Recent Developments in Voltammetric Analysis of Pharmaceuticals Using Disposable Pencil Graphite Electrodes
Abstract
:1. Introduction
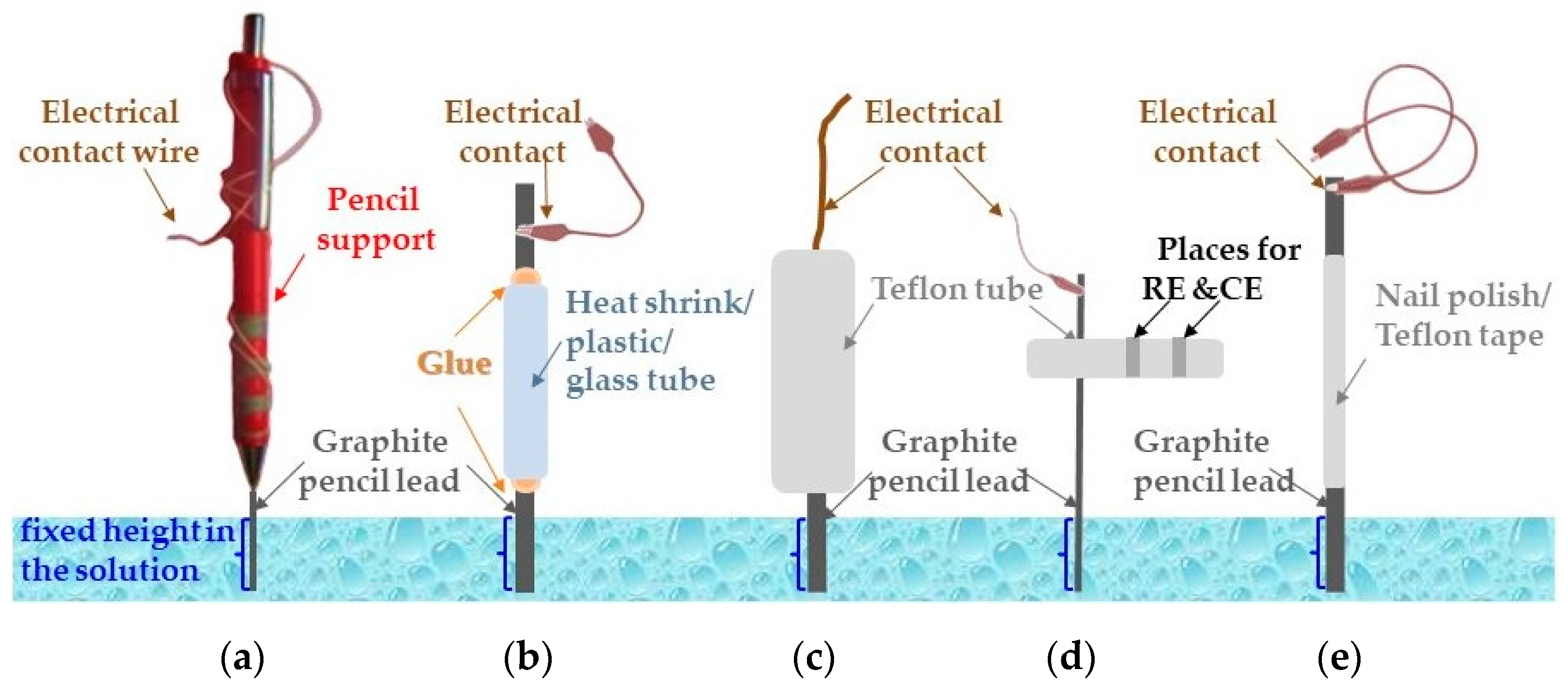
2. PGE Manufacturing Procedures
3. Voltametric Performance Characteristics of PGEs vs. Other Common Bare Working Electrodes
| Analyte (Drug) | PGE Type | Technique | Linear Range (mol/L) | LoD (mol/L) | Sample | Ref. |
|---|---|---|---|---|---|---|
| Acyclovir | 2B | DPV | 1.00 × 10−6–1.00 × 10−4 | 3.00 × 10−7 | Pharmaceutical tablets | [9] |
| 4-Aminophenazone | HB | DPV | 1.00 × 10−6–1.00 × 10−5 | 4.58 × 10−8 | Plasma; urine | [76] |
| Amlodipine | HB | DPV SWV | 2.10 × 10−9–8.96 × 10−8 8.00 × 10−10–5.22 × 10−8 | 4.00 × 10−14 2.00 × 10−14 | Pharmaceutical tablets; spiked human serum | [39] |
| Amoxicicllin | - | DPV | 1.00 × 10−6–6.00 × 10−5 | 2.24 × 10−7 | - | [71] |
| Cefidrin | - | SWV | 0.50–20.00 µg/mL | 0.15 µg/mL | Pharmaceutical tablets and powder | [23] |
| Chloramphenicol | 2H | LSV SWV | 2.50 × 10−6–1.00 × 10−3 2.50 × 10−6–7.50 × 10−4 | 6.09 × 10−7 1.39 × 10−6 | Pharmaceutical capsules | [29] |
| Chlorpromazine | HB | DPV | 1.00 × 10−8–8.00 × 10−8 | 3.00 × 10−9 | Pharmaceutical tablets | [16] |
| Ciprofloxacin | HB | SWV | 1.20 × 10−5–5.50 × 10−5 | 5.60 × 10−6 | Pharmaceutical formulations | [40] |
| Cysteamine | - | DPV | 1.00 × 10−5–1.10 × 10−4 | 4.00 × 10−6 | Urine | [54] |
| Dipyridamole | HB | DPV | 5.00 × 10−7–2.50 × 10−4 | 1.21 × 10−7 | Pharmaceutical tablets | [27] |
| Doxorubicin | - | DPV LSV | 1.00 × 10−5–5.00 × 10−5 1.00 × 10−5–3.00 × 10−5 | 7.20 × 10−6 | Serum | [70] |
| Eugenol | 2B | DPV | 3.00 × 10−7–5.00 × 10−5 | 8.50 × 10−8 | Pharmaceutical liquid | [32] |
| Famotidine | HB | SWV DPV | 5.00 × 10−6–1.80 × 10−4 4.72 × 10−7–4.95 × 10−4 | 1.29 × 10−6 1.51 × 10−7 | Pharmaceutical tablets | [30] [31] |
| Flutamide Irinotecan | 2B | CAdS-SWV | 3.98 × 10−7–6.36 × 10−6 7.94 × 10−8–4.03 × 10−7 | 1.55 × 10−8 1.68 × 10−9 | Serum; urine | [22] |
| Hydroxyurea | - | 1.00 × 10−5–1.00 × 10−3 | 7.89 × 10−6 | Pharmaceutical tablets; urine | [11] | |
| Imipenem | Tb3+-complex deposition + CV | 1.50 × 10−5–7.00 × 10−5 | 2.44 × 10−6 | Pharmaceutical vials; rabbit plasma | [77] | |
| Itraconazole | HB | AdS-DPV AdS-SWV | 32.00–169.00 10.60–127.00 ng/mL | 9.10 8.70 ng/mL | Pharmaceutical capsules; serum; urine | [26] |
| Linezolid | - | SWV | 2.96 × 10−8–5.93 × 10−7 | 1.39 × 10−9 | Pharmaceutical and biological samples | [73] |
| Methylergometrine maleate | HB | DPV | 0.10–1.00 µg/mL | 0.02 µg/mL | Pharmaceutical tablets and ampoules | [24] |
| Niclosamide | 2B | DPV | 5.00 × 10−7–1.00 × 10−5 | 1.50 × 10−9 | Pharmaceutical tablets | [21] |
| Paclitaxel | HB | DPV | 4.00 × 10−7–3.00 × 10−6 | 2.46 × 10−9 | Pharmaceutical injections; plasma; urine | [78] |
| Piroxicam | 4B | DPV | 2.91 × 10−5–1.63 × 10−4 | 2.10 × 10−6 | Pharmaceutical capsules | [42] |
| Strontium Ranelate | HB | DPV SWV | 1.00–10.00 µg/mL 1.00–10.00 µg/mL | 0.17 µg/mL 0.19 µg/mL | Pharmaceutical formulations | [25] |
| Sulfamethoxazole | B | DPV | 1.00 × 10−5–2.50 × 10−4 | 4.04 × 10−6 | Pharmaceutical tablets | [28] |
| Vildagliptin | 2B | SWV | 2.94 × 10−6–4.99 × 10−5 | 8.20 × 10−8 | Pharmaceutical tablets; urine | [13] |
| Vitamin B1 Vitamin B6 | HB | DPV | 1.00 × 10−5–1.00 × 10−3 5.00 × 10−6–1.00 × 10−3 | 5.34 × 10−6 2.81 × 10−6 | Pharmaceutical vials | [33] |
| Vitamin B2 Vitamin B6 | HB | SWV | 1.00 × 10−7–7.50 × 10−4 2.50 × 10−5–2.50 × 10−3 | 7.38 × 10−8 1.10 × 10−5 | Pharmaceutical tablets | [79] |
| Vitamin B12 | - | DPV | 2.50 × 10−9–3.00 × 10−8 | 8.20 × 10−8 | Pharmaceutical tablets | [55] |
4. Voltametric Analysis of Pharmaceutical Active Compounds Using PGEs
4.1. Applications of Unmodified PGEs to the Voltametric Analysis of Pharmaceutical Compounds
4.2. Applications of Electrochemically Pretreated PGEs to the Voltametric Analysis of Pharmaceutical Compounds
4.3. Applications of (Electro)chemically Modified PGEs to the Voltametric Analysis of Pharmaceutical Compounds
| Analyte (Drug) | PGE Type/Modifier | Analysis Technique | Linear Range (mol/L) | LoD (mol/L) | Sample | Ref. |
|---|---|---|---|---|---|---|
| Lomustine | 2B/Hg film | CAdS-SWV | 1.92 × 10−7–1.36 × 10−5 | 8.13 × 10−8 | Human blood and urine | [103] |
| Diazepam | ET/Bi film | DPV | 1.40 × 10−6–1.067 × 10−5 | 1.10 × 10−6 | Pharmaceutical tablets; human urine | [139] |
| Metronidazole | -/Bi film | CV | 3.00 × 10−7–8.00 × 10−5 | Pharmaceutical tablets; human serum | [104] | |
| DPV | 2.00 × 10−7–3.00 × 10−5 | 3.90 × 10−8 | ||||
| Amp | 1.00 × 10−6–1.10 × 10−5 | 2.94 × 10−6 | ||||
| Flutamide Cyproterone acetate | 2B/nanoacetylene black | CAdS-SWV | 2.60 × 10−8–4.77 × 10−7 6.60 × 10−8–1.10 × 10−6 | 1.39 × 10−9 4.74 × 10−9 | Pharmaceutical tablets; human serum and urine | [66] |
| Elbasivir | H/Mn5O8NPs | SWV | 2.00 × 10−7–3.00 × 10−6 | 4.00 × 10−8 | Spiked human plasma | [34] |
| Ledipasivir | H/MnO2NPs | SWV | 2.50 × 10−8–3.60 × 10−6 | 5.10 × 10−9 | Pharmaceutical tablets; rat plasma | [35] |
| Valacyclovir | H/CuMPs | AdS-SWV | 2.00 × 10−9–1.00 × 10−8 | 1.78 × 10−10 | Pharmaceutical tablets | [19] |
| Aceclofenac | 2B/MWCNTs | DPV | 1.00 × 10−6–6.00 × 10−5 | 2.60 × 10−9 | Pharmaceutical tablets; human urine | [47] |
| Buprenorphine | ET/MWCNTs | DPV | 1.00 × 10−12–1.09 × 10−10 1.09 × 10−10–1.10 × 10−7 | 6.00 × 10−13 | Human plasma and urine | [137] |
| Diclofenac sodium | H/MWCNTs | DPV | 4.70 × 10−8–1.29 × 10−5 | 1.70 × 10−8 | Pharmaceutical tablets; human urine | [57] |
| Methadone | H/MWCNTs | DPV | 1.00 × 10−7–1.50 × 10−5 | 8.70 × 10−8 | Human serum and urine | [58] |
| Hydrochlorthiazide | HB/TyB | DPV SWV | 5.00 × 10−7–7.00 × 10−6 1.00 × 10−7–5.00 × 10−6 | 1.33 × 10−7 3.20 × 10−8 | Pharmaceutical tablets | [44] |
| Daclatasivir Vardenafil | H/PXO | SWV | 2.00 × 10−7–7.00 × 10−7 1.00 × 10−7–6.00 × 10−7 | 3.00 × 10−8 2.00 × 10−8 | Rabbit plasma | [46] |
| Domperidone Rabeprazole sodium | -/PEBT | CV AdS-SWV CV AdS-SWV | 5.20 × 10−6–9.00 × 10−5 5.00 × 10−7–8.00 × 10−6 4.10 × 10−6–1.20 × 10−4 7.50 × 10−7–8.00 × 10−6 | 5.20 × 10−6 5.00 × 10−7 4.10 × 10−6 7.50 × 10−6 | Synthetic mixture; human plasma | [111] |
| Domperidone Pantoprazole | -/PEBT | AdS-SWV | 1.00 × 10−8–3.40 × 10−6 4.00 × 10−8–5.50 × 10−6 | 4.00 × 10−9 1.20 × 10−8 | Synthetic mixture; human plasma | [112] |
| Ertapenem Meropenem | -/PBCG | SWV | 3.00 × 10−7–7.50 × 10−5 1.00 × 10−6–6.00 × 10−5 | 8.00 × 10−8 3.20 × 10−7 | Pharmaceutical vials; rabbit plasma | [113] |
| Levofloxacin | 2B/poly(Azure-B) | DPV | 1.00 × 10−6–1.25 × 10−4 | 1.20 × 10−6 | Urine; serum | [45] |
| Acetaminophen | -/2-TBA | DPV | 9.00 × 10−8–5.00 × 10−7 5.00 × 10−7–9.46 × 10−6 9.46 × 10−6–4.23 × 10−4 | 2.10 × 10−8 | Tap and lake water; child syrup | [59] |
| H/PA/AgNPs | DPV | 5.00 × 10−8–8.00 × 10−7 | 1.01 × 10−8 | Pharmaceutical tablets and syrup | [105] | |
| 2H/SWCNTs/PVP | SWV | 1.00 × 10−6–5.00 × 10−4 | 3.80 × 10−7 | [41] | ||
| 2-Thiouracil | -/PA -/PPy | SCV | 1.00 × 10−8–1.30 × 10−7 1.00 × 10−8–1.50 × 10−7 | 1.80 × 10−9 1.60 × 10−9 | Pharmaceutical tablets; serum; urine | [109] |
| 2-Aminobenz-imidazole | HB/MIP (PPy) | DPV | 1.00 × 10−9–3.00 × 10−2 | 1.00 × 10−10 | Chicken, turkey, beef, and fish meat | [114] |
| Benzimidazole | HB/MIP (PPy) | DPV | 3.00 × 10−6–5.00 × 10−3 | 7.00 × 10−7 | Chicken, turkey, beef, lamb, and fish meat | [115] |
| Methimazole | HB/MIP (PPy) | DPV | 7.00 × 10−6–6.00 × 10−3 | 3.00 × 10−6 | Pharmaceutical tablets; human serum | [116] |
| Sulfanilamide | -/MIP (PPy) | DPV | 5.00 × 10−8–1.10 × 10−6 1.10 × 10−6–4.80 × 10−5 | 2.00 × 10−8 | Serum; ground water | [117] |
| Doxicycline | HB/MIP (OPPy) | DPV | 5.00 × 10−5–5.00 × 10−4 3.00 × 10−3–7.00 × 10−3 | 4.35 × 10−5 | Pharmaceutical capsules | [118] |
| Sulfasalazine | 2B/MIP (OPPy) | DPV | 1.00–10.00 μg/mL | 0.26 μg/mL | Pharmaceutical tablets | [119] |
| Primaquine | /C60-MIP (TAT) | AS-DPV | 2.70 × 10−9–8.48 × 10−7 | 8 × 10−10 | Pharmaceuticals; plasma; urine | [48] |
| Lamivudine Zidovudine | /HCS-MIP (TAT) | AS-DPV | 7.26–80.16 ng/mL 4.76–128.76 ng/mL | 2.23 ng/mL 1.26 ng/mL | Pharmaceuticals; serum | [120] |
| Pantoprazole | HB/f-MWCNTs/MIP (PPy) | DPV | 5.00 × 10−6–7.00 × 10−4 | 3.75 × 10−7 | Pharmaceutical capsules; serum | [121] |
| Celecoxib | -/f-MWCNTs/MIP (PPy) | DPV | 5.00 × 10−9–2.00 × 10−5 | 2.34 × 10−9 | Pharmaceutical tablets; serum | [122] |
| Metoprolol | -/f-MWCNTs/MIP (PPy) | DPV | 6.00 × 10−8–4.90 × 10−4 | 2.88 × 10−9 | Pharmaceutical tablets; serum | [106] |
| Triamterene | HB/f-MWCNTs/MIP (PPy) | DPV | 8.00 × 10−8–2.65 × 10−4 | 3.35 × 10−9 | Pharmaceutical tablets; serum | [107] |
| Capecitabine Erlotinib HCl | -/HF-MWCNTs/PU | DPV | 7.70 × 10−6–1.42 × 10−4 1.10 × 10−7–2.35 × 10−5 | 1.10 × 10−7 2.00 × 10−8 | Nail; urine | [133] |
| Flutamide | 2B/HF-GO/HPB | SWV | 1.00 × 10−7–1.10 × 10−4 | 2.90 × 10−8 | Plasma | [140] |
| Imatinib | ET-HB/HF-PAMAM-rGO | DPV | 1.00 × 10−8–1.00 × 10−5 1.00 × 10−5–2.00 × 10−4 | 7.39 × 10−9 | Serum; urine | [88] |
| 6-Thioguanine | HB/ErGO/MIP (PNR) | AS-DPV | 0.124–78 ng/mL | 0.02 ng/mL | Pharmaceuticals; serum; urine | [49] |
| Epinephrine | 2B/MIP (CNT-mer) | AS-DPV | 0.09–5.90 ng/mL | 0.02 ng/mL | Pharmaceutical vials; serum | [50] |
| Folic acid (vitamin B9) | HB/CNDs/MIP (PABSA) | CS-DPV | 2.20–30.80 ng/mL | 2.02 ng/mL | Pharmaceutical tablets; urine | [123] |
| Citaprolam Fluoxetine Setraline | H/PVC/PEDOT-C14 | ITS-LSV | 1.00 × 10−7–1.50 × 10−6 | 3.50 × 10−8 4.50 × 10−8 2.50 × 10−8 | Tap and river water | [138] |
| Cefixime | -/AuNPs/CTAB | DPV | 1.00 × 10−8–3.00 × 10−7 | 1.21 × 10−10 | Pharmaceutical tablets; serum; urine | [141] |
| Ceftizoxime | ET-2B/rGO/PNCs | AdS-DPV | 1.00 × 10−11–3.00 × 10−8 | 1.80 × 10−12 | Pharmaceutical vials; serum | [90] |
| -/rGO/HAuNPs | AdS-DPV | 1.00 × 10−12–1.00 × 10−11 1.00 × 10−11–1.00 × 10−9 | 3.50 × 10−13 | Pharmaceutical vials; plasma | [142] | |
| Ceftazidime | HB/rGO/HPtNPs | AdS-DPV | 5.00 × 10−13–1.00 × 10−9 | 2.20 × 10−13 | Pharmaceutical vials; plasma | [143] |
| Clozapine | -/rGO-X/Nafion (X: Au; Pd; Pt) | DPV | 5.00 × 10−8–1.00 × 10−5 | 1.60 × 10−9 | Serum | [135] |
| Pyridoxine (vitamin B6) | -/β-CD–G/PtNPs | DPV | 5.00 × 10−9–2.05 × 10−7 | 1.20 × 10−9 | Fruit juice | [144] |
| Febuxostat | 2B/AuNPs@f-CNFs | AdS-SWV | 3.00 × 10−8–7.70 × 10−5 | 1.27 × 10−8 | Pharmaceutical tablets; plasma; urine | [17] |
| Propranolol | HB/TiO2/MWCNTs | AdS-DPV | 8.50 × 10−8–6.50 × 10−6 | 2.10 × 10−8 | Pharmaceutical tablets, serum; urine | [132] |
| Riboflavin (vitamin B2) | /AZA/NiHCF | DPV | 4.37 × 10−6–1.23 × 10−3 | 1.45 × 10−6 | Vitamin tablets | [145] |
| 2B/Chit/Sn | SWV | 1.00 × 10−8–1.20 × 10−6 | 5.56 × 10−9 | Pharmaceuticals; milk powder | [69] | |
| SLSMCNTPGCPE | DPV | 2.00 × 10−7–8.00 × 10−7 1.00 × 10−6–5.00 × 10−6 | 1.16 × 10−8 | B-complex pill; food supplement | [72] | |
| Valproic acid | -/APTES-MNPs | DPV | 1.00–100.00 µg/mL | 0.40 µg/mL | Plasma | [67] |
| Ascorbic acid (vitamin C) | ET-2B/MIP (PPy-oPD) | SWV | 1.00 × 10−6–1.00 × 10−3 | 2.63 × 10−7 | Pharmaceutical tablets | [89] |
| -/PIG | SWV | 4.00 × 10−5–4.10 × 10−3 | 2.34 × 10−7 | - | [134] | |
| -/MWCNTs/CeO2NP | CV | 4.20 × 10−8–5.39 × 10−7 | 8.00 × 10−9 | - | [146] | |
| L-Ascorbic acid D-Ascorbic acid | -/MIPANI-FSA/CDs | DPV | 6.00 × 10−9–1.65 × 10−7 6.00 × 10−9–1.55 × 10−7 | 1.00 × 10−12 2.00 × 10−12 | Pharmaceutical tablets; cerebrospinal fluid; plasma | [38] |
| Acetylsalicylic acid Ascorbic acid | 2B/MWCNTs/SMT | AdS-DPV | 8.05 × 10−7–1.00 × 10−4 3.19 × 10−7–6.00 × 10−5 | 2.41 × 10−7 9.60 × 10−8 | Pharmaceutical tablets | [136] |
| Acetaminophen Acetylsalicylic acid Ascorbic acid | ET-2B/MWCNTs/SEP | AdS-DPV | 1.28 × 10−8–1.40 × 10−4 2.13 × 10−8–1.00 × 10−4 2.01 × 10−8–1.20 × 10−4 | 1.80 × 10−8 4.70 × 10−8 4.20 × 10−8 | Pharmaceutical tablets | [85] |
| Acetaminophen Chlorpheniramine maleate Caffeine Ascorbic acid | 2B/P(L-met)/AuNPs | DPV | 3.18 × 10−6–3.00 × 10−3 9.74 × 10−6–7.14 × 10−4 8.30 × 10−6–2.00 × 10−3 1.00 × 10−5–2.00 × 10−3 | 9.50 × 10−7 2.92 × 10−6 2.50 × 10−9 3.03 × 10−6 | Pharmaceutical powder; serum | [130] |
| Guaifenesin | HB/P(L-cys)/AgNPs | DPV | 3.00 × 10−8–3.00 × 10−4 | 6.10 × 10−9 | Pharmaceutical syrup; serum; urine | [53] |
| Ifosfamide Etoposide | ET/Au/Pd@ rGO@P(L-cys) | DPV | 1.00 × 10−7–9.00 × 10−5 1.00 × 10−8–4.00 × 10−5 | 9.21 × 10−9 7.18 × 10−10 | Pharmaceutical injections; serum; urine | [87] |
| Vitamin K | ET/2-A-5-CBP/AgNPs | SWV | 5.00 × 10−8–7.00 × 10−7 | 1.66 × 10−8 | Serum | [60] |
| Mebeverine | HB/MIP (PPy)/AgNPs | DPV | 1.00 × 10−8–1.00 × 10−6 1.00 × 10−5–1.00 × 10−3 | 8.60 × 10−9 | Pharmaceutical capsules; serum | [129] |
| Levodopa | 2B/GO/DHPA@AgNPs | DPV | 3.00 × 10−9–1.00 × 10−7 1.00 × 10−7–1.00 × 10−5 | 7.60 × 10−10 | Pharmaceutical tablets; serum; urine | [147] |
| Methyldopa | HB/GO/PMoA | DPV | 4.90 × 10−10–1.00 × 10−7 | 1.20 × 10−10 | Milk; serum; urine | [148] |
| Furosemid | -/P[Ni(salen)]/Ni(OH)2/C NPs | CV | 2.50 × 10−10–2.70 × 10−9 | 1.40 × 10−10 | - | [149] |
| Insulin | ET/RuO 2-EGO | DPV | 8.00 × 10−10–2.00 × 10−8 2.00 × 10−8–1.00 × 10−6 | 2.40 × 10−11 | Plasma; urine | [150] |
| ET/NiNPs/MBz | DPV | 2.50 × 10−8–4.50 × 10−7 | 8.30 × 10−9 | Serum | [20] | |
| ET/NiNPs/Chit/MWCNTs EA/NiONPs/Chit/MWCNTs | CV | 1.00 × 10−6–5.00 × 10−6 5.00 × 10−8–5.00 × 10−6 | 4.34 × 10−6 2.60 × 10−8 | - | [68] | |
| /CS@Z | DPV | 5.00 × 10−11–5.00 × 10−8 | 1.28 × 10−11 | Pharmaceutical injections | [108] | |
| Avanafil Nimodipine | H/P(SA)/NiONPs | SWV | 4.00 × 10−8–1.00 × 10−6 1.00 × 10−6–1.50 × 10−5 | 3.70 × 10−8 3.20 × 10−7 | Serum | [151] |
| Norepinephrine Uric acid | 2B/DID (TAT)/f-MWCNTs/AuNPs | AS-DPV | 2.83–44.05 ng/mL 1.99–44.31 ng/mL | 0.62 ng/mL 0.43 ng/mL | Pharmaceuticals; serum; urine | [51] |
| Tramadol | H/CNT | DPV | 1.00 × 10−6–2.50 × 10−5 | 7.76 × 10−7 | Blood serum | [75] |
| 2B/f-MWCNTs/AuNPs | DPV | 1.20 × 10−8–1.00 × 10−7 1.00 × 10−7–3.00 × 10−6 | 5.00 × 10−9 | Pharmaceutical tablets; urine | [61] | |
| -/PPy/CuONPs | SWV | 5.00 × 10−9–3.80 × 10−4 | 1.00 × 10−9 | Pharmaceutical tablets and vials | [110] | |
| Raloxifene Tamoxifen | ET/PPy/G/CuO | SWV | 1.00 × 10−8–5.50 × 10−4 5.00 × 10−7–4.50 × 10−4 | 3.00 × 10−9 1.05 × 10−8 | Pharmaceutical tablets; serum | [86] |
| Caffeine | 2B/MIP (PPy)/sol-gel/AuNPs | SWV | 2.00 × 10−9–5.00 × 10−8 5.00 × 10−8–1.00 × 10−6 | 9.00 × 10−10 | Pharmaceutical tablets; urine; plasma green tea; energy and soda drink | [36] |
| Lorazepam | 2B/MIP (PPy)/sol-gel/AuNPs | SWV | 2.00 × 10−10–2.00 × 10−9 2.00 × 10−9–2.00 × 10−8 | 9.00 × 10−11 | Pharmaceutical tablets; plasma; urine | [37] |
| Ranitidine | 2B/f-MWCNTs/MIP/sol–gel/AuNPs | SWV | 5.00 × 10−8–2.00 × 10−6 | 2.00 × 10−8 | Urine | [91] |
| Morphine | ET/f-MWCNTs/MIP (PMTMOS)/AuNPs | SWV | 8.00 × 10−9–5.00 × 10−6 | 2.90 × 10−9 | Plasma; urine | [92] |
| Carbazine | /f-MWCNTs/MIP (PABA)-NSPs | AS-DPV | 0.10–50.80 ng/mL | 0.02 ng/mL | Pharmaceuticals; plasma; urine | [126] |
| Thiamine (vitamin B1) | 2B/ANiNPs/f-MWCNTs/MIP (PNMGA) | AS-DPV | 0.60–19.43 ng/mL | 0.17 ng/mL | Pharmaceutical tablets; plasma; urine | [52] |
| Cyanocobalamine (vitamin B12) | -/MIP (MB-rGO/f-MWCNTs/AU) | AS-DPV | 0.021–1.44 ng/mL | 0.0056 ng/mL | Pharmaceuticals, cerebrospinal fluid; serum; urine | [125] |
| Fexofenadine | HB/NDG/MIP (PAA)/sol-gel | DPV | 5.00 × 10−10–7.80 × 10−6 | 1.50 × 10−10 | Pharmaceutical tablets, blood; urine | [127] |
| Isoniazid | ET/CNQDs/Cu-MOF | DPV | 1.00 × 10−7–6.5 × 10−5 | 6.75 × 10−8 | Pharmaceutical tablets; serum | [62] |
| Setraline | HB/SNDG/Cu-MOF | DPV | 5.00 × 10−8–2.67 × 10−6 | 3.80 × 10−8 | Pharmaceutical tablets; serum | [63] |
| Paroxetine | H/PWA/rGO | DPV | 8.00 × 10−9–1.00 × 10−6 | 9.00 × 10−10 | Pharmaceutical tablets; serum; urine | [64] |
| Sumatripan Paroxetine | H/MIP(PMA)/sol-gel/PWA/rGO | AdS-DPV | 2.00 × 10−8–3.00 × 10−6 5.00 × 10−9–2.20 × 10−6 | 4.00 × 10−9 7.00 × 10−10 | Pharmaceutical tablets; serum; urine | [65] |
| Donepezil | /PSBT/N-CNDs/CoNPs | DPV | 1.50 × 10−9–4.00 × 10−4 | 5.00 × 10−10 | Pharmaceutical tablets; rabbit plasma | [131] |
| 6-mercapto-purine | 2B/IL/N-HCNS@Pd- MIP (N-AAsp) | AS-DPV | 0.80–70.75 ng/mL | 0.11–0.22 ng/mL | Water; pharmaceuticals; plasma; urine | [128] |
| -/MIP (PPy)/sol-gel/ZnO@GQDs | DPV | 1.00 × 10−8–5.00 × 10−5 5.00 × 10−5–7.00 × 10−4 | 5.72 × 10−9 | Pharmaceutical tablets; serum; urine | [124] |
4.4. Simultaneous Voltametric Determination of Drugs Using PGEs
4.5. Pharmacokinetic Studies Performed by Voltammetric Techniques at PGEs
5. PGEs as Electrochemical Detectors in Separation Techniques
6. Development of PGE-Reliant Analytical Procedures Aided by Laborious Calculations
7. PGE Surface Characterization
7.1. PGE Surface Characterization by Electrochemical Techniques
7.2. PGE Surface Characterization by Techniques of Electron Microscopy
7.3. PGE Surface Characterization by Spectroscopic Techniques Using X-rays
7.4. PGE Surface Characterization by Optical Spectroscopic Techniques
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Council of Europe. European Pharmacopoeia; Council of Europe: Strasbourg, France, 2001. [Google Scholar]
- Tasic, Z.Z.; Petrovic Mihajlovic, M.B.; Simonovic, A.T.; Radovanovic, M.B.; Antonijevi, M.M. Review of applied surface modifications of pencil graphite electrodes for paracetamol sensing. Results Phys. 2022, 22, 103911. [Google Scholar] [CrossRef]
- David, I.G.; Litescu, S.C.; Popa, D.E.; Buleandra, M.; Iordache, L.; Albu, C.; Alecu, A.; Penu, R.L. Voltammetric analysis of naringenin at a disposable pencil graphite electrode—Application to polyphenol content determination in citrus juice. Anal. Methods 2018, 10, 5763–5772. [Google Scholar] [CrossRef]
- David, I.G.; Oancea, A.G.; Buleandra, M.; Popa, D.E.; Iorgulescu, E.E.; Ciobanu, A.M. Disposable pencil graphite electrode for diosmin voltammetric analysis. Micromachines 2021, 12, 351. [Google Scholar] [CrossRef] [PubMed]
- David, I.G.; Numan, N.; Buleandra, M.; Popa, D.E.; Lițescu, S.C.; Riga, S.; Ciobanu, A.M. Rapid voltammetric screening method for the assessment of bioflavonoid content using the disposable bare pencil graphite electrode. Chemosensors 2021, 9, 323. [Google Scholar] [CrossRef]
- Buleandra, M.; Popa, D.E.; David, I.G.; Bacalum, E.; David, V.; Ciucu, A.A. Electrochemical behavior study of some selected phenylurea herbicides at activated pencil graphite electrode. Electrooxidation of linuron and monolinuron. Microchem. J. 2019, 147, 1109–1116. [Google Scholar] [CrossRef]
- Wong, A.; Santos, A.M.; da Fonseca Alves, R.; Campanha Vicentini, F.; Fatibello-Filho, O.; Taboada Sotomayor, M.D.P. Simultaneous determination of direct yellow 50, tryptophan, carbendazim, and caffeine in environmental and biological fluid samples using graphite pencil electrode modified with palladium nanoparticles. Talanta 2021, 222, 121539. [Google Scholar] [CrossRef]
- Sharma, S.; Jain, R.; Nitin Raja, A. Review—Pencil graphite electrode: An emerging sensing material. J. Electrochem. Soc. 2020, 167, 037501. [Google Scholar] [CrossRef]
- Dilgin, D.G.; Karakaya, S. Differential pulse voltammetric determination of acyclovir in pharmaceutical preparations using a pencil graphite electrode. Mater. Sci. Eng. C 2016, 63, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Dokur, E.; Gorduk, O.; Sahin, Y. Cost-effective and facile production of a phosphorus doped graphite electrode for the electrochemical determination of pyridoxine. Electroanalysis 2021, 33, 1657–1667. [Google Scholar] [CrossRef]
- Naik, K.M.; Ashi, C.R.; Nandibewoor, S.T. Anodic voltammetric behavior of hydroxyurea and its electroanalytical determination in pharmaceutical dosage form and urine. J. Electroanal. Chem. 2015, 755, 109–114. [Google Scholar] [CrossRef]
- Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R0852&rid=7 (accessed on 25 December 2021).
- Altunkaynak, Y.; Yavuz, O.; Levent, A. Firstly electrochemical examination of vildagliptin at disposable graphite sensor: Sensitive determination in drugs and human urine by square-wave voltammetry. Microchem. J. 2021, 170, 106653. [Google Scholar] [CrossRef]
- David, I.G.; Popa, D.E.; Buleandra, M. Pencil graphite electrodes: A versatile tool in electroanalysis. J. Anal. Methods Chem. 2017, 2017, 1905968. [Google Scholar] [CrossRef] [Green Version]
- Stradolini, F.; Kilic, T.; Di Consiglio, A.; Ozsoz, M.; De Micheli, G.; Carrara, S. Long-term monitoring of propofol and fouling effect on pencil graphite electrodes. Electroanalysis 2018, 30, 1363–1369. [Google Scholar] [CrossRef]
- Purushothama, H.T.; Arthoba Nayaka, Y.; Vinay, M.M.; Manjunatha, P.; Yathisha, R.O.; Basavarajappa, K.V. Pencil graphite electrode as an electrochemical sensor for the voltammetric determination of chlorpromazine. J. Sci. Adv. Mater. Devices 2018, 3, 161–166. [Google Scholar] [CrossRef]
- Ibrahim, H.; Temerk, Y. A novel electrochemical sensor based on gold nanoparticles decorated functionalized carbon nanofibers for selective determination of xanthine oxidase inhibitor febuxostat in plasma of patients with gout. Sens. Actuators B Chem. 2021, 347, 130626. [Google Scholar] [CrossRef]
- Trnkova, L.; Triskova, I.; Cechal, J.; Farka, Z. Polymer pencil leads as a porous nanocomposite graphite material for electrochemical applications: The impact of chemical and thermal treatments. Electrochem. Commun. 2021, 126, 107018. [Google Scholar] [CrossRef]
- Saleh, G.A.; Askal, H.F.; Refaat, I.H.; Naggar, A.H.; Abdel-aal, F.A.M. Adsorptive square wave voltammetric determination of the antiviral drug valacyclovir on a novel sensor of copper microparticles–modified pencil graphite electrode. Arab. J. Chem. 2016, 9, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Kouchakinejad, S.; Babaee, S.; Roshani, F.; Kouchakinejad, R.; Shirmohammadi, N.; Kaki, S. The performance of the new modified pencil graphite electrode in quantifying of insulin. Chem. Phys. Lett. 2020, 759, 137987. [Google Scholar] [CrossRef]
- Dede, E.; Saglam, Ö.; Dilgin, Y. Sensitive voltammetric determination of niclosamide at a disposable pencil graphite electrode. Electrochim. Acta 2014, 127, 20–26. [Google Scholar] [CrossRef]
- Temerk, Y.M.; Ibrahim, H.S.M.; Schuhmann, W. Square wave cathodic adsorptive stripping voltammetric determination of the anticancer drugs flutamide and irinotecan in biological fluids using renewable pencil graphite electrodes. Electroanalysis 2016, 28, 372–379. [Google Scholar] [CrossRef]
- Dinc, E.; Dermiş, S.; Can Akcasoy, S.; Ceren Ertekin, Z. A New Chemometric strategy in electrochemical method optimization for the quantification of cefdinir in tablets, effervescent tablets and suspension samples. Electroanalysis 2020, 32, 613–619. [Google Scholar] [CrossRef]
- Rizk, M.; Hendawy, H.A.M.; Abou El-Alamin, M.M.; Moawad, M.I. Sensitive anodic voltammetric determination of methylergometrine maleate in bulk and pharmaceutical dosage forms using differential pulse voltammetry. J. Electroanal. Chem. 2015, 749, 53–61. [Google Scholar] [CrossRef]
- Rizk, M.; Abou El-Alamin, M.A.; Hendawy, H.A.M.; Moawad, M.I. Highly sensitive differential pulse and square wave voltammetric methods for determination of strontium ranelate in bulk and pharmaceutical dosage form. Electroanalysis 2016, 28, 770–777. [Google Scholar] [CrossRef]
- Shalaby, A.; Hassan, W.S.; Hendawy, H.A.M.; Ibrahim, A.M. Electrochemical oxidation behavior of itraconazole at different electrodes and its anodic stripping determination in pharmaceuticals and biological fluids. J. Electroanal. Chem. 2016, 763, 51–62. [Google Scholar] [CrossRef]
- David, I.G.; Iordache, L.; Popa, D.E.; Buleandra, M.; David, V.; Iorgulescu, E.E. Novel voltammetric investigation of dipyridamole at disposable pencil graphite electrode. Turk. J. Chem. 2019, 43, 1109–1122. [Google Scholar] [CrossRef]
- David, I.G.; Panait, A.L.; Buleandra, M.; Popa, D.E.; Cheregi, M.C. Simple voltammetric analysis of sulfamethoxazole at a disposable pencil graphite electrode. Rev. Roum. Chim. 2022. accepted. [Google Scholar]
- David, I.G.; Buleandră, M.; Popa, D.-E.; Bercea, A.-M.; Ciucu, A.-A. Simple electrochemical chloramphenicol assay at a disposable pencil graphite electrode by square wave voltammetry and linear sweep voltammetry. Anal. Lett. 2021, 1–18. [Google Scholar] [CrossRef]
- David, I.G.; Gâsnac, M.G.; Buleandră, M.; Popa, D.E. Simple and fast square wave voltammetric method for histamine H2-receptor antagonist famotidine quantification. Rev. Roum. Chim. 2021, 66, 573–578. [Google Scholar] [CrossRef]
- David, I.G.; Popa, D.E.; Calin, A.-A.; Buleandră, M.; Iorgulescu, E.E. Voltammetric determination of famotidine on a disposable pencil graphite electrode. Turk. J. Chem. 2016, 40, 125–135. [Google Scholar] [CrossRef]
- Sağlam, Ö.; Dilgin, D.G.; Ertek, B.; Dilgin, Y. Differential pulse voltammetric determination of eugenol at a pencil graphite electrode. Mater. Sci. Eng. C 2016, 60, 156–162. [Google Scholar] [CrossRef]
- David, I.G.; Florea, M.-A.; Cracea, O.G.; Popa, D.E.; Buleandra, M.; Iorgulescu, E.E.; David, V.; Badea, I.A.; Ciucu, A.A. Voltammetric Determination of vitamin B1 and vitamin B6 on a disposable electrode. Chem. Pap. 2015, 69, 901–910. [Google Scholar] [CrossRef]
- Said, M.I.; Abdel-aal, F.A.M.; Rageh, A.H. Novel sponge-like Mn5O8 nanoparticles deposited on graphite electrode for electrochemical study of hepatitis C antiviral drug, elbasvir. Microchem. J. 2020, 157, 105056. [Google Scholar] [CrossRef]
- Abdel-aal, F.A.M.; Rageh, A.H.; Said, M.I.; Saleh, G.A. ε-MnO2-modified graphite electrode as a novel electrochemical sensor for the ultrasensitive detection of the newly FDA approved Hepatitis C antiviral drug ledipasvir. Anal. Chim. Acta 2018, 1038, 29–40. [Google Scholar] [CrossRef]
- Rezaei, B.; Boroujeni, M.K.; Ensafi, A.A. Caffeine electrochemical sensor using imprinted film as recognition element based on polypyrrole, sol-gel, and gold nanoparticles hybrid nanocomposite modified pencil graphite electrode. Biosens. Bioelectron. 2014, 60, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, B.; Boroujeni, M.K.; Ensafi, A.A. A novel electrochemical nanocomposite imprinted sensor for the determination of lorazepam based on modified polypyrrole@sol-gel@gold nanoparticles/pencil graphite electrode. Electrochim. Acta 2014, 123, 332–339. [Google Scholar] [CrossRef]
- Pandey, I.; Jha, S.S. Molecularly imprinted polyaniline-ferrocene-sulfonic acid-carbon dots modified pencil graphite electrodes for chiral selective sensing of D-Ascorbic acid and L-Ascorbic acid: A clinical biomarker for preeclampsia. Electrochim. Acta 2015, 182, 917–928. [Google Scholar] [CrossRef]
- Jadon, N.; Jain, R.; Pandey, A. Electrochemical analysis of amlodipine in some pharmaceutical formulations and biological fluid using disposable pencil graphite electrode. J. Electroanal. Chem. 2017, 788, 7–13. [Google Scholar] [CrossRef]
- Alves, G.F.; Lisboa, T.P.; de Faria, L.V.; de Farias, D.M.; Matos, M.A.C.; Matos, R.C. Disposable pencil graphite electrode for ciprofloxacin determination in pharmaceutical formulations by square wave voltammetry. Electroanalysis 2021, 33, 543–549. [Google Scholar] [CrossRef]
- Pinyou, P.; Blay, V.; Chansaenpak, K.; Lisnund, S. Paracetamol sensing with a pencil lead electrode modified with carbon nanotubes and polyvinylpyrrolidone. Chemosensors 2020, 8, 133. [Google Scholar] [CrossRef]
- de Macêdo, I.Y.L.; Fernandes Alecrim, M.; Oliveira Neto, J.R.; Sapateiro Torres, I.M.; Vieira Thomaz, D.; de Souza Gil, E. Piroxicam voltammetric determination by ultra low cost pencil graphite electrode. Braz. J. Pharm. Sci. 2020, 56, e17344. [Google Scholar] [CrossRef] [Green Version]
- Purushothama, H.T.; Arthoba Nayaka, Y. Electrochemical study of hydrochlorothiazide on electrochemically pretreated pencil graphite electrode as a sensor. Sens. Bio-Sens. Res. 2017, 16, 12–18. [Google Scholar] [CrossRef]
- Purushothama, H.T.; Arthoba Nayaka, Y. Pencil graphite electrode based electrochemical system for the investigation of antihypertensive drug hydrochlorothiazide: An electrochemical study. Chem. Phys. Lett. 2019, 734, 136718. [Google Scholar] [CrossRef]
- Vinay, M.M.; Nayaka, Y.A.; Yatisha, R.O.; Basavarajappa, K.V.; Manjunatha, P.; Purushothama, H.T. Development of Azure-B modified pencil graphite electrode as an electrochemical sensor for the investigation of levofloxacin in pharmaceutical and biological samples. Chem. Data Collect. 2020, 28, 100441. [Google Scholar] [CrossRef]
- Mohamed, A.-M.I.; Rageh, A.H.; Abdel-aal, F.A.M.; Ali, A.-M.B.H. Pencil graphite electrode decorated with xylenol orange flakes for studying possible pharmacokinetic interaction between vardenafil and daclatasvir. Electroanalysis 2020, 32, 635–647. [Google Scholar] [CrossRef]
- Manjunatha, P.; Arthoba Nayaka, Y.; Chethana, B.K.; Vidyasagar, C.C.; Yathisha, R.O. Development of multi-walled carbon nanotubes modified pencil graphite electrode for the electrochemical investigation of aceclofenac present in pharmaceutical and biological samples. Sens. Bio-Sens. Res. 2018, 17, 7–17. [Google Scholar] [CrossRef]
- Prasad, B.B.; Kumar, A.; Singh, K. Molecularly imprinted polymer-based electrochemical sensor using functionalized fullerene as a nanomediator for ultratrace analysis of primaquine. Carbon 2016, 109, 196–207. [Google Scholar] [CrossRef]
- Prasad, B.B.; Singh, R.; Kumar, A. Development of imprinted polyneutral red/electrochemically reduced graphene oxide composite for ultra-trace sensing of 6-thioguanine. Carbon 2016, 102, 86–96. [Google Scholar] [CrossRef]
- Prasad, B.B.; Prasad, A.; Prasad Tiwari, M.; Madhuri, R. Multiwalled carbon nanotubes bearing ‘terminal monomeric unit’ for the fabrication of epinephrine imprinted polymer-based electrochemical sensor. Biosens. Bioelectron. 2013, 45, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Prasad, B.B.; Fatma, S. One MoNomer doubly imprinted dendrimer nanofilm modified pencil graphite electrode for simultaneous electrochemical determination of norepinephrine and uric acid. Electrochim. Acta 2017, 232, 474–483. [Google Scholar] [CrossRef]
- Prasad, B.B.; Singh, R.; Singh, K. Development of highly electrocatalytic and electroconductingimprinted film using Ni nanomer for ultra-trace detection of thiamine. Sens. Actuators B Chem. 2017, 246, 38–45. [Google Scholar] [CrossRef]
- Dehnavi, A.; Soleymanpour, A. Silver nanoparticles/poly(L-cysteine) nanocomposite modified pencil graphite for selective electrochemical measurement of guaifenesin in real samples. Measurement 2021, 175, 109103. [Google Scholar] [CrossRef]
- Rejithamol, R.; Keerthi, P.R.; Beena, S. A novel disposable pencil graphite electrode for the voltammetric determination of cysteamine. Mater. Today Proc. 2019, 18, 5081–5086. [Google Scholar]
- Krishnan, R.G.; Greeshma, S.; Shivon, D.M.; Suryasree, S.R.; Beena, S. Morphological studies of disposable graphite and its effective utilization for vitamin B12 analysis in pharmaceutical formulations. Mater. Today Proc. 2019, 18, 3314–3320. [Google Scholar] [CrossRef]
- Karaboduk, K. Development of a voltammetric method for the determination of rapamycin in pharmaceutical samples at pretreated pencil graphite electrode. J. Chin. Chem. Soc. 2021, 68, 1722–1730. [Google Scholar] [CrossRef]
- Fard, G.P.; Alipour, E.; Sabzi, R.E.A. Modification of a disposable pencil graphite electrode with multiwalled carbon nanotubes: Application to electrochemical determination of diclofenac sodium in some pharmaceutical and biological samples. Anal. Methods 2016, 8, 3966–3974. [Google Scholar] [CrossRef]
- Alipour, E.; Majidi, M.R.; Hoseindokht, O. Development of simple electrochemical sensor for selective determination of methadone in biological samples using multi-walled carbon nanotubes modified pencil graphite electrode. J. Chin. Chem. Soc. 2015, 62, 461–468. [Google Scholar] [CrossRef]
- Tabanlıgil Calam, T. A modified pencil graphite electrode with 2-thiobarbituric acid for the efficient and cheap voltammetric sensing of 4-aminophenol in water samples and child syrup sample. J. Food Compos. Anal. 2021, 98, 103809. [Google Scholar] [CrossRef]
- Rostami-Javanroudi, S.; Babakhanian, A. New electrochemical sensor for direct quantification of vitamin K in human blood serum. Microchem. J. 2021, 163, 105716. [Google Scholar] [CrossRef]
- Kolahi-Ahari, S.; Deiminiat, B.; Rounaghi, G.H. Modification of a pencil graphite electrode with multiwalled carbon nanotubes capped gold nanoparticles for electrochemical determination of tramadol. J. Electroanal. Chem. 2020, 862, 113996. [Google Scholar] [CrossRef]
- Pashazadeh, S.; Habibi, B. Determination of isoniazid by a copper-based metal-organic frameworks/carbon nitride quantum dots modified pencil graphite electrode as a highly sensitive and selective sensor. J. Electroanal. Chem. 2020, 876, 114493. [Google Scholar] [CrossRef]
- Habibi, B.; Pashazadeh, S.; Saghatforoush, L.A.; Pashazadeh, A. Direct electrochemical synthesis of the copper based metal-organic framework on/in the heteroatoms doped graphene/pencil graphite electrode: Highly sensitive and selective electrochemical sensor for sertraline hydrochloride. J. Electroanal. Chem. 2021, 888, 115210. [Google Scholar] [CrossRef]
- Oghli, H.; Soleymanpour, A. Polyoxometalate/reduced graphene oxide modified pencil graphite sensor for the electrochemical trace determination of paroxetine in biological and pharmaceutical media. Mater. Sci. Eng. C 2020, 108, 110407. [Google Scholar] [CrossRef] [PubMed]
- Oghli, H.; Soleymanpour, A. Ultrasensitive electrochemical sensor for simultaneous determination of sumatriptan and paroxetine using molecular imprinted polymer/sol-gel/ polyoxometalate/rGO modified pencil graphite electrode. Sens. Actuators B Chem. 2021, 344, 130215. [Google Scholar] [CrossRef]
- Ibrahim, H.; Temerk, Y. A novel disposable electrochemical sensor based on modifying graphite pencil lead electrode surface with nanoacetylene black for simultaneous determination of antiandrogens flutamide and cyproterone acetate. J. Electroanal. Chem. 2020, 859, 113836. [Google Scholar] [CrossRef]
- Zabardasti, A.; Afrouzi, H.; Talemi, R.P. A simple and sensitive methodology for voltammetric determination of valproic acid in human blood plasma samples using 3-aminopropyletriethoxy silane coatedmagnetic nanoparticles modified pencil graphite electrode. Mater. Sci. Eng. C 2017, 76, 425–430. [Google Scholar] [CrossRef]
- Sisolakova, I.; Hovancova, J.; Orinakova, R.; Orinak, A.; Garcia, D.R.; Shylenko, O.; Radonak, J. Comparison of insulin determination on NiNPs/chitosan-MWCNTs and NiONPs/chitosan-MWCNTs modified pencil graphite electrode. Electroanalysis 2019, 31, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Nagarajan, S.; Vairamuthu, R. Electrochemical detection of riboflavin using tin-chitosan modified pencil graphite electrode. J. Electroanal. Chem. 2021, 891, 115235. [Google Scholar] [CrossRef]
- Deepa, S.; Kumara Swamy, B.E.; Vasantakumar Pai, K. Voltammetric detection of anticancer drug doxorubicin at pencil graphite electrode: A voltammetric study. Sens. Int. 2020, 1, 100033. [Google Scholar] [CrossRef]
- Yen, P.T.H.; Anh, N.H.; Ha, V.T.T.; Hung, L.Q.; Phong, P.H.; Hien, C.T.T. Electrochemical properties of amoxicillin on an economical, simple graphite pencil electrode and the ability of the electrode in amoxicillin detection. Vietnam J. Chem. 2020, 58, 201–205. [Google Scholar] [CrossRef]
- Tigari, G.; Manjunatha, J.G. A surfactant enhanced novel pencil graphite and carbon nanotube composite paste material as an effective electrochemical sensor for determination of riboflavin. J. Sci. Adv. Mater. Dev. 2020, 5, 56–64. [Google Scholar] [CrossRef]
- Aydin, I.; Akgun, H.; Talay Pınar, P. Analytical determination of the oxazolidinone antibiotic linezolid at a pencil graphite and carbon paste electrodes. ChemistrySelect 2019, 4, 9966–9971. [Google Scholar] [CrossRef]
- Sawkar, R.R.; Patil, V.B.; Shanbhag, M.M.; Shetti, N.P.; Tuwar, S.M.; Aminabhavi, T.M. Detection of ketorolac drug using pencil graphite electrode. Biomed. Eng. Adv. 2021, 2, 100009. [Google Scholar] [CrossRef]
- Kojabad, R.N.; Ebrahimiasl, S. Pencil graphite electrode modified nanosensor for detection and determination of tramadol in blood serum. QUID Investig. Cienc. Tecnol. 2017, 1, 597–604. [Google Scholar]
- Gowda, J.I.; Nandibewoor, S.T. Electrochemical behavior of 4-aminophenazone drug at a graphite pencil electrode and its application in real samples. Ind. Eng. Chem. Res. 2012, 51, 15936–15941. [Google Scholar] [CrossRef]
- Gadallah, M.I.; Ali, H.R.H.; Askal, H.F.; Saleh, G.A. Development of terbium based sensor for determination of imipenem in dosage forms and real samples. J. Mol. Liq. 2016, 276, 705–713. [Google Scholar] [CrossRef]
- Gowda, J.I.; Nandibewoor, S.T. Electrochemical characterization and determination of paclitaxel drug using graphite pencil electrode. Electrochim. Acta 2014, 116, 326–333. [Google Scholar] [CrossRef]
- Buleandră, M.; Popa, D.E.; Popa, A.; Codreanu, N.A.M.; David, I.G. Multi-analyte sensor based on pencil graphite electrode for riboflavin and pyridoxine determination. J. Electrochem. Soc. 2022, 169, 017517. [Google Scholar] [CrossRef]
- Parvizi-Fard, G.; Alipour, E.; Sefidi, P.Y.; Sabzi, R.E. Pretreated pencil graphite electrode as a versatile platform for easy measurement of diclofenac sodium in a number of biological and pharmaceutical samples. J. Chin. Chem. Soc. 2018, 65, 472–484. [Google Scholar] [CrossRef]
- Koyun, O.; Gorduk, S.; Arvas, M.B.; Sahin, Y. Electrochemically treated pencil graphite electrodes prepared in one step for the electrochemical determination of paracetamol. Russ. J. Electrochem. 2018, 54, 796–808. [Google Scholar] [CrossRef]
- Khashaba, P.Y.; Ali, H.R.H.; El-Wekil, M.M. Complexation based voltammetric determination of pantoprazole sodium in pharmaceutical formulations and rabbit plasma. Electroanalysis 2017, 29, 890–897. [Google Scholar] [CrossRef]
- Rana, A.; Baig, N.; Saleh, T.A. Electrochemically pretreated carbon electrodes and their electroanalytical applications—A review. J. Electroanal. Chem. 2019, 833, 313–332. [Google Scholar] [CrossRef]
- Levent, A.; Onal, G. Application of a pencil graphite electrode for voltammetric simultaneous determination of ascorbic acid, norepinephrine, and uric acid in real samples. Turk. J. Chem. 2018, 42, 460–471. [Google Scholar] [CrossRef]
- Bayraktepe, D.E.; Yazan, Z. Application of single-use electrode based on nano-clay and MWCNT for simultaneous determination of acetaminophen, ascorbic acid and acetylsalicylic acid in pharmaceutical dosage. Electroanalysis 2020, 32, 1263–1272. [Google Scholar] [CrossRef]
- Fouladgar, M.; Karimi-Maleh, H.; Opoku, F.; Govender, P.P. Electrochemical anticancer drug sensor for determination of raloxifene in the presence of tamoxifen using graphene-CuO-polypyrrole nanocomposite structure modified pencil graphite electrode: Theoretical and experimental investigation. J. Mol. Liq. 2020, 311, 113314. [Google Scholar] [CrossRef]
- Hatamluyi, B.; Lorestani, F.; Es’haghi, Z. Au/Pd@rGO nanocomposite decorated with poly (L-Cysteine) as a probe forsimultaneous sensitive electrochemical determination of anticancer drugs, ifosfamide and etoposide. Biosens. Bioelectron. 2018, 120, 22–29. [Google Scholar] [CrossRef]
- Hatamluyi, B.; Es’haghi, Z. A layer-by-layer sensing architecture based on dendrimer and ionic liquid supported reduced graphene oxide for simultaneous hollow-fiber solid phase microextraction and electrochemical determination of anti-cancer drug imatinib in biological samples. J. Electroanal. Chem. 2017, 801, 439–449. [Google Scholar] [CrossRef]
- Yan, C.; Liu, X.; Zhang, R.; Chen, Y.; Wang, G. A selective strategy for determination of ascorbic acid based on molecular imprinted copolymer of o-phenylenediamine and pyrrole. J. Electroanal. Chem. 2016, 780, 276–281. [Google Scholar] [CrossRef]
- Rouhani, M.; Soleymanpour, A. Ultrasensitive electrochemical determination of trace ceftizoxime using a thin film of Preyssler nanocapsules on pencil graphite electrode surface modified with reduced graphene oxide. Microchem. J. 2021, 165, 106160. [Google Scholar] [CrossRef]
- Rezaei, B.; Lotfi-Forushani, H.; Ensafi, A.A. Modified Au nanoparticles-imprinted sol–gel, multiwall carbon nanotubes pencil graphite electrode used as a sensor for ranitidine determination. Mater. Sci. Eng. C 2014, 37, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, B.; Foroughi-Dehnavi, S.; Ensafi, A.A. Fabrication of electrochemical sensor based on molecularly imprinted polymer and nanoparticles for determination trace amounts of morphine. Ionics 2015, 21, 2969–2980. [Google Scholar] [CrossRef]
- Oghli, H.A.; Alipour, E.; Asadzadeh, M. Development of a novel voltammetric sensor for the determination of methamphetamine in biological samples on the pretreated pencil graphite electrode. RSC Adv. 2015, 5, 9674–9682. [Google Scholar] [CrossRef]
- Alipour, E.; Gasemlou, S. Easy modification of pencil graphite electrode for discrimination and determination of morphine in biological and street samples. Anal. Methods 2012, 4, 2962–2969. [Google Scholar] [CrossRef]
- Gowda, J.I.; Hurakadli, G.S.; Nandibewoor, S.T. Pretreated graphite pencil electrode based voltammetric sensing of albendazole. Anal. Chem. Lett. 2017, 7, 389–401. [Google Scholar] [CrossRef]
- Buleandră, M.; Popa, D.E.; David, I.G.; Ciucu, A.A. A simple and efficient cyclic square wave voltammetric method for simultaneous determination of epinephrine and norepinephrine using an activated pencil graphite electrode. Microchem. J. 2021, 160, 105621. [Google Scholar] [CrossRef]
- Buleandră, M.; Ciucu, A.A.; David, I.G.; Popa, D.E.; Ciobanu, A.M.; Ştefănescu, C.D. Simultaneous determination of epinephrine and norepinephrine by electrochemical reduction at the pre-treated pencil graphite electrode. Rev. Roum. Chim. 2021, 66, 567–572. [Google Scholar] [CrossRef]
- Ciucu, A.A.; Buleandră, M.; Ciurea, T.; Stoica, V.N.; Ştefanescu, C.D.; Ciobanu, A. A new voltammetric approach for electrochemical determination of lamotrigine in pharmaceutical samples. Electroanalysis 2021, 33, 1389–1392. [Google Scholar] [CrossRef]
- Mollarasouli, F.; Zor, E.; Ozcelikay, G.; Ozkan, S.A. Magnetic nanoparticles in developing electrochemical sensors for pharmaceutical and biomedical applications. Talanta 2021, 226, 122108. [Google Scholar] [CrossRef] [PubMed]
- Lawal, A.T. Progress in utilisation of graphene for electrochemical biosensors. Biosens. Bioelectron. 2018, 106, 149–178. [Google Scholar] [CrossRef]
- Akanda, M.R.; Sohail, M.; Aziz, M.A.; Kawde, A.-N. Recent advances in nanomaterial-modified pencil graphite electrodes for electroanalysis. Electroanalysis 2016, 28, 408–424. [Google Scholar] [CrossRef]
- Beluomini, M.A.; da Silva, J.L.; Cardoso de Sa, A.; Buffon, E.; Pereira, T.C.; Stradiotto, N.R. Electrochemical sensors based on molecularly imprinted polymer on nanostructured carbon materials: A review. J. Electroanal. Chem. 2019, 840, 343–366. [Google Scholar] [CrossRef]
- Temerk, Y.; Ibrahim, M.; Ibrahim, H.; Kotb, M. Adsorptive stripping voltammetric determination of anticancer drug lomustine in biological fluids using in situ mercury film coated graphite pencil electrode. J. Electroanal. Chem. 2016, 760, 135–142. [Google Scholar] [CrossRef]
- Asadpour-Zeynali, K.; Majidi, M.R.; Najafi-Marandi, P.; Norysaray, Z. Electrocatalytic reduction of metronidazole on bismuth modified pencil-lead electrode. J. Chin. Chem. Soc. 2013, 60, 1253–1259. [Google Scholar] [CrossRef]
- Güzel, R.; Ekşi, H.; Dinç, E.; Solak, A.O. Determination of acetaminophen in commercial formulations using silver nanostructured aniline modified pencil graphite electrode. J. Electrochem. Soc. 2013, 160, B119–B124. [Google Scholar] [CrossRef]
- Nezhadali, A.; Mojarrab, M. Computational design and multivariate optimization of an electrochemical metoprolol sensor based on molecular imprinting in combination with carbon nanotubes. Anal. Chim. Acta 2016, 924, 86–98. [Google Scholar] [CrossRef]
- Nezhadali, A.; Mojarrab, M. Fabrication of an electrochemical molecularly imprinted polymer triamterene sensor based on multivariate optimization using multi-walled carbon nanotubes. J. Electroanal. Chem. 2015, 744, 85–94. [Google Scholar] [CrossRef]
- Pourbeyram, S.; Moosavifar, M.; Ashtari, L. Ultra-sensitive determination of insulin on pencil graphite electrode modified by cerium salen encapsulated zeolite (CS@Z-PGE). Microporous Mesoporous Mater. 2017, 242, 25–33. [Google Scholar] [CrossRef]
- Pattar, V.P.; Nandibewoor, S.T. Staircase voltammetric determination of 2-thiouracil in pharmaceuticals and human biological fluids at polyaniline and polypyrrole film modified sensors. Sens. Actuators A Phys. 2016, 250, 40–47. [Google Scholar] [CrossRef]
- Arabali, V.; Malekmohammadi, S.; Karimi, F. Surface amplification of pencil graphite electrode using CuO nanoparticle/polypyrrole nanocomposite; a powerful electrochemical strategy for determination of tramadol. Microchem. J. 2020, 158, 105179. [Google Scholar] [CrossRef]
- Khashaba, P.Y.; Refat, H.; Ali, H.; El-Wekil, M.M. A new and cost effective approach for simultaneous voltammetric analysis of two related benzimidazole drugs and their determination in biological fluids. Electroanalysis 2017, 29, 1643–1650. [Google Scholar] [CrossRef]
- Khashaba, P.Y.; Refat, H.; Ali, H.; El-Wekil, M.M. Simultaneous voltammetric analysis of anti-ulcer and D2-antagonist agents in binary mixture using redox sensor and their determination in human serum. Mater. Sci. Eng. C 2017, 75, 733–741. [Google Scholar] [CrossRef]
- Gadallah, M.I.; Ali, H.R.H.; Askal, H.F.; Saleh, G.A. Poly (bromocresol green) flakes-decorated pencil graphite electrode for selective electrochemical sensing applications and pharmacokinetic studies. Mater. Sci. Eng. C 2019, 102, 634–645. [Google Scholar] [CrossRef]
- Nezhadali, A.; Pirayesh, S.; Shadmehri, R. Computer-assisted sensor design and analysis of 2-aminobenzimidazole in biological model samples based on electropolymerized-molecularly imprinted polypyrrole modified pencil graphite electrode. Sens. Actuators B Chem. 2013, 185, 17–23. [Google Scholar] [CrossRef]
- Nezhadali, A.; Mehri, L.; Shadmehri, R. Determination of benzimidazole in biological model samples using electropolymerized-molecularly imprinted polypyrrole modified pencil graphite sensor. Sens. Actuators B Chem. 2012, 171–172, 1125–1131. [Google Scholar] [CrossRef]
- Nezhadali, A.; Mehri, L.; Shadmehri, R. Determination of methimazole based on electropolymerized-molecularly imprinted polypyrrole modified pencil graphite sensor. Mater. Sci. Eng. C 2018, 85, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Tadi, K.K.; Motghare, R.V.; Ganesh, V. Electrochemical detection of sulfanilamide using pencil graphite electrode based on molecular imprinting technology. Electroanalysis 2014, 26, 2328–2336. [Google Scholar] [CrossRef]
- Gürler, B.; Özkorucuklu, S.P.; Kir, E. Voltammetric behavior and determination of doxycycline in pharmaceuticals at molecularly imprinted and non-imprinted overoxidized polypyrrole electrodes. J. Pharm. Biomed. Anal. 2013, 84, 263–268. [Google Scholar] [CrossRef]
- Koseoglu, T.S.; Durgut, A. Development of a novel molecularly imprinted overoxidized polypyrrole electrode for the determination of sulfasalazine. Electroanalysis 2020, 32, 2072–2081. [Google Scholar] [CrossRef]
- Singh, K.; Prasad, B.B. Molecularly imprinted polymer-based core-shells (solid vs hollow) @pencil graphite electrode for electrochemical sensing of certain anti-HIV drugs. Sens. Actuators B Chem. 2017, 244, 167–174. [Google Scholar] [CrossRef]
- Nezhadali, A.; Shadmehri, R. Neuro-genetic multi-objective optimization and computer-aided design of pantoprazole molecularly imprinted polypyrrole sensor. Sens. Actuators B Chem. 2014, 202, 240–251. [Google Scholar] [CrossRef]
- Nezhadali, A.; Sadeghzadeh, S. Experimental design-artificial neural network-genetic algorithm optimization and computer-assisted design of celecoxib molecularly imprinted polymer/carbon nanotube sensor. J. Electroanal. Chem. 2017, 795, 32–40. [Google Scholar] [CrossRef]
- Güney, S. Electrochemical synthesis of molecularly imprinted poly(p-aminobenzene sulphonic acid) on carbon nanodots coated pencil graphite electrode for selective determination of folic acid. J. Electroanal. Chem. 2019, 854, 113518. [Google Scholar] [CrossRef]
- Hatamluyi, B.; Es’haghi, Z. Electrochemical biosensing platform based on molecularly imprinted polymer reinforced by ZnO-graphene capped quantum dots for 6-mercaptopurine detection. Electrochim. Acta 2018, 283, 1170–1177. [Google Scholar] [CrossRef]
- Singh, R.; Jaiswal, S.; Singh, K.; Fatma, S.; Prasad, B.B. Biomimetic polymer-based electrochemical sensor using methyl blue-adsorbed reduced graphene qxide and functionalized multiwalled carbon nanotubes for trace sensing of cyanocobalamin. ACS Appl. Nano Mater. 2018, 1, 4652–4660. [Google Scholar] [CrossRef]
- Prasad, B.B.; Pathak, P.K. Development of surface imprinted nanospheres using the inverse suspension polymerization method for electrochemical ultra sensing of dacarbazine. Anal. Chim. Acta 2017, 974, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Oghli, A.H.; Soleymanpour, A. Pencil graphite electrode modified with nitrogen-doped graphene and molecular imprinted polyacrylamide/sol-gel as an ultrasensitive electrochemical sensor for the determination of fexofenadine in biological media. Biochem. Eng. J. 2021, 167, 107920. [Google Scholar] [CrossRef]
- Kumar, A.; Pathak, P.K.; Prasad, B.B. Electrocatalytic imprinted polymer of N-doped hollow carbon nanosphere−palladium nanocomposite for ultratrace detection of anticancer drug 6-mercaptopurine. ACS Appl. Mater. Interfaces 2019, 11, 16065–16074. [Google Scholar] [CrossRef] [PubMed]
- Nezhadali, A.; Bonakdar, G.A. Multivariate optimization of mebeverine analysis using molecularly imprinted polymer electrochemical sensor based on silver nanoparticles. J. Food Drug Anal. 2019, 27, 305–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayraktepe, D.E.; Inal, E.K.; Yazan, Z. Preparation and characterization of a pencil graphite electrode modified with gold nanoparticles decorated poly (L-methionine) and its use in the simultaneous sensitive electrochemical analysis of ascorbic acid, acetaminophen, chlorpheniramine maleate, and caffeine. Microchem. J. 2021, 171, 106812. [Google Scholar] [CrossRef]
- Mohamed, F.A.; Khashaba, P.Y.; Shahin, R.Y.; El-Wekil, M.M. Tunable ternary nanocomposite prepared by electrodeposition for biosensing of centrally acting reversible acetyl cholinesterase inhibitor donepezil hydrochloride in real samples. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 567, 76–85. [Google Scholar] [CrossRef]
- Dehnavi, A.; Soleymanpou, A. Titanium dioxide/multi-walled carbon nanotubes composite modified pencil graphite sensor for sensitive voltammetric determination of propranolol in real samples. Electroanalysis 2021, 33, 355–364. [Google Scholar] [CrossRef]
- Es’haghi, Z.; Moeinpour, F. Carbon nanotube/polyurethane modified hollow fiber-pencil graphite electrode for in situ concentration and electrochemical quantification of anticancer drugs capecitabine and erlotinib. Eng. Life Sci. 2019, 19, 302–314. [Google Scholar] [CrossRef] [Green Version]
- Das, T.R.; Jena, S.K.; Madhuri, R.; Sharma, P.K. Polymeric iron oxide-graphene nanocomposite as a trace level sensor of vitamin C. Appl. Surf. Sci. 2018, 449, 304–313. [Google Scholar] [CrossRef]
- Senel, M.; Durmus, Z.; Alachkar, A. Measurement of the antipsychotic clozapine using reduced graphene oxide nanocomposites-Au/Pd/Pt electrodes. Electroanalysis 2021, 33, 1585–1595. [Google Scholar] [CrossRef]
- Bayraktepe, D.E.; Yazan, Z.; Onal, M. Sensitive and cost effective disposable composite electrode based on graphite, nano-smectite and multiwall carbon nanotubes for the simultaneous trace level detection of ascorbic acid and acetylsalicylic acid in pharmaceuticals. Talanta 2019, 203, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Ensafi, A.A.; Khoddami, E.; Rezaei, B. A combined liquid three-phase micro-extraction and differential pulse voltammetric method for preconcentration and detection of ultra-trace amounts of buprenorphine using a modified pencil electrode. Talanta 2013, 116, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Izadyar, A.; Arachchige, D.R.; Cornwell, H.; Hershberger, J.C. Ion transfer stripping voltammetry for the detection of nanomolar levels of fluoxetine, citalopram, and sertraline in tap and river water samples. Sens. Actuators B Chem. 2016, 223, 226–233. [Google Scholar] [CrossRef]
- Dehghanzade, M.; Alipour, E. Voltammetric determination of diazepam using a bismuth modified pencil graphite electrode. Anal. Methods 2016, 8, 1995–2004. [Google Scholar] [CrossRef]
- Rezaeifar, Z.; Rounaghi, G.H.; Es’haghi, Z.; Chamsaz, M. Electrochemical determination of anticancer drug, flutamide in human plasma sample using a microfabricated sensor based on hyperbranchedpolyglycerol modified graphene oxide reinforced hollow fiber-pencil graphite electrode. Mater. Sci. Eng. C 2018, 91, 10–18. [Google Scholar] [CrossRef]
- Manjunatha, P.; Nayaka, Y.A. Cetyltrimethylammonium bromide-gold nanoparticles composite modified pencil graphite electrode for the electrochemical investigation of cefixime present in pharmaceutical formulations and biology. Chem. Data Collect. 2019, 21, 100217. [Google Scholar] [CrossRef]
- Azadmehr, F.; Zarei, K. Ultrasensitive determination of ceftizoxime using pencil graphite electrode modified by hollow gold nanoparticles/reduced graphene oxide. Arab. J. Chem. 2020, 13, 1890–1900. [Google Scholar] [CrossRef]
- Akbari Hasanjani, H.R.; Zarei, K. Electrochemical sensor for ultrasensitive determination of ceftazidime using hollow platinum nanoparticles/reduced graphene oxide/pencil graphite electrode. Chem. Pap. 2018, 72, 1935–1944. [Google Scholar] [CrossRef]
- Rison, S.; Mathew, A.T.; George, L.; Maiyalagan, T.; Hegde, G.; Varghese, A. Pt nanospheres decorated graphene-β-CD modified pencil graphite electrode for the electrochemical determination of vitamin B6. Top Catal. 2022. [Google Scholar] [CrossRef]
- Sangeetha, N.S.; Narayanan, S.S. Effective electrochemical detection of riboflavin and butylated hydroxy anisole based on azure A and nickel hexacyanoferrate framework on graphite electrode. Chem. Data Collect. 2020, 30, 100544. [Google Scholar] [CrossRef]
- Sangsefidi, F.S.; Salavati-Niasari, M.; Mazaheri, S.; Sabet, M. Controlled green synthesis and characterization of CeO2 nanostructures as materials for the determination of ascorbic acid. J. Mol. Liq. 2017, 241, 772–781. [Google Scholar] [CrossRef]
- Rouhani, M.; Soleymanpour, A. Preparation of Dawson heteropolyacid-embedded silver nanoparticles/graphene oxide nanocomposite thin film used to modify pencil graphite electrode as a sensor for trace electrochemical sensing of levodopa. Mater. Sci. Eng. C 2020, 117, 111287. [Google Scholar] [CrossRef] [PubMed]
- Dehnavi, A.; Soleymanpour, A. Highly sensitive voltammetric electrode for the trace measurement of methyldopa based on a pencil graphite modified with phosphomolibdate/graphene oxide. Microchem. J. 2020, 157, 104969. [Google Scholar] [CrossRef]
- Martins, T.S.; Bott-Neto, J.L.; Raymundo-Pereira, P.A.; Ticianelli, E.A.; Machado, S.A.S. An electrochemical furosemide sensor based on pencil graphite surface modified with polymer film Ni-salen and Ni(OH)2/C nanoparticles. Sens. Actuators B Chem. 2018, 276, 378–387. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Khoddami, E.; Rezaei, B.; Jafari-Asl, M. A supported liquid membrane for microextraction of insulin, and its determination with a pencil graphite electrode modified with RuO2-graphene oxide. Mikrochim. Acta 2015, 182, 1599–1607. [Google Scholar] [CrossRef]
- Ali, A.-M.B.H.; Abdel-aal, F.A.M.; Rageh, A.H.; Mohamed, A.-M.I. Hybrid NiO nanostructured/sulfanilamide polymeric film for studying possible pharmacokinetic interaction between avanafil and nimodipine in real human serum by their simultaneous determination using square-wave voltammetry. Microchem. J. 2022, 172, 106895. [Google Scholar] [CrossRef]
- Singh, K.; Jaiswal, S.; Singh, R.; Fatma, S.; Prasad, B.B. One-by-one imprinting in two eccentric layers of hollow core-shells: Sequential electroanalysis of anti-HIV drugs. Biosens. Bioelectron. 2018, 111, 82–89. [Google Scholar] [CrossRef]
- Fakahari, A.R.; Sahragard, A.; Ahmar, H.; Tabani, H. A novel platform sensing based on combination of electromembrane-assisted solid phase microextraction with linear sweep voltammetry for the determination of tramadol. J. Electroanal. Chem. 2015, 747, 12–19. [Google Scholar] [CrossRef]
- Riman, D.; Rozsypal, J.; Halouzka, V.; Hrbac, J.; Jirovsky, D. The use of micro carbon pencil lead electrode for sensitive HPLC-ED analysis of selected antipsychotic drugs. Microchem. J. 2020, 154, 104406. [Google Scholar] [CrossRef]
- Available online: www.scopus.com (accessed on 5 February 2022).

| Analyte (Drug) | PGE Type/ET Conditions | Analysis Technique | Linear Range (mol/L) | LoD (mol/L) | Sample | Ref. |
|---|---|---|---|---|---|---|
| Ascorbic acid Norepinephrine Uric acid | 1.40 V, 60 s | DPV | 1.00 × 10−7–8.00 × 10−7 2.00 × 10−8–1.70 × 10−7 4.00 × 10−8–1.75 × 10−7 | 2.70 × 10−8 4.00 × 10−9 1.00 × 10−8 | Pharmaceutical formulations; urine | [84] |
| Diclofenac sodium | H/−0.50 V, 300 s; 0.50 mol/L ABS pH 4.80 + 0.10 mol/L NaCl | DPV | 2.30 × 10−7–1.29 × 10−5 | 1.20 × 10−7 | Pharmaceutical tablets; urine | [80] |
| Methamphetamine | 1.70 V, 10 min; 0.10 mol/L BRB pH 11.00 | DPV | 7.40 × 10−8–5.40 × 10−5 | 5.00 × 10−8 | Serum; urine | [93] |
| Morphine | +1.80 V, 300 s; 0.50 mol/L PBS pH 7.00 | DPV Amp | 1.00 × 10−6–1.00 × 10−4 | 2.60 × 10−7 | Street drugs; urine | [94] |
| Pantoprazole | HB/+1.30 V, 30 s; BRB pH 7.00 | Co2+-complexation + AdS-SWV | 1.00 × 10−10–9.00 × 10−9 | 4.00 ×10−11 | Pharmaceutical tablets and vials; spiked rabbit plasma | [82] |
| Vitamin B6 | 2.00 V, 100 s; 0.10 mol/L H3PO4 | DPV | 5.00 × 10−7–3.00 × 10−4 | 2.19 × 10−7 | Energy drink; vitamin water | [10] |
| Acetaminophen | HB/CV: 0.00–2.10 V; n = 10; 0.50 mol/L H2SO4 | DPV | 1.00 × 10−6–1.00 × 10−3 | 1.74 × 10−7 | Pharmaceutical tablets | [81] |
| Albendazole | HB/CV: −2.00 to 2.00 V; n = 5; 0.05 V/s; 0.10 M HCl | DPV | 2.50 × 10−4–1.45 × 10−3 | 5.42 × 10−9 | Pharmaceutical tablets; urine | [95] |
| Epinephrine Norepinephrine Epinephrine Norepinephrine | HB/CV: −0.20 to 2.00 V; n = 10; 0.50 V/s; BRB pH 2.21 | CSWV SWV | 2.50 × 10−6–2.50 × 10−4 2.50 × 10−6–2.50 × 10−4 2.50 × 10−6–2.50 × 10−4 2.50 × 10−6–2.40 × 10−4 | 8.32 × 10−7 9.92 × 10−7 1.46 × 10−6 1.46 × 10−6 | Pharmaceutical vials; plasma Human plasma | [96,97] |
| Hydrochlorthiazide | HB/CV: 0.40 to 1.20 V; n = 50; 0.10 V/s; 0.10 mol/L PBS pH 7.00 | DPV SWV | 4.00 × 10−6–1.40 × 10−4 1.00 × 10−6–2.00 × 10−5 | 3.25 × 10−6 4.21 × 10−7 | Pharmaceutical tablets; urine | [43] |
| Ketorolac | CV: 0.60 to 1.60 V; n = 10; 0.20 mol/L PBS pH 3.00 | DPV | 2.00 × 10−6–1.00 × 10−3 | 4.59 × 10−7 | Pharmaceutical tablets; urine | [74] |
| Lamotrigine | HB/CV: −0.20 to 2.00 V; n = 10; 0.50 V/s; BRB pH 2.21 | LSV | 2.50 × 10−5–1.00 × 10−3 | 1.94 × 10−5 | Pharmaceutical tablets | [98] |
| Rapamycin | 2B/CV: 1.50 to 2.00 V; n = 50; 0.05 V/s; 0.10 mol/L PBS pH 7.00 | DPV | 1.00 × 10−8–2.50 × 10−4 | 7.50 × 10−9 | Pharmaceutical tablets | [56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
David, I.G.; Buleandra, M.; Popa, D.E.; Cheregi, M.C.; David, V.; Iorgulescu, E.E.; Tartareanu, G.O. Recent Developments in Voltammetric Analysis of Pharmaceuticals Using Disposable Pencil Graphite Electrodes. Processes 2022, 10, 472. https://doi.org/10.3390/pr10030472
David IG, Buleandra M, Popa DE, Cheregi MC, David V, Iorgulescu EE, Tartareanu GO. Recent Developments in Voltammetric Analysis of Pharmaceuticals Using Disposable Pencil Graphite Electrodes. Processes. 2022; 10(3):472. https://doi.org/10.3390/pr10030472
Chicago/Turabian StyleDavid, Iulia Gabriela, Mihaela Buleandra, Dana Elena Popa, Mihaela Carmen Cheregi, Vasile David, Emilia Elena Iorgulescu, and Georgiana Oana Tartareanu. 2022. "Recent Developments in Voltammetric Analysis of Pharmaceuticals Using Disposable Pencil Graphite Electrodes" Processes 10, no. 3: 472. https://doi.org/10.3390/pr10030472
APA StyleDavid, I. G., Buleandra, M., Popa, D. E., Cheregi, M. C., David, V., Iorgulescu, E. E., & Tartareanu, G. O. (2022). Recent Developments in Voltammetric Analysis of Pharmaceuticals Using Disposable Pencil Graphite Electrodes. Processes, 10(3), 472. https://doi.org/10.3390/pr10030472








