; Garcia-Belmonte, G.; Kang, Y.S.; Mora-Sero, I.; Bisquert, J. Photoinduced giant dielectric constant in lead halide perovskite solar cells. J. Phys. Chem. Lett. 2014, 5, 2390–2394. [Google Scholar] [CrossRef]
- Yuan, Y.; Xiao, Z.; Yang, B.; Huang, J. Arising applications of ferroelectric materials in photovoltaic devices. J. Mater. Chem. A 2014, 2, 6027–6041. [Google Scholar] [CrossRef] [Green Version]
- Stroppa, A.; Di Sante, D.; Barone, P.; Bokdam, M.; Kresse, G.; Franchini, C.; Whangbo, M.-H.; Picozzi, S. Tunable ferroelectric polarization and its interplay with spin–orbit coupling in tin iodide perovskites. Nat. Commun. 2014, 5, 5900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asif, M.; Aziz, A.; Dao, A.Q.; Hakeem, A.; Wang, H.; Dong, S.; Zhang, G.; Xiao, F.; Liu, H. Real-time tracking of hydrogen peroxide secreted by live cells using MnO2 nanoparticles intercalated layered doubled hydroxide nanohybrids. Anal. Chim. Acta 2015, 898, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Wasylishen, R.E.; Knop, O.; Macdonald, J.B. Cation rotation in methylammonium lead halides. Solid State Commun. 1985, 56, 581–582. [Google Scholar] [CrossRef]
- Mashiyama, H.; Kawamura, Y.; Kubota, Y. The Anti-Polar Structure of CH~3NH~3PbBr~3. J. Korean Phys. Soc. 2007, 51, 850. [Google Scholar] [CrossRef]
- Liu, T.; Li, Y.; Xu, R.; Lou, L.; Gao, J.; Zhang, K.; Su, M.; Qian, W.; Wang, G.; Xiao, S.; et al. Building Bulk Heterojunction to Enhance Hole Extraction for High-Performance Printable Carbon-Based Perovskite Solar Cells. Solar RRL 2022, 6, 2200206. [Google Scholar] [CrossRef]
- Guerrero, A.; Juarez-Perez, E.J.; Bisquert, J.; Mora-Sero, I.; Garcia-Belmonte, G. Electrical field profile and doping in planar lead halide perovskite solar cells. Appl. Phys. Lett. 2014, 105, 133902. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-S.; Mora-Sero, I.; Gonzalez-Pedro, V.; Fabregat-Santiago, F.; Juarez-Perez, E.J.; Park, N.-G.; Bisquert, J. Mechanism of carrier accumulation in perovskite thin-absorber solar cells. Nat. Commun. 2013, 4, 2242. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Chung, Y.-H.; Ma, Y.; Zhang, L.; Xiao, L.; Chen, Z.; Wang, S.; Qu, B.; Gong, Q. A hydrophobic hole transporting oligothiophene for planar perovskite solar cells with improved stability. ChemComm 2014, 50, 11196–11199. [Google Scholar] [CrossRef]
- Shkrob, I.A.; Marin, T.W. Charge trapping in photovoltaically active perovskites and related halogenoplumbate compounds. J. Phys. Chem. Lett. 2014, 5, 1066–1071. [Google Scholar] [CrossRef]
- Sum, T.C.; Mathews, N. Advancements in perovskite solar cells: Photophysics behind the photovoltaics. Energy Environ. Sci. 2014, 7, 2518–2534. [Google Scholar] [CrossRef] [Green Version]
- Wehrenfennig, C.; Eperon, G.E.; Johnston, M.B.; Snaith, H.J.; Herz, L.M. High charge carrier mobilities and lifetimes in organolead trihalide perovskites. Adv. Mater. 2014, 26, 1584–1589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Saliba, M.; Stranks, S.D.; Sun, Y.; Shi, X.; Wiesner, U.; Snaith, H.J. Enhancement of perovskite-based solar cells employing core–shell metal nanoparticles. Nano Lett. 2013, 13, 4505–4510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stolterfoht, M.; Caprioglio, P.; Wolff, C.M.; Márquez, J.A.; Nordmann, J.; Zhang, S.; Rothhardt, D.; Hörmann, U.; Amir, Y.; Redinger, A. The impact of energy alignment and interfacial recombination on the internal and external open-circuit voltage of perovskite solar cells. Energy Environ. Sci. 2019, 12, 2778–2788. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Wang, Y.; Zheng, H.; Wu, J.; Ji, L.; Zhang, P.; Ahmad, W.; Chen, H.; Chen, Z.; Li, S. Improved stability of perovskite solar cells with enhanced moisture-resistant hole transport layers. Electrochim. Acta 2019, 296, 508–516. [Google Scholar] [CrossRef]
- Bu, I.Y.-Y.; Lu, Y.-C.; Fu, Y.-S.; Hung, C.-T. Novel CuAlO2/polyaniline hole transport layer for industrial production of perovskite solar cells. Optik 2020, 210, 164505. [Google Scholar] [CrossRef]
- Noh, J.H.; Im, S.H.; Heo, J.H.; Mandal, T.N.; Seok, S.I. Chemical Management for Colorful, Efficient, and Stable Inorganic–Organic Hybrid Nanostructured Solar Cells. Nano Lett. 2013, 13, 1764–1769. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Lin, J. Layered Organic–Inorganic Hybrid Perovskites: Structure, Optical Properties, Film Preparation, Patterning and Templating Engineering. CrystEngComm 2010, 12, 2646–2662. [Google Scholar] [CrossRef]
- Cao, D.H.; Stoumpos, C.C.; Farha, O.K.; Hupp, J.T.; Kanatzidis, M.G. 2D Homologous Perovskites as Light-Absorbing Materials for Solar Cell Applications. J. Am. Chem. Soc. 2015, 137, 7843–7850. [Google Scholar] [CrossRef]
- Smith, I.C.; Hoke, E.T.; Solis-Ibarra, D.; McGehee, M.D.; Karunadasa, H.I. A Layered Hybrid Perovskite Solar-Cell Absorber with Enhanced Moisture Stability. Angew. Chem. Int. Ed. 2014, 53, 11232–11235. [Google Scholar] [CrossRef]
- Jiang, Q.; Rebollar, D.; Gong, J.; Piacentino, E.L.; Zheng, C.; Xu, T. Pseudohalide-Induced Moisture Tolerance in Perovskite CH3NH3Pb(SCN)2I Thin Films. Angew. Chem. 2015, 127, 7727–7730. [Google Scholar] [CrossRef]
- Saliba, M.; Matsui, T.; Seo, J.-Y.; Domanski, K.; Correa-Baena, J.-P.; Khaja Nazeeruddin, M.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; et al. Cesium-Containing Triple Cation Perovskite Solar Cells: Improved Stability, Reproducibility and High Efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.-Y.; Chen, S.; Xu, P.; Xiang, H.; Gong, X.-G.; Walsh, A.; Wei, S.-H. Intrinsic Instability of the Hybrid Halide Perovskite Semiconductor CH3NH3PbI3\ast. Chin. Phys. Lett. 2018, 35, 036104. [Google Scholar] [CrossRef] [Green Version]
- Yin, W.-J.; Yan, Y.; Wei, S.-H. Anomalous Alloy Properties in Mixed Halide Perovskites. J. Phys. Chem. Lett. 2014, 5, 3625–3631. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Yin, W.-J. Thermodynamic Stability Trend of Cubic Perovskites. J. Am. Chem. Soc. 2017, 139, 14905–14908. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Park, B.-W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H.; et al. Iodide Management in Formamidinium-Lead-Halide–Based Perovskite Layers for Efficient Solar Cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional Engineering of Perovskite Materials for High-Performance Solar Cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef]
- Hu, H.; Dong, B.; Zhang, W. Low-Toxic Metal Halide Perovskites: Opportunities and Future Challenges. J. Mater. Chem. A 2017, 5, 11436–11449. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, N.; Tang, B.; Li, M.; Zhang, Y.-W.; Yu, Z.G.; Gong, H. Highly Stable New Organic—Inorganic Hybrid 3D Perovskite CH3NH3PdI3 and 2D Perovskite (CH3NH3)3Pd2I7: DFT Analysis, Synthesis, Structure, Transition Behavior, and Physical Properties. J. Phys. Chem. Lett. 2018, 9, 5862–5872. [Google Scholar] [CrossRef]
- Aharon, S.; Cohen, B.E.; Etgar, L. Hybrid Lead Halide Iodide and Lead Halide Bromide in Efficient Hole Conductor Free Perovskite Solar Cell. J. Phys. Chem. C 2014, 118, 17160–17165. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, G.; Xu, X.; Alsaedi, A.; Hayat, T.; Pan, X.; Dai, S. Acquiring High-Performance and Stable Mixed-Dimensional Perovskite Solar Cells by Using a Transition-Metal-Substituted Pb Precursor. ChemSusChem 2018, 11, 3269–3275. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, Y.; Zhou, X.; Fu, R.; Li, Q.; Zhao, Y.; Liu, K.; Yu, D.; Zhao, Q. Light-Independent Ionic Transport in Inorganic Perovskite and Ultrastable Cs-Based Perovskite Solar Cells. J. Phys. Chem. Lett. 2017, 8, 4122–4128. [Google Scholar] [CrossRef]
- Akbulatov, A.F.; Luchkin, S.Y.; Frolova, L.A.; Dremova, N.N.; Gerasimov, K.L.; Zhidkov, I.S.; Anokhin, D.V.; Kurmaev, E.Z.; Stevenson, K.J.; Troshin, P.A. Probing the Intrinsic Thermal and Photochemical Stability of Hybrid and Inorganic Lead Halide Perovskites. J. Phys. Chem. Lett. 2017, 8, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Chen, S.; Xiang, H.-J.; Gong, X.-G.; Wei, S.-H. Influence of Defects and Synthesis Conditions on the Photovoltaic Performance of Perovskite Semiconductor CsSnI3. Chem. Mater. 2014, 26, 6068–6072. [Google Scholar] [CrossRef]
- Yi, C.; Luo, J.; Meloni, S.; Boziki, A.; Ashari-Astani, N.; Grätzel, C.; Zakeeruddin, S.M.; Röthlisberger, U.; Grätzel, M. Entropic Stabilization of Mixed A-Cation ABX3 Metal Halide Perovskites for High Performance Perovskite Solar Cells. Energy Environ. Sci. 2016, 9, 656–662. [Google Scholar] [CrossRef]
- Eperon, G.E.; Stranks, S.D.; Menelaou, C.; Johnston, M.B.; Herz, L.M.; Snaith, H.J. Formamidinium Lead Trihalide: A Broadly Tunable Perovskite for Efficient Planar Heterojunction Solar Cells. Energy Environ. Sci. 2014, 7, 982–988. [Google Scholar] [CrossRef]
- Ono, L.K.; Juarez-Perez, E.J.; Qi, Y. Progress on Perovskite Materials and Solar Cells with Mixed Cations and Halide Anions. ACS Appl. Mater. Interfaces 2017, 9, 30197–30246. [Google Scholar] [CrossRef] [Green Version]
- Tress, W.; Domanski, K.; Carlsen, B.; Agarwalla, A.; Alharbi, E.A.; Graetzel, M.; Hagfeldt, A. Performance of Perovskite Solar Cells under Simulated Temperature-Illumination Real-World Operating Conditions. Nat. Energy 2019, 4, 568–574. [Google Scholar] [CrossRef]
- Wu, R.; Yang, J.; Xiong, J.; Liu, P.; Zhou, C.; Huang, H.; Gao, Y.; Yang, B. Efficient electron-blocking layer-free planar heterojunction perovskite solar cells with a high open-circuit voltage. Org. Electron. 2015, 26, 265–272. [Google Scholar] [CrossRef]
- Liu, C.; Cai, M.; Yang, Y.; Arain, Z.; Ding, Y.; Shi, X.; Shi, P.; Ma, S.; Hayat, T.; Alsaedi, A. AC 60/TiOx bilayer for conformal growth of perovskite films for UV stable perovskite solar cells. J. Mater. Chem. A 2019, 7, 11086–11094. [Google Scholar] [CrossRef]
- Uddin, A.; Yi, H. Progress and challenges of SnO2 electron transport layer for perovskite solar cells: A critical review. Solar RRL 2022, 6, 2100983. [Google Scholar] [CrossRef]
- Liu, G.; Zheng, H.; Zhang, L.; Xu, H.; Xu, S.; Xu, X.; Liang, Z.; Pan, X. Tailoring multifunctional passivation molecules with halogen functional groups for efficient and stable perovskite photovoltaics. Chem. Eng. J. 2021, 407, 127204. [Google Scholar] [CrossRef]
- Liao, J.-F.; Wu, W.-Q.; Jiang, Y.; Zhong, J.-X.; Wang, L.; Kuang, D.-B. Understanding of carrier dynamics, heterojunction merits and device physics: Towards designing efficient carrier transport layer-free perovskite solar cells. Chem. Soc. Rev. 2020, 49, 354–381. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Rahman, N.; Khan, W.; Wang, Y.; Fu, S.; Ahmed, G.; Akhtar, M.; Wu, M. Hollow 3D TiO2 sub-microspheres as an electron transporting layer for highly efficient perovskite solar cells. Mater. Today Energy 2021, 19, 100614. [Google Scholar] [CrossRef]
- Bhatt, S.; Shukla, R.; Pathak, C.; Pandey, S.K. Evaluation of performance constraints and structural optimization of a core-shell ZnO nanorod based eco-friendly perovskite solar cell. Sol. Energy 2021, 215, 473–481. [Google Scholar] [CrossRef]
- Ahmad, S.; Abbas, H.; Khan, M.B.; Nagal, V.; Hafiz, A.; Khan, Z.H. ZnO for stable and efficient perovskite bulk heterojunction solar cell fabricated under ambient atmosphere. Sol. Energy 2021, 216, 164–170. [Google Scholar] [CrossRef]
- Wang, H.; Liu, H.; Ye, F.; Chen, Z.; Ma, J.; Liang, J.; Zheng, X.; Tao, C.; Fang, G. Hydrogen peroxide-modified SnO2 as electron transport layer for perovskite solar cells with efficiency exceeding 22%. J. Power Sources 2021, 481, 229160. [Google Scholar] [CrossRef]
- Zeng, X.; Zhou, T.; Leng, C.; Zang, Z.; Wang, M.; Hu, W.; Tang, X.; Lu, S.; Fang, L.; Zhou, M. Performance improvement of perovskite solar cells by employing a CdSe quantum dot/PCBM composite as an electron transport layer. J. Mater. Chem. A 2017, 5, 17499–17505. [Google Scholar] [CrossRef]
- Dunlap-Shohl, W.A.; Younts, R.; Gautam, B.; Gundogdu, K.; Mitzi, D.B. Effects of Cd diffusion and doping in high-performance perovskite solar cells using CdS as electron transport layer. J. Phys. Chem. C 2016, 120, 16437–16445. [Google Scholar] [CrossRef]
- Ono, L.K.; Raga, S.R.; Remeika, M.; Winchester, A.J.; Gabe, A.; Qi, Y. Pinhole-free hole transport layers significantly improve the stability of MAPbI3-based perovskite solar cells under operating conditions. J. Mater. Chem. A 2015, 3, 15451–15456. [Google Scholar] [CrossRef] [Green Version]
- Elnaggar, M.M.; Frolova, L.A.; Gordeeva, A.M.; Ustinova, M.I.; Laurenzen, H.; Akkuratov, A.V.; Nikitenko, S.L.; Solov’eva, E.A.; Luchkin, S.Y.; Fedotov, Y.S.; et al. Improving stability of perovskite solar cells using fullerene-polymer composite electron transport layer. Synth. Met. 2022, 286, 117028. [Google Scholar] [CrossRef]
- Zheng, Y.; Kong, J.; Huang, D.; Shi, W.; McMillon-Brown, L.; Katz, H.E.; Yu, J.; Taylor, A.D. Spray coating of the PCBM electron transport layer significantly improves the efficiency of pin planar perovskite solar cells. Nanoscale 2018, 10, 11342–11348. [Google Scholar] [CrossRef] [PubMed]
- Bera, A.; Wu, K.; Sheikh, A.; Alarousu, E.; Mohammed, O.F.; Wu, T. Perovskite oxide SrTiO3 as an efficient electron transporter for hybrid perovskite solar cells. J. Phys. Chem. C 2014, 118, 28494–28501. [Google Scholar] [CrossRef]
- Shin, S.S.; Yeom, E.J.; Yang, W.S.; Hur, S.; Kim, M.G.; Im, J.; Seo, J.; Noh, J.H.; Seok, S.I. Colloidally prepared La-doped BaSnO3 electrodes for efficient, photostable perovskite solar cells. Science 2017, 356, 167–171. [Google Scholar] [CrossRef]
- Zhu, L.; Ye, J.; Zhang, X.; Zheng, H.; Liu, G.; Pan, X.; Dai, S. Performance enhancement of perovskite solar cells using a La-doped BaSnO3 electron transport layer. J. Mater. Chem. A 2017, 5, 3675–3682. [Google Scholar] [CrossRef]
- Shin, S.S.; Yang, W.S.; Noh, J.H.; Suk, J.H.; Jeon, N.J.; Park, J.H.; Kim, J.S.; Seong, W.M.; Seok, S.I. High-performance flexible perovskite solar cells exploiting Zn2SnO4 prepared in solution below 100 °C. Nat. Commun. 2015, 6, 7410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mali, S.S.; Shim, C.S.; Hong, C.K. Highly porous Zinc Stannate (Zn2 SnO4) nanofibers scaffold photoelectrodes for efficient methyl ammonium halide perovskite solar cells. Sci. Rep. 2015, 5, 11424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leijtens, T.; Eperon, G.E.; Noel, N.K.; Habisreutinger, S.N.; Petrozza, A.; Snaith, H.J. Stability of metal halide perovskite solar cells. Adv. Energy Mater. 2015, 5, 1500963. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, N.; Wei, P.; Cui, M.; Li, X.; Qin, C. Ultraviolet-ozone modification on TiO2 surface to promote both efficiency and stability of low-temperature planar perovskite solar cells. Chem. Eng. J. 2020, 393, 124731. [Google Scholar] [CrossRef]
- Qin, P.; Tanaka, S.; Ito, S.; Tetreault, N.; Manabe, K.; Nishino, H.; Nazeeruddin, M.K.; Grätzel, M. Inorganic hole conductor-based lead halide perovskite solar cells with 12.4% conversion efficiency. Nat. Commun. 2014, 5, 3834. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Niu, G.; Dong, Q.; Liu, J.; Li, N.; Li, J.; Wang, L. The role of interface between electron transport layer and perovskite in halogen migration and stabilizing perovskite solar cells with Cs4SnO4. J. Mater. Chem. A 2018, 6, 23797–23804. [Google Scholar] [CrossRef]
- Niu, G.; Li, W.; Meng, F.; Wang, L.; Dong, H.; Qiu, Y. Study on the stability of CH3NH3 PbI3 films and the effect of post-modification by aluminum oxide in all-solid-state hybrid solar cells. J. Mater. Chem. A 2014, 2, 705–710. [Google Scholar] [CrossRef]
- Berhe, T.A.; Su, W.-N.; Chen, C.-H.; Pan, C.-J.; Cheng, J.-H.; Chen, H.-M.; Tsai, M.-C.; Chen, L.-Y.; Dubale, A.A.; Hwang, B.-J. Organometal halide perovskite solar cells: Degradation and stability. Energy Environ. Sci. 2016, 9, 323–356. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, X.; You, J. SnO2: A wonderful electron transport layer for perovskite solar cells. Small 2018, 14, 1801154. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, Z.; Sun, Q.; Lee, E.-C. Morphology control of SnO2 layer by solvent engineering for efficient perovskite solar cells. Sol. Energy 2021, 214, 280–287. [Google Scholar] [CrossRef]
- Cao, J.-J.; Wang, K.-L.; Dong, C.; Li, X.-M.; Yang, W.-F.; Wang, Z.-K. Bottom-contact passivation for high-performance perovskite solar cells using TaCl5-doped SnO2 as electron-transporting layer. Org. Electron. 2021, 88, 105972. [Google Scholar] [CrossRef]
- Nozik, A.J.; Memming, R. Physical chemistry of semiconductor− liquid interfaces. J. Phys. Chem. 1996, 100, 13061–13078. [Google Scholar] [CrossRef]
- Rus, S.F.; Ward, T.Z.; Herklotz, A. Strain-induced optical band gap variation of SnO2 films. Thin Solid Films 2016, 615, 103–106. [Google Scholar] [CrossRef] [Green Version]
- Jarzebski, Z.; Marton, J. Physical properties of SnO2 materials: II. Electrical properties. J. Electrochem. Soc. 1976, 123, 299C. [Google Scholar] [CrossRef]
- Tibayan, E.B., Jr.; Muflikhun, M.A.; Al Rey, C.V.; Kumar, V.; Santos, G.N.C. Structures and UV resistance of Ag/SnO2 nanocomposite materials synthesized by horizontal vapor phase growth for coating applications. J. Mater. Res. Technol. 2020, 9, 4806–4816. [Google Scholar] [CrossRef]
- Ko, Y.; Kim, Y.; Lee, C.; Kim, T.; Kim, S.; Yun, Y.J.; Gwon, H.J.; Lee, N.H.; Jun, Y. Self-Aggregation-Controlled Rapid Chemical Bath Deposition of SnO2 Layers and Stable Dark Depolarization Process for Highly Efficient Planar Perovskite Solar Cells. ChemSusChem 2020, 13, 4051–4063. [Google Scholar] [CrossRef]
- Jiang, X.; Xiong, Y.; Zhang, Z.; Rong, Y.; Mei, A.; Tian, C.; Zhang, J.; Zhang, Y.; Jin, Y.; Han, H. Efficient hole-conductor-free printable mesoscopic perovskite solar cells based on SnO2 compact layer. Electrochim. Acta 2018, 263, 134–139. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, X.; Guo, J.; Li, X.; Weng, Z.; Liu, F.; Wu, L.; Ahmed, I.; Akram, A.; Javed, S. [(C8H17)4N]4 [SiW12O40](TASiW-12)-Modified SnO2 Electron Transport Layer for Efficient and Stable Perovskite Solar Cells. Sol. RRL 2020, 4, 2000406. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Z.; Mei, A.; Jiang, Y.; Hou, X.; Wang, Q.; Du, K.; Rong, Y.; Zhou, Y.; Xu, G. Improved performance of printable perovskite solar cells with bifunctional conjugated organic molecule. Adv. Mater. 2018, 30, 1705786. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, R.; Xia, X.; Steichen, P.; Liu, N.; Yang, J.; Chu, L.; Li, X.A. Room Temperature Processed Double Electron Transport Layers for Efficient Perovskite Solar Cells. Nanomaterials 2021, 11, 329. [Google Scholar] [CrossRef]
- Wang, W.-T.; Sharma, J.; Chen, J.-W.; Kao, C.-H.; Chen, S.-Y.; Chen, C.-H.; Feng, Y.-C.; Tai, Y. Nanoparticle-induced fast nucleation of pinhole-free PbI2 film for ambient-processed highly-efficient perovskite solar cell. Nano Energy 2018, 49, 109–116. [Google Scholar] [CrossRef]
- Mali, S.S.; Patil, J.V.; Hong, C.K. Simultaneous improved performance and thermal stability of planar metal ion incorporated CsPbI2Br all-inorganic perovskite solar cells based on MgZnO nanocrystalline electron transporting layer. Adv. Energy Mater. 2020, 10, 1902708. [Google Scholar] [CrossRef]
- Mali, S.S.; Patil, J.V.; Arandiyan, H.; Luque, R.; Hong, C.K. Stability of Unstable Perovskites: Recent Strategies for Making Stable Perovskite Solar Cells. ECS J. Solid State Sci. Technol. 2019, 8, Q111. [Google Scholar] [CrossRef]
- Wu, S.-H.; Lin, M.-Y.; Chang, S.-H.; Tu, W.-C.; Chu, C.-W.; Chang, Y.-C. A design based on a charge-transfer bilayer as an electron transport layer for improving the performance and stability in planar perovskite solar cells. J. Phys. Chem. C 2018, 122, 236–244. [Google Scholar] [CrossRef]
- Zhang, W.; Ren, Z.; Guo, Y.; He, X.; Li, X. Improved the long-term air stability of ZnO-based perovskite solar cells prepared under ambient conditions via surface modification of the electron transport layer using an ionic liquid. Electrochim. Acta 2018, 268, 539–545. [Google Scholar] [CrossRef]
- Tavakoli, M.M.; Tavakoli, R.; Yadav, P.; Kong, J. A graphene/ZnO electron transfer layer together with perovskite passivation enables highly efficient and stable perovskite solar cells. J. Mater. Chem. A 2019, 7, 679–686. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Lin, Z.; Guo, X.; Zhou, L.; Su, J.; Zhang, C.; Yang, Z.; Chang, J.; Liu, S.; Hao, Y. Low-temperature solution-processed ZnO electron transport layer for highly efficient and stable planar perovskite solar cells with efficiency over 20%. Sol. RRL 2019, 3, 1900096. [Google Scholar] [CrossRef]
- Zhang, W.; Xiong, J.; Jiang, L.; Wang, J.; Mei, T.; Wang, X.; Gu, H.; Daoud, W.A.; Li, J. Thermal stability-enhanced and high-efficiency planar perovskite solar cells with interface passivation. ACS Appl. Mater. Interfaces 2017, 9, 38467–38476. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wu, B.; Chen, R.; Wu, Y.; Hui, Y.; Mao, B.W.; Zheng, N. Efficient, hysteresis-free, and stable perovskite solar cells with ZnO as electron-transport layer: Effect of surface passivation. Adv. Mater. 2018, 30, 1705596. [Google Scholar] [CrossRef] [PubMed]
- Coutts, T.J.; Young, D.L.; Li, X.; Mulligan, W.; Wu, X. Search for improved transparent conducting oxides: A fundamental investigation of CdO, Cd2SnO4, and Zn2SnO4. J. Vac. Sci. Technol. A 2000, 18, 2646–2660. [Google Scholar] [CrossRef]
- Hu, L.; Sun, K.; Wang, M.; Chen, W.; Yang, B.; Fu, J.; Xiong, Z.; Li, X.; Tang, X.; Zang, Z. Inverted planar perovskite solar cells with a high fill factor and negligible hysteresis by the dual effect of NaCl-doped PEDOT: PSS. ACS Appl. Mater. Interfaces 2017, 9, 43902–43909. [Google Scholar] [CrossRef]
- Lim, J.W.; Wang, H.; Choi, C.H.; Quan, L.N.; Chung, K.; Park, W.-T.; Noh, Y.-Y.; Kim, D.H. Polyethylenimine ethoxylated interlayer-mediated ZnO interfacial engineering for high-performance and low-temperature processed flexible perovskite solar cells: A simple and viable route for one-step processed CH3NH3PbI3. J. Power Sources 2019, 438, 226956. [Google Scholar] [CrossRef]
- Kwon, S.-N.; Yu, J.-H.; Na, S.-I. A systematic approach to ZnO nanoparticle-assisted electron transport bilayer for high efficiency and stable perovskite solar cells. J. Alloys Compd. 2019, 801, 277–284. [Google Scholar] [CrossRef]
- Jiang, T.; Fu, W. Improved performance and stability of perovskite solar cells with bilayer electron-transporting layers. RSC Adv. 2018, 8, 5897–5901. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Li, S.; Li, X.; Wang, R.; Li, X. Green low-temperature-solution-processed in situ HI modified TiO2/SnO2 bilayer for efficient and stable planar perovskite solar cells build at ambient air conditions. Electrochim. Acta 2019, 326, 134924. [Google Scholar] [CrossRef]
- Hou, Y.; Du, X.; Scheiner, S.; McMeekin, D.P.; Wang, Z.; Li, N.; Killian, M.S.; Chen, H.; Richter, M.; Levchuk, I. A generic interface to reduce the efficiency-stability-cost gap of perovskite solar cells. Science 2017, 358, 1192–1197. [Google Scholar] [CrossRef] [Green Version]
- Chavan, R.D.; Yadav, P.; Nimbalkar, A.; Bhoite, S.P.; Bhosale, P.N.; Hong, C.K. Ruthenium doped mesoporous titanium dioxide for highly efficient, hysteresis-free and stable perovskite solar cells. Sol. Energy 2019, 186, 156–165. [Google Scholar] [CrossRef]
- Zhao, X.; Shen, H.; Zhang, Y.; Li, X.; Zhao, X.; Tai, M.; Li, J.; Li, J.; Li, X.; Lin, H. Aluminum-doped zinc oxide as highly stable electron collection layer for perovskite solar cells. ACS Appl. Mater. Interfaces 2016, 8, 7826–7833. [Google Scholar] [CrossRef] [PubMed]
- Spalla, M.; Planes, E.; Perrin, L.; Matheron, M.; Berson, S.; Flandin, L. Alternative electron transport layer based on Al-Doped ZnO and SnO2 for perovskite solar cells: Impact on microstructure and stability. ACS Appl. Energy Mater. 2019, 2, 7183–7195. [Google Scholar] [CrossRef]
- Dong, Q.; Ho, C.H.Y.; Yu, H.; Salehi, A.; So, F. Defect passivation by fullerene derivative in perovskite solar cells with aluminum-doped zinc oxide as electron transporting layer. Chem. Mater. 2019, 31, 6833–6840. [Google Scholar] [CrossRef]
- Mahmood, K.; Khalid, A.; Ahmad, S.W.; Mehran, M.T. Indium-doped ZnO mesoporous nanofibers as efficient electron transporting materials for perovskite solar cells. Surf. Coat. Technol. 2018, 352, 231–237. [Google Scholar] [CrossRef]
- Aydin, E.; De Bastiani, M.; De Wolf, S. Defect and contact passivation for perovskite solar cells. Adv. Mater. 2019, 31, 1900428. [Google Scholar] [CrossRef]
- Hermes, I.M.; Hou, Y.; Bergmann, V.W.; Brabec, C.J.; Weber, S.A. The interplay of contact layers: How the electron transport layer influences interfacial recombination and hole extraction in perovskite solar cells. J. Phys. Chem. Lett. 2018, 9, 6249–6256. [Google Scholar] [CrossRef]
- Zuo, L.; Chen, Q.; De Marco, N.; Hsieh, Y.-T.; Chen, H.; Sun, P.; Chang, S.-Y.; Zhao, H.; Dong, S.; Yang, Y. Tailoring the interfacial chemical interaction for high-efficiency perovskite solar cells. Nano Lett. 2017, 17, 269–275. [Google Scholar] [CrossRef]
- Wang, Q.; Dong, Q.; Li, T.; Gruverman, A.; Huang, J. Thin insulating tunneling contacts for efficient and water-resistant perovskite solar cells. Adv. Mater. 2016, 28, 6734–6739. [Google Scholar] [CrossRef]
- Qiu, L.; Ono, L.K.; Jiang, Y.; Leyden, M.R.; Raga, S.R.; Wang, S.; Qi, Y. Engineering interface structure to improve efficiency and stability of organometal halide perovskite solar cells. J. Phys. Chem. B 2017, 122, 511–520. [Google Scholar] [CrossRef] [Green Version]
- Duan, J.; Liu, Y.; Chen, X.; Huang, S.; Ou-Yang, W.; Zhu, G.; Zhang, S.; Sun, Z. Efficient and stable mesoporous perovskite solar cells using p-type poly (9-vinylcarbazole) modified the interface of perovskite/mesoporous TiO2 layers. Org. Electron. 2020, 82, 105737. [Google Scholar] [CrossRef]
- Li, W.; Wang, D.; Hou, W.; Li, R.; Sun, W.; Wu, J.; Lan, Z. High-Efficiency, Low-Hysteresis Planar Perovskite Solar Cells by Inserting the NaBr Interlayer. ACS Appl. Mater. Interfaces 2021, 13, 20251–20259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, H. Reduced energy loss enabled by thiophene-based interlayers for high performance and stable perovskite solar cells. J. Mater. Chem. A 2021, 9, 4138–4149. [Google Scholar] [CrossRef]
- Lei, T.; Xu, J.; Chen, J.; Zhu, X.; Jiao, B.; Wu, Z. An ultra-thin inorganic interlayer strategy for achieving efficient inverted planar perovskite solar cells and modules with high fill factor. Org. Electron. 2020, 87, 105937. [Google Scholar] [CrossRef]
- Fakharuddin, A.; Schmidt-Mende, L.; Garcia-Belmonte, G.; Jose, R.; Mora-Sero, I. Interfaces in perovskite solar cells. Adv. Energy Mater. 2017, 7, 1700623. [Google Scholar] [CrossRef]
- Karavioti, A.; Vitoratos, E.; Stathatos, E. Improved performance and stability of hole-conductor-free mesoporous perovskite solar cell with new amino-acid iodide cations. J. Mater. Sci. Mater. Electron. 2020, 31, 6109–6117. [Google Scholar] [CrossRef]
- Ono, L.K.; Qi, Y. Research progress on organic–inorganic halide perovskite materials and solar cells. J. Phys. D Appl. Phys. 2018, 51, 093001. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef]
- Green, M.A.; Hishikawa, Y.; Dunlop, E.D.; Levi, D.H.; Hohl-Ebinger, J.; Ho-Baillie, A.W. Solar cell efficiency tables (version 52). Prog. Photovolt. Res. Appl. 2018, 26, 427–436. [Google Scholar] [CrossRef]
- Dong, W.; Xiong, S.; Yang, J.; Qiao, W.; Zeng, Q.; Wang, X.; Yao, Y.; Bao, Q. Black phosphorus doped Poly (triarylamine) as hole transport layer for highly efficient perovskite solar cells. Org. Electron. 2021, 89, 106052. [Google Scholar] [CrossRef]
- Xu, L.; Wang, H.; Feng, X.; Zhou, Y.; Chen, Y.; Chen, R.; Huang, W. Vanadium Oxide-modified Triphenylamine-Based Hole-Transport Layer for Highly Reproducible and Efficient Inverted Perovskite Solar Cells. Adv. Photonics Res. 2021, 2, 2000132. [Google Scholar] [CrossRef]
- de Sousa, A.L.M.D.; Dos Santos, W.M.; de Souza, M.L.; Silva, L.C.P.B.B.; Yun, A.E.H.K.; Aguilera, C.S.B.; de França Chagas, B.; Rolim, L.A.; da Silva, R.M.F.; Neto, P.J.R. Layered Double Hydroxides as Promising Excipients for Drug Delivery Purposes. Eur. J. Pharm. Sci. 2021, 165, 105922. [Google Scholar] [CrossRef] [PubMed]
- Hawash, Z.; Ono, L.K.; Raga, S.R.; Lee, M.V.; Qi, Y. Air-exposure induced dopant redistribution and energy level shifts in spin-coated spiro-MeOTAD films. Chem. Mater. 2015, 27, 562–569. [Google Scholar] [CrossRef]
- Ghasemi, M.; Zhang, L.; Yun, J.H.; Hao, M.; He, D.; Chen, P.; Bai, Y.; Lin, T.; Xiao, M.; Du, A. Dual-Ion-Diffusion Induced Degradation in Lead-Free Cs2AgBiBr6 Double Perovskite Solar Cells. Adv. Funct. Mater. 2020, 30, 2002342. [Google Scholar] [CrossRef]
- Pham, N.D.; Shang, J.; Yang, Y.; Hoang, M.T.; Tiong, V.T.; Wang, X.; Fan, L.; Chen, P.; Kou, L.; Wang, L. Alkaline-earth bis (trifluoromethanesulfonimide) additives for efficient and stable perovskite solar cells. Nano Energy 2020, 69, 104412. [Google Scholar] [CrossRef]
- Zhou, X.; Qiu, L.; Fan, R.; Wang, A.; Ye, H.; Tian, C.; Hao, S.; Yang, Y. Metal–Organic Framework-Derived N-Rich Porous Carbon as an Auxiliary Additive of Hole Transport Layers for Highly Efficient and Long-Term Stable Perovskite Solar Cells. Sol. RRL 2020, 4, 1900380. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Y.; Zhang, X.; Zeng, P.; Li, F.; Wang, B.; Yang, Q.; Liu, M. Inhibited aggregation of lithium salt in spiro-OMeTAD toward highly efficient perovskite solar cells. Nano Energy 2020, 70, 104483. [Google Scholar] [CrossRef]
- Caliò, L.; Salado, M.; Kazim, S.; Ahmad, S. A generic route of hydrophobic doping in hole transporting material to increase longevity of perovskite solar cells. Joule 2018, 2, 1800–1815. [Google Scholar] [CrossRef] [Green Version]
- Pellaroque, A.; Noel, N.K.; Habisreutinger, S.N.; Zhang, Y.; Barlow, S.; Marder, S.R.; Snaith, H.J. Efficient and stable perovskite solar cells using molybdenum tris (dithiolene) s as p-dopants for spiro-OMeTAD. ACS Energy Lett. 2017, 2, 2044–2050. [Google Scholar] [CrossRef]
- Li, Z.; Tinkham, J.; Schulz, P.; Yang, M.; Kim, D.H.; Berry, J.; Sellinger, A.; Zhu, K. Acid additives enhancing the conductivity of Spiro-OMeTAD toward high-efficiency and hysteresis-less planar perovskite solar cells. Adv. Energy Mater. 2017, 7, 1601451. [Google Scholar] [CrossRef]
- Khairulaman, F.L.; Yap, C.C.; Jumali, M.H.H. Improved performance of inverted type organic solar cell using copper iodide-doped P3HT: PCBM as active layer for low light application. Mater. Lett. 2021, 283, 128827. [Google Scholar] [CrossRef]
- Zhao, S.; Zhuang, J.; Liu, X.; Zhang, H.; Zheng, R.; Peng, X.; Gong, X.; Guo, H.; Wang, H.; Li, H. F4-TCNQ doped strategy of nickel oxide as high-efficient hole transporting materials for invert perovskite solar cell. Mater. Sci. Semicond. Proc. 2021, 121, 105458. [Google Scholar] [CrossRef]
- Luo, J.; Jia, C.; Wan, Z.; Han, F.; Zhao, B.; Wang, R. The novel dopant for hole-transporting material opens a new processing route to efficiently reduce hysteresis and improve stability of planar perovskite solar cells. J. Power Sources 2017, 342, 886–895. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, J.; Hua, Y.; Liu, P.; Wang, L.; Ruan, C.; Li, Y.; Boschloo, G.; Johansson, E.M.; Kloo, L. Tailor-making low-cost spiro [fluorene-9, 9′-xanthene]-based 3D oligomers for perovskite solar cells. Chem 2017, 2, 676–687. [Google Scholar] [CrossRef] [Green Version]
- Christians, J.A.; Schulz, P.; Tinkham, J.S.; Schloemer, T.H.; Harvey, S.P.; de Villers, B.J.T.; Sellinger, A.; Berry, J.J.; Luther, J.M. Tailored interfaces of unencapsulated perovskite solar cells for >1000 hour operational stability. Nat. Energy 2018, 3, 68–74. [Google Scholar] [CrossRef]
- Ebadi, M.; Bullo, S.; Buskara, K.; Hussein, M.Z.; Fakurazi, S.; Pastorin, G. Release of a liver anticancer drug, sorafenib from its PVA/LDH-and PEG/LDH-coated iron oxide nanoparticles for drug delivery applications. Sci. Rep. 2020, 10, 21521. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Lim, J.; Yun, H.-J.; Kim, Y.-H.; Park, T. A diketopyrrolopyrrole-containing hole transporting conjugated polymer for use in efficient stable organic–inorganic hybrid solar cells based on a perovskite. Energy Environ. Sci. 2014, 7, 1454–1460. [Google Scholar] [CrossRef]
- Arora, N.; Dar, M.I.; Hinderhofer, A.; Pellet, N.; Schreiber, F.; Zakeeruddin, S.M.; Grätzel, M. Perovskite solar cells with CuSCN hole extraction layers yield stabilized efficiencies greater than 20%. Science 2017, 358, 768–771. [Google Scholar] [CrossRef] [Green Version]
- Christians, J.A.; Fung, R.C.; Kamat, P.V. An inorganic hole conductor for organo-lead halide perovskite solar cells. Improved hole conductivity with copper iodide. J. Am. Chem. Soc. 2014, 136, 758–764. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, L.; Li, P.; Fan, B.; Qiu, Y. A modeled perovskite solar cell structure with a Cu2O hole-transporting layer enabling over 20% efficiency by low-cost low-temperature processing. J. Phys. Chem. Solids 2019, 124, 205–211. [Google Scholar] [CrossRef]
- Chen, W.; Wu, Y.; Yue, Y.; Liu, J.; Zhang, W.; Yang, X.; Chen, H.; Bi, E.; Ashraful, I.; Grätzel, M. Efficient and stable large-area perovskite solar cells with inorganic charge extraction layers. Science 2015, 350, 944–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etgar, L.; Gao, P.; Xue, Z.; Peng, Q.; Chandiran, A.K.; Liu, B.; Nazeeruddin, M.K.; Grätzel, M. Mesoscopic CH3NH3PbI3/TiO2 heterojunction solar cells. J. Am. Chem. Soc. 2012, 134, 17396–17399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Shi, Y.; Dong, Q.; Zhang, H.; Xing, Y.; Wang, K.; Du, Y.; Ma, T. Hole-conductor-free, metal-electrode-free TiO2/CH3NH3PbI3 heterojunction solar cells based on a low-temperature carbon electrode. J. Phys. Chem. Lett. 2014, 5, 3241–3246. [Google Scholar] [CrossRef] [PubMed]
- Mei, A.; Li, X.; Liu, L.; Ku, Z.; Liu, T.; Rong, Y.; Xu, M.; Hu, M.; Chen, J.; Yang, Y. A hole-conductor–free, fully printable mesoscopic perovskite solar cell with high stability. Science 2014, 345, 295–298. [Google Scholar] [CrossRef]
- Priyadarshi, A.; Haur, L.J.; Murray, P.; Fu, D.; Kulkarni, S.; Xing, G.; Sum, T.C.; Mathews, N.; Mhaisalkar, S.G. A large area (70 cm2) monolithic perovskite solar module with a high efficiency and stability. Energy Environ. Sci. 2016, 9, 3687–3692. [Google Scholar] [CrossRef]
- Chen, J.; Rong, Y.; Mei, A.; Xiong, Y.; Liu, T.; Sheng, Y.; Jiang, P.; Hong, L.; Guan, Y.; Zhu, X. Hole-Conductor-Free Fully Printable Mesoscopic Solar Cell with Mixed-Anion Perovskite CH3NH3PbI(3−x)(BF4)x. Adv. Energy Mater. 2016, 6, 1502009. [Google Scholar] [CrossRef]
- Xu, M.; Rong, Y.; Ku, Z.; Mei, A.; Liu, T.; Zhang, L.; Li, X.; Han, H. Highly ordered mesoporous carbon for mesoscopic CH3NH3PbI3/TiO2 heterojunction solar cell. J. Mater. Chem. A 2014, 2, 8607–8611. [Google Scholar] [CrossRef]
- Duan, M.; Rong, Y.; Mei, A.; Hu, Y.; Sheng, Y.; Guan, Y.; Han, H. Efficient hole-conductor-free, fully printable mesoscopic perovskite solar cells with carbon electrode based on ultrathin graphite. Carbon 2017, 120, 71–76. [Google Scholar] [CrossRef]
- Hou, X.; Hu, Y.; Liu, H.; Mei, A.; Li, X.; Duan, M.; Zhang, G.; Rong, Y.; Han, H. Effect of guanidinium on mesoscopic perovskite solar cells. J. Mater. Chem. A 2017, 5, 73–78. [Google Scholar] [CrossRef]
- Hu, Y.; Si, S.; Mei, A.; Rong, Y.; Liu, H.; Li, X.; Han, H. Stable large-area (10 × 10 cm2) printable mesoscopic perovskite module exceeding 10% efficiency. Sol. RRL 2017, 1, 1600019. [Google Scholar] [CrossRef]
- Rong, Y.; Hou, X.; Hu, Y.; Mei, A.; Liu, L.; Wang, P.; Han, H. Synergy of ammonium chloride and moisture on perovskite crystallization for efficient printable mesoscopic solar cells. Nat. Commun. 2017, 8, 14555. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, K.; Sarwar, S.; Mehran, M.T. Current status of electron transport layers in perovskite solar cells: Materials and properties. RSC Adv. 2017, 7, 17044–17062. [Google Scholar] [CrossRef] [Green Version]
- Domanski, K.; Correa-Baena, J.-P.; Mine, N.; Nazeeruddin, M.K.; Abate, A.; Saliba, M.; Tress, W.; Hagfeldt, A.; Grätzel, M. Not All That Glitters Is Gold: Metal-Migration-Induced Degradation in Perovskite Solar Cells. ACS Nano 2016, 10, 6306–6314. [Google Scholar] [CrossRef] [PubMed]
- Tisdale, J.T.; Muckley, E.; Ahmadi, M.; Smith, T.; Seal, C.; Lukosi, E.; Ivanov, I.N.; Hu, B. Dynamic impact of electrode materials on interface of single-crystalline methylammonium lead bromide perovskite. Adv. Mater. Interfaces 2018, 5, 1800476. [Google Scholar] [CrossRef]
- Zhao, J.; Zheng, X.; Deng, Y.; Li, T.; Shao, Y.; Gruverman, A.; Shield, J.; Huang, J. Is Cu a stable electrode material in hybrid perovskite solar cells for a 30-year lifetime? Energy Environ. Sci. 2016, 9, 3650–3656. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, Y.; Shen, H.; Luo, Q.; Zhao, X.; Li, J.; Lin, H. Working from Both Sides: Composite Metallic Semitransparent Top Electrode for High Performance Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 4523–4531. [Google Scholar] [CrossRef]
- Docampo, P.; Ball, J.M.; Darwich, M.; Eperon, G.E.; Snaith, H.J. Efficient organometal trihalide perovskite planar-heterojunction solar cells on flexible polymer substrates. Nat. Commun. 2013, 4, 2761. [Google Scholar] [CrossRef] [Green Version]
- Kaltenbrunner, M.; Adam, G.; Głowacki, E.D.; Drack, M.; Schwödiauer, R.; Leonat, L.; Apaydin, D.H.; Groiss, H.; Scharber, M.C.; White, M.S.; et al. Flexible high power-per-weight perovskite solar cells with chromium oxide—Metal contacts for improved stability in air. Nat. Mater. 2015, 14, 1032–1039. [Google Scholar] [CrossRef]
- Lai, W.-C.; Lin, K.-W.; Guo, T.-F.; Chen, P.; Liao, Y.-Y. Efficient CH3NH3PbI3 perovskite/fullerene planar heterojunction hybrid solar cells with oxidized Ni/Au/Cu transparent electrode. Appl. Phys. Lett. 2018, 112, 071103. [Google Scholar] [CrossRef]
- Peng, J.; Wu, Y.; Ye, W.; Jacobs, D.A.; Shen, H.; Fu, X.; Wan, Y.; Wu, N.; Barugkin, C.; Nguyen, H.T. Interface passivation using ultrathin polymer–fullerene films for high-efficiency perovskite solar cells with negligible hysteresis. Energy Environ. Sci. 2017, 10, 1792–1800. [Google Scholar] [CrossRef] [Green Version]
- Moia, D.; Gelmetti, I.; Calado, P.; Fisher, W.; Stringer, M.; Game, O.; Hu, Y.; Docampo, P.; Lidzey, D.; Palomares, E. Ionic-to-electronic current amplification in hybrid perovskite solar cells: Ionically gated transistor-interface circuit model explains hysteresis and impedance of mixed conducting devices. Energy Environ. Sci. 2019, 12, 1296–1308. [Google Scholar] [CrossRef]
- Han, F.; Hao, G.; Wan, Z.; Luo, J.; Xia, J.; Jia, C. Bifunctional electron transporting layer/perovskite interface linker for highly efficient perovskite solar cells. Electrochim. Acta 2019, 296, 75–81. [Google Scholar] [CrossRef]
- Zhong, Y.; Hufnagel, M.; Thelakkat, M.; Li, C.; Huettner, S. Role of PCBM in the suppression of hysteresis in perovskite solar cells. Adv. Funct. Mater. 2020, 30, 1908920. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Liu, C.; Ding, Y.; Arain, Z.; Wang, S.; Liu, X.; Hayat, T.; Alsaedi, A.; Dai, S. Eliminating charge accumulation via interfacial dipole for efficient and stable perovskite solar cells. ACS Appl. Mater. Interfaces 2019, 11, 34964–34972. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhang, D.; Bu, S.; Peng, R.; Wei, Q.; Ge, Z. Synergistic Interface Energy Band Alignment Optimization and Defect Passivation toward Efficient and Simple-Structured Perovskite Solar Cell. Adv. Sci. 2020, 7, 1902656. [Google Scholar] [CrossRef]
- Lee, J.-W.; Kim, S.-G.; Yang, J.-M.; Yang, Y.; Park, N.-G. Verification and mitigation of ion migration in perovskite solar cells. APL Mater. 2019, 7, 041111. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Z.; Yuan, Y.; Shao, Y.; Wang, Q.; Dong, Q.; Bi, C.; Sharma, P.; Gruverman, A.; Huang, J. Giant switchable photovoltaic effect in organometal trihalide perovskite devices. Nat. Mater. 2015, 14, 193–198. [Google Scholar] [CrossRef]
- Calado, P.; Telford, A.M.; Bryant, D.; Li, X.; Nelson, J.; O’Regan, B.C.; Barnes, P.R. Evidence for ion migration in hybrid perovskite solar cells with minimal hysteresis. Nat. Commun. 2016, 7, 13831. [Google Scholar] [CrossRef]
- Paul, G.; Chatterjee, S.; Bhunia, H.; Pal, A.J. Self-Doping in hybrid halide perovskites via precursor stoichiometry: To probe the type of conductivity through scanning tunneling spectroscopy. J. Phys. Chem. C 2018, 122, 20194–20199. [Google Scholar] [CrossRef]
- Xie, H.; Liu, X.; Lyu, L.; Niu, D.; Wang, Q.; Huang, J.; Gao, Y. Effects of precursor ratios and annealing on electronic structure and surface composition of CH3NH3PbI3 perovskite films. J. Phys. Chem. C 2016, 120, 215–220. [Google Scholar] [CrossRef]
- Wang, Q.; Shao, Y.; Xie, H.; Lyu, L.; Liu, X.; Gao, Y.; Huang, J. Qualifying composition dependent p and n self-doping in CH3NH3PbI3. Appl. Phys. Lett. 2014, 105, 163508. [Google Scholar] [CrossRef] [Green Version]
- Abdi-Jalebi, M.; Dar, M.I.; Sadhanala, A.; Senanayak, S.P.; Grätzel, M.; Friend, R.H. Monovalent cation doping of CH3NH3PbI3 for efficient perovskite solar cells. J. Vis. Exp. JoVE 2017, 121, e55307. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.; Lin, Z.; Zhu, H.; Isikgor, F.H.; Xu, Q.-H.; Zhang, C.; Hao, Y.; Ouyang, J. Enhancing the photovoltaic performance of planar heterojunction perovskite solar cells by doping the perovskite layer with alkali metal ions. J. Mater. Chem. A 2016, 4, 16546–16552. [Google Scholar] [CrossRef]
- Chen, C.; Song, Z.; Xiao, C.; Zhao, D.; Shrestha, N.; Li, C.; Yang, G.; Yao, F.; Zheng, X.; Ellingson, R.J. Achieving a high open-circuit voltage in inverted wide-bandgap perovskite solar cells with a graded perovskite homojunction. Nano Energy 2019, 61, 141–147. [Google Scholar] [CrossRef]
- Okamoto, Y.; Sumiya, M.; Suzuki, Y. Perovskite Solar Cells with >19% Efficiency Achieved by an Advanced Three-Step Method Using Additional HC(NH2)2I–NaI Spin-Coating. ACS Appl. Energy Mater. 2019, 2, 1823–1831. [Google Scholar] [CrossRef]
- Qiao, H.W.; Yang, S.; Wang, Y.; Chen, X.; Wen, T.Y.; Tang, L.J.; Cheng, Q.; Hou, Y.; Zhao, H.; Yang, H.G. A gradient heterostructure based on tolerance factor in high-performance perovskite solar cells with 0.84 fill factor. Adv. Mater. 2019, 31, 1804217. [Google Scholar] [CrossRef]
- Jiang, Q.; Ni, Z.; Xu, G.; Lin, Y.; Rudd, P.N.; Xue, R.; Li, Y.; Li, Y.; Gao, Y.; Huang, J. Interfacial molecular doping of metal halide perovskites for highly efficient solar cells. Adv. Mater. 2020, 32, 2001581. [Google Scholar] [CrossRef]
- Zhang, W.; Xiong, J.; Li, J.; Daoud, W.A. Guanidinium induced phase separated perovskite layer for efficient and highly stable solar cells. J. Mater. Chem. A 2019, 7, 9486–9496. [Google Scholar] [CrossRef]
- Pham, N.D.; Zhang, C.; Tiong, V.T.; Zhang, S.; Will, G.; Bou, A.; Bisquert, J.; Shaw, P.E.; Du, A.; Wilson, G.J. Tailoring crystal structure of FA0.83Cs0.17PbI3 perovskite through guanidinium doping for enhanced performance and tunable hysteresis of planar perovskite solar cells. Adv. Funct. Mater. 2019, 29, 1806479. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.; Xiao, J.; Lin, Z.; Zhu, H.; Xu, Q.-H.; Zeng, K.; Hao, Y.; Ouyang, J. Elucidating the charge carrier transport and extraction in planar heterojunction perovskite solar cells by Kelvin probe force microscopy. J. Mater. Chem. A 2016, 4, 17464–17472. [Google Scholar] [CrossRef]
- Wang, C.; Li, C.; Wang, G.; Wang, C.; Ma, P.; Huang, L.; Wen, S.; Guo, W.; Shen, L.; Ruan, S. Employing Pentacene To Balance the Charge Transport in Inverted Organic Solar Cells. J. Phys. Chem. C 2018, 122, 17110–17117. [Google Scholar] [CrossRef]
- Song, J.; Zhang, W.; Wang, D.; Deng, K.; Wu, J.; Lan, Z. Colloidal synthesis of Y-doped SnO2 nanocrystals for efficient and slight hysteresis planar perovskite solar cells. Sol. Energy 2019, 185, 508–515. [Google Scholar] [CrossRef]
- Kim, D.H.; Heo, J.H.; Im, S.H. Hysteresis-less CsPbI2Br mesoscopic perovskite solar cells with a high open-circuit voltage exceeding 1.3 V and 14.86% of power conversion efficiency. ACS Appl. Mater. Interfaces 2019, 11, 19123–19131. [Google Scholar] [CrossRef]
- Mahmood, K.; Swain, B.S.; Amassian, A. Core–shell heterostructured metal oxide arrays enable superior light-harvesting and hysteresis-free mesoscopic perovskite solar cells. Nanoscale 2015, 7, 12812–12819. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Xu, L.; Li, C.; Zhang, B.; Wu, W. Needle coke: A predominant carbon black alternative for printable triple mesoscopic perovskite solar cells. Carbon 2019, 153, 602–608. [Google Scholar] [CrossRef]
- Heo, J.H.; Han, H.J.; Kim, D.; Ahn, T.K.; Im, S.H. Hysteresis-less inverted CH3NH3PbI3 planar perovskite hybrid solar cells with 18.1% power conversion efficiency. Energy Environ. Sci. 2015, 8, 1602–1608. [Google Scholar] [CrossRef]
- Yin, X.; Que, M.; Xing, Y.; Que, W. High efficiency hysteresis-less inverted planar heterojunction perovskite solar cells with a solution-derived NiOx hole contact layer. J. Mater. Chem. A 2015, 3, 24495–24503. [Google Scholar] [CrossRef]
- Forgács, D.; Sessolo, M.; Bolink, H.J. Lead acetate precursor based pin perovskite solar cells with enhanced reproducibility and low hysteresis. J. Mater. Chem. A 2015, 3, 14121–14125. [Google Scholar] [CrossRef]
- Liang, P.W.; Chueh, C.C.; Williams, S.T.; Jen, A.K.Y. Roles of fullerene-based interlayers in enhancing the performance of organometal perovskite thin-film solar cells. Adv. Energy Mater. 2015, 5, 1402321. [Google Scholar] [CrossRef]
- Yao, K.; Li, F.; He, Q.; Wang, X.; Jiang, Y.; Huang, H.; Jen, A.K.-Y. A copper-doped nickel oxide bilayer for enhancing efficiency and stability of hysteresis-free inverted mesoporous perovskite solar cells. Nano Energy 2017, 40, 155–162. [Google Scholar] [CrossRef]
- Syed, A.A.; Poon, C.Y.; Li, H.W.; Zhu, F. A sodium citrate-modified-PEDOT: PSS hole transporting layer for performance enhancement in inverted planar perovskite solar cells. J. Master. Chem. C 2019, 7, 5260–5266. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.-G.; Chiang, C.-H.; Tseng, Z.-L.; Nazeeruddin, M.K.; Hagfeldt, A.; Grätzel, M. High efficiency stable inverted perovskite solar cells without current hysteresis. Energy Environ. Sci. 2015, 8, 2725–2733. [Google Scholar] [CrossRef]
- Kim, H.-S.; Lee, C.-R.; Im, J.-H.; Lee, K.-B.; Moehl, T.; Marchioro, A.; Moon, S.-J.; Humphry-Baker, R.; Yum, J.-H.; Moser, J.E.; et al. Lead Iodide Perovskite Sensitized All-Solid-State Submicron Thin Film Mesoscopic Solar Cell with Efficiency Exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Chen, Q.; Li, G.; Luo, S.; Song, T.; Duan, H.-S.; Hong, Z.; You, J.; Liu, Y.; Yang, Y. Interface Engineering of Highly Efficient Perovskite Solar Cells. Science 2014, 345, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Fr, S.; Ad, B.; Ba, E.; Gj, J. Iodine Alters Gene Expression in the MCF7 Breast Cancer Cell Line: Evidence for an Anti-Estrogen Effect of Iodine. Int. J. Med. Sci. 2008, 5, 189–196. [Google Scholar]
- Guarnera, S.; Abate, A.; Zhang, W.; Foster, J.M.; Richardson, G.; Petrozza, A.; Snaith, H.J. Improving the Long-Term Stability of Perovskite Solar Cells with a Porous Al2O3 Buffer Layer. J. Phys. Chem. Lett. 2015, 6, 432–437. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-W.; Kim, D.-H.; Kim, H.-S.; Seo, S.-W.; Cho, S.M.; Park, N.-G. Formamidinium and Cesium Hybridization for Photo- and Moisture-Stable Perovskite Solar Cell. Adv. Energy Mater. 2015, 5, 1501310. [Google Scholar] [CrossRef]
- Ramos, F.J.; Cortés, D.; Aguirre, A.; Castaño, F.J.; Ahmad, S. Fabrication and Encapsulation of Perovskites Sensitized Solid State Solar Cells. In Proceedings of the 2014 IEEE 40th Photovoltaic Specialist Conference (PVSC), Denver, CO, USA, 8–13 June 2014; pp. 2584–2587. [Google Scholar]
- Han, Y.; Meyer, S.; Dkhissi, Y.; Weber, K.; Pringle, J.M.; Bach, U.; Spiccia, L.; Cheng, Y.-B. Degradation Observations of Encapsulated Planar CH3NH3PbI3 Perovskite Solar Cells at High Temperatures and Humidity. J. Mater. Chem. A 2015, 3, 8139–8147. [Google Scholar] [CrossRef]
- Jørgensen, M.; Norrman, K.; Gevorgyan, S.A.; Tromholt, T.; Andreasen, B.; Krebs, F.C. Stability of Polymer Solar Cells. Adv. Mater. 2012, 24, 580–612. [Google Scholar] [CrossRef]
- Krebs, F.C. Encapsulation of Polymer Photovoltaic Prototypes. Sol. Energy Mater. Sol. Cells 2006, 90, 3633–3643. [Google Scholar] [CrossRef]
- Lewis, J. Material Challenge for Flexible Organic Devices. Mater. Today 2006, 9, 38–45. [Google Scholar] [CrossRef]
- Schuller, S.; Schilinsky, P.; Hauch, J.; Brabec, C.J. Determination of the Degradation Constant of Bulk Heterojunction Solar Cells by Accelerated Lifetime Measurements. Appl. Phys. A 2004, 79, 37–40. [Google Scholar] [CrossRef]
- Jørgensen, M.; Norrman, K.; Krebs, F.C. Stability/Degradation of Polymer Solar Cells. Sol. Energy Mater. Sol. Cells 2008, 92, 686–714. [Google Scholar] [CrossRef]
- Krebs, F.C.; Spanggaard, H. Significant Improvement of Polymer Solar Cell Stability. Chem. Mater. 2005, 17, 5235–5237. [Google Scholar] [CrossRef]
- Krebs, F.C.; Gevorgyan, S.A.; Alstrup, J. A Roll-to-Roll Process to Flexible Polymer Solar Cells: Model Studies, Manufacture and Operational Stability Studies. J. Mater. Chem. 2009, 19, 5442–5451. [Google Scholar] [CrossRef]
- Hwang, I.; Jeong, I.; Lee, J.; Ko, M.J.; Yong, K. Enhancing Stability of Perovskite Solar Cells to Moisture by the Facile Hydrophobic Passivation. ACS Appl. Mater. Interfaces 2015, 7, 17330–17336. [Google Scholar] [CrossRef]
- Idígoras, J.; Aparicio, F.J.; Contreras-Bernal, L.; Ramos-Terrón, S.; Alcaire, M.; Sánchez-Valencia, J.R.; Borras, A.; Barranco, Á.; Anta, J.A. Enhancing Moisture and Water Resistance in Perovskite Solar Cells by Encapsulation with Ultrathin Plasma Polymers. ACS Appl. Mater. Interfaces 2018, 10, 11587–11594. [Google Scholar] [CrossRef]
- Jin, J.; Chen, S.; Zhang, J. Investigation of UV Aging Influences on the Crystallization of Ethylene-Vinyl Acetate Copolymer via Successive Self-Nucleation and Annealing Treatment. J. Polym. Res. 2010, 17, 827–836. [Google Scholar] [CrossRef]
- Cuddihy, E.; Coulbert, C.; Gupta, A.; Liang, R. Electricity from Photovoltaic Solar Cells: Flat-Plate Solar Array Project Final Report. Volume VII: Module Encapsulation. Available online: https://resolver.caltech.edu/JPLpub86-31-volumeVII (accessed on 4 January 2022).
- Chen, Z.; Wang, H.; Wang, X.; Chen, P.; Liu, Y.; Zhao, H.; Zhao, Y.; Duan, Y. Low-Temperature Remote Plasma Enhanced Atomic Layer Deposition of ZrO2/Zircone Nanolaminate Film for Efficient Encapsulation of Flexible Organic Light-Emitting Diodes. Sci. Rep. 2017, 7, 40061. [Google Scholar] [CrossRef] [Green Version]
- Heuer, H.; Wenzel, C.; Herrmann, D.; Hübner, R.; Zhang, Z.L.; Bartha, J.W. Thin Tantalum–Silicon–Oxygen/Tantalum–Silicon–Nitrogen Films as High-Efficiency Humidity Diffusion Barriers for Solar Cell Encapsulation. Thin Solid Films 2006, 515, 1612–1617. [Google Scholar] [CrossRef]
- Dong, Q.; Liu, F.; Wong, M.K.; Tam, H.W.; Djurišić, A.B.; Ng, A.; Surya, C.; Chan, W.K.; Ng, A.M.C. Encapsulation of Perovskite Solar Cells for High Humidity Conditions. ChemSusChem 2016, 9, 2597–2603. [Google Scholar] [CrossRef] [PubMed]
- Wilderspin, T.J.; De Rossi, F.; Watson, T.M. A Simple Method to Evaluate the Effectiveness of Encapsulation Materials for Perovskite Solar Cells. Solar Energy 2016, 139, 426–432. [Google Scholar] [CrossRef] [Green Version]
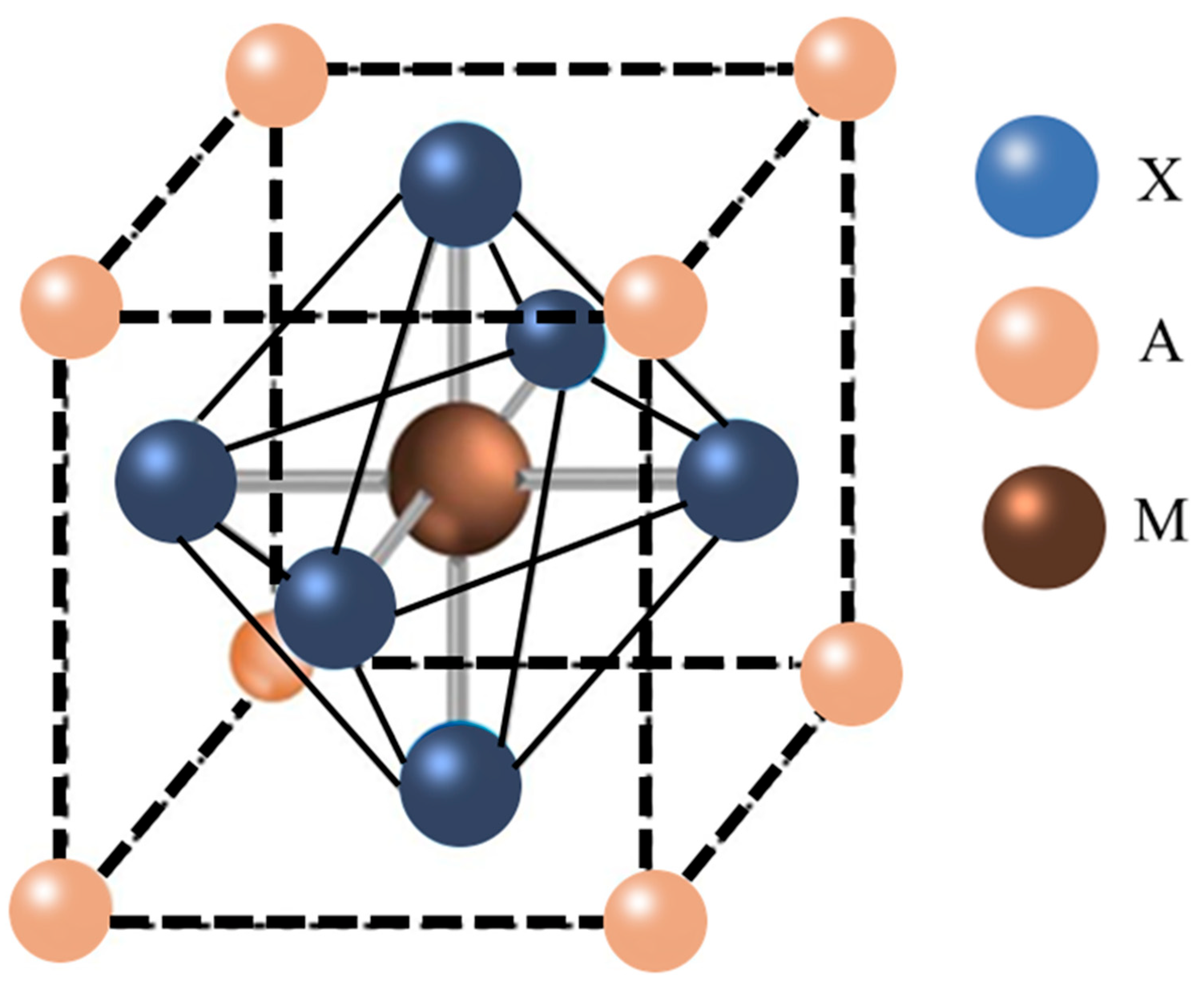
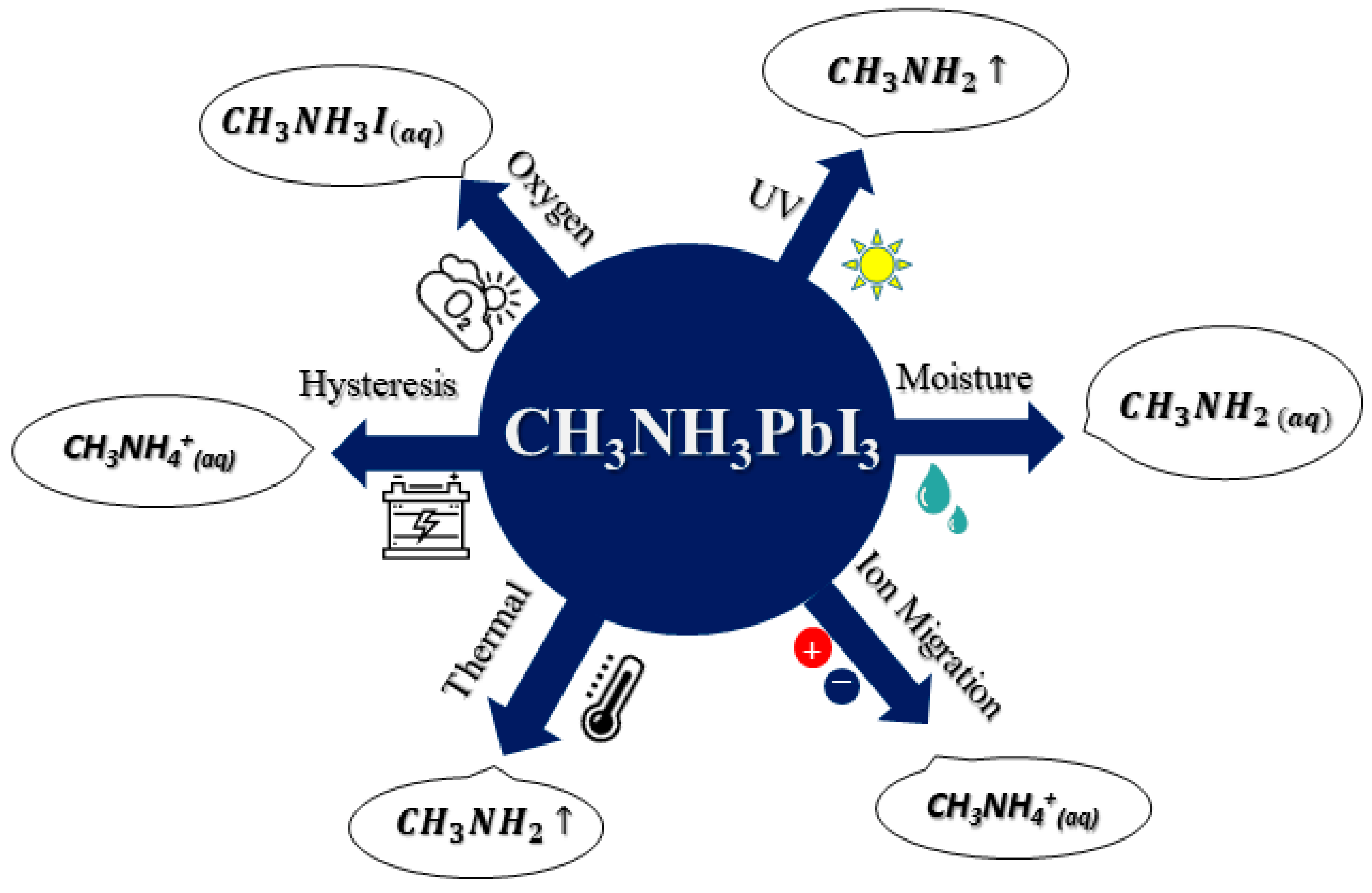
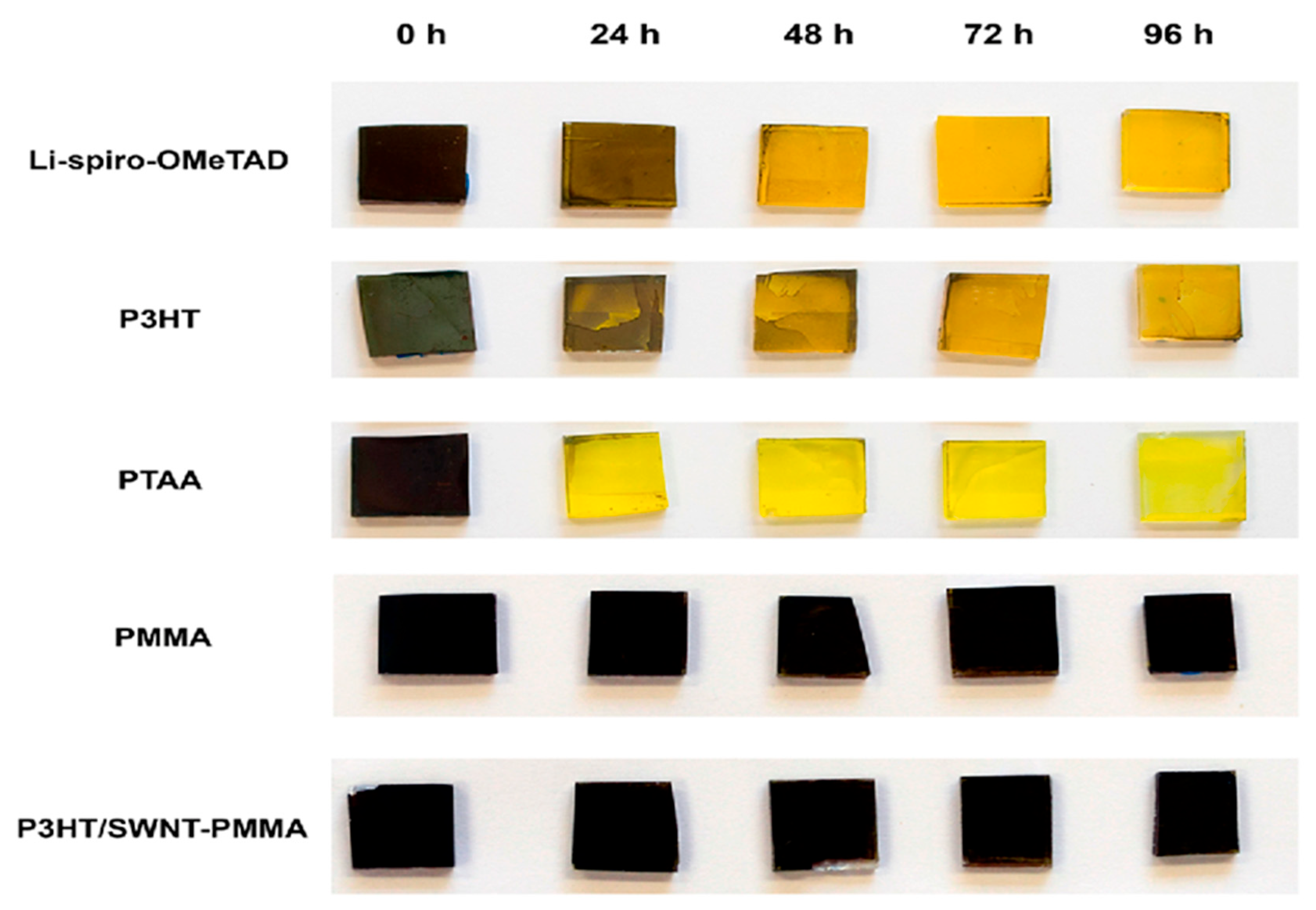
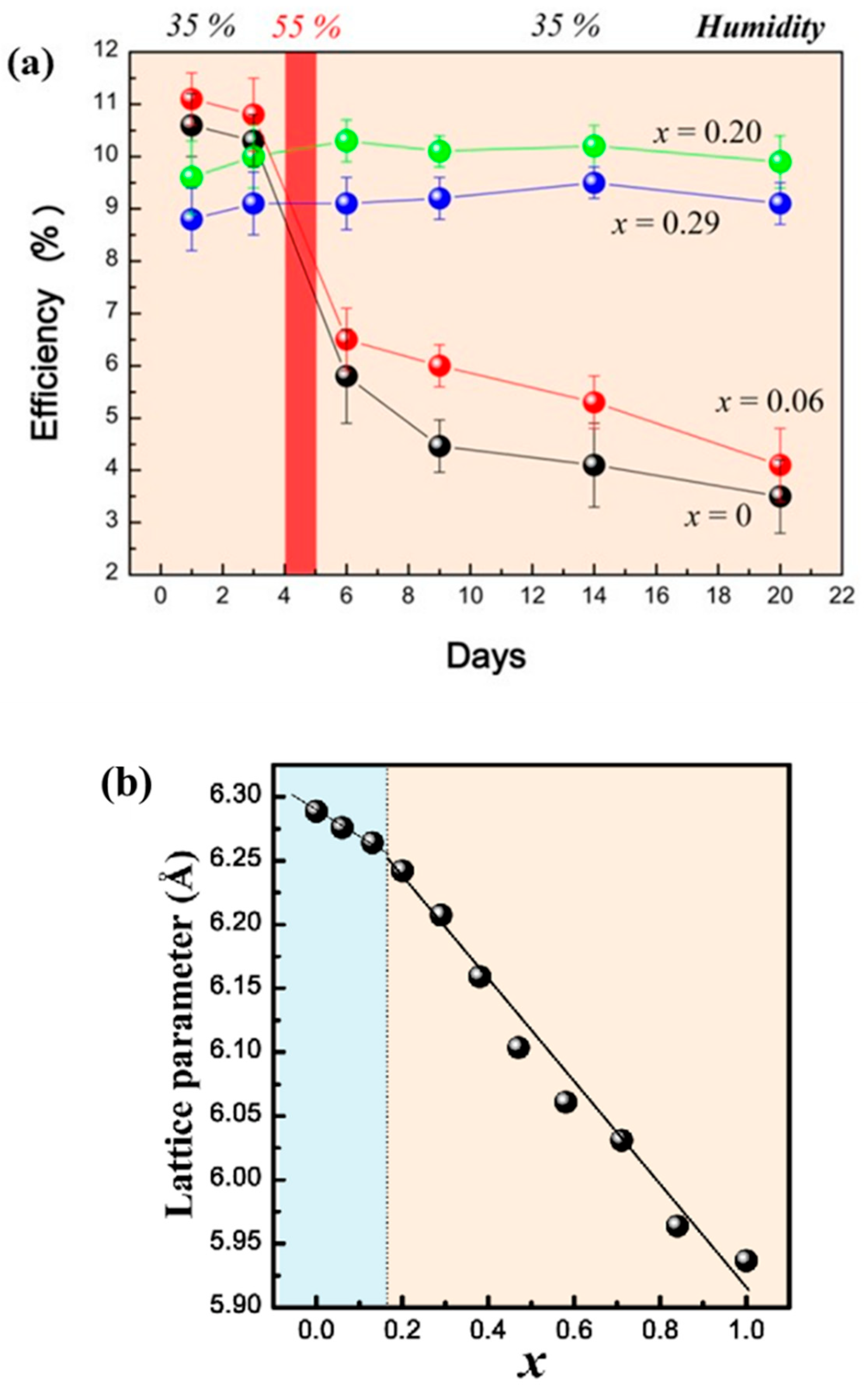

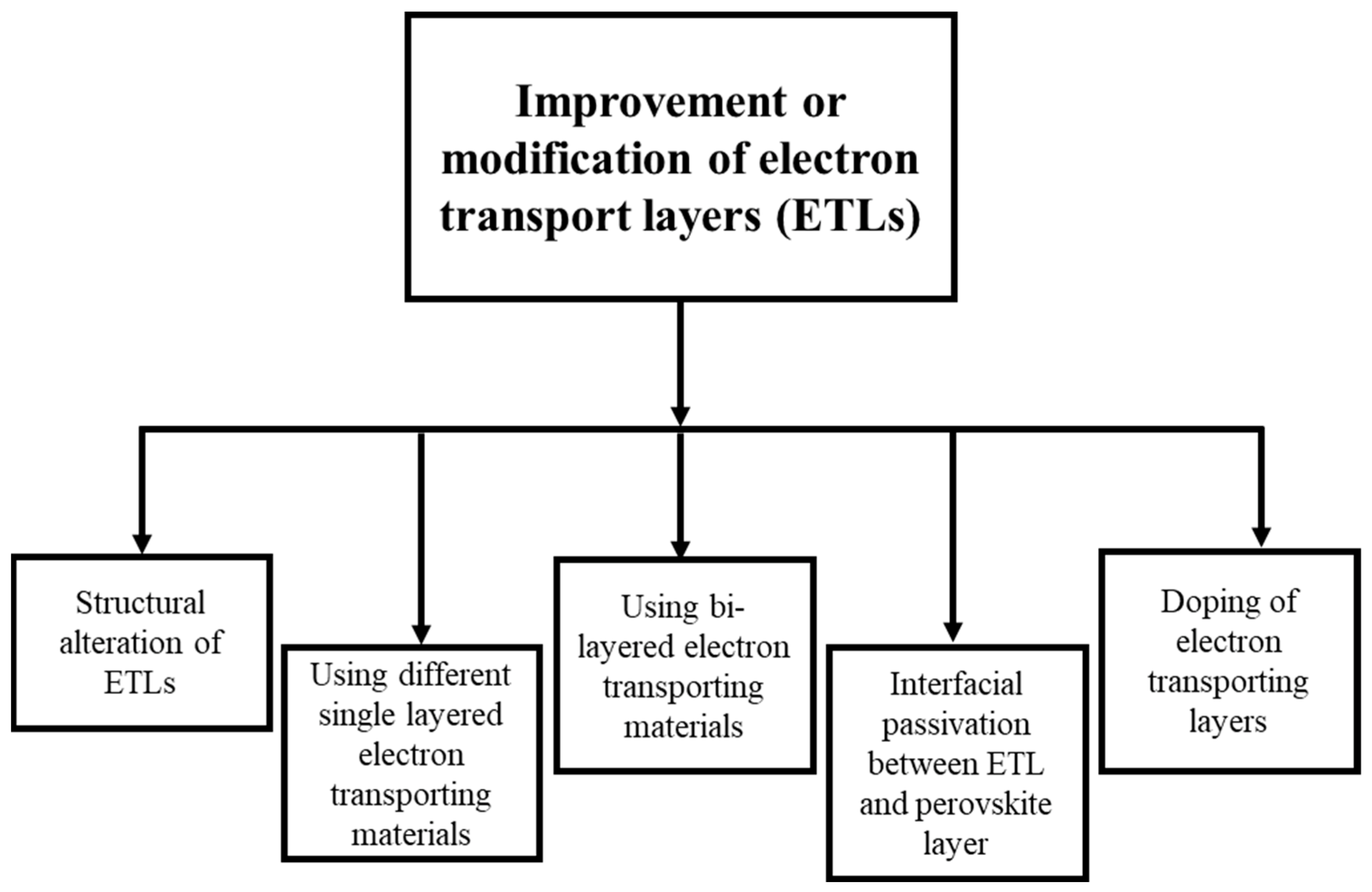


| Device Structure | Testing Condition | Initial PCE | Remaining PCE | Ref. |
|---|---|---|---|---|
| FTO/c-TiO2/mp-Al2O3/MAPbI3xClx/spiroMeOTAD/Au | 1000 h at 40 °C | 12.3 | 50% | [94] |
| FTO/C60/mp-Al2O3/MAPbI3−xClx/spiroMeOTAD/Au | 500 h at 60 °C | 10.4 | 55% | [94] |
| FTO/PEDOT:PSS/(BA)2(MA)3Pb4I13/PCBM/Al | Under 1 sun 2250 h, 65% RH | 12.52 | 100% | [94] |
| FTO/c-TiO2/mp-TiO2/(FAPbI3)x (MAPbBr3)1−x /spiroMeOTAD/Au | 3 months outdoors | 18.7 | 95% | [94] |
| FTO/LiNiO/Cs0.05 FA0.7MA0.25PbI3/C60/Al | 1 sun | 20.5 | 85% | [94] |
| FA0.83Cs0.17Pb(I0.6Br0.4)3 | 4000 h in air | 17.5 | 80% | [94] |
| FTO/c-TiO2/MAPbI3/spiro-MeOTAD/Au | 500 h at 45 °C | 13.9 | 90% | [94] |
| FTO/TiO2 (ZnO)/MAPbI3/spiro-MeOTAD/MoO3/Al | Under 1 sun 144 h, 85% RH | 14.0 | 85% | [344] |
| ITO/PEDOT:PSS/MAPbI3−xClx/PCBM/Ca/Ag | 2 month in ambient conditions | 12.25 | 90% | [345] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, M.S.; Alom, J.; Asaduzzaman, M.; Ahmed, M.B.; Hossain, M.D.; Saem, A.; Masud, J.; Thakare, J.; Hossain, M.A. Recent Criterion on Stability Enhancement of Perovskite Solar Cells. Processes 2022, 10, 1408. https://doi.org/10.3390/pr10071408
Hasan MS, Alom J, Asaduzzaman M, Ahmed MB, Hossain MD, Saem A, Masud J, Thakare J, Hossain MA. Recent Criterion on Stability Enhancement of Perovskite Solar Cells. Processes. 2022; 10(7):1408. https://doi.org/10.3390/pr10071408
Chicago/Turabian StyleHasan, Md Saif, Jahangir Alom, Md Asaduzzaman, Mohammad Boshir Ahmed, Md Delowar Hossain, ASM Saem, Jahangir Masud, Jivan Thakare, and Md Ashraf Hossain. 2022. "Recent Criterion on Stability Enhancement of Perovskite Solar Cells" Processes 10, no. 7: 1408. https://doi.org/10.3390/pr10071408
APA StyleHasan, M. S., Alom, J., Asaduzzaman, M., Ahmed, M. B., Hossain, M. D., Saem, A., Masud, J., Thakare, J., & Hossain, M. A. (2022). Recent Criterion on Stability Enhancement of Perovskite Solar Cells. Processes, 10(7), 1408. https://doi.org/10.3390/pr10071408










