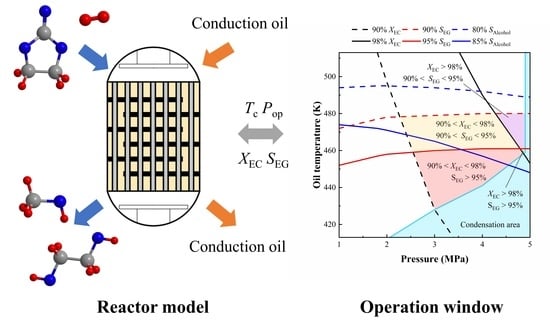
Model-Based Analysis for Ethylene Carbonate Hydrogenation Operation in Industrial-Type Tubular Reactors
Abstract
:1. Introduction
2. Methods
2.1. Method Description
- Reactants are fed into the adiabatic reactor through inlet manifolds, which are simplified into a ring.
- The inlet gas distributor of the heat-exchange multi-tubular reactor ensures uniform distribution of the reactants to all reaction tubes, so that only a single reaction tube is modeled as representative.
- The wall temperatures of all reaction tubes of the boiling water-cooled reactor are equal to the boiling temperature of pressurized water.
- The coolant temperature and external heat transfer coefficient are the same for all reaction tubes of the oil-cooled reactor.
2.2. Governing Equations
2.3. Bed Voidage and Pressure Drop
2.4. Heat and Mass Transfer in the Catalyst Bed
2.5. Heat and Mass Transfer of Catalyst Particles
2.6. Coolant Heat Transfer
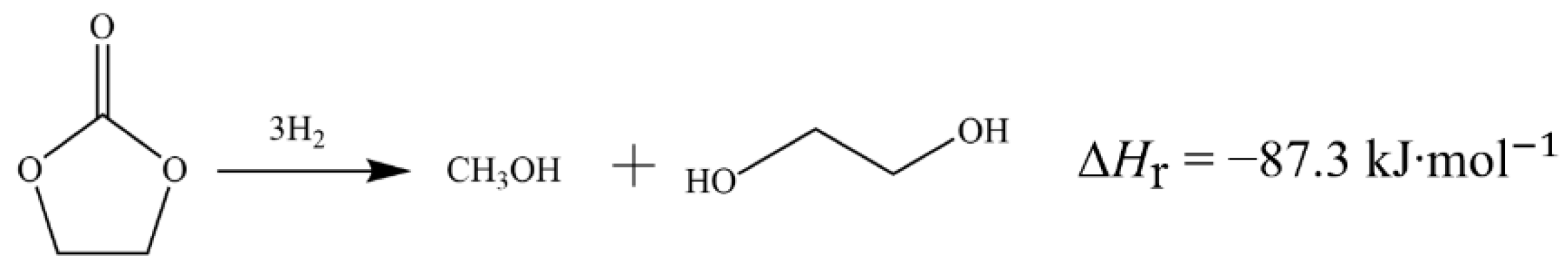
2.7. Chemical Reactions
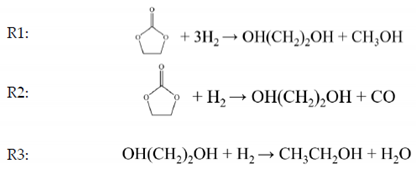
2.8. Model Implementation
3. Results and Discussion
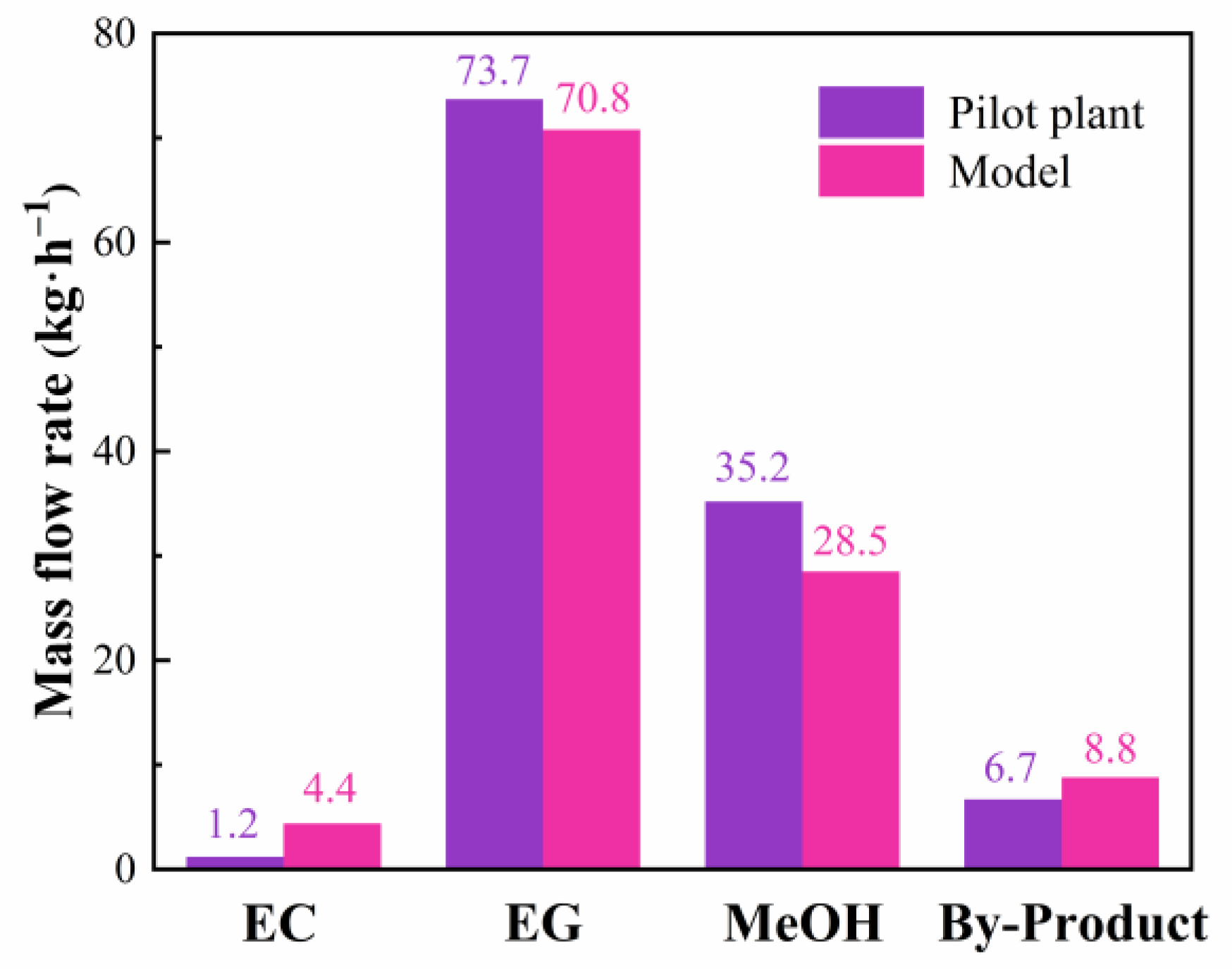
3.1. Model Validation
3.2. Reactor Profiles
3.2.1. Adiabatic
3.2.2. Boiling Water Cooling
3.2.3. Conduction Oil Cooling
3.3. Effects of Key Operating Variables
3.3.1. Temperature
3.3.2. H2/EC
3.3.3. Pressure and Space Velocity
3.4. Operation Windows
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Notations
| Surface area of particles per unit volume (m−1) | |
| Specific heat capacity of fluid (J·kg−1·K−1) | |
| Specific heat capacity of conduction oil (J·kg−1·K−1) | |
| Inertial loss coefficient (m−1) | |
| Concentration of EC in the center of catalyst (kmol·m−3) | |
| Concentration of EC in the gas phase (kmol·m−3) | |
| Concentration of EC on the surface of catalyst (kmol·m−3) | |
| Diameter of sphere (m) | |
| Diameter of sphere with equal specific surface area (m) | |
| Diameter of sphere with equal surface area (m) | |
| Diameter of sphere with equal volume (m) | |
| Bed or tube diameter (m) | |
| Effective axis diffusion coefficient (m2·s−1) | |
| Effective radial diffusion coefficient (m2·s−1) | |
| Effective diffusion coefficient of EC in the catalyst particle (m2·s−1) | |
| Molecular diffusivity of EC (m2·s−1) | |
| Thermal diffusion coefficient (kg·m−1·s−1) | |
| Total fluid energy (m2·s−2) | |
| Total solid energy (m2·s−2) | |
| Enthalpy of formation of species (J·kmol−1) | |
| H2/EC | Molar ratio of EC to H2 |
| Diffusion flux of species vector (kg·m−2·s−1) | |
| Turbulence kinetic energy | |
| Effective axial thermal conductivity (W·m−1·K−1) | |
| Effective radial thermal conductivity (W·m−1·K−1) | |
| Thermal conductivity of fluid (W·m−1·K−1) | |
| Gas-solid mass transfer coefficient (m·s−1) | |
| Thermal conductivity of catalyst particle (W·m−1·K−1) | |
| Pre-exponential factor | |
| Bed length (m) | |
| EC inlet mass flow (kg·h−1) | |
| EC outlet mass flow (kg·h−1) | |
| EG outlet mass flow (kg·h−1) | |
| MeOH outlet mass flow (kg·h−1) | |
| Conduction oil mass flow (kg·h−1) | |
| Molecular weight (kg·kmol−1) | |
| Reaction order | |
| Nusselt number | |
| Static pressure (Pa) | |
| EC partial pressure (Pa) | |
| EG partial pressure (Pa) | |
| Operating pressure (MPa) | |
| Fluid Peclet number for heat transfer | |
| Peclet number for axial heat conduction | |
| Peclet radial heat transfer for fully developed turbulence flow | |
| Peclet number for axial mass dispersion | |
| Peclet number for radial mass dispersion | |
| Prandtl number | |
| Heat flux to oil (W) | |
| Reaction heat (W) | |
| Intrinsic reaction rate (kmol·m−3·s−1) | |
| Effective reaction rate (kmol·m−3·s−1) | |
| Ratio of tube diameter to catalyst’s volume-equivalent diameter | |
| Effective consumption rate of EC at the particle surface (kmol·m−3·s−1) | |
| Volumetric rate of creation of species (kmol·m−3·s−1) | |
| Reynolds number based on particle diameter | |
| Total alcohol selectivity | |
| Surface area of catalyst particle (m2) | |
| EG selectivity | |
| Energy source term (W·m−3) | |
| Mass source term (kg·m−3·s−1) | |
| MeOH selectivity | |
| Surface area of wall (m2) | |
| Momentum source term (kg·m−2·s−2) | |
| Schmidt number | |
| Sherwood number | |
| Space velocity (gEC·gcat−1·h−1) | |
| Time (s) or adjacent baffle plate space (m) | |
| Temperature (K) | |
| Boiling point of pressurized water (K) | |
| Coolant temperature (K) | |
| Reactant inlet temperature (K) | |
| Conduction oil temperature (K) | |
| Catalyst surface temperature (K) | |
| Wall temperature (K) | |
| Superficial velocity (m·s−1) | |
| Fluid flow velocity vector (m·s−1) | |
| Volume of catalyst particle (m3) | |
| X position along the bed axial direction (m) | |
| EC conversion | |
| Mass fraction of species | |
| Mole fraction of species | |
| Greek letters | |
| Gas-solid heat transfer coefficient (W·m−2·K−1) | |
| Enthalpy of reaction (J·kmol−1) | |
| Bed pressure drop (Pa) | |
| Temperature difference between catalyst surface and gas phase (K) | |
| Temperature difference between catalyst surface and center (K) | |
| Bed voidage | |
| Internal porosity of catalyst particle | |
| Effectiveness factor for EC internal mass transfer | |
| Effective thermal conductivity of catalyst particle (W·m−1·K−1) | |
| Fluid viscosity (Pa·s) | |
| Fluid density (kg·m−3) | |
| Stress tensor (Pa) | |
| Generalized Thiele modulus of EC | |
| Specific dissipation rate |
References
- Hepburn, C.; Adlen, E.; Beddington, J.; Carter, E.A.; Fuss, S.; Mac Dowell, N.; Minx, J.C.; Smith, P.; Williams, C.K. The technological and economic prospects for CO2 utilization and removal. Nature 2019, 575, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Pan, S.; Li, H.; Cai, J.; Olabi, A.G.; Anthony, E.J.; Manovic, V. Recent advances in carbon dioxide utilization. Renew. Sustain. Energy Rev. 2020, 125, 109799. [Google Scholar] [CrossRef]
- Ronda-Lloret, M.; Rothenberg, G.; Shiju, N.R. A Critical Look at Direct Catalytic Hydrogenation of Carbon Dioxide to Olefins. ChemSusChem 2019, 12, 3896–3914. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Sun, J.; Ge, Q.; Tsubaki, N. Recent advances in direct catalytic hydrogenation of carbon dioxide to valuable C2+ hydrocarbons. J. Mater. Chem. A 2018, 6, 23244–23262. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, C.; Gao, P.; Wang, H.; Li, X.; Zhong, L.; Wei, W.; Sun, Y. A review of the catalytic hydrogenation of carbon dioxide into value-added hydrocarbons. Catal. Sci. Technol. 2017, 7, 4580–4598. [Google Scholar] [CrossRef]
- Ye, K.; Zhou, Z.; Shao, J.; Lin, L.; Gao, D.; Ta, N.; Si, R.; Wang, G.; Bao, X. In Situ Reconstruction of a Hierarchical Sn-Cu/SnOx Core/Shell Catalyst for High-Performance CO2 Electroreduction. Angew. Chem. Int. Ed. 2020, 59, 4814–4821. [Google Scholar] [CrossRef]
- Wu, C.; Lin, L.; Liu, J.; Zhang, J.; Zhang, F.; Zhou, T.; Rui, N.; Yao, S.; Deng, Y.; Yang, F.; et al. Inverse ZrO2/Cu as a highly efficient methanol synthesis catalyst from CO2 hydrogenation. Nat. Commun. 2020, 11, 5767. [Google Scholar] [CrossRef]
- Pedersen, J.K.; Batchelor, T.A.A.; Bagger, A.; Rossmeisl, J. High-Entropy Alloys as Catalysts for the CO2 and CO Reduction Reactions. ACS Catal. 2020, 10, 2169–2176. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, T.; Pei, J.; Shang, H.; Zhou, D.; Li, H.; Dong, J.; Wang, Y.; Cao, R.; Zhuang, Z.; et al. Discovery of main group single Sb–N4 active sites for CO2 electroreduction to formate with high efficiency. Energy Environ. Sci. 2020, 13, 2856–2863. [Google Scholar] [CrossRef]
- Hu, J.; Yu, L.; Deng, J.; Wang, Y.; Cheng, K.; Ma, C.; Zhang, Q.; Wen, W.; Yu, S.; Pan, Y.; et al. Sulfur vacancy-rich MoS2 as a catalyst for the hydrogenation of CO2 to methanol. Nat. Catal. 2021, 4, 242–250. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.; Li, Z.; Tang, C.; Feng, Z.; An, H.; Liu, H.; Liu, T.; Li, C. A highly selective and stable ZnO-ZrO2 solid solution catalyst for CO2 hydrogenation to methanol. Sci. Adv. 2017, 3, e1701290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sen, R.; Goeppert, A.; Kar, S.; Prakash, G.K.S. Hydroxide Based Integrated CO2 Capture from Air and Conversion to Methanol. J. Am. Chem. Soc. 2020, 142, 4544–4549. [Google Scholar] [CrossRef] [PubMed]
- Hartadi, Y.; Widmann, D.; Behm, R.J. CO2 Hydrogenation to Methanol on Supported Au Catalysts under Moderate Reaction Conditions: Support and Particle Size Effects. ChemSusChem 2015, 8, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Frei, M.S.; Mondelli, C.; Cesarini, A.; Krumeich, F.; Hauert, R.; Stewart, J.A.; Curulla Ferré, D.; Pérez-Ramírez, J. Role of Zirconia in Indium Oxide-Catalyzed CO2 Hydrogenation to Methanol. ACS Catal. 2020, 10, 1133–1145. [Google Scholar] [CrossRef]
- Dang, S.; Qin, B.; Yang, Y.; Wang, H.; Cai, J.; Han, Y.; Li, S.; Gao, P.; Sun, Y. Rationally designed indium oxide catalysts for CO2 hydrogenation to methanol with high activity and selectivity. Sci. Adv. 2020, 6, eaaz2060. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, S.; Ma, X.; Gong, J. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhao, L.; Wang, W.; Zhao, Y.; Zhang, G.; Ma, X.; Gong, J. Morphology control of ceria nanocrystals for catalytic conversion of CO2 with methanol. Nanoscale 2013, 5, 5582–5588. [Google Scholar] [CrossRef]
- Song, Q.; Zhou, Z.; He, L. Efficient, selective and sustainable catalysis of carbon dioxide. Green Chem. 2017, 19, 3707–3728. [Google Scholar] [CrossRef]
- Tamura, M.; Kitanaka, T.; Nakagawa, Y.; Tomishige, K. Cu Sub-Nanoparticles on Cu/CeO2 as an Effective Catalyst for Methanol Synthesis from Organic Carbonate by Hydrogenation. ACS Catal. 2016, 6, 376–380. [Google Scholar] [CrossRef]
- Li, Y.; Junge, K.; Beller, M. Improving the Efficiency of the Hydrogenation of Carbonates and Carbon Dioxide to Methanol. ChemCatChem 2013, 5, 1072–1074. [Google Scholar] [CrossRef]
- Du, X.; Sun, X.; Jin, C.; Jiang, Z.; Su, D.; Wang, J. Efficient Hydrogenation of Alkyl Formate to Methanol over Nanocomposite Copper/Alumina Catalysts. ChemCatChem 2014, 6, 3075–3079. [Google Scholar] [CrossRef]
- Balaraman, E.; Gunanathan, C.; Zhang, J.; Shimon, L.J.W.; Milstein, D. Efficient hydrogenation of organic carbonates, carbamates and formates indicates alternative routes to methanol based on CO2 and CO. Nat. Chem. 2011, 3, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Jiang, Z.; Su, D.; Wang, J. Research Progress on the Indirect Hydrogenation of Carbon Dioxide to Methanol. ChemSusChem 2016, 9, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.-Y.; Chen, M.-K.; Chien, I.L. Assessment on CO2 Utilization through Rigorous Simulation: Converting CO2 to Dimethyl Carbonate. Ind. Eng. Chem. Res. 2018, 57, 639–652. [Google Scholar] [CrossRef]
- Gu, X.; Zhang, X.; Zhang, X.; Deng, C. Simulation and assessment of manufacturing ethylene carbonate from ethylene oxide in multiple process routes. Chin. J. Chem. Eng. 2021, 31, 135–144. [Google Scholar] [CrossRef]
- Han, Z.; Rong, L.; Wu, J.; Zhang, L.; Wang, Z.; Ding, K. Catalytic Hydrogenation of Cyclic Carbonates: A Practical Approach from CO2 and Epoxides to Methanol and Diols. Angew. Chem. Int. Ed. 2012, 51, 13041–13045. [Google Scholar] [CrossRef]
- Wu, X.; Ji, L.; Ji, Y.; Elageed, E.H.M.; Gao, G. Hydrogenation of ethylene carbonate catalyzed by lutidine-bridged N-heterocyclic carbene ligands and ruthenium precursors. Catal. Commun. 2016, 85, 57–60. [Google Scholar] [CrossRef]
- Lian, C.; Ren, F.; Liu, Y.; Zhao, G.; Ji, Y.; Rong, H.; Jia, W.; Ma, L.; Lu, H.; Wang, D.; et al. Heterogeneous selective hydrogenation of ethylene carbonate to methanol and ethylene glycol over a copper chromite nanocatalyst. Chem. Commun. 2015, 51, 1252–1254. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Y.; Li, A.; Yao, D.; Gao, Y.; Fayisa, B.A.; Wang, M.-Y.; Huang, S.; Lv, J.; Wang, Y.; et al. Nanoflower-like Cu/SiO2 Catalyst for Hydrogenation of Ethylene Carbonate to Methanol and Ethylene Glycol: Enriching H2 Adsorption. ChemCatChem 2020, 12, 3670–3678. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, D.; Zhang, M.; Li, A.; Gao, Y.; Fayisa, B.A.; Wang, M.; Huang, S.; Wang, Y.; Ma, X. Efficient hydrogenation of CO2-derived ethylene carbonate to methanol and ethylene glycol over Mo-doped Cu/SiO2 catalyst. Catal. Today 2021, 371, 113–119. [Google Scholar] [CrossRef]
- Ding, Y.; Tian, J.; Chen, W.; Guan, Y.; Xu, H.; Li, X.; Wu, H.; Wu, P. One-pot synthesized core/shell structured zeolite@copper catalysts for selective hydrogenation of ethylene carbonate to methanol and ethylene glycol. Green Chem. 2019, 21, 5414–5426. [Google Scholar] [CrossRef]
- Zhou, M.; Shi, Y.; Ma, K.; Tang, S.; Liu, C.; Yue, H.; Liang, B. Nanoarray Cu/SiO2 Catalysts Embedded in Monolithic Channels for the Stable and Efficient Hydrogenation of CO2-Derived Ethylene Carbonate. Ind. Eng. Chem. Res 2018, 57, 1924–1934. [Google Scholar] [CrossRef]
- Chen, X.; Cui, Y.; Wen, C.; Wang, B.; Dai, W. Continuous synthesis of methanol: Heterogeneous hydrogenation of ethylene carbonate over Cu/HMS catalysts in a fixed bed reactor system. Chem. Commun. 2015, 51, 13776–13778. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, L.; Han, X.; Cao, Y.; He, P.; Li, H. Selective hydrogenation of ethylene carbonate to methanol and ethylene glycol over Cu/SiO2 catalysts prepared by ammonia evaporation method. Int. J. Hydrogen Energy 2017, 42, 2144–2156. [Google Scholar] [CrossRef]
- Chen, W.; Song, T.; Tian, J.; Wu, P.; Li, X. An efficient Cu-based catalyst for the hydrogenation of ethylene carbonate to ethylene glycol and methanol. Catal. Sci. Technol. 2019, 9, 6749–6759. [Google Scholar] [CrossRef]
- Tian, J.; Chen, W.; Wu, P.; Zhu, Z.; Li, X. Cu–Mg–Zr/SiO2 catalyst for the selective hydrogenation of ethylene carbonate to methanol and ethylene glycol. Catal. Sci. Technol. 2018, 8, 2624–2635. [Google Scholar] [CrossRef]
- Song, T.; Qi, Y.; Jia, A.; Ta, N.; Lu, J.; Wu, P.; Li, X. Continuous hydrogenation of CO2-derived ethylene carbonate to methanol and ethylene glycol at Cu-MoOx interface with a low H2/ester ratio. J. Catal. 2021, 399, 98–110. [Google Scholar] [CrossRef]
- Chen, X.; Wang, L.; Zhang, C.; Tu, W.; Cao, Y.; He, P.; Li, J.; Li, H. The effective and stable Cu–C@SiO2 catalyst for the syntheses of methanol and ethylene glycol via selective hydrogenation of ethylene carbonate. Int. J. Hydrogen Energy 2021, 46, 17209–17220. [Google Scholar] [CrossRef]
- Deng, F.; Li, N.; Tang, S.; Liu, C.; Yue, H.; Liang, B. Evolution of active sites and catalytic consequences of mesoporous MCM-41 supported copper catalysts for the hydrogenation of ethylene carbonate. Chem. Eng. J. 2018, 334, 1943–1953. [Google Scholar] [CrossRef]
- Poels, E.K.; Brands, D.S. Modification of Cu/ZnO/SiO2 catalysts by high temperature reduction. Appl. Catal. A: Gen. 2000, 191, 83–96. [Google Scholar] [CrossRef]
- Meyer, J.J.; Tan, P.; Apfelbacher, A.; Daschner, R.; Hornung, A. Modeling of a Methanol Synthesis Reactor for Storage of Renewable Energy and Conversion of CO2—Comparison of Two Kinetic Models. Chem. Eng. Technol. 2016, 39, 233–245. [Google Scholar] [CrossRef]
- Bozzano, G.; Manenti, F. Efficient methanol synthesis: Perspectives, technologies and optimization strategies. Prog. Energy Combust. Sci. 2016, 56, 71–105. [Google Scholar] [CrossRef]
- Haid, J.; Koss, U. Lurgi’s Mega-Methanol technology opens the door for a new era in down-stream applications. In Studies in Surface Science and Catalysis; Iglesia, E., Spivey, J.J., Fleisch, T.H., Eds.; Elsevier Science Pub. Co. Inc.: Amsterdam, The Netherlands, 2001; Volume 136, pp. 399–404. [Google Scholar]
- Samimi, F.; Feilizadeh, M.; Ranjbaran, M.; Arjmand, M.; Rahimpour, M.R. Phase stability analysis on green methanol synthesis process from CO2 hydrogenation in water cooled, gas cooled and double cooled tubular reactors. Fuel Processing Technol. 2018, 181, 375–387. [Google Scholar] [CrossRef]
- Cui, X.; Kær, S.K. A comparative study on three reactor types for methanol synthesis from syngas and CO2. Chem. Eng. J. 2020, 393, 124632. [Google Scholar] [CrossRef]
- Kuo, K.K. Principles of Combustion; Elsevier Science Pub. Co. Inc.: Amsterdam, The Netherlands, 1986. [Google Scholar]
- Verman, L.C.; Banerjee, S. Effect of Container Walls on Packing Density of Particles. Nature 1946, 157, 584. [Google Scholar] [CrossRef]
- Theuerkauf, J.; Witt, P.; Schwesig, D. Analysis of particle porosity distribution in fixed beds using the discrete element method. Powder Technol. 2006, 165, 92–99. [Google Scholar] [CrossRef]
- Roshani, S. Elucidation of Local and Global Structural Properties of Packed Bed Configurations; The University of Leeds: Leeds, UK, 1990. [Google Scholar]
- de Klerk, A. Voidage variation in packed beds at small column to particle diameter ratio. AIChE J. 2003, 49, 2022–2029. [Google Scholar] [CrossRef]
- Ergun, S. Fluid Flow Through Packed Columns. Chem. Eng. Prog. 1952, 48, 89–94. [Google Scholar]
- Koning, B. Heat and Mass Transport in Tubular Packed Bed Reactors at Reacting and Non-Reacting Conditions; University of Twente: Enschede, The Netherlands, 2002. [Google Scholar]
- Agnew, J.B.; Potter, O. Heat transfer properties of packed tubes of small diameter. Trans. Inst. Chem. Eng. 1970, 48, T15. [Google Scholar]
- Bauer, R.; Schluender, E.U. Effective Radial Thermal Conductivity of Packings in Gas Flow-1. Convective Transport Coefficient. Int. Chem. Eng. 1978, 18, 181–188. [Google Scholar]
- Bauer, R.; Schluender, E.U. Effective Radial Thermal Conductivity of Packings in Gas Flow-2. Thermal Conductivity of the Packing Fraction without Gas Flow. Int. Chem. Eng. 1978, 18, 189–204. [Google Scholar]
- Westerterp, K.R.; Swaaij, W.P.M.V.; Beenackers, A.A.C.M. Chemical Reactor Design and Operation, 2nd ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1987; p. 800. [Google Scholar]
- Fahien, R.W.; Smith, J.M. Mass transfer in packed beds. AIChE J. 1955, 1, 28–37. [Google Scholar] [CrossRef]
- Specchia, V.; Baldi, G.; Sicardi, S. Heat transfer in packed bed reactors with one phase flow. Chem. Eng. Commun. 1980, 4, 361–380. [Google Scholar] [CrossRef]
- Wakao, N.; Funazkri, T. Effect of fluid dispersion coefficients on particle-to-fluid mass transfer coefficients in packed beds: Correlation of sherwood numbers. Chem. Eng. Sci. 1978, 33, 1375–1384. [Google Scholar] [CrossRef]
- Bai, P.T.; Manokaran, V.; Saiprasad, P.S.; Srinath, S. Studies on Heat and Mass Transfer Limitations in Oxidative Dehydrogenation of Ethane Over Cr2O3/Al2O3 Catalyst. Procedia Eng. 2015, 127, 1338–1345. [Google Scholar] [CrossRef] [Green Version]
- Soomro, M.; Hughes, R. The thermal conductivity of porous catalyst pellets. Can. J. Chem. Eng. 1979, 57, 24–28. [Google Scholar] [CrossRef]
- McCabe, W.L.; Smith, J.C.; Harriott, P. Heat Tranfer to Fluids without Phase Change. In Unit Operations in Chemical Engineering; Clark, B.J., Castellano, E., Eds.; Mcgraw Hill, Inc.: New York, NY, USA, 1993; pp. 647–685. [Google Scholar]
- Ross, J.R.H. Chapter 8-Mass and Heat Transfer Limitations and Other Aspects of the Use of Large-Scale Catalytic Reactors. In Contemporary Catalysis; Ross, J.R.H., Ed.; Elsevier Science Pub. Co. Inc.: Amsterdam, The Netherlands, 2019; pp. 187–213. [Google Scholar]
- Plessis, J.P.; Diedericks, G. Fluid transport in porous media: Pore-scale modelling of interstitial transport phenomena. Comput. Mech. Publ. 1997, 11, 61–104. [Google Scholar]
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N. Transport Phenomena; John Wiley & Sons, Ltd.: New York, NY, USA, 1960. [Google Scholar]

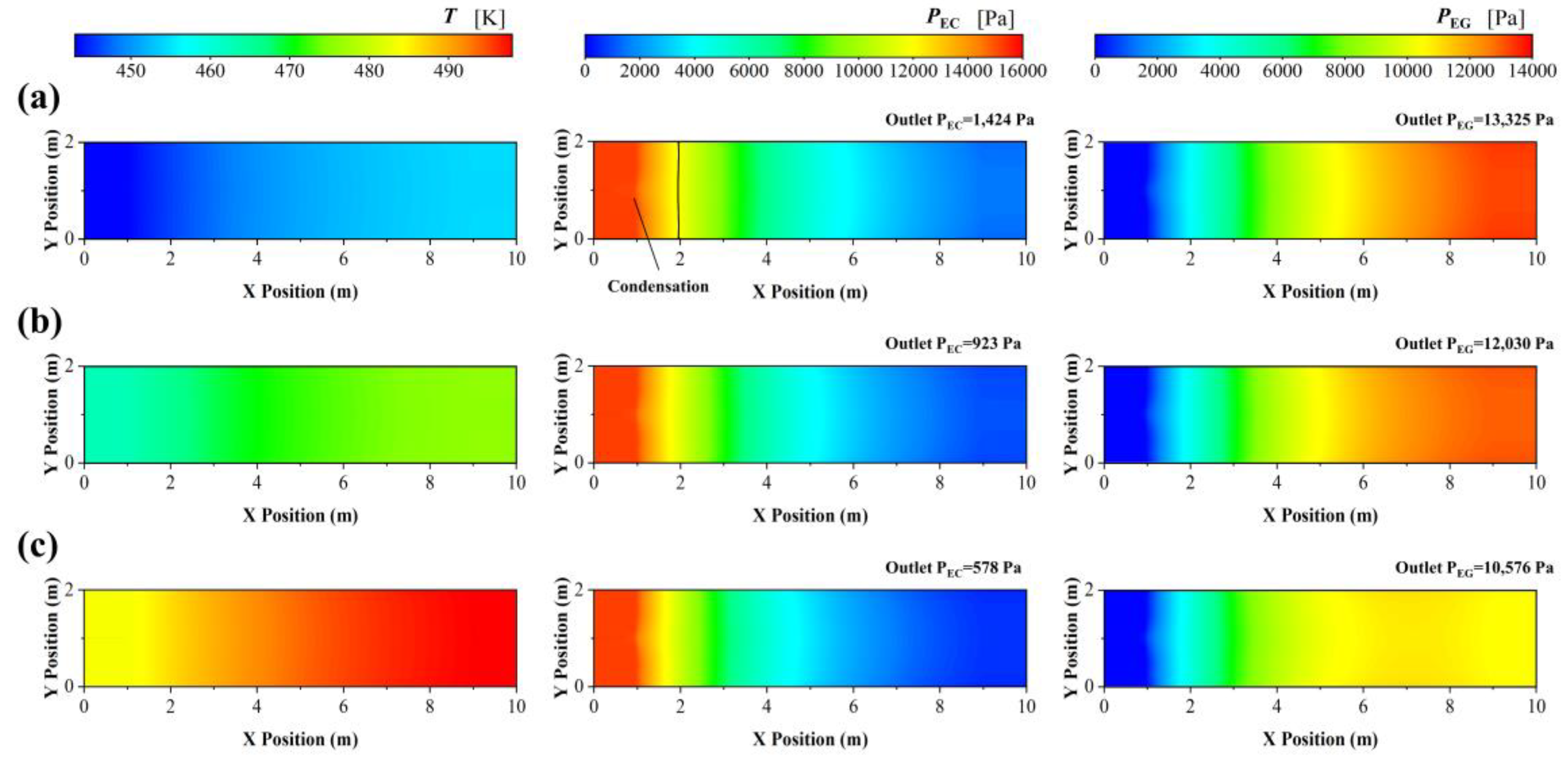
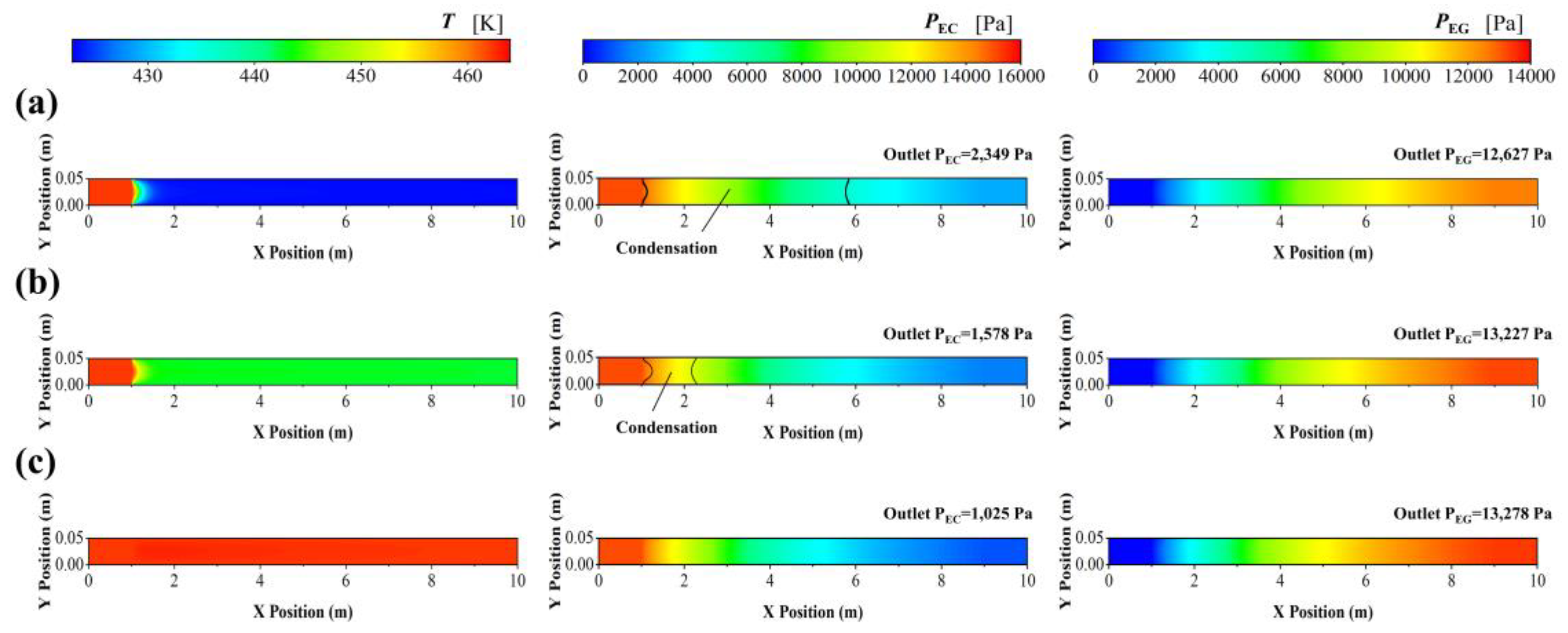

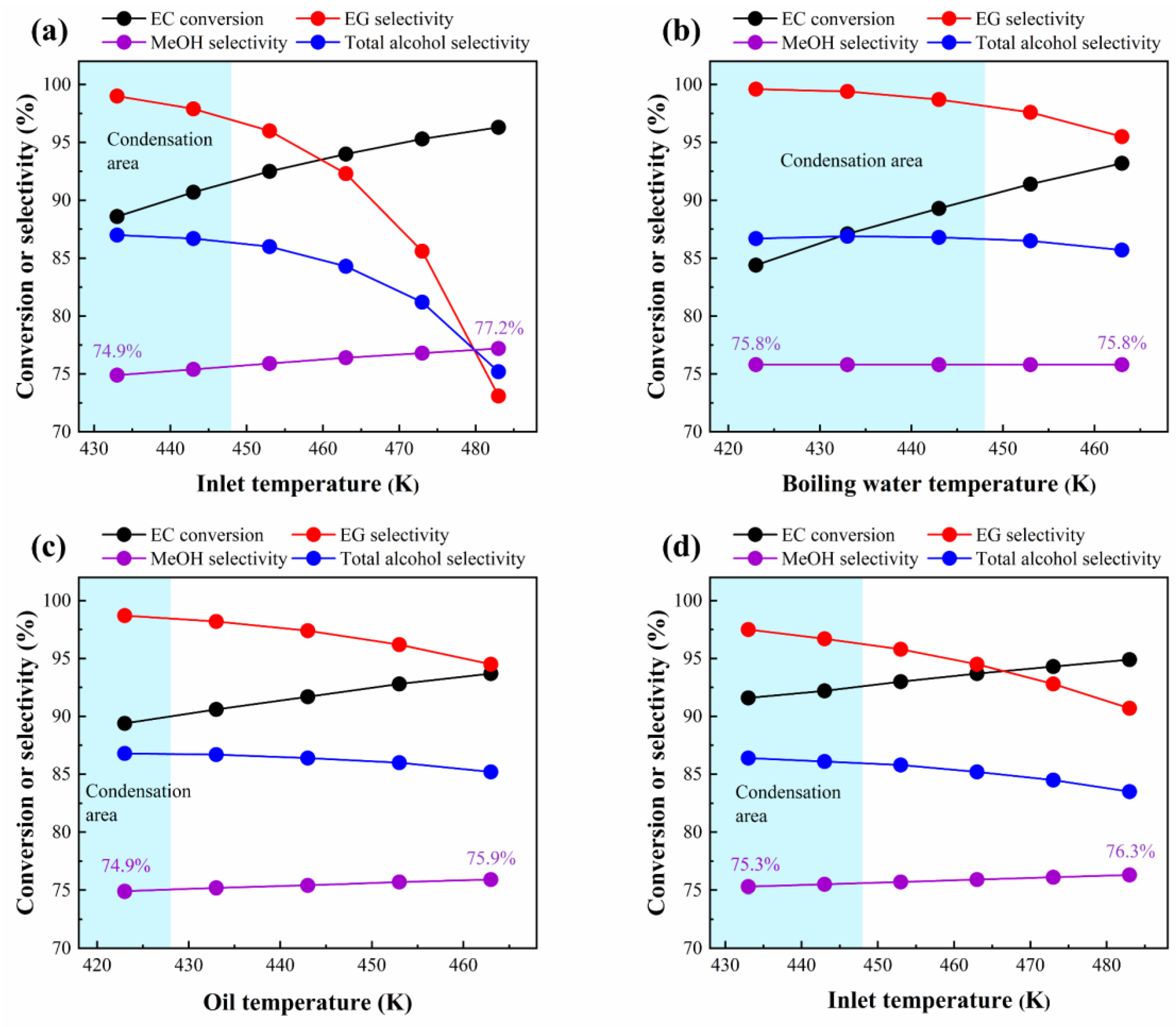


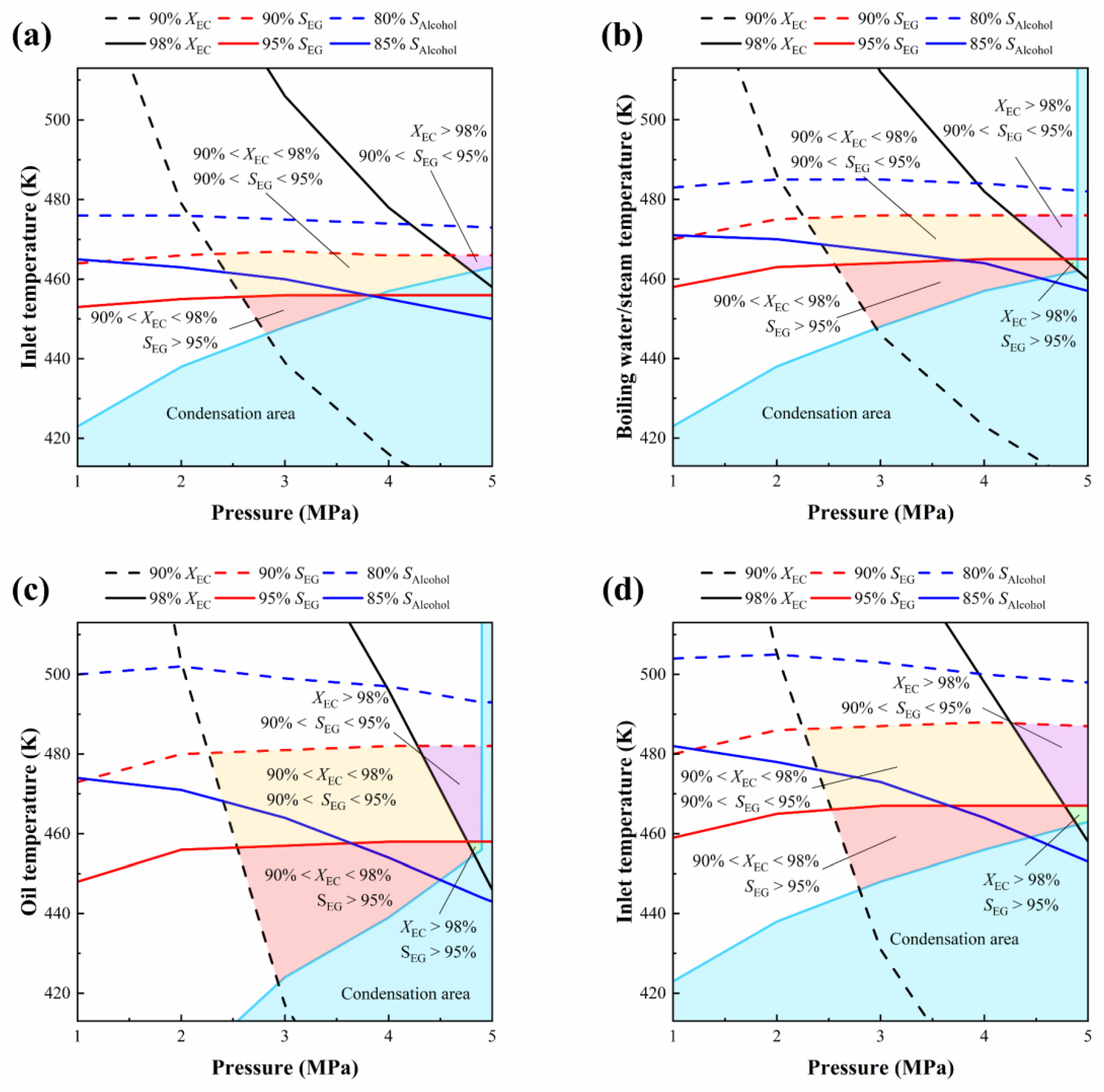

| Geometric Parameters | Adiabatic FBR | Heat-Exchange FBR |
|---|---|---|
| (m) | 2 | 0.05 |
| Developing zone length | 2 m | |
| Reaction zone length | 8 m | |
| Catalyst type | Cu/SiO2 [30] | |
| Catalyst geometry | 3/5/7 mm (sphere); 3 × 3/5 × 5/7 × 7 mm (cylinder) | |
| Catalyst loading mass (kg) | 13730 kg for (5 mm sphere) | |
| Average bed voidage | 0.4 (5 mm sphere) | 0.43 (5 mm sphere) |
| Gas distributor geometry | 1 m diameter, ring | / |
| Operating conditions (Nominal value/range) | ||
| (K) | 463/433–483 | |
| (K) | / | 463/423–463 |
| (MPa) | 3/1–5 | |
| (gEC·gcat−1·h−1) | 0.3/0.1–0.5 | |
| Molar ratio of H2 to EC | 200/80–200 | |
| Adiabatic Reactor Model | ||||
| Mesh Size | 1250 | 10,000 | 20,000 | |
| EC conversions (%) | 20% length (sphere) | 37.6 | 39.0 | 39.1 |
| outlet (sphere) | 94.1 | 94.0 | 94.0 | |
| 20% length (cylinder) | 29.7 | 30.2 | 30.1 | |
| outlet (cylinder) | 86.0 | 85.1 | 84.8 | |
| Heat-Exchange Reactor Model | ||||
| Mesh Size | 2500 | 10,000 | 40,000 | |
| EC conversions (%) | 20% length (sphere) | 36.8 | 38.0 | 38.3 |
| outlet (sphere) | 93.2 | 93.2 | 93.2 | |
| 20% length (cylinder) | 33.7 | 34.8 | 35.1 | |
| outlet (cylinder) | 89.4 | 89.4 | 89.4 | |
| Pilot Reactor Configuration | |
| Tube Inner Diameter (m) | 0.034 |
| Tube length (m) | 4 |
| Tube number | 95 |
| Operating Conditions | |
| EC mass flow (kg·h−1) | 107.3 |
| Feed EC to H2 molar ratio | 170 |
| Feed temperature (K) | 458.2 |
| Coolant type | Conduction oil |
| Coolant mass flow rate (kg·h−1) | 20,000 |
| Coolant temperature (K) | 453.2 |
| Catalyst Information | |
| Catalyst composition | Cu/SiO2 |
| Size and shape | 3 × 5 mm cylinder |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Cao, C.; Wang, Y.; Yang, Y.; Lv, J.; Xu, J. Model-Based Analysis for Ethylene Carbonate Hydrogenation Operation in Industrial-Type Tubular Reactors. Processes 2022, 10, 688. https://doi.org/10.3390/pr10040688
Huang H, Cao C, Wang Y, Yang Y, Lv J, Xu J. Model-Based Analysis for Ethylene Carbonate Hydrogenation Operation in Industrial-Type Tubular Reactors. Processes. 2022; 10(4):688. https://doi.org/10.3390/pr10040688
Chicago/Turabian StyleHuang, Hai, Chenxi Cao, Yue Wang, Youwei Yang, Jianning Lv, and Jing Xu. 2022. "Model-Based Analysis for Ethylene Carbonate Hydrogenation Operation in Industrial-Type Tubular Reactors" Processes 10, no. 4: 688. https://doi.org/10.3390/pr10040688
APA StyleHuang, H., Cao, C., Wang, Y., Yang, Y., Lv, J., & Xu, J. (2022). Model-Based Analysis for Ethylene Carbonate Hydrogenation Operation in Industrial-Type Tubular Reactors. Processes, 10(4), 688. https://doi.org/10.3390/pr10040688