Preparation of a Mulberry-like MnO Specimen and Its Lithium Property
Abstract
:1. Introduction
2. Experimental
2.1. Fabrication Process of the Mulberry-like MnO
2.2. Material Characterization
2.3. Electrochemical Measurements
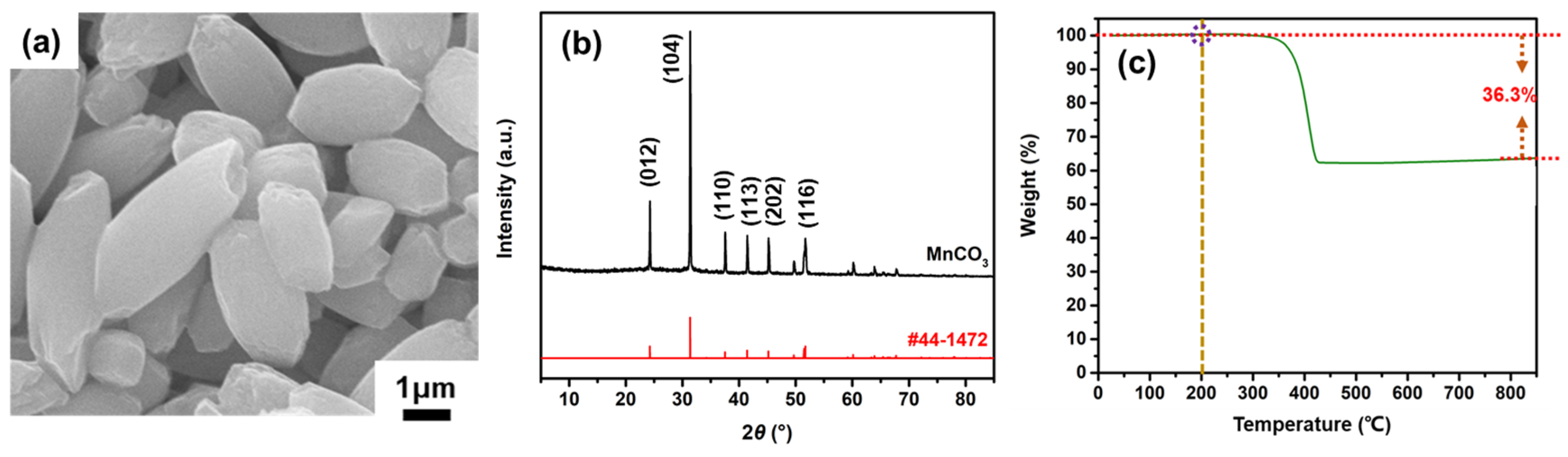
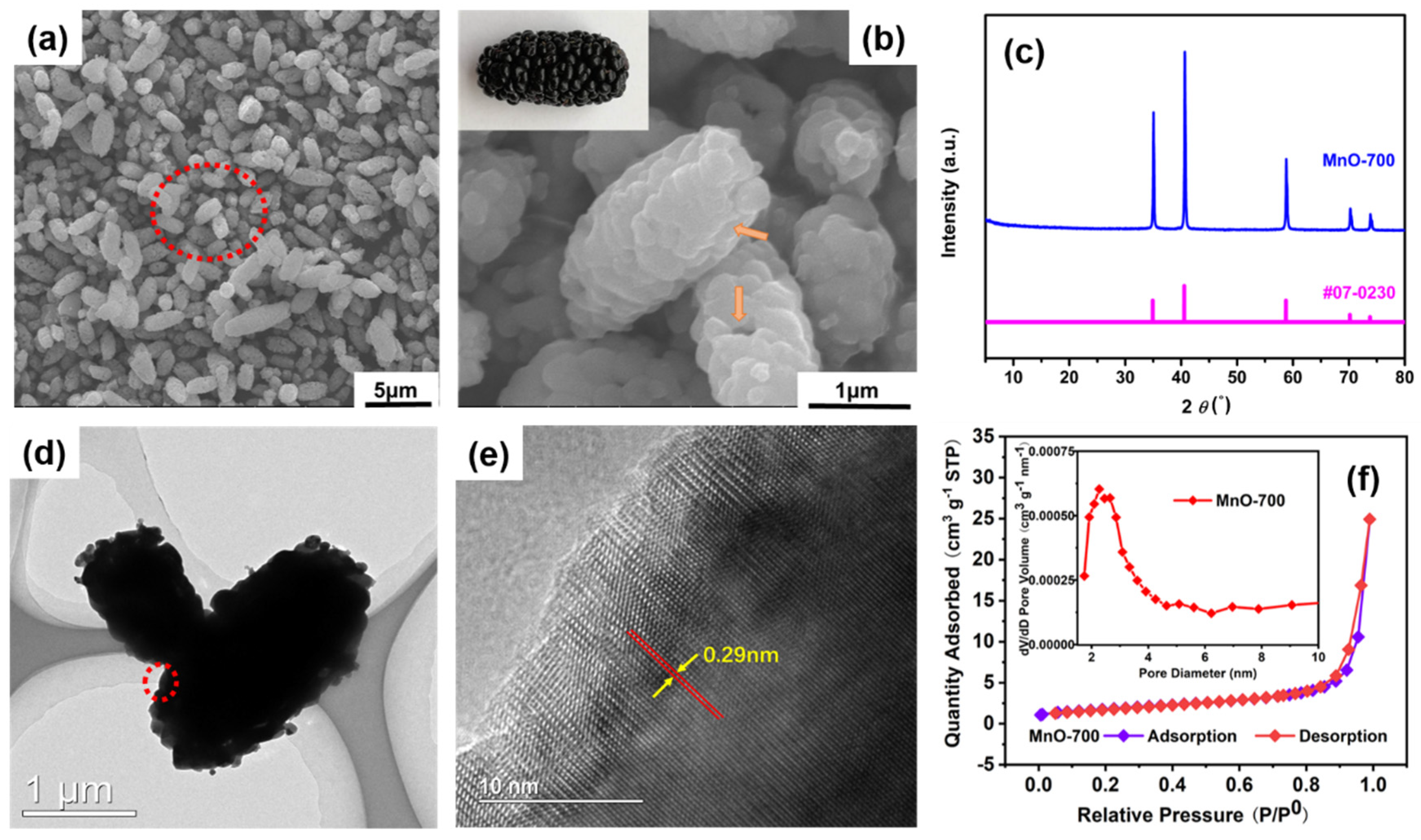
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sun, Y.-H.; Liu, S.; Zhou, F.-C.; Nan, J.-M. Electrochemical performance and structure evolution of core-shell nano-ring α-Fe2O3@Carbon anodes for lithium-ion batteries. Appl. Surf. Sci. 2016, 390, 175–184. [Google Scholar] [CrossRef]
- Cao, W.; Hu, A.; Chen, X.; Liu, X.; Liu, P.; Tang, Q.; Zhao, X. NiO hollow microspheres interconnected by carbon nanotubes as an anode for lithium ion batteries. Electrochim. Acta 2016, 213, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Guo, J.; Jiang, F.; Su, Q.; Zhang, J.; Du, G. Growth of hierarchal porous CoO nanowire arrays on carbon cloth as binder-free anodes for high-performance flexible lithium-ion batteries. J. Alloy. Compd. 2016, 655, 372–377. [Google Scholar] [CrossRef]
- Xie, K.; Wu, P.; Zhou, Y.; Ye, Y.; Wang, H.; Tang, Y.; Zhou, Y.; Lu, T. Nitrogen-Doped Carbon-Wrapped Porous Single-Crystalline CoO Nanocubes for High-Performance Lithium Storage. ACS Appl. Mater. Interfaces 2014, 6, 10602–10607. [Google Scholar] [CrossRef]
- Wang, G.; Wen, W.; Chen, S.; Yu, R.; Wang, X.; Yang, X. Improving the electrochemical performances of spherical LiNi0.5Mn1.5O4 by Fe2O3 surface coating for lithium-ion batteries. Electrochim. Acta 2016, 212, 791–799. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, W.; Jiang, D.; Huang, X. Mesoporous activated carbon decorated with MnO as anode materials for lithium ion batteries. J. Mater. Sci. 2016, 51, 3536–3544. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, L.; Qi, H.; Yue, H.; Zhang, T.; Zhao, X.; Chen, G.; Wei, Y.; Wang, C. Nanosheet assembled hollow ZnFe2O4 microsphere as anode for lithium-ion batteries. J. Alloy. Compd. 2018, 762, 480–487. [Google Scholar] [CrossRef]
- Li, H.; Ma, H.; Yang, M.; Wang, B.; Shao, H.; Wang, L.; Yu, R.; Wang, D. Highly controlled synthesis of multi-shelled NiO hollow microspheres for enhanced lithium storage properties. Mater. Res. Bull. 2017, 87, 224–229. [Google Scholar] [CrossRef]
- Zhao, S.; Guo, J.; Jiang, F.; Su, Q.; Du, G. Porous CoFe2O4 nanowire arrays on carbon cloth as binder-free anodes for flexible lithium-ion batteries. Mater. Res. Bull. 2016, 79, 22–28. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Hu, X.; Luo, W.; Xia, F.; Huang, Y. Reconstruction of Conformal Nanoscale MnO on Graphene as a High-Capacity and Long-Life Anode Material for Lithium Ion Batteries. Adv. Funct. Mater. 2013, 23, 2436–2444. [Google Scholar] [CrossRef]
- Lee, S.M.; Choi, S.H.; Lee, J.-K.; Kang, Y.C. Electrochemical properties of graphene-MnO composite and hollow-structured MnO powders prepared by a simple one-pot spray pyrolysis process. Electrochim. Acta 2014, 132, 441–447. [Google Scholar] [CrossRef]
- Zang, J.; Qian, H.; Wei, Z.; Cao, Y.; Zheng, M.; Dong, Q. Reduced Graphene Oxide Supported MnO Nanoparticles with Excellent Lithium Storage Performance. Electrochim. Acta 2014, 118, 112–117. [Google Scholar] [CrossRef]
- Li, S.R.; Sun, Y.; Ge, S.Y.; Qiao, Y.; Chen, Y.M.; Liebervvirth, I.; Yu, Y.; Chen, C.H. A facile route to synthesize nano-MnO/C composites and their application in lithium ion batteries. Chem. Eng. J. 2012, 192, 226–231. [Google Scholar] [CrossRef]
- Yang, C.; Gao, Q.; Tian, W.; Tan, Y.; Zhang, T.; Yang, K.; Zhu, L. Superlow load of nanosized MnO on a porous carbon matrix from wood fibre with superior lithium ion storage performance. J. Mater. Chem. A 2014, 2, 19975–19982. [Google Scholar] [CrossRef]
- Zhang, Q.; Dai, Q.; Li, M.; Wang, X.; Li, A. Incorporation of MnO nanoparticles inside porous carbon nanotubes originated from conjugated microporous polymers for lithium storage. J. Mater. Chem. A 2016, 4, 19132–19139. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, X.; Wang, F.; Li, J.; Song, S.; Zhang, H. Nanoconfined nitrogen-doped carbon-coated MnO nanoparticles in graphene enabling high performance for lithium-ion batteries and oxygen reduction reaction. Chem. Sci. 2016, 7, 4284–4290. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Zhu, L.; Song, H.; Chen, X.; Zhou, J. Enhanced electrochemical performance of MnO nanowire/graphene composite during cycling as the anode material for lithium-ion batteries. Nano Energy 2014, 10, 172–180. [Google Scholar] [CrossRef]
- Liu, B.; Li, D.; Liu, Z.; Gu, L.; Xie, W.; Li, Q.; Guo, P.; Liu, D.; He, D. Carbon-wrapped MnO nanodendrites interspersed on reduced graphene oxide sheets as anode materials for lithium-ion batteries. Appl. Surf. Sci. 2017, 394, 1–8. [Google Scholar] [CrossRef]
- Qiu, J.X.; Lai, C.; Wang, Y.Z.; Li, S.; Zhang, S.Q. Resilient mesoporous TiO2/graphene nanocomposite for high rate performance lithium-ion batteries. Chem. Eng. J. 2014, 256, 247–254. [Google Scholar] [CrossRef]
- Bai, T.; Zhou, H.C.; Zhou, X.Y.; Liao, Q.C.; Chen, S.M.; Yang, J. N-doped carbon-encapsulated MnO@ graphene nanosheet as high-performance anode material for lithium-ion batteries. J. Mater. Sci. 2017, 52, 11608–11619. [Google Scholar] [CrossRef]
- Wu, T.; Tu, F.; Liu, S.; Zhuang, S.; Jin, G.; Pan, C. MnO nanorods on graphene as an anode material for high capacity lithium ion batteries. J. Mater. Sci. 2013, 49, 1861–1867. [Google Scholar] [CrossRef]
- Li, D.L.; Zhang, Y.J.; Li, L.; Hu, F.; Yang, H.C.; Wang, C.H.; Wang, Q.B. Polydopamine directed MnO@C microstructures as electrode for lithium ion battery. Sci. China Chem. 2016, 59, 122–127. [Google Scholar] [CrossRef]
- Wang, S.B.; Xing, Y.L.; Xiao, C.L.; Xu, H.Z.; Zhang, S.C. A peapod-inspired MnO@C core-shell design for lithium ion batteries. J. Power Sources 2016, 307, 11–16. [Google Scholar] [CrossRef]
- Wang, S.B.; Ren, Y.B.; Liu, G.R.; Xing, Y.L.; Zhang, S.C. Peanut-like MnO@C core-shell composites as anode electrodes for high-performance lithium ion batteries. Nanoscale 2014, 6, 3508–3512. [Google Scholar] [CrossRef]
- Wang, J.; Li, C.; Yang, Z.; Chen, D. Chemical vapor deposition-assisted fabrication of a graphene-wrapped MnO/carbon nanofibers membrane as a high-rate and long-life anode for lithium ion batteries. RSC Adv. 2017, 7, 50973–50980. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Shang, X.; Li, D.; Yue, H.; Wang, S.; Qiao, L.; He, D. Facile Synthesis of Porous MnO Microspheres for High-Performance Lithium-Ion Batteries. Part. Part. Syst. Charact. 2014, 31, 1001–1007. [Google Scholar] [CrossRef]
- Fan, X.; Li, S.; Lu, L. Porous micrometer-sized MnO cubes as anode of lithium ion battery. Electrochim. Acta 2016, 200, 152–160. [Google Scholar] [CrossRef]
- Pei, X.-Y.; Mo, D.-C.; Lyu, S.-S.; Zhang, J.-H.; Fu, Y.-X. Preparation of novel two-stage structure MnO micrometer particles as lithium-ion battery anode materials. RSC Adv. 2018, 8, 28518–28524. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Zi, Z.; Chen, B.; Zhao, B.; Zhu, X.; Liang, C.; Ma, X.; Dai, J. Facile synthesis of hollow MnO microcubes as superior anode materials for lithium-ion batteries. J. Alloy. Compd. 2018, 756, 93–102. [Google Scholar] [CrossRef]
- Zhong, K.; Zhang, B.; Luo, S.; Wen, W.; Li, H.; Huang, X.; Chen, L. Investigation on porous MnO microsphere anode for lithium ion batteries. J. Power Sources 2011, 196, 6802–6808. [Google Scholar] [CrossRef]
- Li, X.; Li, D.; Qiao, L.; Wang, X.; Sun, X.; Wang, P.; He, D. Interconnected porous MnO nanoflakes for high-performance lithium ion battery anodes. J. Mater. Chem. 2012, 22, 9189–9194. [Google Scholar] [CrossRef]
- Pei, X.-Y.; Mo, D.-C.; Lyu, S.-S.; Zhang, J.-H.; Fu, Y.-X. Preparation of a fusiform shape MnO/C composite as anode materials for lithium-ion batteries. J. Mater. Sci. Mater. Electron. 2018, 29, 11982–11990. [Google Scholar] [CrossRef]
- De las Casas, C.; Li, W. A review of application of carbon nanotubes for lithium ion battery anode material. J. Power Sources 2012, 208, 74–85. [Google Scholar] [CrossRef]
- Gu, X.; Yue, J.; Chen, L.; Liu, S.; Xu, H.Y.; Yang, J.; Qian, Y.T.; Zhao, X.B. Coaxial MnO/N-doped carbon nanorods for advanced lithium-ion battery anodes. J. Mater. Chem. A 2015, 3, 1037–1041. [Google Scholar] [CrossRef]
- Ma, S.; Chen, D.; Wang, W.-L. MnO nanoparticles embedded in a carbon matrix as high performance lithium-ion battery anodes: Preparation, microstructure and electrochemistry. Phys. Chem. Chem. Phys. 2016, 18, 19130–19136. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, X.; Luo, W.; Huang, Y. Porous carbon-modified MnO disks prepared by a microwave-polyol process and their superior lithium-ion storage properties. J. Mater. Chem. 2012, 22, 19190–19195. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.; Li, F.; Xia, D. Facile synthesis of MnO/C anode materials for lithium-ion batteries. Electrochim. Acta 2011, 56, 6448–6452. [Google Scholar] [CrossRef]
- Xia, P.; Lin, H.; Tu, W.; Chen, X.; Cai, X.; Zheng, X.; Xu, M.; Li, W. A Novel Fabrication for Manganese Monoxide/Reduced Graphene Oxide Nanocomposite as High Performance Anode of Lithium Ion Battery. Electrochim. Acta 2016, 198, 66–76. [Google Scholar] [CrossRef]
- Li, F.; Ma, J.; Ren, H.; Wang, H.; Wang, G. Fabrication of MnO nanowires implanted in graphene as an advanced anode material for sodium-ion batteries. Mater. Lett. 2017, 206, 132–135. [Google Scholar] [CrossRef]
- Ding, P.; Xu, Y.L.; Sun, X.F. Synthesis and Performance of Nano MnO as an Anode Material for Lithium-Ion Batteries. Acta Phys. Chim. Sin. 2013, 29, 293–297. [Google Scholar]
- Zhang, Y.; Steiner, J.D.; Uzodinma, J.; Walsh, J.; Zydlewski, B.; Lin, F.; Chen, Y.; Tang, J.; Pradhan, N.; Dai, Q. Thermally synthesized MnO nanoparticles for magnetic properties and lithium batteries. Mater. Res. Express 2019, 6, 025015. [Google Scholar] [CrossRef]
- Qi, J.; Zhu, Y.-Y.; Zhang, J.-Z.; Jiao, M.-L.; Wang, C.-Y. Synthesis of porous hollow six-branched star-like MnO and its enhanced electrochemical properties as a lithium ion anode material. Ceram. Int. 2020, 46, 20878–20884. [Google Scholar] [CrossRef]
- Zhong, K.; Xia, X.; Zhang, B.; Li, H.; Wang, Z.; Chen, L. MnO powder as anode active materials for lithium ion batteries. J. Power Sources 2010, 195, 3300–3308. [Google Scholar] [CrossRef]
- Zhang, L.; Shen, L.; Liu, Y.; Suo, G. Urchin-like MnO/C microspheres as high-performance lithium-ion battery anode. Ionics 2021, 27, 1423–1428. [Google Scholar] [CrossRef]
- Zhu, W.J.; Hu, Y.H.; Yu, Y.Z. Biotemplate Synthesis of MnO/C Composite and Its Lithium-Storage Property. Rare Met. Mat. Eng. 2020, 49, 1273–1276. [Google Scholar]
- Qin, Y.; Wang, B.; Jiang, S.; Jiang, Q.; Huang, C.; Chen, H.C. Strongly anchored MnO nanoparticles on graphene as high-performance anode materials for lithium-ion batteries. Ionics 2020, 26, 3315–3323. [Google Scholar] [CrossRef]
- Jia, Y.; Yang, Z.-W.; Li, H.-J.; Wang, Y.-Z.; Wang, X.-M. Reduced graphene oxide encapsulated MnO microspheres as an anode for high-rate lithium ion capacitors. New Carbon Mater. 2021, 36, 573–584. [Google Scholar] [CrossRef]
- Ding, Y.; Du, J.; Guo, L.; Zhou, H.; Yang, H.; Feng, W. Nanoscale MnO and natural graphite hybrid materials as high-performance anode for lithium ion batteries. Electrochim. Acta 2015, 170, 9–15. [Google Scholar] [CrossRef]
- Zeng, S.; Zhao, R.; Li, A.; Xue, S.; Lv, D.; Luo, Q.; Shu, D.; Chen, H. MnO/Carbon fibers prepared by an electrospinning method and their properties used as anodes for lithium ion batteries. Appl. Surf. Sci. 2019, 463, 211–216. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, Y.; Jiang, Z.-G.; Zeng, X.; Ji, J.; Li, Z.; Gao, X.; Sun, M.; Lin, Z.; Ling, M.; et al. Exploring competitive features of stationary sodium ion batteries for electrochemical energy storage. Energy Environ. Sci. 2019, 12, 1512–1533. [Google Scholar] [CrossRef]


| Sample Name | Current Density (mA g−1) | Capacity Retention (mAh g−1) | Cycle Number (Times) | References |
|---|---|---|---|---|
| Mulberry-like MnO | 200 | 702 | 120 | This work |
| Nano MnO | 141.1 | 581.5 | 50 | [40] |
| MnO cubes | 200 | 615.9 | 10 | [27] |
| MnO-nanoparticle | 151.2 | 428 | 100 | [41] |
| star-like MnO | 500 | 844.8 | 200 | [42] |
| MnO micrometer particles | 100 | 747 | 100 | [28] |
| MnO particles | 95 | 650 | 150 | [43] |
| u-MnO/C | 152 | 723 | 80 | [44] |
| MnO/C composite | 200 | 664 | 100 | [45] |
| MnO/RGO composite | 300 | 544 | 60 | [46] |
| MnO/rGO | 100 | 846 | 110 | [47] |
| MnO/natural graphite | 152 | 497 | 120 | [48] |
| MnO/Carbon fibers | 400 | 680 | 100 | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.-X.; Huang, H.-S.; Ge, W.-J.; Qiu, W.; Pei, X.-Y. Preparation of a Mulberry-like MnO Specimen and Its Lithium Property. Processes 2022, 10, 1110. https://doi.org/10.3390/pr10061110
Fu Y-X, Huang H-S, Ge W-J, Qiu W, Pei X-Y. Preparation of a Mulberry-like MnO Specimen and Its Lithium Property. Processes. 2022; 10(6):1110. https://doi.org/10.3390/pr10061110
Chicago/Turabian StyleFu, Yuan-Xiang, Hong-Shen Huang, Wu-Jie Ge, Wei Qiu, and Xian-Yinan Pei. 2022. "Preparation of a Mulberry-like MnO Specimen and Its Lithium Property" Processes 10, no. 6: 1110. https://doi.org/10.3390/pr10061110
APA StyleFu, Y.-X., Huang, H.-S., Ge, W.-J., Qiu, W., & Pei, X.-Y. (2022). Preparation of a Mulberry-like MnO Specimen and Its Lithium Property. Processes, 10(6), 1110. https://doi.org/10.3390/pr10061110





