How Does Long-Term Storage Influence the Physical Stability and Dissolution of Bicalutamide from Solid Dispersions and Minitablets?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Ball Milling
2.2.2. Final Blend Preparation and Characterization
2.2.3. Preparation of Minitablets by Direct Compression and Evaluation of Their Properties
2.3. Stability Evaluation
2.3.1. Storage of Solid Dispersions and Minitablets
2.3.2. Visual Inspection of Sample Appearance
2.3.3. Laser Diffraction Measurements
2.3.4. Powder X-ray Diffraction (XRPD)
2.3.5. Dissolution Studies
3. Results and Discussions
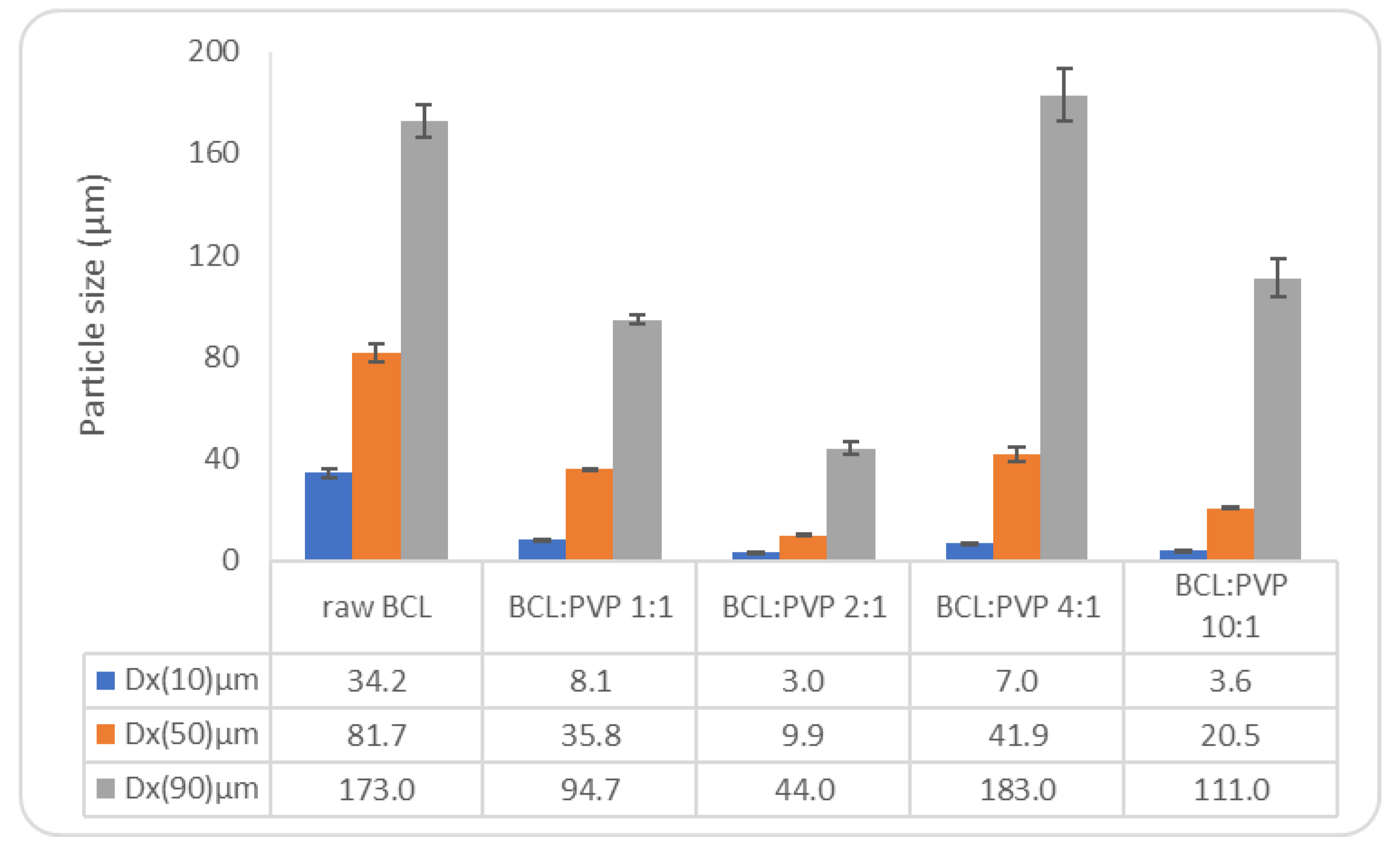
3.1. Bicalutamide and PVP K29/32 Solid Dispersions
3.1.1. Appearance of the Samples
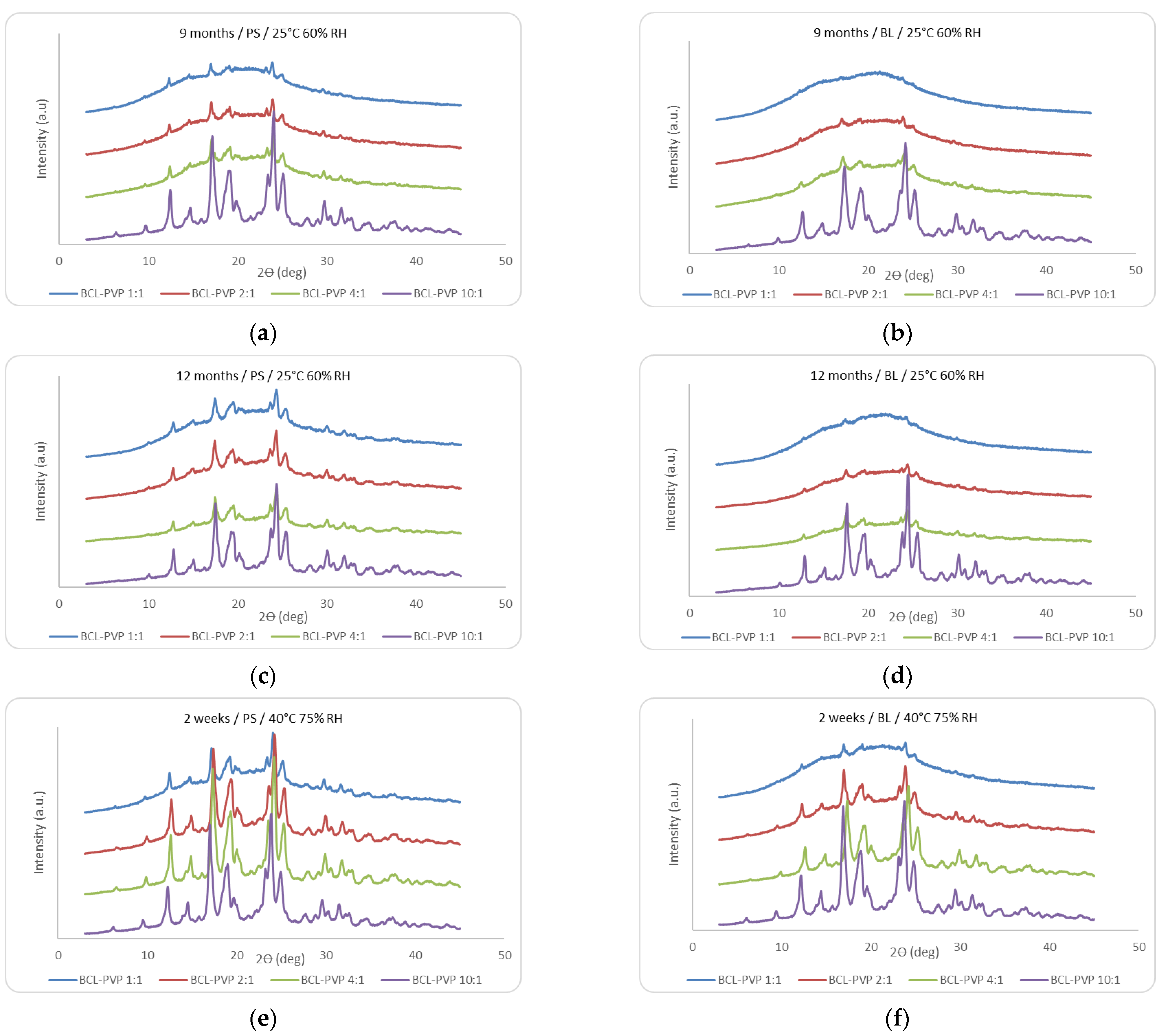
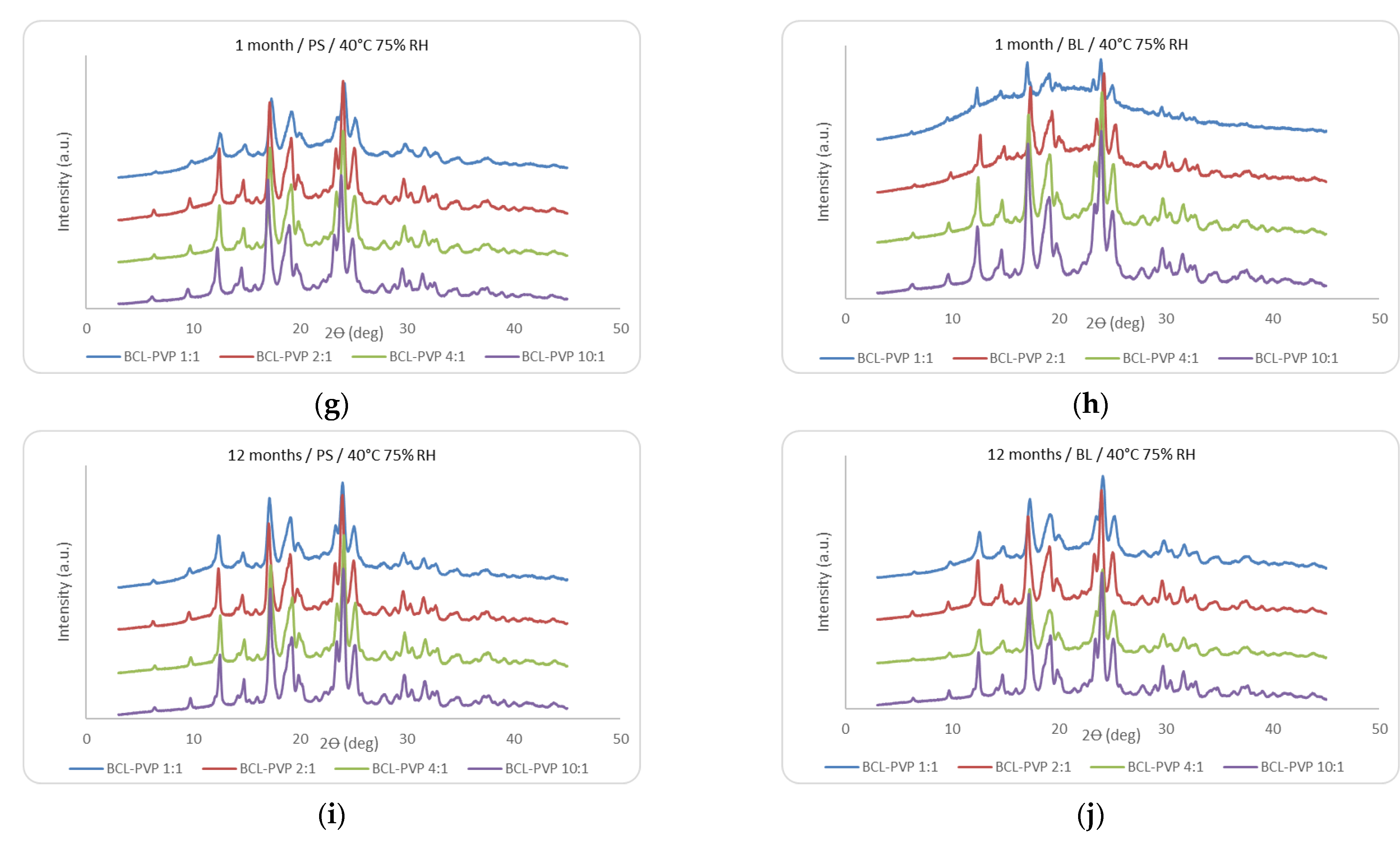
3.1.2. X-ray Analysis
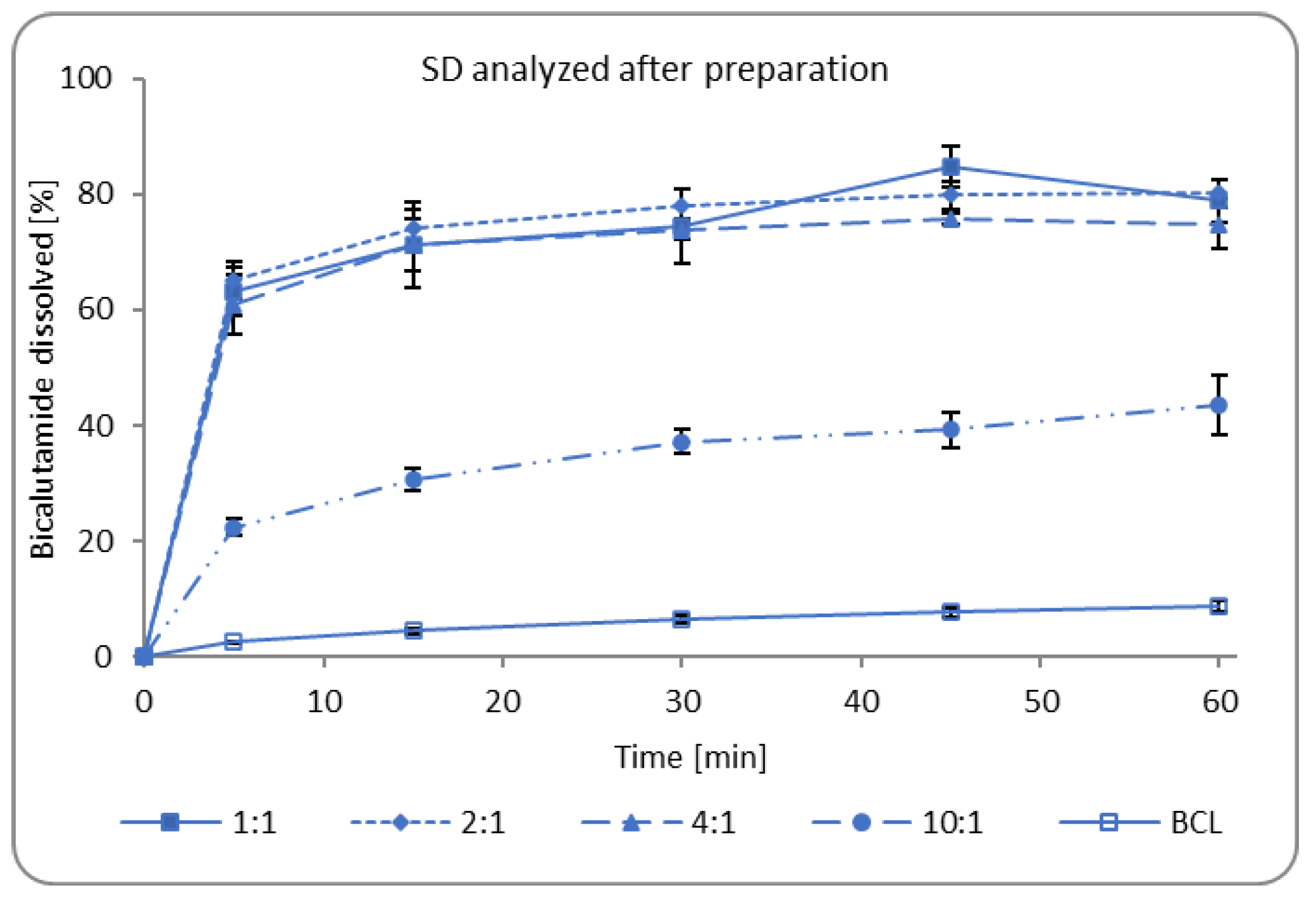
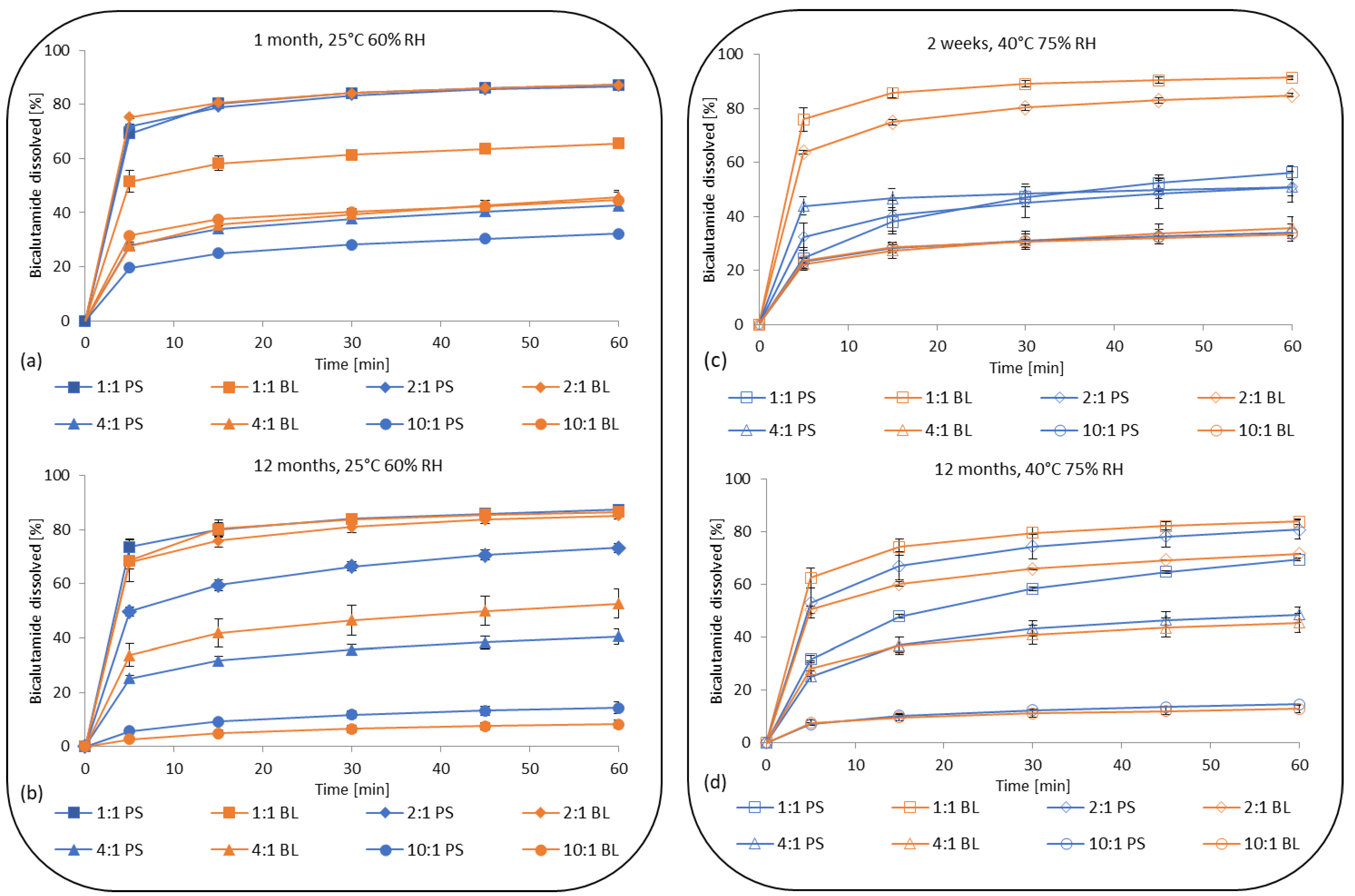
3.1.3. Dissolution of Bicalutamide from Solid Dispersions
3.2. Final Blend and Minitablet Characterization
3.2.1. Physical Characteristics of the Final Blend and Minitablets
3.2.2. X-ray Diffraction Studies
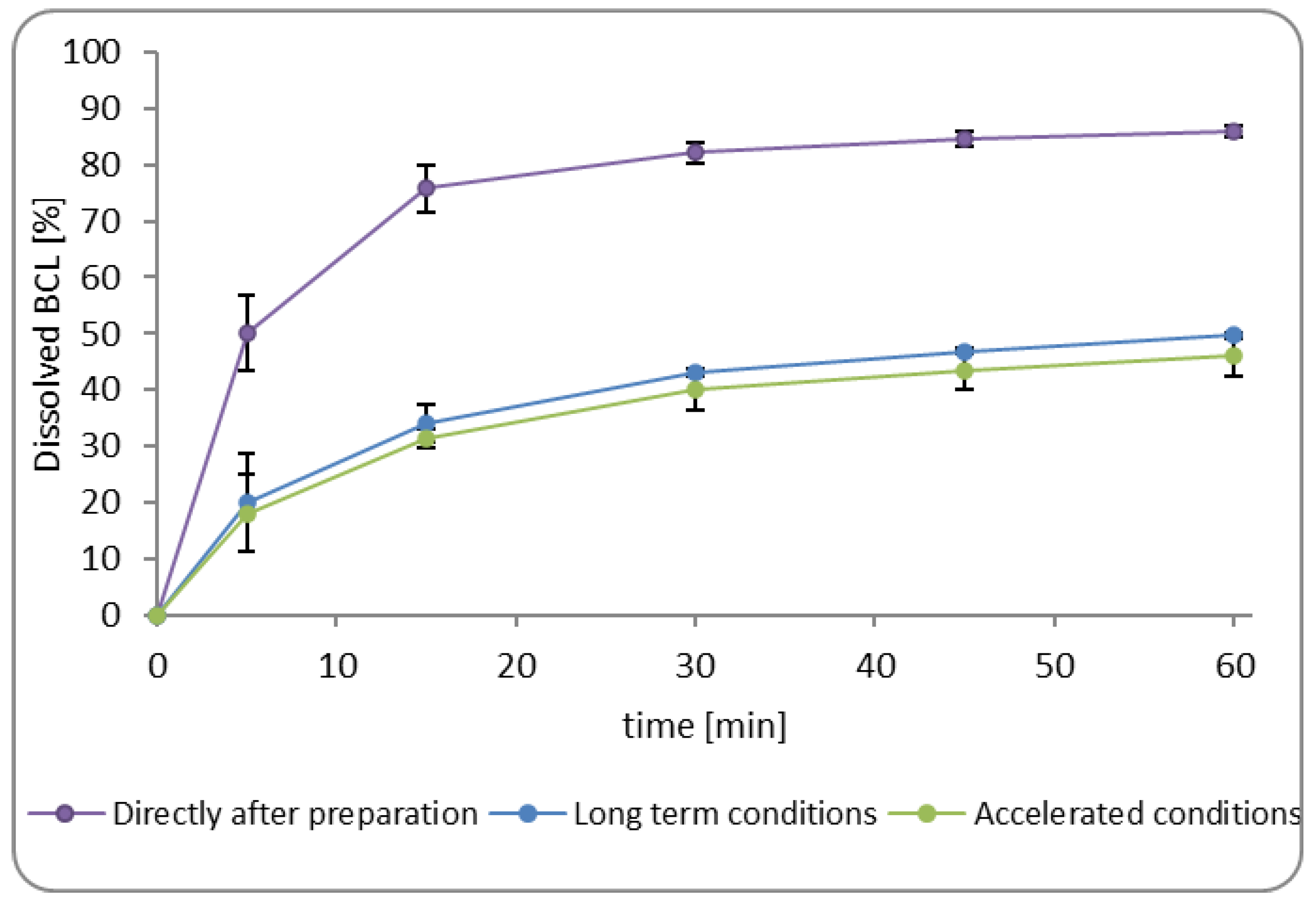
3.2.3. Dissolution of Bicalutamide from Minitablets
3.2.4. Kinetics Data
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Long, B.; Ryan, K.M.; Pardela, L. From batch to continuous—New opportunities for supercritical CO2 technology in pharmaceutical manufacturing. Eur. J. Pharm. Sci. 2019, 137, 104971. [Google Scholar] [CrossRef]
- Peltonen, L. Practical guidelines for the characterization and quality control of pure drug nanoparticles and nano-crystals in the pharmaceutical industry. Adv. Drug Deliv. Rev. 2018, 131, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Mendonsa, N.; Almutairy, B.; Kallakunta, V.R.; Sarabu, S.; Thipsay, P.; Bandari, S.; Repka, M.A. Manufacturing strategies to develop amorphous solid dispersions: An overview. J. Drug Deliv. Sci. Technol. 2020, 55, 101459. [Google Scholar] [CrossRef] [PubMed]
- Szafraniec, J.; Antosik, A.; Knapik-Kowalczuk, J.; Chmiel, K.; Kurek, M.; Gawlak, K.; Paluch, M.; Jachowicz, R. Enhanced dissolution of solid dispersions containing bicalutamide subjected to mechanical stress. Int. J. Pharm. 2018, 542, 18–26. [Google Scholar] [CrossRef]
- Rodriguez-Aller, M.; Guillarme, D.; Veuthey, J.-L.; Gurny, R. Strategies for formulating and delivering water-soluble drugs. J. Drug Deliv. Sci. Technol. 2015, 30, 342–351. [Google Scholar] [CrossRef]
- Al-Obaidi, H.; Lawrence, M.J.; Shah, S.; Moghul, H.; Al-Saden, N.; Bari, F. Effect of drug–polymer interactions on the aqueous solubility of milled solid dispersions. Int. J. Pharm. 2013, 446, 100–105. [Google Scholar] [CrossRef]
- Luebbert, C.; Stoyanov, E.; Sadowski, G. Phase behavior of ASDs based on hydroxypropyl cellulose. Int. J. Pharm. X 2021, 3, 100070. [Google Scholar] [CrossRef]
- Wang, B.; Wang, D.; Zhao, S.; Huang, X.; Zhang, J.; Lv, Y.; Liu, X.; Lv, G.; Ma, X. Evaluate the ability of PVP to inhibit crystallization of amorphous solid dispersions by density functional theory and experimental verify. Eur. J. Pharm. Sci. 2017, 96, 45–52. [Google Scholar] [CrossRef]
- Taylor, L.S.; Zografi, G. Spectroscopic Characterization of Interactions Between PVP and Indomethacin in Amorphous Molecular Dispersions. Pharm. Res. 1997, 14, 1691–1698. [Google Scholar] [CrossRef]
- Schittny, A.; Huwyler, J.; Puchkov, M. Mechanisms of increased bioavailability through amorphous solid dispersions: A review. Drug Deliv. 2020, 27, 110–127. [Google Scholar] [CrossRef]
- Yoshioka, M.; Hancock, B.C.; Zografi, G. Inhibition of Indomethacin Crystallization in Poly(vinylpyrrolidone) coprecipitates. J. Pharm. Sci. 1995, 84, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Lehmkemper, K.; Kyeremateng, S.O.; Bartels, M.; Degenhardt, M.; Sadowski, G. Physical Stability of API/Polymer-Blend Amorphous Solid Dispersions. Eur. J. Pharm. Biopharm. 2018, 124, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Theil, F.; Anantharaman, S.; Kyeremateng, S.O.; van Lishaut, H.; Dreis-Kühne, S.H.; Rosenberg, J.; Mägerlein, M.; Woehrle, G.H. Frozen in Time: Kinetically Stabilized Amorphous Solid Dispersions of Nifedipine Stable after a Quarter Century of Storage. Mol. Pharm. 2017, 14, 182–192. [Google Scholar] [CrossRef]
- Hanada, M.; Jermain, S.V.; Williams, R.O. Enhanced dissolution of a Porous Carrier-Containing Ternary Amorphous Solid Dispersion System Prepared by a Hot Melt Method. J. Pharm. Sci. 2018, 17, 362–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.G.Z.; Law, D.; Schmitt, E.A.; Qiu, Y. Phase transformation considerations during process development and manufacture of solid oral dosage forms. Adv. Drug Deliv. Rev. 2004, 56, 371–390. [Google Scholar] [CrossRef]
- Dengale, S.J.; Grohanz, H.; Rades, T.; Löbmann, K. Recent advances in co-amorphous drug formulations. Adv. Drug Deliv. Rev. 2016, 100, 116–125. [Google Scholar] [CrossRef]
- Włodarski, K.; Tajber, L.; Sawicki, W. Physicochemical properties of direct compression tablets with spray dried and ball milled solid dispersions of tadalafil in PVP-VA. Eur. J. Pharm. Biopharm. 2016, 109, 14–23. [Google Scholar] [CrossRef]
- Zhang, D.; Rumondor, A.C.F.; Zhu, W.; Colace, T.; Marota, M.; Mora, J.; Liu, Z.; Li, Y. The Development of Minitablets for a Pediatric Dosage Form for a Combination Therapy. J. Pharm. Sci. 2020, 109, 3590–3597. [Google Scholar] [CrossRef]
- Cheol-Hee, C.; Ju-Youn, K.; Eun-Seok, P. Utilization of a compaction simulator to formulate mini-tablets containing high dose of acyclovir. J. Drug Deliv. Sci. Technol. 2021, 64, 102602. [Google Scholar] [CrossRef]
- Lura, A.; Tardy, G.; Kleinebudde, P.; Breikreutz, J. Tableting of mini-tablets in comparison with conventionally sized tablets: A comparison of tableting properties and tablets dimensions. Int. J. Pharm. X 2020, 2, 100061. [Google Scholar] [CrossRef]
- Ren, F.; Jing, Q.; Tang, Y.; Shen, Y.; Chen, J.; Gao, F.; Ciu, J. Characteristics of Bicalutamide Solid Disprsions and Improvement of the Dissolution. Drug Dev. Ind. Pharm. 2006, 32, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Sancheti, P.P.; Vyas, V.M.; Shah, M.; Karekar, P.; Pore, Y.V. Development and characterization of bicalutamide-poloxamer F68 solid dispersion system. Pharmazie 2008, 63, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.L.; Pore, Y.V.; Kuchekar, B.S.; Late, S.G. Solid-state characterization and dissolution properties of bicalutamide-β-cyclodextrin inclusion complex. Pharmazie 2008, 63, 282–285. [Google Scholar] [CrossRef]
- Li, C.; Li, C.; Le, Y.; Chen, J.-F. Formation of bicalutamide nanodispersion for dissolution rate enhancement. Int. J. Pharm. 2011, 404, 236–257. [Google Scholar] [CrossRef]
- Varona, S.; Fernandez, J.; Rossmann, M.; Braeuer, A. Solubility of Paracetamol and Polyvinylpyrrolidone in Mixtures of Carbon Dioxide, Ethanol, and Acetone at Elevated Pressures. J. Chem. Eng. Data 2013, 58, 1054–1061. [Google Scholar] [CrossRef]
- Szafraniec, J.; Antosik, A.; Knapik-Kowalczuk, J.; Kurek, M.; Syrek, K.; Chmiel, K.; Paluch, M.; Jachowicz, R. Planetary ball milling and supercritical fluid technology as a way to enhance dissolution of bicalutamide. Int. J. Pharm. 2017, 533, 470–479. [Google Scholar] [CrossRef]
- Antosik-Rogóż, A.; Szafraniec-Szczęsny, J.; Gawlak, K.; Knapik-Kowalczuk, J.; Paluch, M.; Jachowicz, R. Tabletting solid dispersions of bicalutamide prepared using ball-milling or supercritical carbon dioxide: The interrelationship between phase transition and in-vitro dissolution. Pharm. Dev. Technol. 2020, 25, 1109–1117. [Google Scholar] [CrossRef]
- Andrews, G.P.; AbuDiak, O.A.; Jones, D.S. Physicochemical Characterization of Hot Melt Extruded Bicalutamide–Polyvinylpyrrolidone Solid Dispersions. J. Pharm. Sci. 2010, 99, 1322–1335. [Google Scholar] [CrossRef]
- Mendyk, A.; Jachowicz, R.; Fijorek, K.; Dorożyński, P.; Kulinowski, P.; Polak, S. KinetDS: An oper source software for dissolution test data analysis. Dissolution Technol. 2012, 19, 6–11. [Google Scholar] [CrossRef]


| Time (Months) | Storage Conditions | BCL:PVP Weight Ratio | Polystyrene Container | PVC/Al Blister |
|---|---|---|---|---|
| Properties | ||||
| 0.5 | 25 °C/60% RH | 1:1 | White, free-flowing powder | White, free-flowing powder |
| 2:1 | ||||
| 4:1 | ||||
| 10:1 | ||||
| 40 °C/75% RH | 1:1 | Yellowish-beige, agglomerated | White, free-flowing powder | |
| 2:1 | Slightly beige, agglomerated (less than 1:1) | |||
| 4:1 | White, free-flowing powder | |||
| 10:1 | ||||
| 1 | 25 °C/60% RH | 1:1 | White, free-flowing powder | White, free-flowing powder |
| 2:1 | ||||
| 4:1 | ||||
| 10:1 | ||||
| 40 °C/75% RH | 1:1 | Beige, compacted, rubbery | Agglomerated, brittle, easy to pulverize | |
| 2:1 | Slightly beige, lumpy powder | White, free-flowing powder | ||
| 4:1 | White, free-flowing powder | |||
| 10:1 | ||||
| 3 | 25 °C/60% RH | 1:1 | White, free-flowing powder without clods | White, free-flowing powder without clods |
| 2:1 | ||||
| 4:1 | ||||
| 10:1 | ||||
| 40 °C/75% RH | 1:1 | Beige, agglomerated, rubbery | Yellowish, agglomerated | |
| 2:1 | Slightly beige, agglomerated | White, free-flowing powder | ||
| 4:1 | White, free-flowing powder | |||
| 10:1 | ||||
| 6 | 25 °C/60% RH | 1:1 | White, free-flowing powder | White, free-flowing powder |
| 2:1 | ||||
| 4:1 | ||||
| 10:1 | ||||
| 40 °C/75% RH | 1:1 | Beige-yellow, agglomerated, rubbery, difficult to pulverize | Beige-yellow, agglomerated, rubbery, difficult to pulverize | |
| 2:1 | White, slightly agglomerated, easy to pulverize | White, slightly sticky lumps | ||
| 4:1 | White, slightly sticky, pulverize after shaking | White, slightly sticky, pulverized after shaking | ||
| 10:1 | ||||
| 9 | 25 °C/60% RH | 1:1 | White, free-flowing powder | White, free-flowing powder |
| 2:1 | ||||
| 4:1 | ||||
| 10:1 | ||||
| 40 °C/75% RH | 1:1 | Beige-yellow, agglomerated, rubbery, difficult to pulverize | Beige-yellow, strongly agglomerated, rubbery, difficult to pulverize | |
| 2:1 | White, slightly sticky, easy to pulverize by spatula | White, slightly sticky, easy to pulverize by spatula | ||
| 4:1 | Slightly agglomerated, easy to pulverize | Slightly agglomerated, easy to pulverize | ||
| 10:1 | ||||
| 12 | 25 °C/60% RH | 1:1 | White, slightly agglomerated, easy to pulverize | White, slightly agglomerated |
| 2:1 | ||||
| 4:1 | White, free-flowing powder | |||
| 10:1 | ||||
| 40 °C/75% RH | 1:1 | Yellowish, sticky, rubbery, hardly to pulverize | Beige, sticky, rubbery, hardly to pulverize | |
| 2:1 | Beige, agglomerated, pulverize by mortar | Beige, agglomerated, easy to pulverize by spatula | ||
| 4:1 | White, slightly agglomerated, easy to pulverize | Yellowish, slightly agglomerated | ||
| 10:1 | White, free-flowing powder | Yellowish, agglomerated, easy to pulverize by spatula | ||
| Attribute | Angle of Repose (°) | Hausner Ratio | Compressibility Index | |
|---|---|---|---|---|
| Sample | ||||
| Final blend | 55 | 1.43 | 30.1 | |
| Parameter | Mass (mg) | Hardness (N) | Thickness (mm) | Disintegration Time (min:s) | |
|---|---|---|---|---|---|
| Minitablets | |||||
| Immediately after preparation | 16.3 ± 0.7 | 38.95 ± 2.94 | 2.01 ± 0.03 | 14:29 | |
| Long-term conditions | 16.0 ±1.1 | 5.20 ± 0.49 | 2.93 ±0.02 | 4:33 | |
| Accelerated conditions | 17.3 ± 0.5 | 26.29 ± 3.83 | 2.95 ± 0.09 | 4:25 | |
| Kinetic Model | Korsmeyer–Peppas | Weibull | |||||
|---|---|---|---|---|---|---|---|
| Minitablets | A | n | R2 | a | b | R2 | |
| Immediately after preparation | 2.81 | 1.04 | 0.9956 | 1.98 | 1.06 | 0.9973 | |
| Long-term conditions | 1.52 | 1.01 | 0.9971 | 5.31 | 1.02 | 0.9978 | |
| Accelerated conditions | 1.42 | 1.01 | 0.9972 | 5.81 | 1.02 | 0.9978 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antosik-Rogóż, A.; Szafraniec-Szczęsny, J.; Knapik-Kowalczuk, J.; Kurek, M.; Gawlak, K.; Paluch, M.; Jachowicz, R. How Does Long-Term Storage Influence the Physical Stability and Dissolution of Bicalutamide from Solid Dispersions and Minitablets? Processes 2022, 10, 1002. https://doi.org/10.3390/pr10051002
Antosik-Rogóż A, Szafraniec-Szczęsny J, Knapik-Kowalczuk J, Kurek M, Gawlak K, Paluch M, Jachowicz R. How Does Long-Term Storage Influence the Physical Stability and Dissolution of Bicalutamide from Solid Dispersions and Minitablets? Processes. 2022; 10(5):1002. https://doi.org/10.3390/pr10051002
Chicago/Turabian StyleAntosik-Rogóż, Agata, Joanna Szafraniec-Szczęsny, Justyna Knapik-Kowalczuk, Mateusz Kurek, Karolina Gawlak, Marian Paluch, and Renata Jachowicz. 2022. "How Does Long-Term Storage Influence the Physical Stability and Dissolution of Bicalutamide from Solid Dispersions and Minitablets?" Processes 10, no. 5: 1002. https://doi.org/10.3390/pr10051002
APA StyleAntosik-Rogóż, A., Szafraniec-Szczęsny, J., Knapik-Kowalczuk, J., Kurek, M., Gawlak, K., Paluch, M., & Jachowicz, R. (2022). How Does Long-Term Storage Influence the Physical Stability and Dissolution of Bicalutamide from Solid Dispersions and Minitablets? Processes, 10(5), 1002. https://doi.org/10.3390/pr10051002










