Cladium mariscus Saw-Sedge versus Sawdust—Efficient Biosorbents for Removal of Hazardous Textile Dye C.I. Basic Blue 3 from Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adsorbents and Adsorbate Characteristics
2.2. Kinetic Studies
2.3. Isotherm Studies
2.4. Desorption Study
3. Results and Discussion
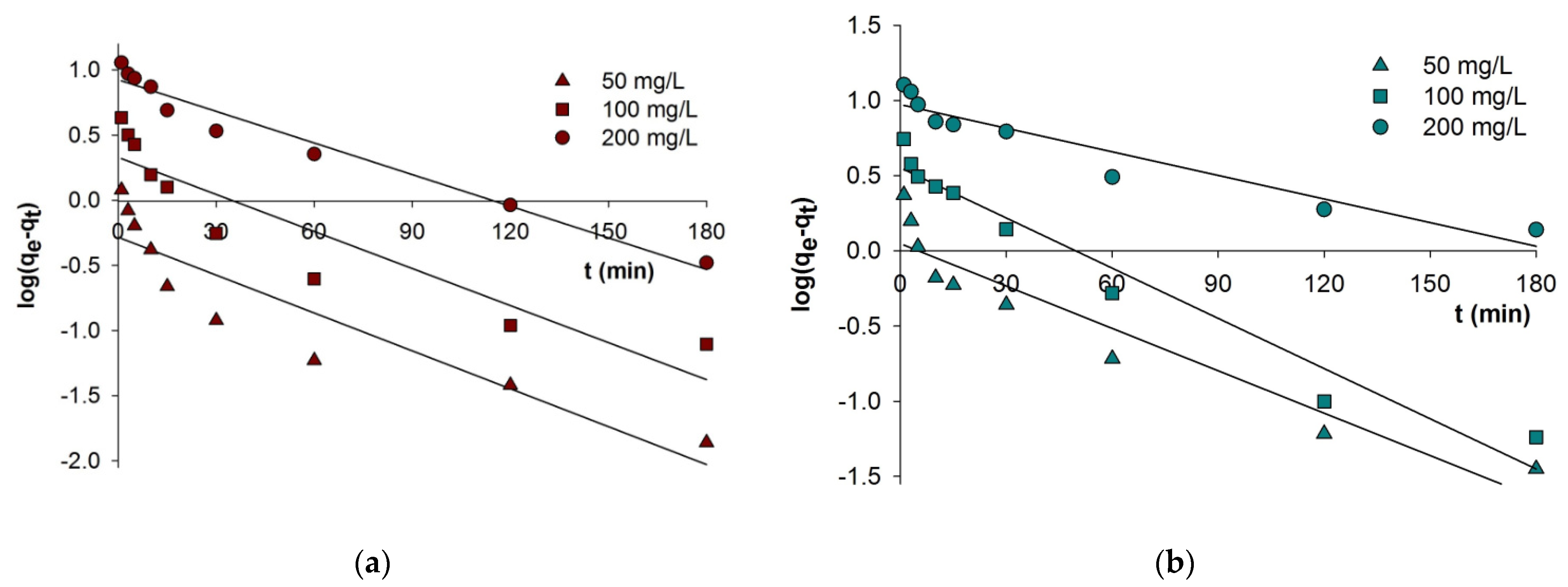
3.1. Kinetic Adsorption
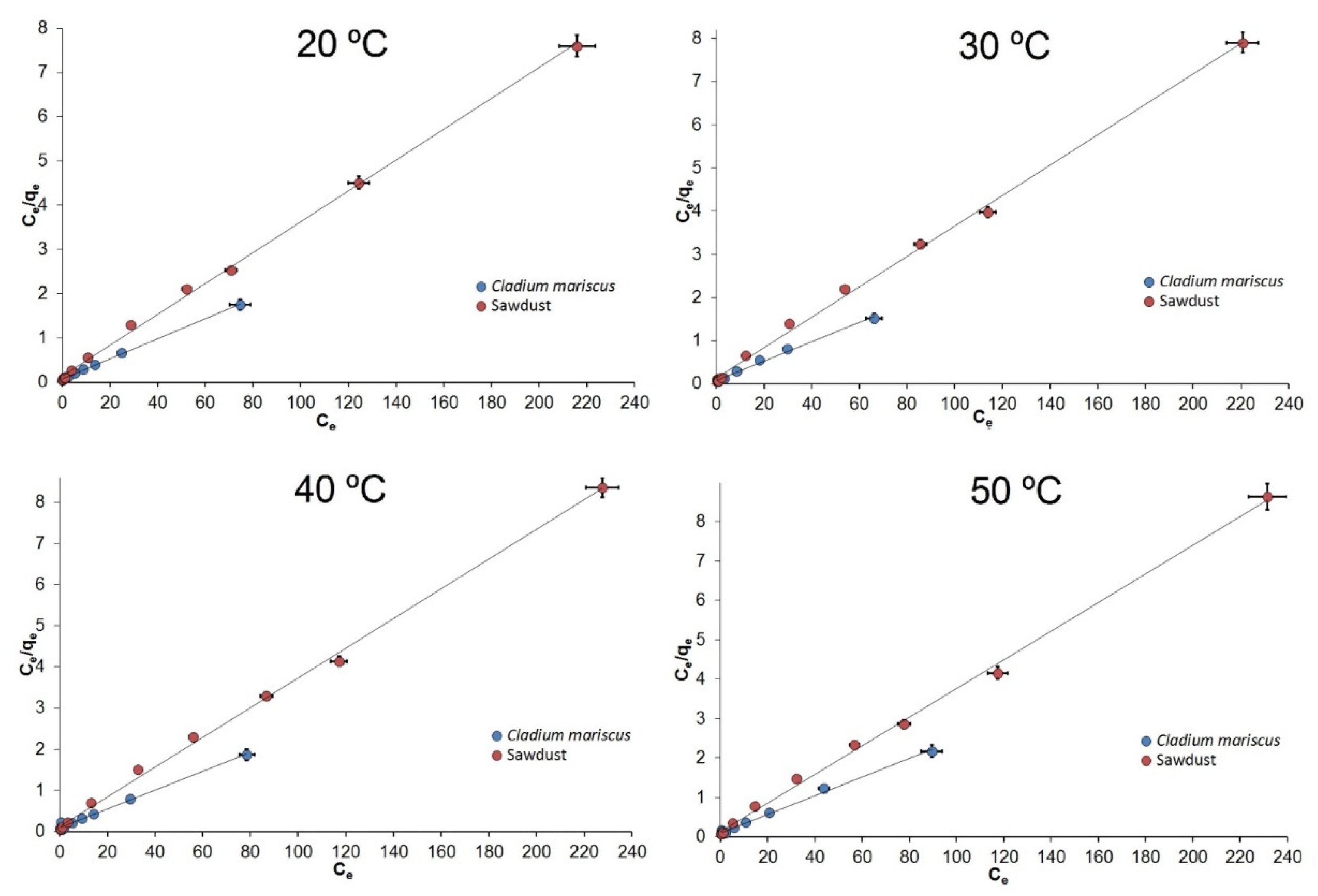
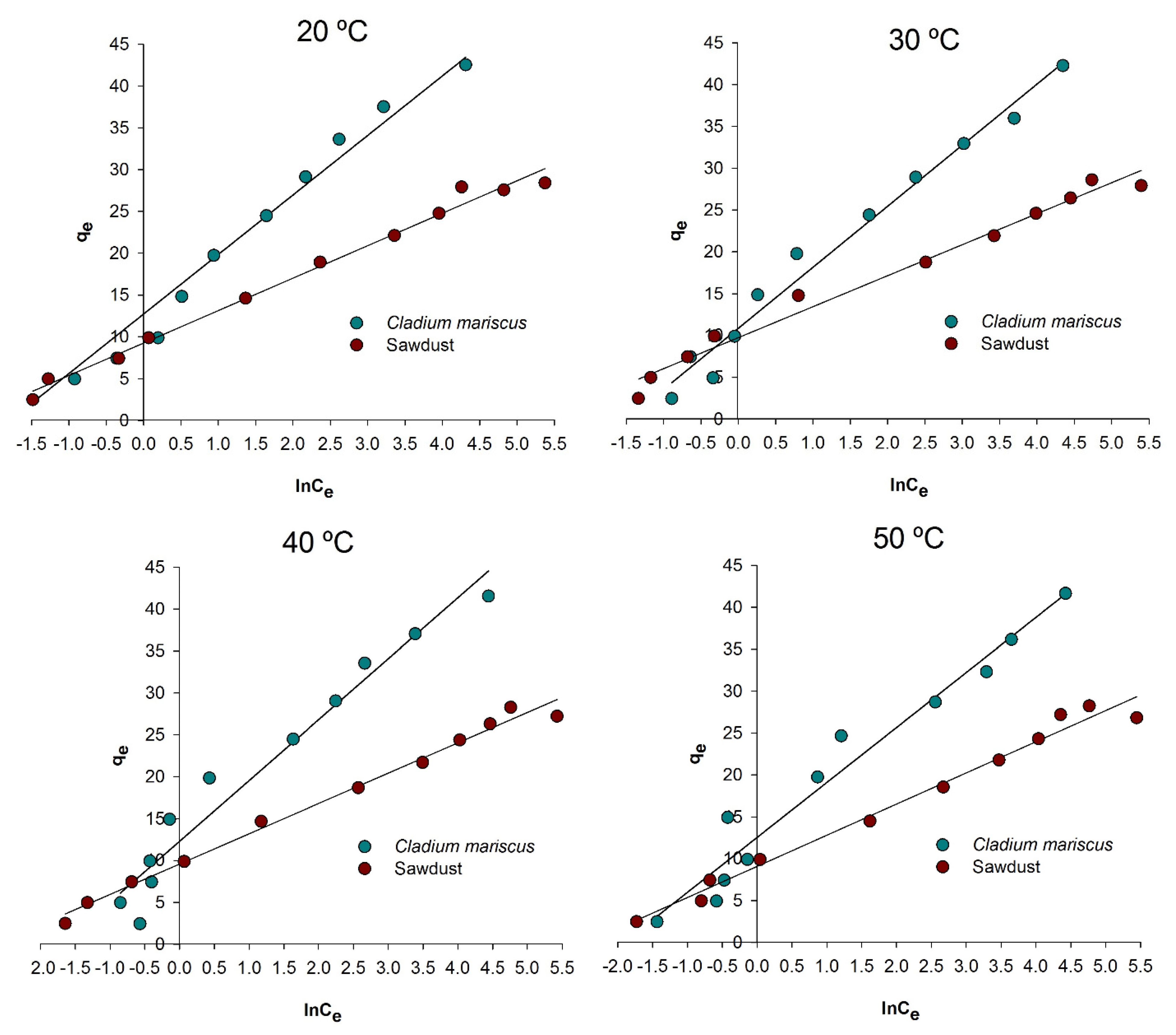
3.2. Isotherm of Adsorption
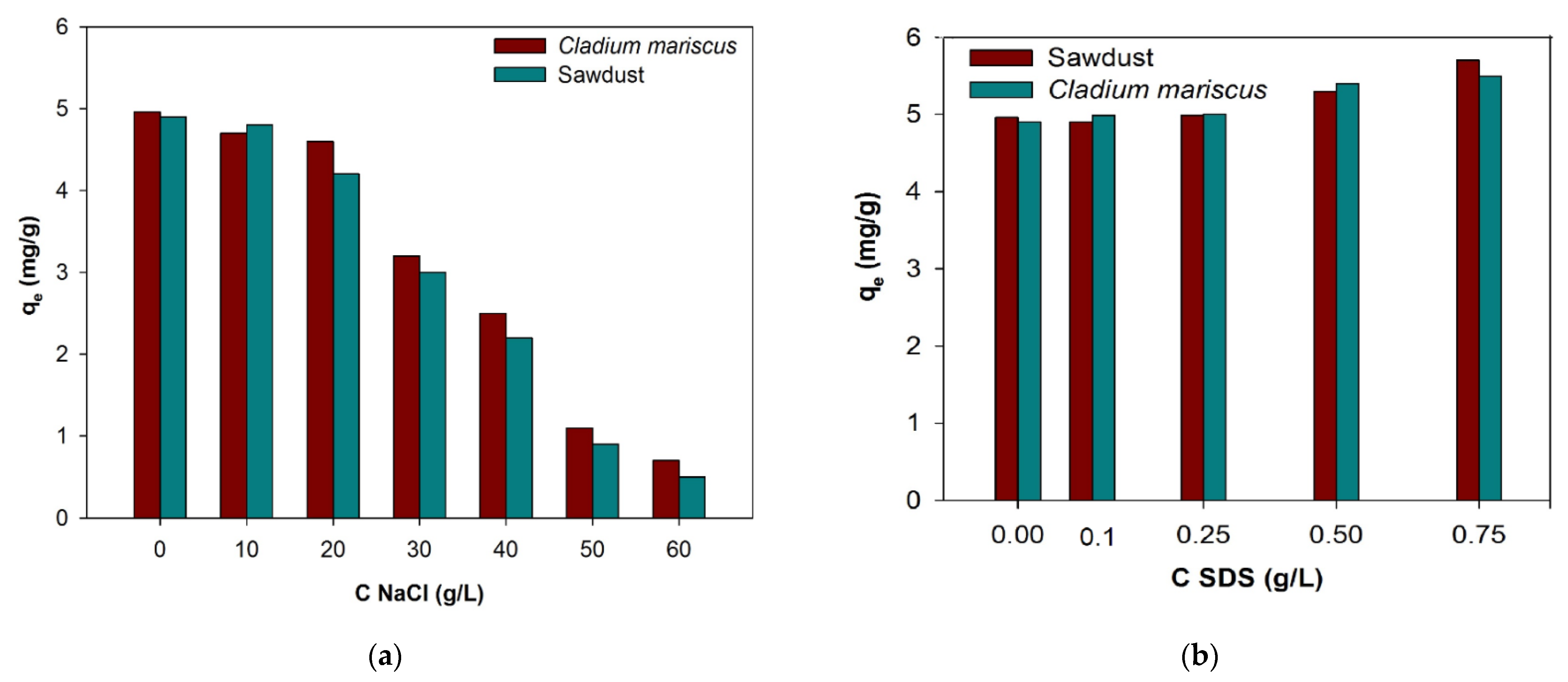
3.3. Salt and Surfactant Impact on BB3 Uptake
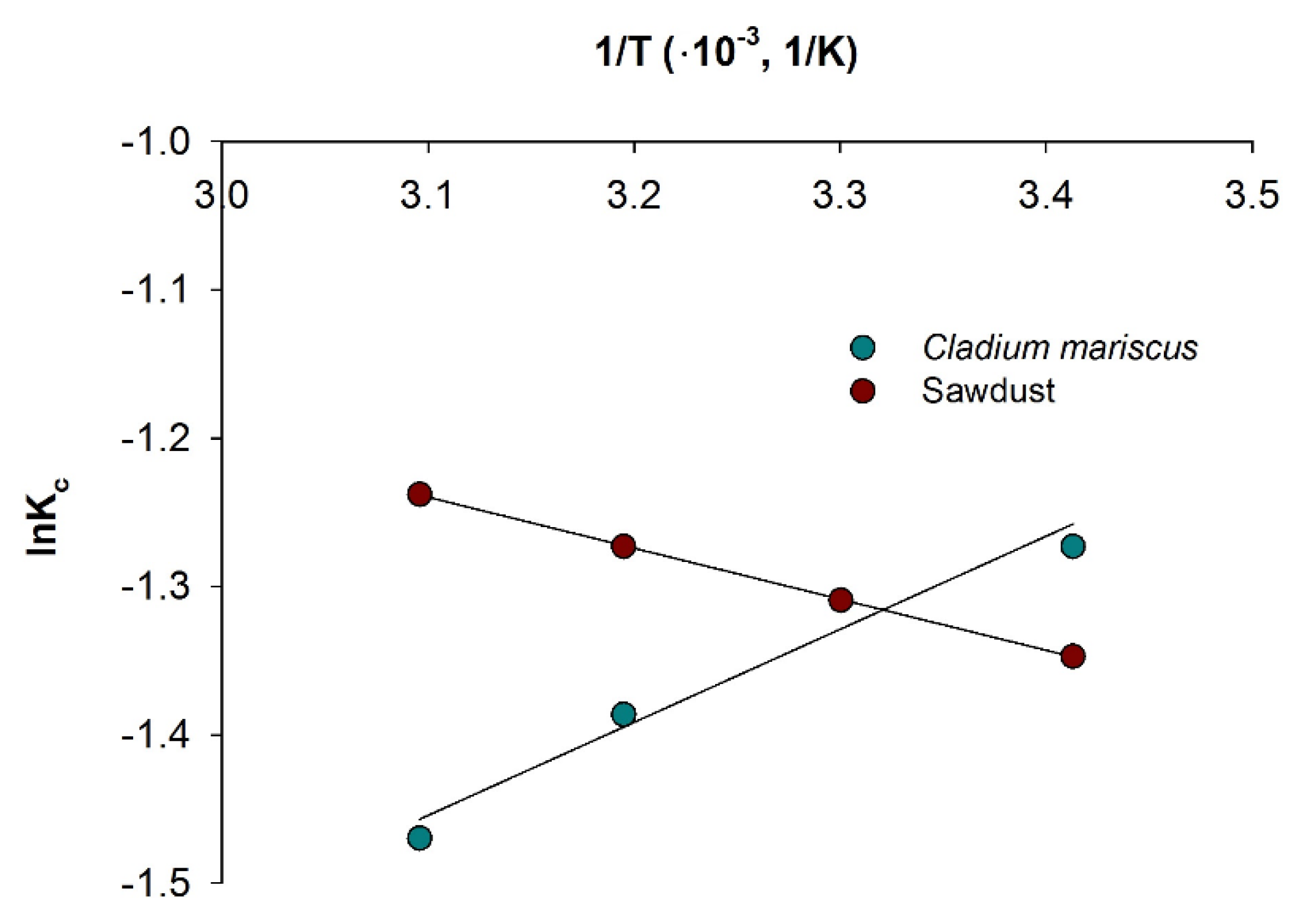
3.4. Thermodynamic Parameters of the Adsorption
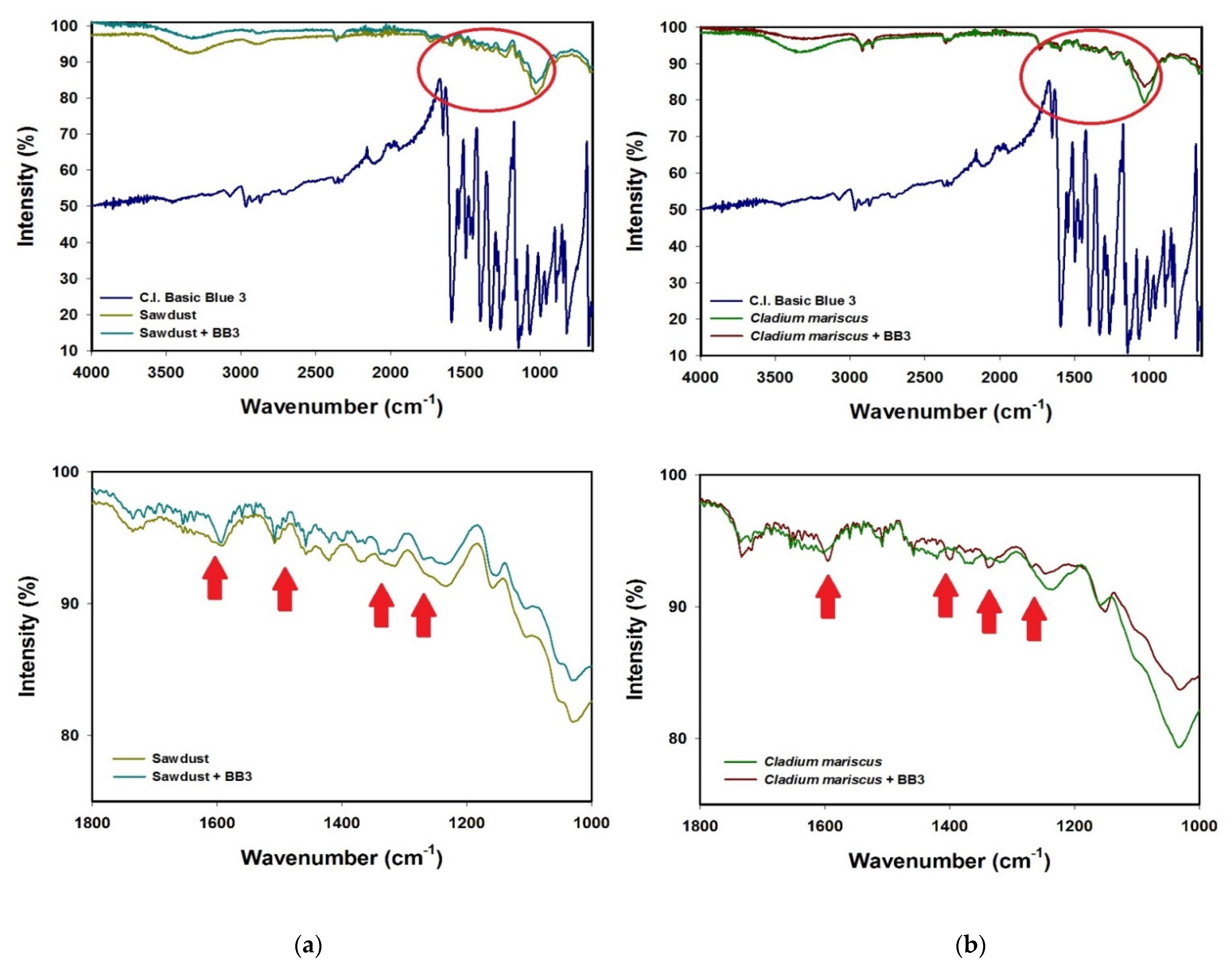
3.5. FT-IR Studies and Mechanism of Biosorption
3.6. Desorption Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chu, H.C.; Chen, K.M. Reuse of activated sludge biomass: I. Removal of basic dyes from wastewater biomass. Process Biochem. 2002, 37, 595–600. [Google Scholar] [CrossRef]
- Wong, S.Y.; Tan, Y.P.; Abdullah, A.H.; Ong, S.T. The removal of basic and reactive dyes using quartenised sugar cane bagasse. J. Phys. Sci. 2009, 20, 59–74. [Google Scholar]
- Adegoke, K.A.; Bello, O.S. Dye sequestration using agricultural wastes as adsorbents. Water Resour. Ind. 2015, 12, 8–24. [Google Scholar] [CrossRef] [Green Version]
- Lacasse, K.; Baumann, W. Textile Chemicals: Environmental Data and Facts; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Ong, S.T.; Lee, C.K.; Zainal, Z. A comparison of sorption and photodegradation study in the removal of basic and reactive dyes. Aust. J. Basic Appl. Sci. 2009, 3, 3408–3416. [Google Scholar]
- Ong, S.T.; Keng, P.; Lee, S.; Leong, M.; Hung, Y. Equilibrium studies for the removal of basic dye by sunflower seed husk (Helianthus annuus). Int. J. Phys. Sci. 2010, 5, 1270–1276. [Google Scholar]
- Ciesielczyk, F.; Bartczak, P.; Jesionowski, T. A comprehensive study of Cd(II) ions removal utilizing high-surface-area binary Mg-Si hybrid oxide adsorbent. Int. J. Environ. Sci. Technol. 2015, 12, 3613–3626. [Google Scholar] [CrossRef] [Green Version]
- Pathania, D.; Sharma, A.; Siddiqi, Z.M. Removal of congo red dye from aqueous system using Phoenix dactylifera seeds. J. Mol. Liq. 2016, 219, 359–367. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, D.; Pandey, L.; Gaur, J.P. Methylene blue sorption capacity of some common waste plant materials. Chem. Eng. Commun. 2010, 197, 1435–1444. [Google Scholar] [CrossRef]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V. A critical review on textile wastewaters: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef]
- Hasanbeigi, A.; Price, L. A technical review of emerging technologies for energy and water efficiency and pollution reduction in the textile industry. J. Clean. Prod. 2015, 95, 30–44. [Google Scholar] [CrossRef] [Green Version]
- Royer, B.; Lima, E.C.; Cardoso, N.F.; Calvete, T.; Bruns, R.E. Statistical design of experiments for optimization of batch adsorption conditions for removal of reactive red 194 textile dye from aqueous effluents. Chem. Eng. Commun. 2010, 197, 775–790. [Google Scholar] [CrossRef]
- Wawrzkiewicz, M.; Polska-Adach, E. Physicochemical interactions in systems C.I. Direct Yellow 50–weakly basic resins: Kinetic, equilibrium, and auxiliaries addition aspects. Water 2021, 13, 385. [Google Scholar] [CrossRef]
- Zhigang, J.; Ziyu, L.; Tao, N.; Shengbiao, L. Adsorption of low-cost absorption materials based on biomass (Cortaderia selloana flower spikes) for dye removal: Kinetics, isotherms and thermodynamic studies. J. Mol. Liq. 2017, 229, 285–292. [Google Scholar]
- Kim, S.Y.; Jin, M.R.; Chung, C.H.; Yun, Y.S.; Jahng, K.Y.; Yu, K.Y. Biosorption of cationic basic dye and cadmium by the novel biosorbent Bacillus catenulatus JB-022 strain. J. Biosci. Bioeng. 2015, 119, 433–439. [Google Scholar] [CrossRef]
- Contreras, E.G.; Martinez, B.E.; Sepúlveda, L.A.; Palma, C.L. Kinetics of basic dye adsorption onto Sphagnum magellanicum peat. Ads. Sci. Technol. 2007, 25, 637–646. [Google Scholar] [CrossRef] [Green Version]
- Khataee, A.R.; Vafaei, F.; Jannatkhah, M. Biosorption of three textile dyes from contaminated water by filamentous green algal Spirogyra sp.: Kinetic, isotherm and thermodynamic studies. Int. Biodeter. Biodegr. 2013, 83, 33–40. [Google Scholar] [CrossRef]
- Liew, S.-W.; Ong, S.-T. Removal of basic blue 3 dye using pomelo peel. Asian J. Chem. 2014, 26, 3808–3814. [Google Scholar] [CrossRef]
- Aber, S.; Sheydaei, M. Application of physicochemically prepared activated carbon fiber for the removal of Basic Blue 3 from water. Desal. Water Treat. 2011, 28, 97–102. [Google Scholar] [CrossRef]
- Vasanth Kumar, K.; Sivanesan, S. Isotherm parameters for basic dyes onto activated carbon: Comparison of linear and non-linear method. J. Hazard. Mater. B 2006, 129, 147–150. [Google Scholar] [CrossRef]
- Nassar, M.N.; Daifullah, A.H.; Magdy, Y.H.; Ebrahiem, E.E. Uptake of cationic dyes by cement kiln dust: Sorption mechanism and equilibrium isotherm. Ads. Sci. Technol. 2002, 20, 657–668. [Google Scholar] [CrossRef] [Green Version]
- Karagozoglu, B.; Tasdemir, M.; Demirbas, E.; Kobya, M. The adsorption of basic dye (Astrazon Blue FGRL) from aqueous solutions onto sepiolite, fly ash and apricot shell activated carbon: Kinetic and equilibrium studies. J. Hazard. Mater. 2007, 147, 297–306. [Google Scholar] [CrossRef]
- Barsanescu, A.; Buhaceanu, R.; Dulman, V. Removal of basic blue 3 by sorption onto a weak acid acrylic resin. J. Appl. Polym. Sci. 2009, 113, 607–614. [Google Scholar] [CrossRef]
- Wawrzkiewicz, M. Removal of C.I Basic Blue 3 dye by sorption onto cation exchange resin, functionalized and non-functionalized polymeric sorbents from aqueous solutions and wastewaters. Chem. Eng. J. 2013, 217, 414–425. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. K. Sven. Vetensk. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W.; Morris, J. Kinetics of adsorption on carbon from solutions. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Temkin, M.I. Adsorption equilibrium and kinetics of processes on heterogeneous surfaces and at interaction between molecules. Russ. J. Phys. Chem. 1941, 15, 296–303. [Google Scholar]
- Vasanth Kumar, K. Linear and non-linear regression analysis for the sorption kinetics of methylene blue onto activated carbon. J. Hazard. Mater. B 2006, 137, 1538–1544. [Google Scholar] [CrossRef]
- Crini, G.; Gimbert, F.; Robert, C.; Martel, B.; Adama, O.; Morin-Crini, N.; De Giorgi, F.; Badot, P.-M. The removal of Basic Blue 3 from aqueous solutions by chitosan-based adsorbent: Batch studies. J. Hazard. Mater. 2008, 153, 96–106. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modelling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Dogan, M.; Karaoglu, M.H.; Alkan, M. Adsorption kinetics of maxilon yellow 4GL and maxilon red GRL dyes on kaolinite. J. Hazard. Mater. 2009, 165, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Genc, A.; Oguz, A. Sorption of acid dyes from aqueous solution by using non-ground ash and slag. Desalination 2010, 264, 78–83. [Google Scholar] [CrossRef]
- Janoš, P.; Šmídová, V. Effects of surfactants on the adsorptive removal of basic dyes from water using an organomineral sorbent-iron humate. J. Colloid Interface Sci. 2005, 291, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Janoš, P.; Šedivý, P.; Rýznarová, M.; Grötschelová, S. Sorption of basic and acid dyes from aqueous solutions onto oxihumolite. Chemosphere 2005, 59, 881–886. [Google Scholar] [CrossRef]
- Janoš, P.; Buchtová, H.; Rýznarová, M. Sorption of dyes from aqueous solutions onto fly ash. Water Res. 2003, 37, 4938–4944. [Google Scholar] [CrossRef]
- Janoš, P. Sorption of basic dyes onto iron humate. Environ. Sci. Technol. 2003, 37, 5792–5798. [Google Scholar] [CrossRef]
- Can, M. Investigation of the factors affecting Acid Blue 256 adsorption from aqueous solutions onto red pine sawdust: Equilibrium, kinetics, process design, and spectroscopic analysis. Desalin. Water Treat. 2016, 57, 5636–5653. [Google Scholar] [CrossRef]
- Dai, D.; Fan, M. Preparation of bio-composite from wood sawdust and gypsum. Ind. Crops Prod. 2015, 74, 417–424. [Google Scholar] [CrossRef]
- Ouazene, N.; Lounis, A. Adsorption characteristics of C.I. Basic Blue 3 from aqueous solution onto Aleppo pine-tree sawdust. Color. Technol. 2011, 128, 21–27. [Google Scholar] [CrossRef]




| Parameter | Cladium mariscus Saw-Sedge | Sawdust |
|---|---|---|
| Elemental analysis | 48% C, 7.1% H, 0.95% N, 0.4% S | 49.5% C, 8.8% H, 0.4% N, 0.5% S |
| Specific surface area BET (m2/g) | 0.60 | 1.17 |
| Volume of the pores (cm3/g) | 0.01 | 0.58 |
| Kinetic Model | Parameter | Initial Concentration of BB3 (mg/L) | |||||
|---|---|---|---|---|---|---|---|
| 50 | 100 | 200 | 50 | 100 | 200 | ||
| Sawdust | Cladium mariscus | ||||||
| qe,exp (mg/g) | 4.96 | 9.77 | 18.26 | 4.87 | 9.51 | 18.28 | |
| PFO | qe,cal (mg/g) | 0.52 | 2.13 | 8.40 | 1.12 | 3.57 | 9.40 |
| k1 (1/min) | 0.022 | 0.022 | 0.019 | 0.022 | 0.026 | 0.012 | |
| r2 | 0.844 | 0.854 | 0.966 | 0.906 | 0.959 | 0.914 | |
| PSO | qe,cal (mg/g) | 4.96 | 9.82 | 18.50 | 4.90 | 9.64 | 18.24 |
| k2 (g/mg min) | 0.27 | 0.06 | 0.01 | 0.11 | 0.03 | 0.01 | |
| h (mg/g min) | 6.74 | 5.52 | 3.31 | 2.564 | 2.732 | 2.201 | |
| r2 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.996 | |
| IP | kip (mg/g min0.5) | 0.069 | 0.290 | 1.010 | 0.103 | 0.485 | 0.896 |
| Cip | 4.41 | 7.39 | 8.66 | 3.88 | 5.29 | 7.88 | |
| r2 | 0.781 | 0.926 | 0.844 | 0.999 | 0.986 | 0.929 | |
| Adsorbent | Type of Kinetic | r2 | k1 or k2 (1/min) or (g/mg min) | BB3 Concentration (mg/g) | Ref. |
|---|---|---|---|---|---|
| Bacillus catenulatus | PFO | 0.986 | 0.17 | 500 | [15] |
| Sphagnum Magellanicum peat | 0.999 | 0.08 | 50 | ||
| PSO | 0.999 | 0.02 | 100 | [16] | |
| 0.999 | 0.01 | 200 | |||
| Spirogyra sp. biomass | PSO | 0.999 | 0.03 | 15 | [17] |
| Pomelo peel | 0.997 | 0.04 | 20 | ||
| PSO | 0.999 | 0.03 | 30 | [18] | |
| 0.996 | 0.01 | 40 | |||
| Activated carbon | PSO | 0.990 | 0.001 | 20 | [19] |
| Chitosan-based adsorbent | PSO | 0.994 | 0.02 | 20 | [32] |
| 0.999 | 0.27 | 50 | |||
| Sawdust | PSO | 0.999 | 0.06 | 100 | This study |
| 0.999 | 0.01 | 200 | |||
| 0.999 | 0.11 | 50 | |||
| Cladium mariscus saw- sedge | PSO | 0.999 | 0.03 | 100 | This study |
| 0.996 | 0.01 | 200 |
| Adsorbent | T (°C) | Langmuir Parameters | Freundlich Parameters | Temkin Parameters | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r2 | Q0 (mg/g) | b (L/mg) | r2 | kF (mg/g) | n | r2 | bT (J/mol) | A (L/g) | ||
| Sawdust | 20 | 0.999 | 28.69 | 0.26 | 0.891 | 7.27 | 3.25 | 0.990 | 638.02 | 10.87 |
| 30 | 0.998 | 28.35 | 0.27 | 0.848 | 7.64 | 3.45 | 0.982 | 668.02 | 13.81 | |
| 40 | 0.998 | 27.73 | 0.28 | 0.888 | 7.47 | 3.44 | 0.990 | 684.09 | 14.06 | |
| 50 | 0.998 | 27.50 | 0.29 | 0.901 | 7.14 | 3.32 | 0.983 | 665.29 | 11.47 | |
| Cladium mariscus | 20 | 0.999 | 44.29 | 0.28 | 0.929 | 9.12 | 2.16 | 0.966 | 347.91 | 5.98 |
| 30 | 0.994 | 44.62 | 0.27 | 0.819 | 9.49 | 2.24 | 0.970 | 337.51 | 5.77 | |
| 40 | 0.991 | 43.93 | 0.25 | 0.740 | 9.32 | 2.30 | 0.958 | 331.55 | 5.24 | |
| 50 | 0.995 | 42.07 | 0.23 | 0.795 | 8.48 | 2.31 | 0.975 | 352.66 | 4.74 | |
| Kind of Adsorbent | qm (mg/g) | Ref. |
|---|---|---|
| Green algal Spirogyra sp. | 71.4 | [17] |
| Cladium mariscus sew-sedge | 44.4 | This study |
| Sphagnum Magellanicum Peat | 40.6 | [16] |
| Quaternized sugar cane bagasse | 37.6 | [2] |
| Activated sludge biomass | 36.5 | [1] |
| Sawdust | 28.7 | This study |
| Pomelo peel | 23.9 | [18] |
| Sugarcane bagasse | 23.6 | [18] |
| Ethylenediamine-modified rice hull | 3.29 | [5] |
| Wood activated charcoal | 0.59–0.64 | [20] |
| Adsorbent | ΔH° (kJ/mol) | ΔS° (J/mol K) | ΔG° (kJ/mol) | |||
|---|---|---|---|---|---|---|
| 20 °C | 30 °C | 40 °C | 50 °C | |||
| Sawdust | 2.83 | −1.43 | 3.28 | 3.30 | 3.31 | 3.32 |
| Cladium mariscus | −4.30 | −28.28 | 3.10 | 3.30 | 3.61 | 3.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartczak, P.; Wawrzkiewicz, M.; Borysiak, S.; Jesionowski, T. Cladium mariscus Saw-Sedge versus Sawdust—Efficient Biosorbents for Removal of Hazardous Textile Dye C.I. Basic Blue 3 from Aqueous Solutions. Processes 2022, 10, 586. https://doi.org/10.3390/pr10030586
Bartczak P, Wawrzkiewicz M, Borysiak S, Jesionowski T. Cladium mariscus Saw-Sedge versus Sawdust—Efficient Biosorbents for Removal of Hazardous Textile Dye C.I. Basic Blue 3 from Aqueous Solutions. Processes. 2022; 10(3):586. https://doi.org/10.3390/pr10030586
Chicago/Turabian StyleBartczak, Przemysław, Monika Wawrzkiewicz, Sławomir Borysiak, and Teofil Jesionowski. 2022. "Cladium mariscus Saw-Sedge versus Sawdust—Efficient Biosorbents for Removal of Hazardous Textile Dye C.I. Basic Blue 3 from Aqueous Solutions" Processes 10, no. 3: 586. https://doi.org/10.3390/pr10030586
APA StyleBartczak, P., Wawrzkiewicz, M., Borysiak, S., & Jesionowski, T. (2022). Cladium mariscus Saw-Sedge versus Sawdust—Efficient Biosorbents for Removal of Hazardous Textile Dye C.I. Basic Blue 3 from Aqueous Solutions. Processes, 10(3), 586. https://doi.org/10.3390/pr10030586