1. Introduction
Botrytis cinerea is a fungal plant pathogen that causes the grey mold (or Botrytis bunch rot) in mature berries and is the largest encountered fungal infection in vineyards, with a fast-spreading rate. Its occurrence and development are favored by cold and humid weather.
Botrytis cinerea has devastating economic effects and it represents a hard problem for grape growers and wine producers worldwide. Currently, preventive fungicide treatments are performed in vineyards according to the specific weather conditions in order to reduce the impact of the
Botrytis cinerea. In the last decades, several studies have been carried out to find an organic alternative to fungicides, including the use of yeast as a biocontrol agent against
Botrytis cinerea [
1].
Botrytis cinerea acts by excreting enzymes that degrade the cellular walls of grapes, progressing to metabolic changes and necrosis of the grapes.
Laccase is considered an indicator of a grape’s fungal infection with
Botrytis cinerea, with an activity higher than 3 U/mL indicating susceptibility to the oxidative enzymatic degradation of wine [
2]. Appropriate treatment of the must with sulfur dioxide should be performed promptly to obtain high quality wines [
2]. Moreover, if the degree of infection is higher than 50%, the grapes should not be processed further as the damage is too great and the irreversible deterioration of wine quality cannot be prevented.
Various approaches have been considered for the detection of
Botrytis cinerea [
3], including gigahertz ultrasonic imaging of fungal spores [
4], the fingerprint of the Raman spectrum of the fungal spores [
5], detection from the mycelia using antibodies specific for
Botrytis species [
6], detection of the fungal DNA [
7,
8,
9], or indirect detection through the measurement of indicators such as laccase activity [
10], glycerol and gluconic acid [
10], or plant hormones (i.e., the combined detection of salicylic, azelaic, and jasmonic acids) [
11]. It is important to note that not all strains of
Botrytis cinerea produce laccase. However, due to its importance and relative simplicity, laccase activity evaluation is the most convenient and widely used test for screening for grape infection by
Botrytis cinerea.
Despite the various methods that have been developed, there are still no quantitative, fast procedures to be deployed in the vineyard and in the grape processing centers to screen grapes for fungal attack by
Botrytis cinerea. For the evaluation of laccase activity by observing color formation, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and syringaldazine are the most used enzymatic substrates [
12,
13,
14]. Various analytical kits are available commercially and some are very sensitive, with detection limit down to 2 mU/mL; nonetheless, many involve laboratory-based equipment for colorimetric or fluorimetry-based measurements [
15,
16]. Syringaldazine is oxidized by laccase into a pink-purple compound, while ABTS is transformed in ABTS
+, a green-blue colored compound. ABTS has the advantage that is water soluble, while syringaldazine was used in the studies that defined the 3 U/mL critical activity threshold for the high risk of irreversible browning of wines. Semi-quantitative kits for laccase based on syringaldazine are also available, enabling the simple detection of laccase activity in less than 15 min [
17]. There are also several research studies that focused on finding a simple and fast method for detecting laccase. For example, Dias et al. [
18] adsorbed ABTS on filter paper disks which were placed in a reaction well and reacted for 10 min at 30 °C with the sample added to the well. The approach was used to identify, with the naked eye, the fractions containing laccase during the purification of laccase extracted from
Ganoderma resinaceum [
18]. While simple and effective for screening a large number of purification fractions, the method was not developed further into a semi-quantitative procedure (and was not used to observe grape contamination).
The colorimetric measurement of laccase activity based either on the green-blue ABTS or pink-purple syringaldazine requires clear, colorless samples. The recommended sample preparation approach, which also eliminates the phenolic compounds that are inhibitors of laccase, is to treat the grape musts with polyvinylpolypyrollidone (PVPP) [
19].
Electrochemical detection is an attractive alternative to optical methods due to its compatibility with turbid or colored samples, high sensitivity, and relatively simple equipment. The enzymatic activity of several enzymes was evaluated via electrochemistry [
20,
21]. Moreover, we had previously described a fast electrochemical assay for laccase activity based on the enzymatic substrate ABTS and amperometric detection. The detection was based on the electrochemical reduction of enzymatically generated ABTS
+ at a potential of 0 V vs. Ag/AgCl, according to the reaction:
The test required only 5 min and is characterized by a detection limit of 0.5 U/mL [
22].
Furthermore, the combination of paper membranes impregnated with various reagents (enzymes, cofactors, and/or electrochemical mediators) with electrochemical sensors has brought many benefits in terms of cost, simplicity, and speed, e.g., in microfluidic systems and “origami” [
23,
24] or “kirigami” systems [
25]. There are many examples in the literature of such devices for determining the activity of enzymes or their inhibitors [
20,
21]. For example, the activity of acetylcholinesterase was measured using an electrochemically active enzyme–substrate adsorbed on paper [
20].
Considering all the above, and with the aim to increase reliability and further simplify the assay of laccase, we developed a dual optical and electrochemical method where the enzymatic substrate ABTS is pre-adsorbed on a paper sensor and placed on top of a screen-printed electrochemical cell. The laccase activity (i.e., the amount of oxidized ABTS) is then measured via chronoamperometry. The assay was optimized and applied to monitor the artificial infection of different types of grapes with Botrytis cinerea. The influence of the fungal strain and grape variety was examined, as was the correlation of the infection degree with the laccase activity. Moreover, the results obtained with the new method were compared to the classic spectrophotometric method based on syringaldazine.
2. Material and Methods
2.1. Reagents and Materials
The reagents and materials used in the study include sodium acetate (Sigma-Aldrich Merck, Darmstadt, Germany), glacial acetic acid AnalaR Normapur grade (VWR International S.A.S, Baire, France), monobasic and dibasic sodium phosphate and polyvinylpyrrolidone—PVPP (Sigma-Aldrich Merck, Darmstadt, Germany), laccase from Trametes versicolor and chitosan (Sigma-Aldrich Merck, Darmstadt, Germany), 2,2′-azino-bis (3-ethylbenzothiazolin-6-sulfonic acid)—ABTS (Roche Diagnostics, Mannheim, Germany), and medium porosity filter paper and screen-printed DRP 220 AT three-electrode systems printed on a ceramic support (Metrohm Dropsens, Oviedo, Spain), which contain a 4 mm Au working electrode, an Au counter electrode, and an Ag reference electrode. The buffer solutions used for this study were the 0.1 M acetate of pH 4.5, which was needed for the preparation of laccase solutions and ABTS solution, pre-conditioning the PVPP cartridges, and the control sample (i); the 0.1 M acetate of pH 5.5 for pre-conditioning the PVPP cartridges (ii); and the 0.1 M phosphate buffer of pH 6.5, which was used for the preparation of the laccase stock solution (iii). To reach the desired laccase activities for calibration and spiking experiments, appropriate dilutions were made with 0.1 M acetate buffer of pH 4.5 from a 1 mg/mL (112 U/mL) stock laccase solution.
2.2. Equipment
The electrochemical experiments were conducted using a VSP potentiostat equipped with EC-Lab software (BioLogic SAS, France) and a Dropsens μStat 8000 potentiostat with Dropview 8400 software (Metrohm Dropsens, Oviedo, Spain). The experiments were performed with the electrodes placed horizontally. A 7 mm × 7 mm piece of ABTS-containing paper was placed on the surface of the electrode, over which 30 μL of laccase solution or must sample was added. Chronoamperometric measurements were performed at 0 V for up to 10 min. The difference between the current intensity at 30 s and at 300 s was taken as the analytical signal to be correlated with laccase activity in the must sample.
Spectrophotometric measurements were performed with a UV-VIS Evolution 600 spectrophotometer (Thermo Scientific, Loughborough, United Kingdom) equipped with VISION PRO software. All measurements were performed at room temperature.
2.3. Development of the Paper Sensor
The ABTS solution (0.4 mg/mL in the 0.1 M acetate buffer of pH 4.5. unless mentioned otherwise) or the ABTS-chitosan mixture (0.4 mg/mL ABTS and 0.5 mg/mL chitosan in 0.1 M acetate buffer of pH 4.5) was poured over a piece of round filter paper with a diameter of 12 cm in a petri dish. The paper was soaked in the ABTS-containing solution for 20 min and turned once to favor the uniform adsorption of the reagent. Next, the paper was placed it in a clean bowl and dried at 50 °C. Square pieces measuring 7 mm × 7 mm were cut from the treated paper and used in the electrochemical and optical assays.
2.4. Laccase Activity Testing by the Spectrophotometric Method with Syringaldazine
The laccase activity was measured based on the absorbance of oxidized syringaldazine at 530 nm, according to the method of Grassin [
2].
2.5. Image Processing Applied to the Paper Sensor
Images of the paper sensor (for different reaction times with laccase, different activities of laccase, or sensors obtained with different impregnating solutions) were taken with a Huawei P Smart 2018 model FIX-LX1 smartphone, equipped with Android 9.0 operating software and dual camera (a back camera of 13 MP + 2 MP, used to take the pictures of the sensors, and a 8 MP front camera). The “Autoexposure” function of the camera was, by default, set to “on”. All images were taken in ambient light, with the flash off, and included controls. The pictures were processed using ImageJ. Three regions of approximately equal areas were measured on each sensor. The difference in the mean grey value between the sample containing laccase and one containing only acetate buffer (the control sample, tested in parallel in the same conditions), divided to the mean grey value of the control sample, was taken as the analytical signal to be correlated with laccase activity.
2.6. Artificial Infection and Sample Preparation Procedure
The artificial inoculation of grapes with
Botrytis cinerea was studied in three separate experiments in Spain and Romania. In the first experiment, the berries of the Tempranillo variety of grapes were first sterilized with a solution of sodium hypochlorite, rinsed three times with sterile water, and dried. A small, superficial cut was made near the distal end of the berry and 10 µL of a solution of 10
6 CFU spores of
Botrytis cinerea were placed in the cut. The berries were stored in an incubator at 24 °C for 20 days. Samples were taken regularly, more often in the first 12 days, and then directly after 20 days. The second experiment was similar, except that the storage of the berries following inoculation was at 24 °C and 55% RH (relative humidity), and the experiment was allowed to proceed for 13 days, after which the berries were grouped according to the degree of infection, and then analyzed for their laccase activity. In the third experiment, red (Moldova variety), rosé (Red Globe), and white grapes (unknown variety) were acquired from a local supermarket. The healthiest berries of similar size were selected and further sterilized with ethyl alcohol, rinsed with sterile water, and dried. For each type of grapes, the berries were split into two groups which were infected with different strains of
Botrytis cinerea. After proceeding in a similar manner as for the Tempranillo grapes for inoculation with
Botrytis cinerea, the berries were placed in an incubator at 24 °C and 90% RH (ensured by placing several small containers with water inside the incubator). The white grapes were removed after 13 days, while the rosé and red ones were left for two more days. After this time, the berries were sorted according to the degree of infection. To analyze the laccase activity of musts, the berries were crushed in sterile bags and the must was filtered through a PVPP cartridge [
22] prior to analysis.
3. Results
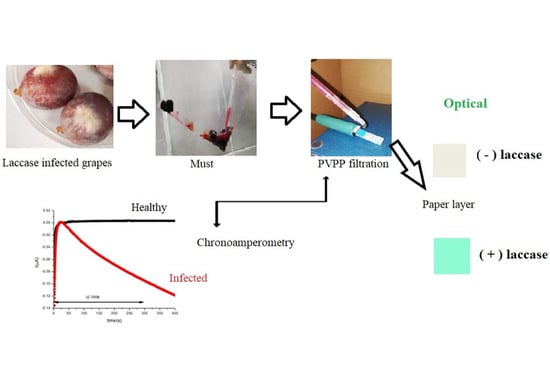
3.1. Principle of the Assay
The principle of the dual optical and electrochemical assay used to assess laccase activity is depicted in
Figure 1.
After filtration through a PVPP cartridge (to remove interfering phenolic compounds which interfere both with the ABTS—enzyme reaction and with the color readout), a drop of filtered must (approximately 30 µL) is placed on an ABTS-impregnated filter paper (7 mm× 7 mm) covering a three-electrode screen-printed cell, which includes Au working and counter electrodes and an Ag reference electrode. The enzymatic oxidation of ABTS in the paper by the laccase in the sample leads to the formation of the oxidized (green-blue) ABTS. Chronoamperometry measurements at 0 V are started immediately after depositing the must. At the chosen applied potential, the oxidized ABTS is reduced back and can re-enter in the enzymatic reaction. The cathodic current generated in a specific time interval through the reduction of the oxidized ABTS is proportional with the amount of oxidized ABTS, and, hence, with the activity of laccase. The difference between the current intensity at a specified time (typically 300 s) and at 30 s is taken as an analytical parameter that is correlated with the laccase activity of the must.
3.2. Optimization of the Paper-Assisted Electrochemical Assay
Aiming to develop a fast, simple, and robust method for laccase activity determination, we first investigated the composition of the ABTS solution used for impregnating the paper sensor in relation to the sensitivity and the duration of the assay. As optimization criteria, the best combination of highest sensitivity to oenology-relevant laccase activity (i.e., 1 U/mL), short test duration, and adequate reproducibility were considered. The paper sensors were used not only as reservoirs of enzymatic, electrochemically active substrate ABTS for the amperometric assay, but also for their usefulness for the visual evaluation of laccase based on the color change of ABTS into its green-blue, oxidized form, with the aim of also using the paper sensor independently from the electrochemical assay.
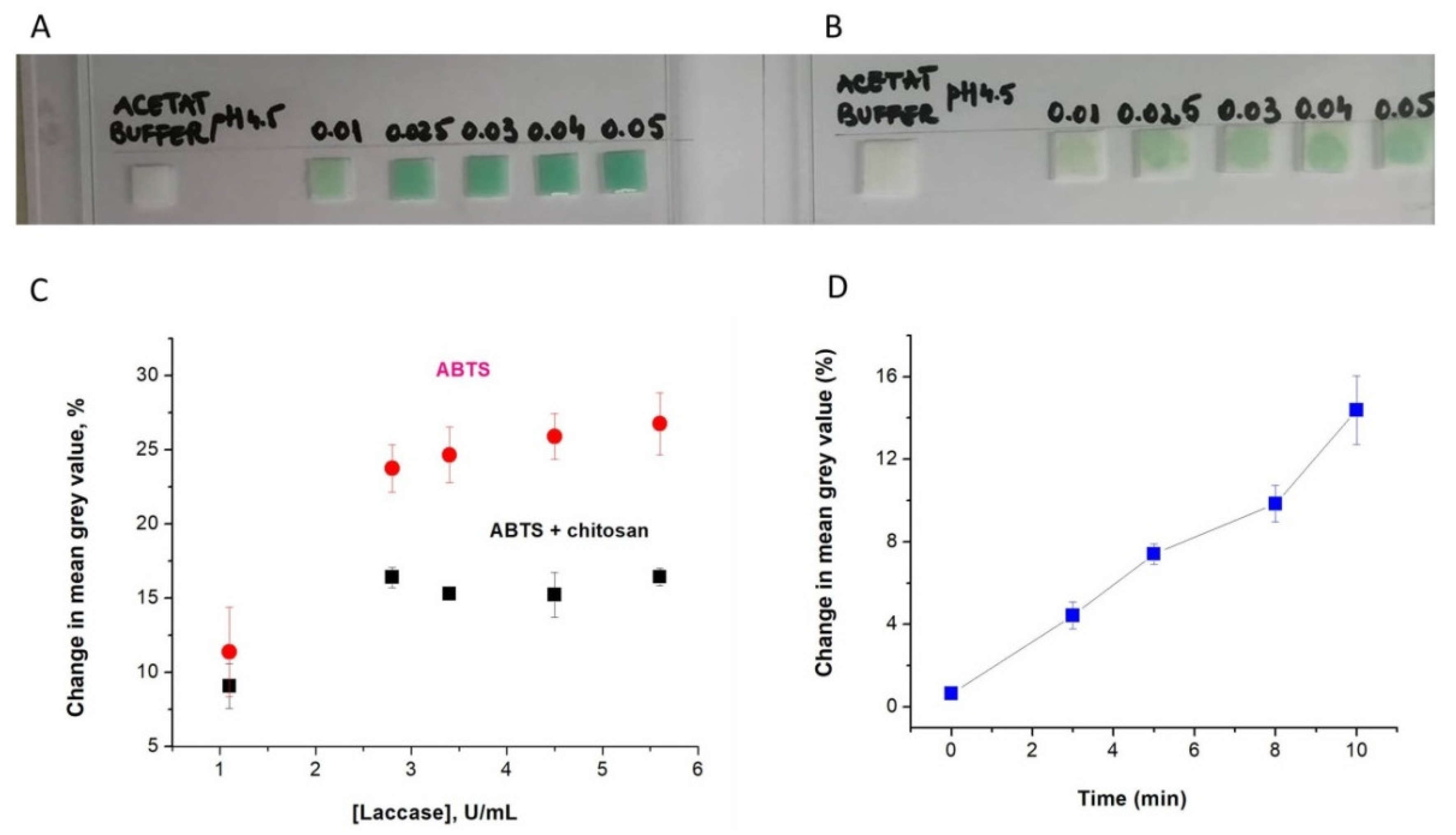
In the first series of experiments, two types of paper sensors were compared, which differed in the composition of the impregnating solution: the first was impregnated with a 0.4 mg/mL ABTS solution (a), and the second with a mixture of 0.4 mg/mL ABTS and 0.5 mg/mL chitosan in a 0.1 M acetate buffer of pH 4.5 (b). The optical detection of laccase was studied by taking pictures of the paper sensors in the presence of laccase solutions of different activities, at regular time intervals, up to 10 min. The interpretation of the changes in color was made by measuring the mean grey value of the paper sensors via ImageJ (as described in
Section 2.5).
Based on the images of the paper sensors impregnated with ABTS only or with a mixture of ABTS and chitosan (
Figure 2A), it was apparent that the addition of chitosan slowed down the diffusion of laccase through the impregnated paper. The color change could be easily seen in the sample application spot compared to the rest of the ABTS–chitosan paper, which can serve as a visual aid. The net change in color was more intense for the paper containing only ABTS (
Figure 2A–C). The change in color intensity increased with time, as shown for the ABTS paper sensors tested with 1.1 U/mL laccase (
Figure 2D). For a clearly (visually) observable change, i.e., defined as 10% of the mean grey value of the paper sensor, at least 8 min was necessary for detecting 1.1 U/mL laccase.
The sensitivity to laccase in the range of activities (1.1–5.6 U/mL) relevant for indicating the grapes’ infection by Botrytis cinerea clearly shows the possibility of using both paper sensors to screen the laccase activity of musts in a simple, semi-quantitative manner, independent of the electrochemical assay.
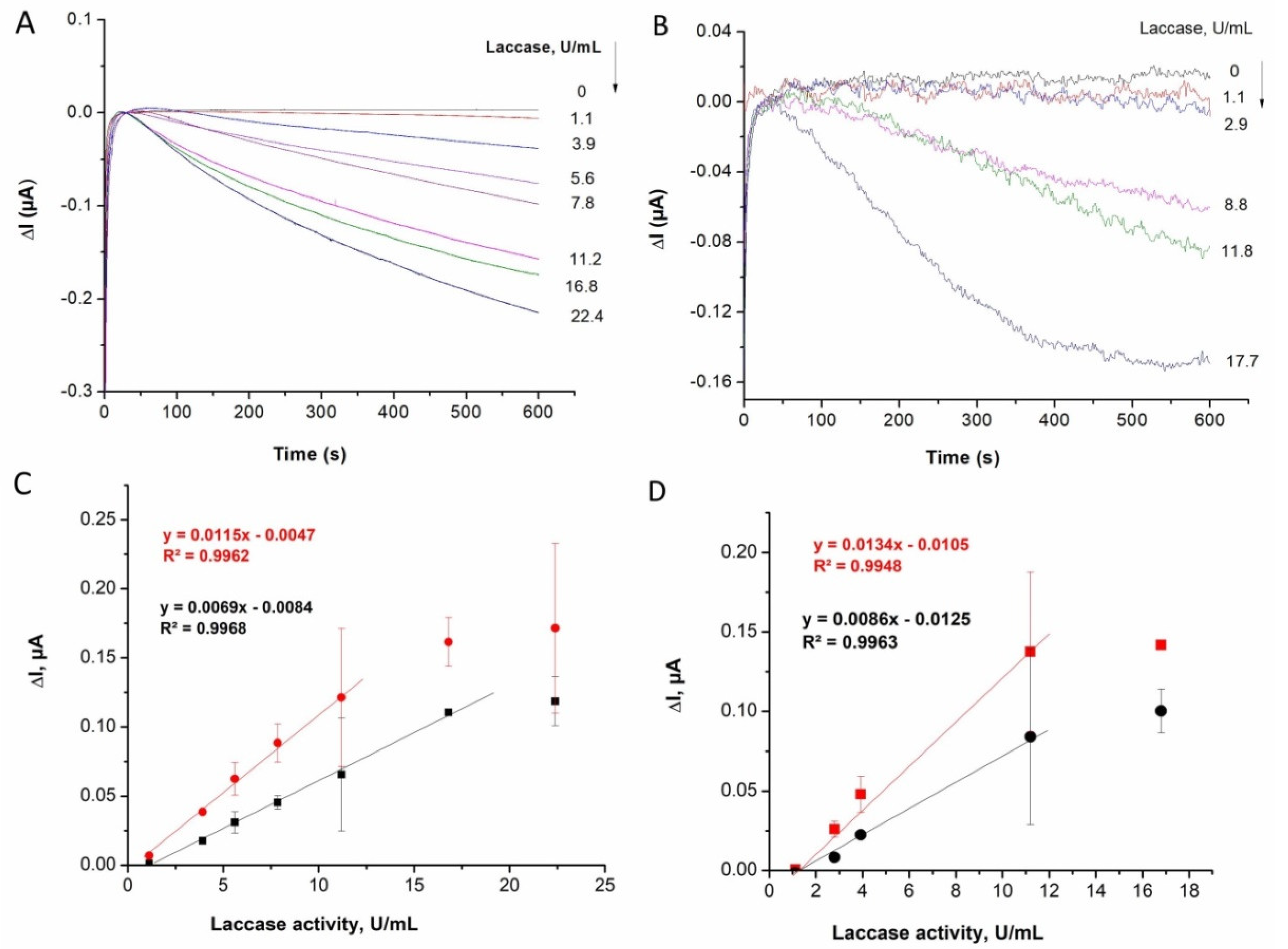
For a faster, quantitative evaluation of laccase activity, the paper sensors with ABTS and ABTS–chitosan were further interfaced with the Au screen-printed electrodes (described in
Section 2.1). Amperometric measurements were performed by measuring the cathodic current increase at 0 V vs. Ag/AgCl for laccase samples of different activities from 1 to 20 U/mL prepared in a 0.1 M acetate buffer of pH 4.5. The cathodic current due to the reduction of enzymatically produced oxidized ABTS was monitored for up to 10 min. Typical current versus time plots are given in
Figure 3A,B, while the corresponding calibration plots seen in
Figure 3C,D are based on the current intensity measured after 5 and 10 min.
By comparing the results obtained using ABTS-only impregnated paper sensors with those of the ABTS and chitosan mixture, the sensitivity of the electrochemical assays appears similar. While there is an apparent sensitivity increase when using paper sensors with ABTS and chitosan, it is not statistically significant. For a 10 min assay, regardless of the paper type, the sensitivity is higher than for the 5 min assay, as through the advancement of the enzymatic reaction, more oxidized ABTS is formed. The detection limit, calculated based on three times the standard deviation of the blank divided by the slope of the calibration curve, varies from 0.26 U/mL to 0.70 U/mL (
Table 1). The evolution of the color (see
Figure 2A versus
Figure 2B) and the evolution of the amperometric current signal (see
Figure 3A versus
Figure 3B) suggest that part of the ABTS impregnated into the paper becomes unavailable for the enzyme-catalyzed reaction when chitosan is also loaded into the paper. As a consequence, the ABTS and chitosan-based sensors also tend to saturate at lower enzyme concentrations/activities (see
Figure 3C versus
Figure 3D and
Table 1).
The linear range is appropriate for screening grapes before processing, considering the threshold of 3 U/mL indicative of musts prone to enzymatic oxidative browning and the various activities in infected grapes reported in the literature. It is also adequate for screening artificially infected grapes (investigated further in this work).
Compared to other methods in the literature discussed in the Introduction, this procedure has the advantage of not requiring laboratory-based equipment (e.g., the µSTAT 8000 potentiostat is portable, but several smaller potentiostats are commercially available and can be used as well). It is simple and can quickly measure laccase activity. The best compromise between high sensitivity and short assay time should be chosen in relation to each application, e.g., if screening a high number of samples, requiring a short time or when aiming to measure low laccase activities. The paper sensor provides a confirmation of the electrochemical assay, and ideally, one should be able to use it independently from the electrochemical assay. Considering the results of both electrochemical and optical investigations, the rest of the studies were conducted using ABTS-only paper.
3.3. Optimization of the Amount of ABTS in the Paper Sensor
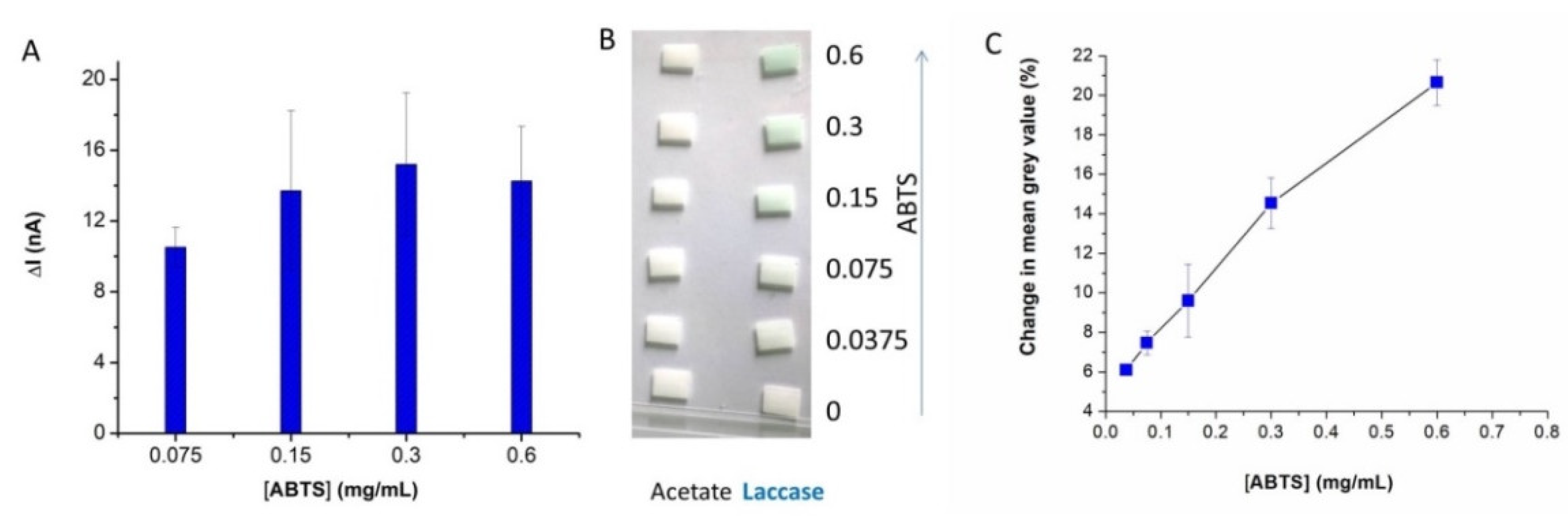
The amount of ABTS in the paper sensor is an important parameter for both the optical and electrochemical assay of laccase. To verify the adequate amount of ABTS in the paper, sensors made by impregnating the filter paper with 0.075–0.6 mg/mL ABTS solutions in a 0.1 M acetate buffer of pH 4.5 were used for the amperometric testing of laccase solutions of 3.5 U/mL. The assay time was 5 min and five replicates were measured for each variant. As illustrated in
Figure 4, the analytical signal increases with the concentration of ABTS in the impregnating solution from 0.075 to 0.15 mg/mL. More concentrated ABTS solutions lead to only small increases of the cathodic current. This is similar to the observations made with our previous paper-free assay when the ABTS reagent was added in the solution [
22]. Notably, this “insensitivity” of the method to the concentration of ABTS over the 0.15–0.3 mg/mL range is very important for achieving a robust testing method for laccase activity.
Next, the study focused on the influence of the ABTS concentration on the sensitivity of obtained optical paper sensors. The sensors obtained from filter paper impregnated with 0.0375–0.6 mg/mL ABTS were tested with 1.1 U/mL laccase solutions, and the change in color was monitored by taking pictures of the sensors every minute for 10 min. The images taken after 10 min were analyzed, using ImageJ, by measuring the mean grey value of the paper sensors. The results in
Figure 4B,C show that the color change increases with the concentration of ABTS. Based on the combined results of the electrochemical and optical study, 0.4 mg/mL ABTS was selected as the optimum concentration and the following optical and electrochemical studies were conducted exclusively with the paper sensors obtained in these conditions.
3.4. Evaluation of the Paper Sensor for Screening the Laccase Activity of Musts
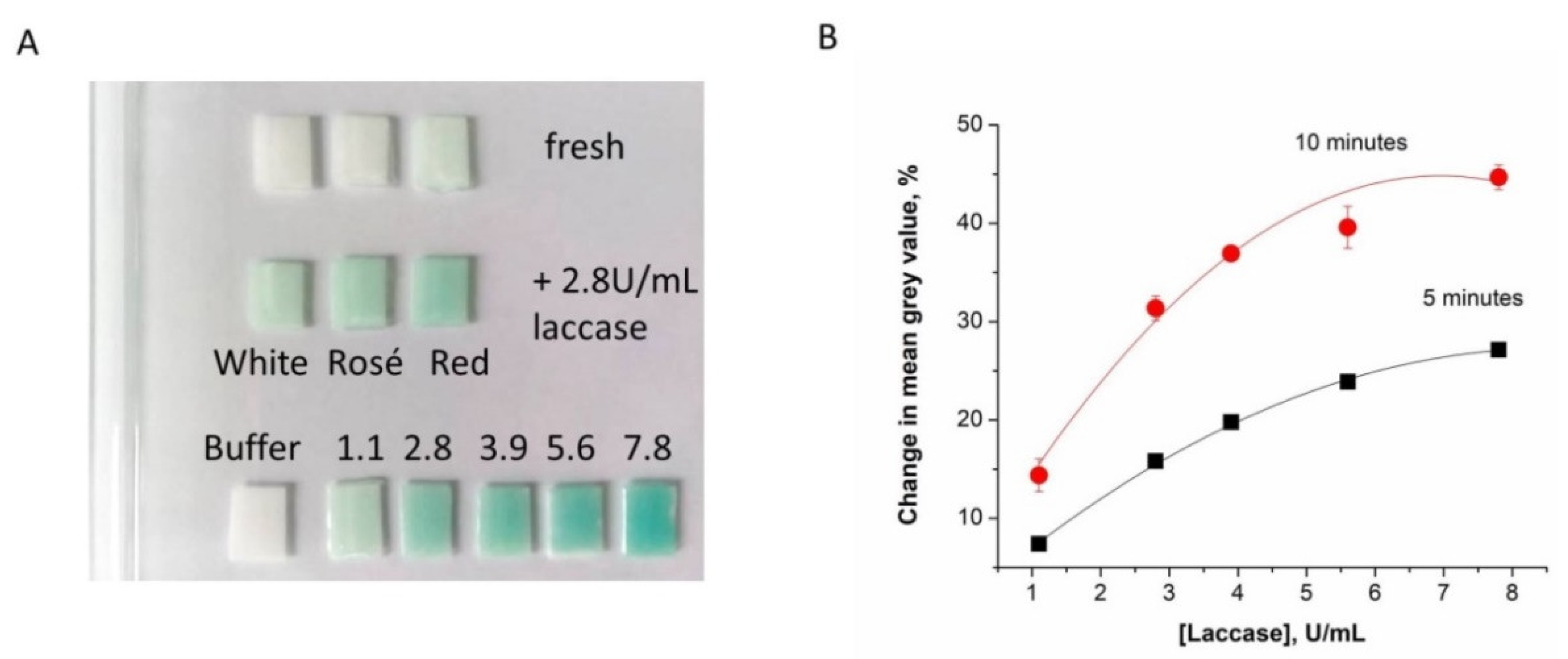
The paper sensor was tested with a series of laccase solutions with activities of 1–8 U/mL and with red, rosé, and white grape musts to which laccase was added to obtain an activity of 2.8 U/mL. The enzymatic reaction was monitored for 10 min. The analysis of the images taken after 0, 5 and 10 min respectively illustrates that the method readily distinguishes between 1 U/mL, 2.8 U/mL, and higher activities (
Figure 5A). Nonetheless, the color intensity changes in the range of 3.9–7.8 U/mL laccase are small. The color intensity increases in time and the sensitivity of the test (the slope of the curves in
Figure 5B) is almost double at 10 min compared to 5 min. The red, rosé, and white musts obtained from healthy grapes do not show any change in color as they do not contain laccase, while the musts with added laccase show similar (albeit somewhat reduced) color as the corresponding standard laccase solutions of the same activity (see
Figure 5A).
The paper sensor provides a semi-quantitative assay and may represent a simple and affordable tool for winemakers, particularly when used as a limit test, e.g., to verify if the laccase activity is lower or higher than a specific threshold value, e.g., 3 U/mL. The sensitivity of the assay may be increased if, instead of analyzing the grey value intensity of the composite image, the color is split into red, blue, and green channels in ImageJ (
Supplementary Information Figure S1). Measuring the color intensity change on the red channel provides a higher sensitivity, denoted by the larger slope of the change in grey value versus laccase activity. More precise quantitative evaluations would be possible by performing calibrations at specific light and distances, and at set exposures. An appropriate software application should be developed for interpreting small changes in color corresponding to small laccase activities. While the assay time of 10 min required for low laccase activity samples remains relatively long for screening a large number of musts, the software application could potentially be made available on a smartphone to simplify the assay and encourage its adoption by winemakers. Nonetheless, it needs to be particularized for the type of camera and smartphone used, as differences do exist between phones, even when the image-taking conditions are the same (
Supplementary Information Figure S2). Such technical development is beyond the purpose of this work, where we present a proof-of-concept application of the paper sensor as an independent, semi-quantitative tool for laccase activity evaluation, which serves also as a confirmatory assay and as an ABTS reservoir when combined with the electrochemical assay.
Therefore, the next studies focused on combining the paper sensor with electrochemical detection for quantitative evaluations of laccase activity, which is useful for monitoring the advancement of Botrytis cinerea infection during the grape development cycle.
3.5. Recovery Studies in Musts and Comparison with the Spectrophotometric Method Based on Syringaldazine
To examine the applicability of the paper-assisted electrochemical assay for the evaluation of laccase activity in grape musts, two approaches were considered. First, musts from healthy grapes and from grapes infected with
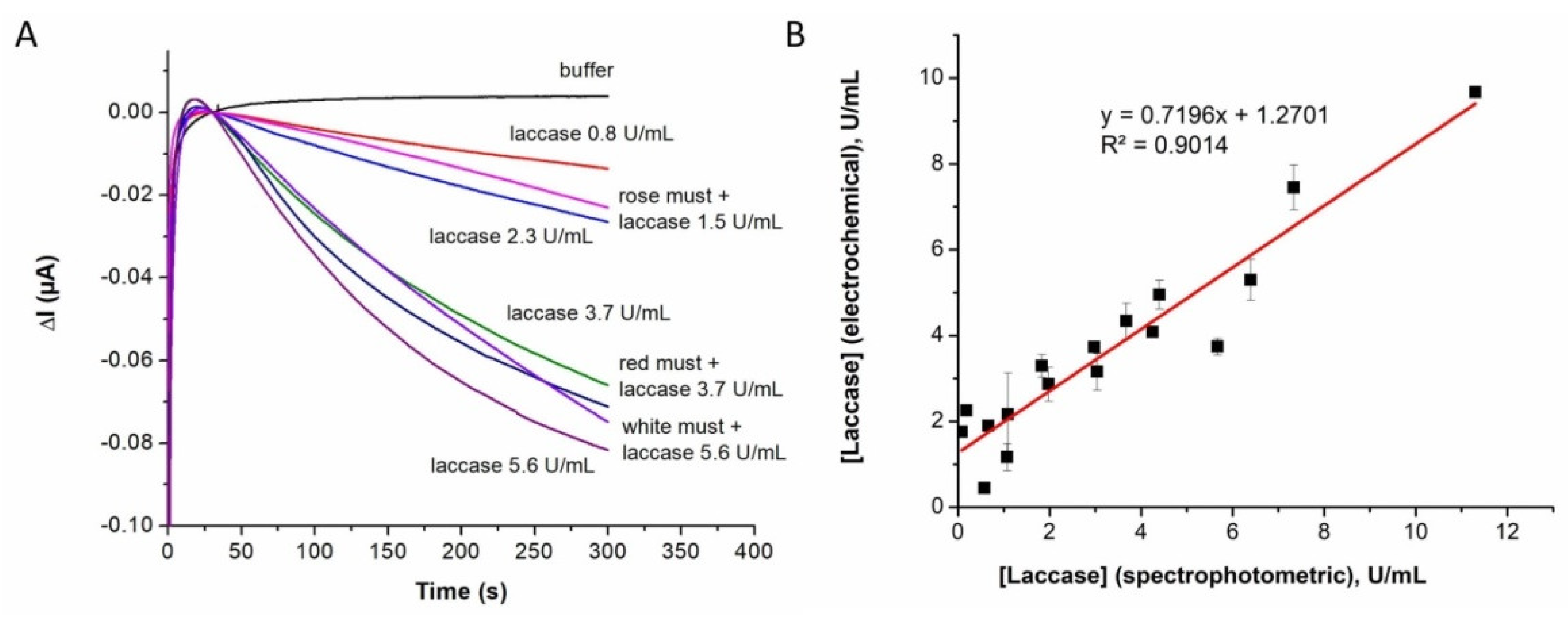
Botrytis cinerea were analyzed. Additional measurements were made for musts to which laccase was intentionally added at three activity levels, relevant in oenology. Fresh red, rosé, and white musts from healthy grapes did not show any change in the current intensity at 0 V (
Supplementary Information Figure S3 for red must). Instead, when spiked with laccase at three activity levels (1.5, 3.7, and 5.6 U/mL), the analytical signals recorded were proportional to the laccase activity (
Figure 6A).
The recovery factors calculated as “Recovered activity/Theoretical activity of spiked samples × 100” ranged between 77.3% and 130.7% (
Supplementary Information Table S1). These results clearly indicate that the proposed paper-assisted electrochemical assay is adequate for evaluating the activity of laccase in must samples.
The spectrophotometric method that is based on syringaldazine as an enzymatic substrate [
2] is widely used in oenology laboratories for measuring the laccase activity of musts and wine. Moreover, the threshold given in the literature (3 U/mL) as indicative of the risk of enzymatic browning of musts and wine was established by this method [
19]. Consequently, we used this method as a reference to compare it with the paper-assisted electrochemical assay. In this experiment, 17 samples of red, rosé, and white musts were analyzed, in parallel, by the two methods. As illustrated in
Figure 6B, there is a good agreement between the two methods, although there are some differences for a few samples.
The same observation was previously made for the electrochemical assay performed in the absence of the paper sensor by using the reagent ABTS mixed with the sample in solution on the electrode surface [
22]. In that work, we showed that the electrochemical assay provides the same results as a spectrophotometric determination using ABTS, and we concluded that the difference between the results of the syringaldazine method and the one using ABTS originate, for the most part, in the different natures of the enzymatic substrate and the laccase interacting differently with the two substrates. Therefore, the introduction of the paper sensor does not fundamentally change the assay, and it provides a simplification and a double confirmation (via the color change of the paper) of the assay.
3.6. Application to the Analysis of Artificially Infected Grapes
Red, rosé, and white grapes with various sugar contents, pH levels, total acidities, and total phenolic indices (
Supplementary Information Table S2) were inoculated with a suspension of spores of
Botrytis cinerea and kept in conditions favoring the development of
Botrytis cinerea for up to 20 days (see
Supplementary Information Figures S4–S6). Two types of experiments were conducted: in the first, we examined the influence of time over the development of the fungi and the change in the laccase activity of musts. In the second experiment, the fungal infection was allowed to develop for a long enough time to cover a significant proportion of the berries’ surfaces and the grapes were analyzed at a single time point. The results are summarized in
Table 2.
The results shown for Tempranillo grapes incubated at 24 °C with no control of humidity emphasize the slow increase in laccase activity in the first 7–10 days of the study (also confirmed by parallel measurements using the spectrophotometric method with syringaldazine, see results in
Supplementary Information Table S3). From an activity level below the detection limit in the first 7 days, in the 10th day of artificial infection of Tempranillo grapes at 24 °C, the activity of the must from the infected grapes rose to 2.26 ± 0.01 U/mL. After two more days, the activity was surprisingly smaller, 1.76 ± 0.1 U/mL. This result is attributed to the large differences between the individual berries. Even if the berries had a similar size and weight and were inoculated in the same way and kept in the same conditions, large variations still exist. For this reason, we did not observe a steady increase in the laccase activity with time. Moreover, without ensuring adequate humidity, 20 days were necessary for the berries to be covered entirely by the fungus. After this period, again, large variations between individual berries were observed, from berries with low development of mycelium, localized at the inoculation site, to berries whose surface was completely covered by mycelium.
To explore the correlation between the degree of infection and the enzymatic activity of laccase in the corresponding musts, a second strategy was to analyze musts obtained from grapes with a similar degree of infection by Botrytis cinerea. The grapes were grouped by degree of infection (I0 = 0% infection, I1 ≤ 25% infection, I2 = 25–50% infection, I3 = 51–75%, and I4 ≥ 75% infection). In addition, a second trial with Tempranillo grapes was conducted in conditions of controlled humidity where the berries were kept at 24 °C and 55% RH for 13 days, and then grouped by the degree of infection. In parallel with the studies performed on wine grapes, we studied the artificial inoculation of three types of table grapes (white, rosé, and red). In these experiments, the grapes acquired from the supermarket were split into two groups, and each was inoculated with a different strain of Botrytis cinerea. The inoculated berries were kept at 25 °C and 90% RH for 14 days (white grapes) and 17 days (rosé and red grapes) until significant development of the mycelium was observed.
Considering the results obtained with all types of grapes, inoculation, and incubation conditions (
Table 2), there was, overall, a good correlation between the degree of infection with
Botrytis cinerea and the laccase activity, which increased in the order I1 < I2 < I3 < I4, in accordance with the data in the literature. For two samples, the activity of laccase in I4 musts was, however, smaller than in the corresponding I3 musts. Considering the observed variability between berries, more in-depth studies should be performed using a larger number of berries. Among the types of grapes used in the study, the red varieties (i.e., Tempranillo and Moldova) have shown the highest laccase activities. For white grapes, inoculation with the 8C strain of
Botrytis cinerea led to higher enzymatic activities compared to the strain 1C, for the same degree of infection (e.g., for 2.87 U/mL for I4-8C compared to 2.16 U/mL for I4-1C and 3.73 U/mL for I3-8C compared to 1.17 U/mL for I3-1C (
Table 2)). The range of enzymatic activities determined for the artificially infected must is well covered by the linear range of the paper-assisted electrochemical assay. The above results emphasize that while larger studies need to be conducted to explore the differences between fungal strains and grape varieties with respect to the activity of laccase produced by infection with
Botrytis cinerea, the proposed analytical method is suitable for such studies.
3.7. Practical Considerations for Applying the Method for the Fast Evaluation of Musts
The pH of the electrolyte influences the activity of laccase and the results of the assay [
22]. The white, rosé, and red wines used in this study had pH values of 2.8–3.6 (
Supplementary Information Table S2), while the optimum pH of the ABTS-based test was previously determined to be 4.5 [
22]. To ensure that the samples are brought to the optimum pH while maintaining the simplicity of the assay, the paper sensor and the filtration cartridge with PVPP, were pre-conditioned with acetate buffer. Thus, the ABTS solution used for the filter paper was prepared in a 0.1 M acetate buffer of pH 4.5. Rinsing the PVPP cartridge with 2 mL 0.1 M acetate buffer of pH 5.5 and drying is efficient in bringing the pH of even very acidic musts into the optimum range, e.g., the pH of a red must have changed from 3.08 ± 0.01 to 4.41 ± 0.01 after the filtration through the pre-conditioned PVPP cartridge. Combined with the acetate conditioned filter paper, the buffering capacity is enough to prevent any significant variations in the assay results due to the sample pH. Elimination of phenolic compounds prior to the electrochemical assay is paramount for obtaining accurate results. The grapes contain phenolic compounds that are readily oxidized by laccase (e.g., caffeic acid, ferulic acid, and malvidin 3-O-glucoside [
26]), as well as others, such as tannins, which are inhibitors of laccase [
27].
The total phenolic index of red, rosé, and white must samples (based on the optical density at 280 nm) changed in order from 12.4 ± 0.0 to 6.0 ± 0.0, from 14.7 ± 0.3 to 6.8 ± 0.3, and from 14.2 ± 0.1 to 6.7 ± 0.2, respectively. The PVPP-filtered musts are colorless; therefore, they do not interfere with any colorimetric assays or with the optical paper sensor. Moreover, musts lacking laccase give no change in the cathodic current at 0 V (
Supplementary Information Figure S3), hence there is no electrochemical interference with the proposed electrochemical assay. Nonetheless, since the phenolic compounds are not completely eliminated, there is a chance they can interfere, to a certain extent, with the evaluation of the enzymatic activity of laccase. While this possibility deserves further investigation, the recovery study of red, rosé, and white grape musts spiked with laccase (discussed in
Section 3.5 above) emphasized acceptable recovery factors. This supports the idea that the proposed sample pre-treatment by filtration through the PVPP cartridge is adequate.
Temperature is another parameter that drastically influences the laccase activity and the assay, as it was emphasized through measurements with the reagents (ABTS and laccase) in the solution (
Supplementary Information Figure S7). Nonetheless, by taking into consideration the change in response according to temperature, corrections can be implemented in the software interface so that a user will get an accurate estimation of laccase activity.
3.8. Robustness, Repeatability, and Reproducibility of the Assay
Repeatability was evaluated by performing five assays with the same electrode for a solution of 5.9 U/mL laccase. The RSD of the change in current after 5 min at 0 V was 10.9%. The reproducibility was determined by measuring the change in the current produced in the presence of a solution of 3.5 U/mL laccase using three different electrodes and five replicates for each electrode. The RSD of the response for the three electrodes was 4.11%.
The robustness was evaluated by changing the volume in the range 25–40 µL and the potential in the range from −0.05 to +0.05 V vs. Ag/AgCl. The results did not show any statistically significant difference (
Supplementary Information Figure S6) compared to the experimental parameters used in the study, namely 30 µL sample volume, paper sensor obtained using a 0.4 mg/mL ABTS solution, and amperometry measurements at an applied potential of 0 V vs. Ag/AgCl. The concentration of ABTS in the filter paper was evaluated in
Section 3.3, and it was found that variations in the range of 0.3–0.6 mg/mL do not have a major impact on the response of the sensor. All these results create the premise of a robust assay.
4. Conclusions
This work reports a paper-assisted electrochemical assay adapted for on-site testing, which provides additional reliability to and simplifies an electrochemical assay previously developed by some of the authors of the present study [
22]. The method is versatile and more reliable, as the paper sensor provides an optical confirmation of the electrochemical evaluation of laccase activity in grapes. Based on its change in color, the ABTS-impregnated paper sensor can be used independently from the electrochemical assay, providing a semi-quantitative assessment of enzymatic activity. Low laccase activities require 10 min for the color to develop significantly. Given the development of smartphone-assisted analytical devices and smartphone dedicated applications, it is reasonable to expect that the optical test can be developed in this direction to make it accurate even for low laccase activities (low color changes), and attractive to users. Further increases in test sensitivity are possible, e.g., by analyzing only the red component of the color.
While easy to use, the paper-based optical assay also has a limitation concerning the analysis time, which prevents its adoption for testing a large number of samples, e.g., an operator inspecting the vineyard and wanting to test many locations in a day. However, screen-printed electrodes such as those used in the present study are also sold as arrays of eight electrode systems. Such arrays could significantly boost the throughput of our method. Nonetheless, the developed paper sensor is adequate for testing laccase activity at the grape processing points to ensure that the laccase activity is below the threshold value of 3 U/mL, which indicates the risk of irreversible damage of the grape, impacting the quality of the produced wine. When combined with a screen-printed cell including an Au working electrode, the paper impregnated with ABTS serves as substrate reservoir, diffusion media for laccase and ABTS, and buffering aid (given that the ABTS solution used for impregnation is made in a 0.1 M acetate buffer of pH 4.5). The combination between the paper sensor and the screen-printed electrode enables the quantitative detection of laccase activities down to 1 U/mL in 5 min. As demonstrated, the assay is applicable to red, rosé, and white must samples, and the recovery of enzymatic activities from musts spiked with laccase is quantitative, indicating a good accuracy of the assay. The method was tested with samples of musts from grapes that were artificially inoculated with Botrytis cinerea. The artificial inoculation experiments, conducted in various storage conditions using different fungal strains and different types of grapes, emphasized the slow development of the mycelium in the absence of adequate humidity. The laccase activity in grapes with a low degree of infection was very small or undetectable. Despite being of similar size, from the same grape, and inoculated and stored in the same way, there was a large variation between the individual berries. When grouped according to the degree of infection, a correlation was found between the severity of the infection with Botrytis cinerea and the laccase activity of the corresponding musts. The study enabled us to observe differences between the fungal strains and the grapes’ susceptibility to infection, which was also dependent on the grape variety.
Moreover, the paper-assisted electrochemical assay was characterized with respect to its repeatability, reproducibility, and robustness, and found to be adequate to be used on-site in the vineyard by making some adaptations, such as using a portable potentiostat and a dedicated software platform for data acquisition and interpretation. With all the precautions taken (i.e., using a pre-conditioned PVPP filtration cartridge, implementing the temperature correction, and using a preloaded calibration) the method has the appropriate robustness and simplicity to be used for quantitative measurements in the vineyard or at the grape processing points by operators with no special training. The ongoing work by our team in the frame of the ERA-Net project pursues this study direction.
One important aspect of the grapes’ infection by Botrytis cinerea is that not all fungal strains produce laccase. In addition to focusing future work on further improving the sensitivity of the assay to detect an attack on the vine by Botrytis cinerea at a very early stage, other indicators besides laccase can be considered. Consequently, specific electrochemical assays can be envisaged, starting from the herein described approach.