Oxidative Desulfurization of Real High-Sulfur Diesel Using Dicarboxylic Acid/H2O2 System
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
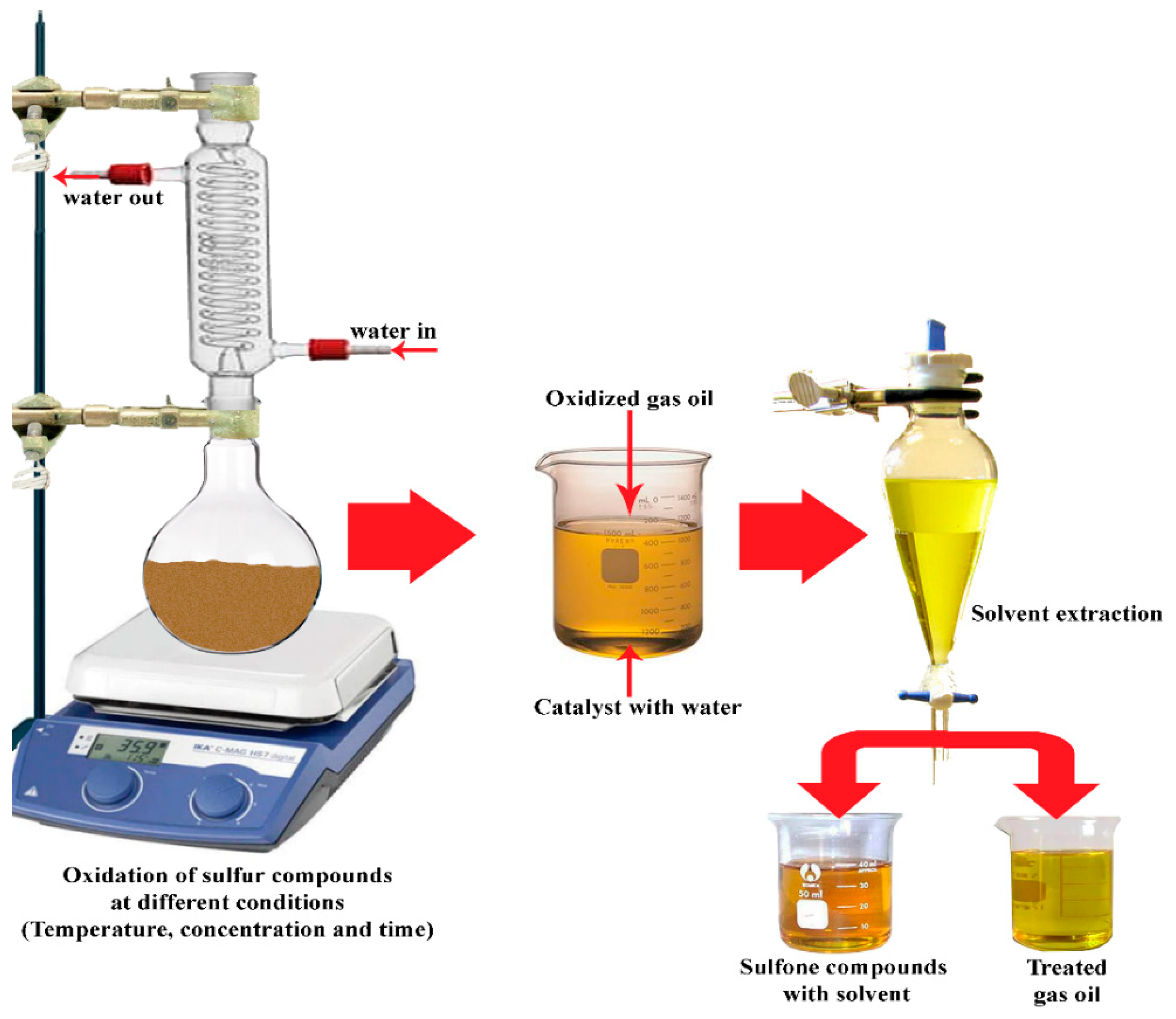
2.2. Desulfurization Activity Testing
2.3. Analysis of Real Diesel
3. Results and Discussion
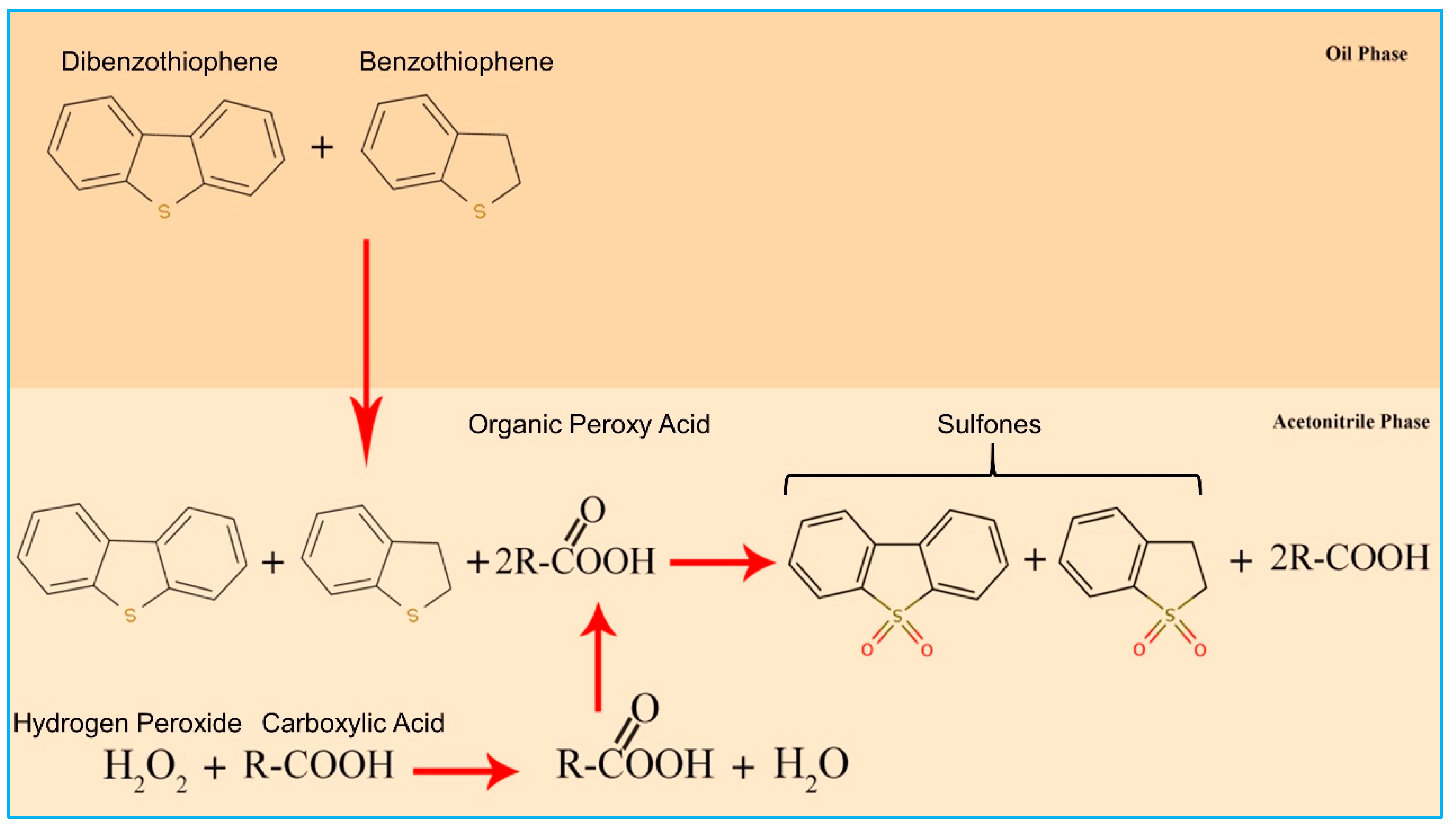
3.1. Oxidation Desulfurization of Real Diesel
3.2. Effect of Parameters on Desulfurization Efficiency
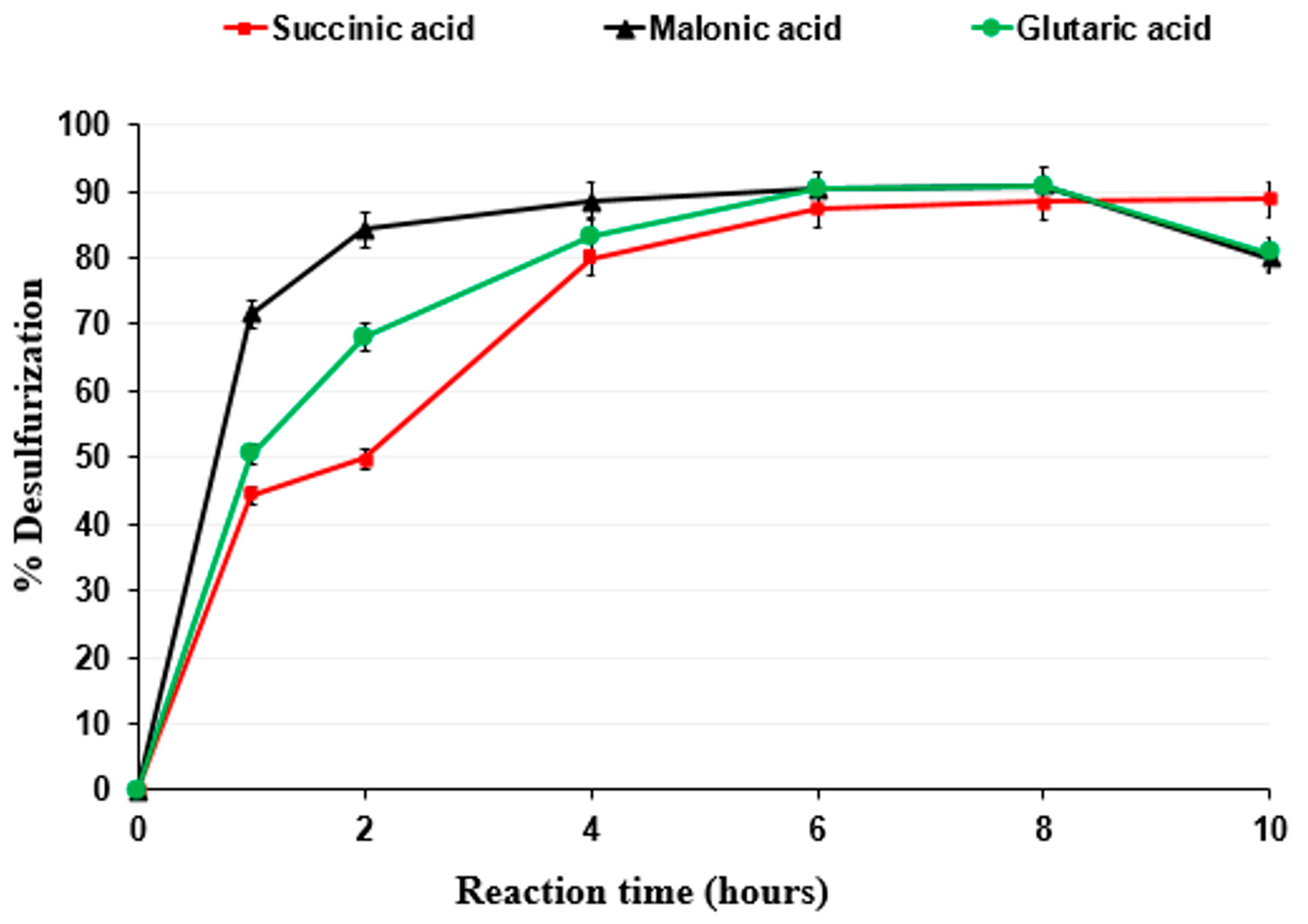
3.2.1. Influence of Time on Reaction
3.2.2. Effect of Temperature on Desulfurization Efficiency
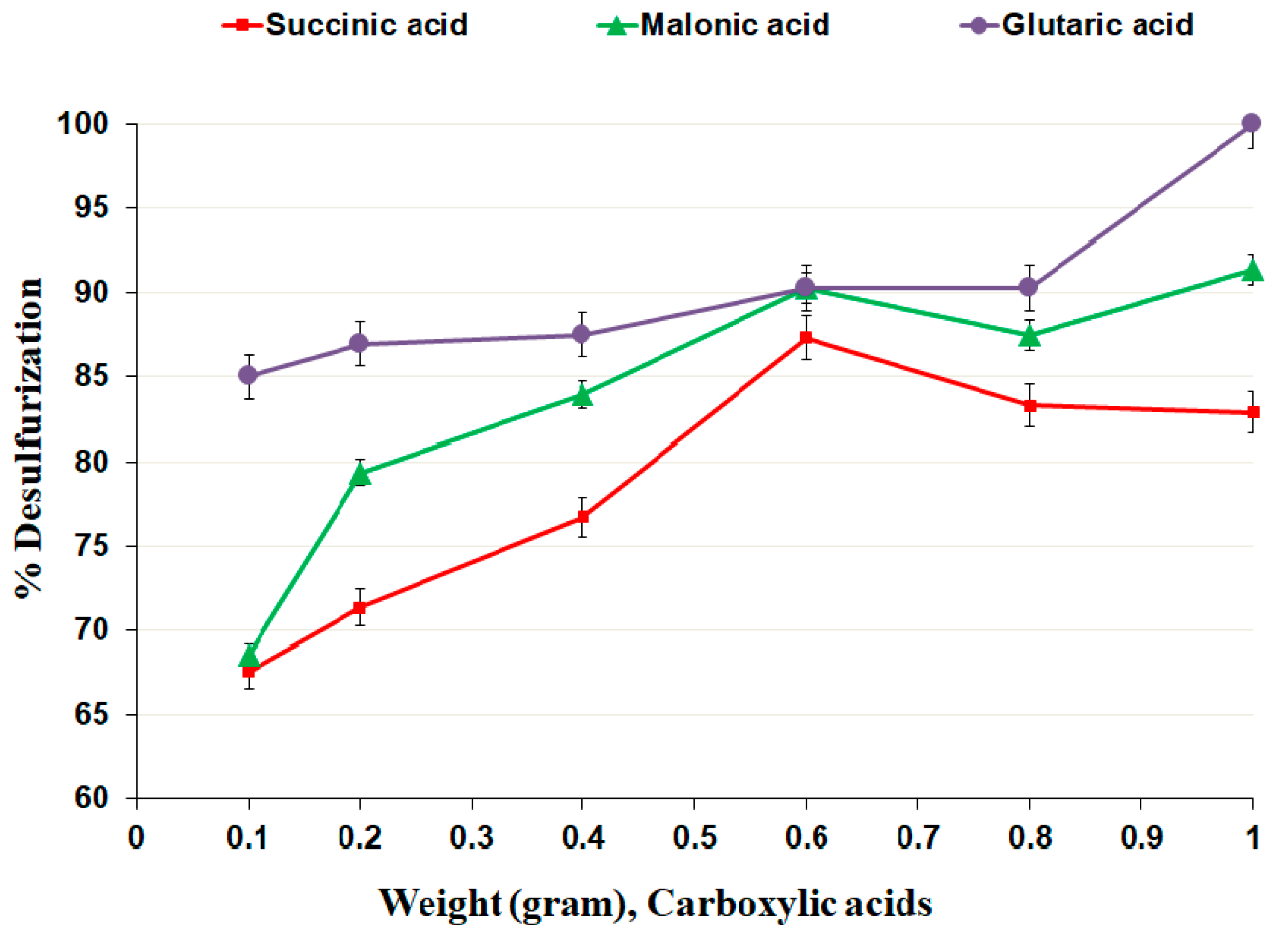
3.2.3. Effect of Dicarboxylic Acid Dosage
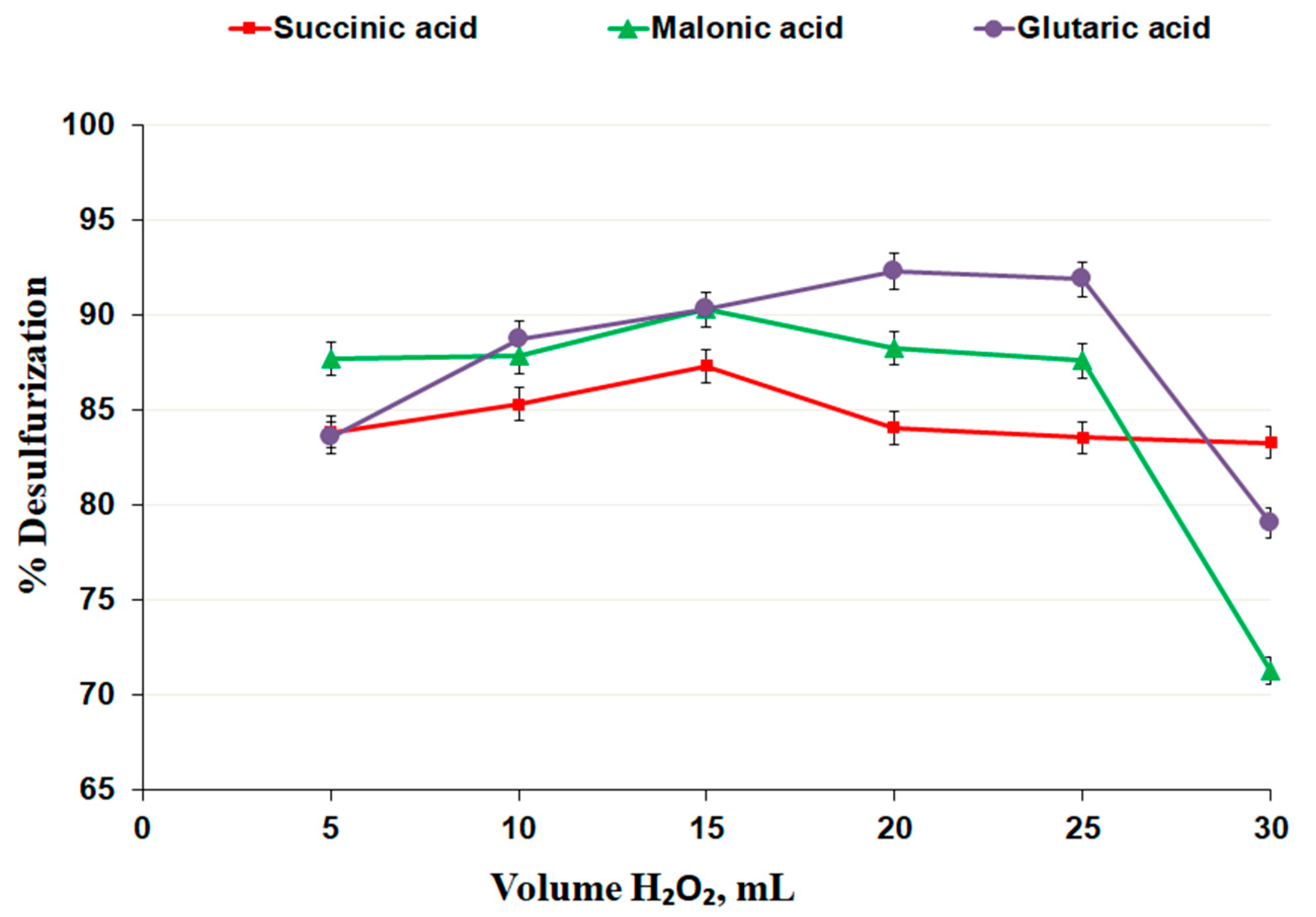
3.2.4. Effect of Hydrogen Peroxide Dosage
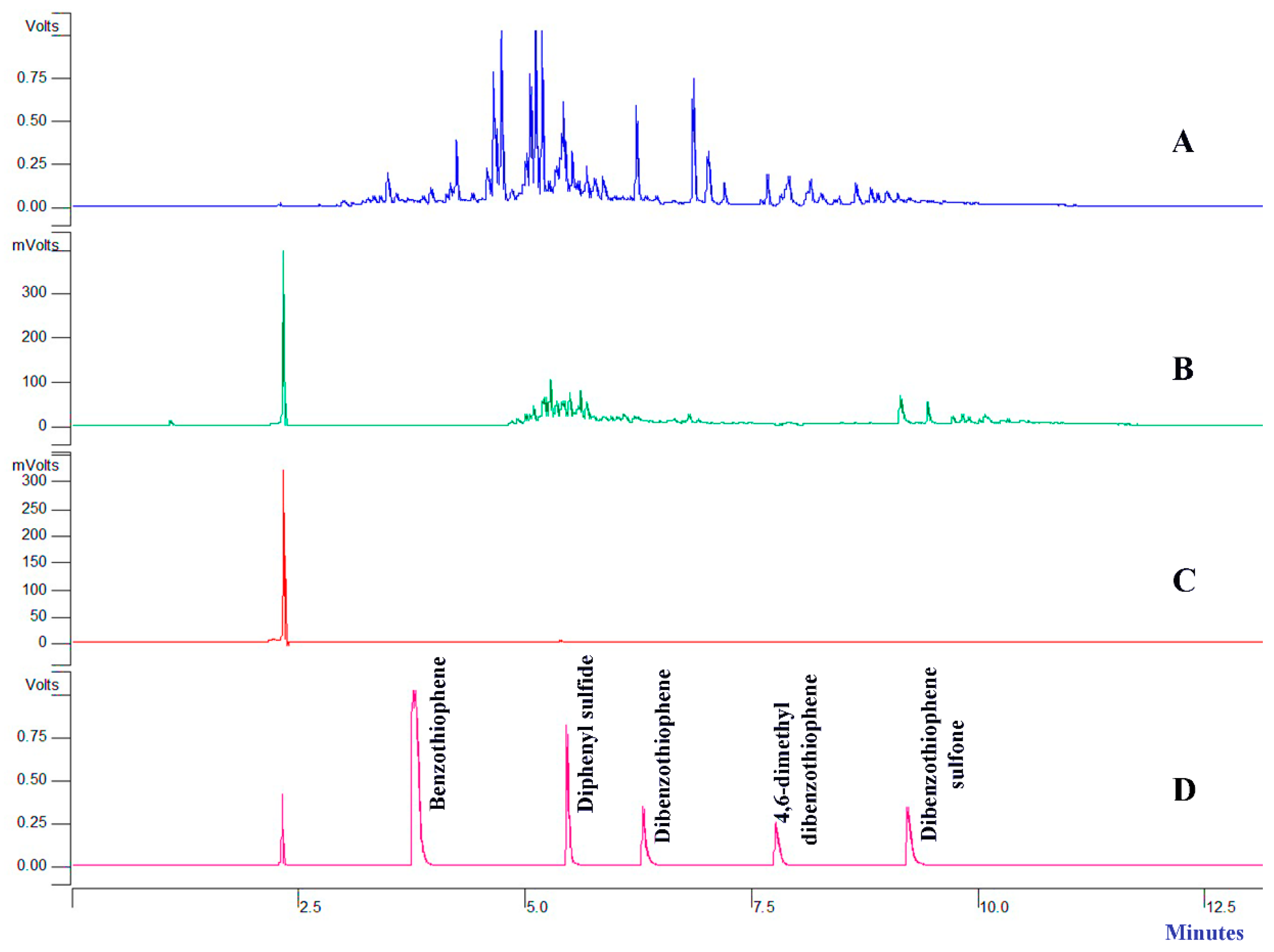
3.3. Characterizations of Treated Diesel
3.4. Kinetic Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hai, T.; Aziz, K.H.H.; Zhou, J.; Dhahad, H.A.; Sharma, K.; Almojil, S.F.; Almohana, A.I.; Alali, A.F.; Kh, T.I.; Mehrez, S.; et al. Neural network-based optimization of hydrogen fuel production energy system with proton exchange electrolyzer supported nanomaterial. Fuel 2023, 332, 125827. [Google Scholar] [CrossRef]
- Chandran, D.; Khalid, M.; Walvekar, R.; Mubarak, N.M.; Dharaskar, S.; Wong, W.Y.; Gupta, T.C.S.M. Deep eutectic solvents for extraction-desulphurization: A review. J. Mol. Liq. 2019, 275, 312–322. [Google Scholar] [CrossRef]
- Mwangi, J.K.; Lee, W.-J.; Chang, Y.-C.; Chen, C.-Y.; Wang, L.-C. An overview: Energy saving and pollution reduction by using green fuel blends in diesel engines. Appl. Energy 2015, 159, 214–236. [Google Scholar] [CrossRef]
- Fraile, J.M.; Gil, C.; Mayoral, J.A.; Muel, B.; Roldán, L.; Vispe, E.; Calderón, S.; Puente, F. Heterogeneous titanium catalysts for oxidation of dibenzothiophene in hydrocarbon solutions with hydrogen peroxide: On the road to oxidative desulfurization. Appl. Catal. B Environ. 2016, 180, 680–686. [Google Scholar] [CrossRef]
- De Leon, J.D. Binary γ-Al2O3–α-Ga2O3 as supports of NiW catalysts for hydrocarbon sulfur removal. Appl. Catal. B Environ. 2016, 181, 524–533. [Google Scholar] [CrossRef]
- Saleh, T.A. Carbon nanotube-incorporated alumina as a support for MoNi catalysts for the efficient hydrodesulfurization of thiophenes. Chem. Eng. J. 2021, 404, 126987. [Google Scholar] [CrossRef]
- Shafiq, I.; Shafique, S.; Akhter, P.; Yang, W.; Hussain, M. Recent developments in alumina supported hydrodesulfurization catalysts for the production of sulfur-free refinery products: A technical review. Catal. Rev. 2022, 64, 1–86. [Google Scholar] [CrossRef]
- Houda, S.; Lancelot, C.; Blanchard, P.; Poinel, L.; Lamonier, C. Oxidative desulfurization of heavy oils with high sulfur content: A review. Catalysts 2018, 8, 344. [Google Scholar] [CrossRef]
- Shafiq, I.; Hussain, M.; Shafique, S.; Rashid, R.; Akhter, P.; Ahmed, A.; Jeon, J.-K.; Park, Y.-K. Oxidative desulfurization of refinery diesel pool fractions using LaVO4 photocatalyst. J. Ind. Eng. Chem. 2021, 98, 283–288. [Google Scholar] [CrossRef]
- Saha, B.; Vedachalam, S.; Dalai, A.K. Review on recent advances in adsorptive desulfurization. Fuel Process. Technol. 2020, 214, 106685. [Google Scholar] [CrossRef]
- Dharaskar, S.A.; Wasewar, K.L.; Varma, M.N.; Shende, D.Z.; Yoo, C. Synthesis, characterization and application of 1-butyl-3-methylimidazolium tetrafluoroborate for extractive desulfurization of liquid fuel. Arab. J. Chem. 2016, 9, 578–587. [Google Scholar] [CrossRef]
- Bu, J.; Loh, G.; Gwie, C.G.; Dewiyanti, S.; Tasrif, M.; Borgna, A. Desulfurization of diesel fuels by selective adsorption on activated carbons: Competitive adsorption of polycyclic aromatic sulfur heterocycles and polycyclic aromatic hydrocarbons. Chem. Eng. J. 2011, 166, 207–217. [Google Scholar] [CrossRef]
- Seredych, M.; Bandosz, T.J. Removal of dibenzothiophenes from model diesel fuel on sulfur rich activated carbons. Appl. Catal. B Environ. 2011, 106, 133–141. [Google Scholar] [CrossRef]
- Srivastav, A.; Srivastava, V.C. Adsorptive desulfurization by activated alumina. J. Hazard. Mater. 2009, 170, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Hoguet, J.-C.; Karagiannakis, G.P.; Valla, J.; Agrafiotis, C.; Konstandopoulos, A.G. Gas and liquid phase fuels desulphurization for hydrogen production via reforming processes. Int. J. Hydrogen Energy 2009, 34, 4953–4962. [Google Scholar] [CrossRef]
- Ahmad, W.; Ahmad, I.; Ishaq, M.; Ihsan, K. Adsorptive desulfurization of kerosene and diesel oil by Zn impregnated montmorollonite clay. Arab. J. Chem. 2017, 10, S3263–S3269. [Google Scholar] [CrossRef]
- Yu, G.; Lu, S.; Chen, H.; Zhu, Z. Diesel fuel desulfurization with hydrogen peroxide promoted by formic acid and catalyzed by activated carbon. Carbon 2005, 43, 2285–2294. [Google Scholar] [CrossRef]
- Triantafyllidis, K.S.; Deliyanni, E.A. Desulfurization of diesel fuels: Adsorption of 4, 6-DMDBT on different origin and surface chemistry nanoporous activated carbons. Chem. Eng. J. 2014, 236, 406–414. [Google Scholar] [CrossRef]
- Mokhtar, W.N.A.W.; Abu Bakar, W.A.W.; Ali, R.; Kadir, A.A.A. Development of bimetallic and trimetallic oxides doped on molybdenum oxide based material on oxidative desulfurization of diesel. Arab. J. Chem. 2018, 11, 1201–1208. [Google Scholar] [CrossRef]
- Saleh, T.A. Simultaneous adsorptive desulfurization of diesel fuel over bimetallic nanoparticles loaded on activated carbon. J. Clean. Prod. 2018, 172, 2123–2132. [Google Scholar] [CrossRef]
- Sun, L.; Zhu, Z.; Su, T.; Liao, W.; Hao, D.; Chen, Y.; Zhao, Y.; Ren, W.; Ge, H.; Lü, H. Novel acidic eutectic mixture as peroxidase mimetics for oxidative desulfurization of model diesel. Appl. Catal. B Environ. 2019, 255, 117747. [Google Scholar] [CrossRef]
- Hao, L.; Wang, M.; Shan, W.; Deng, C.; Ren, W.; Shi, Z.; Lü, H. L-proline-based deep eutectic solvents (DESs) for deep catalytic oxidative desulfurization (ODS) of diesel. J. Hazard. Mater. 2017, 339, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Su, T.; Hao, D.; Deng, C.; Ren, W.; Lü, H. Oxidative desulfurization of diesel fuel with caprolactam-based acidic deep eutectic solvents: Tailoring the reactivity of DESs by adjusting the composition. Chin. J. Catal. 2018, 39, 1552–1559. [Google Scholar] [CrossRef]
- Li, A.; Song, H.; Meng, H.; Lu, Y.; Li, C. Ultrafast desulfurization of diesel oil with ionic liquid based PMoO catalysts and recyclable NaClO oxidant. Chem. Eng. J. 2020, 380, 122453. [Google Scholar] [CrossRef]
- Jiang, W.; Zhu, K.; Li, H.; Zhu, L.; Hua, M.; Xiao, J.; Wang, C.; Yang, Z.; Chen, G.; Zhu, W.; et al. Synergistic Effect of Dual Brønsted Acidic Deep Eutectic Solvents for Oxidative Desulfurization of Diesel Fuel. Chem. Eng. J. 2020, 394, 124831. [Google Scholar] [CrossRef]
- Yue, D.; Lei, J.; Zhou, L.; Du, X.; Guo, Z.; Li, J. Oxidative desulfurization of fuels at room temperature using ordered meso/macroporous H3PW12O40/SiO2 catalyst with high specific surface areas. Arab. J. Chem. 2020, 13, 2649–2658. [Google Scholar] [CrossRef]
- Aguiar, A.; Ribeiro, S.; Silva, A.M.; Cunha-Silva, L.; de Castro, B.; Silva, A.M.; Balula, S.S. An efficient eco-sustainable oxidative desulfurization process using μ-oxo-bridged Fe (III) complex of meso-tetrakis (pentafluorophenyl) porphyrin. Appl. Catal. A Gen. 2014, 478, 267–274. [Google Scholar] [CrossRef]
- Sampanthar, J.T.; Xiao, H.; Dou, J.; Nah, T.Y.; Rong, X.; Kwan, W.P. A novel oxidative desulfurization process to remove refractory sulfur compounds from diesel fuel. Appl. Catal. B Environ. 2006, 63, 85–93. [Google Scholar] [CrossRef]
- Ghubayra, R.; Yahya, R.; Kozhevnikova, E.F.; Kozhevnikov, I.V. Oxidative desulfurization of model diesel fuel catalyzed by carbon-supported heteropoly acids: Effect of carbon support. Fuel 2021, 301, 121083. [Google Scholar] [CrossRef]
- Ghubayra, R.; Nuttall, C.; Hodgkiss, S.; Craven, M.; Kozhevnikova, E.F.; Kozhevnikov, I.V. Oxidative desulfurization of model diesel fuel catalyzed by carbon-supported heteropoly acids. Appl. Catal. B Environ. 2019, 253, 309–316. [Google Scholar] [CrossRef]
- Timbila, S.; Donoso, C.; Mejía, F. Oxidative Desulfurization of Diesel from the Topping Plant using 1-Butyl-3-Methylimidazolium Hydrogen Sulfate [Bmim][HSO4] as Catalyst. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021. [Google Scholar]
- Gao, H.; Guo, C.; Xing, J.; Zhao, J.; Liu, H. Extraction and oxidative desulfurization of diesel fuel catalyzed by a Brønsted acidic ionic liquid at room temperature. Green Chem. 2010, 12, 1220–1224. [Google Scholar] [CrossRef]
- Zhu, Z.; Lin, H.; Chi, M.; Gao, X.; Feng, Y.; Yang, K.; Lü, H. Unveiling structure-function relationships in deep eutectic solvents based biomimetic catalysis for aerobic oxidative desulfurization. Fuel 2022, 308, 122070. [Google Scholar] [CrossRef]
- Rafiee, E.; Joshaghani, M.; Abadi, P.G.-S. Oxidative desulfurization of diesel by potato based-carbon as green support for H5PMo10V2O40: Efficient composite nanorod catalyst. J. Saudi Chem. Soc. 2017, 21, 599–609. [Google Scholar] [CrossRef]
- Yaseen, M.; Khattak, S.; Ullah, S.; Subhan, F.; Ahmad, W.; Shakir, M.; Tong, Z. Oxidative desulfurization of model and real petroleum distillates using Cu or Ni impregnated banana peels derived activated carbon–NaClO catalyst–oxidant system. Chem. Eng. Res. Des. 2022, 179, 107–118. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Leus, K.; Sun, Z.; Li, X.; Depauw, H.; Wang, A.; Zhang, J.; Van Der Voort, P. Catalytic oxidative desulfurization of model and real diesel over a molybdenum anchored metal-organic framework. Microporous Mesoporous Mater. 2019, 277, 245–252. [Google Scholar] [CrossRef]
- Li, A.; Song, H.; Meng, H.; Lu, Y.; Li, C. Poly (ionic liquid) s based nano core-shell catalyst SiO2@ V-PIL for efficient oxidative desulfurization of diesel. Appl. Catal. A Gen. 2021, 616, 118096. [Google Scholar] [CrossRef]
- Kargar, H.; Ghahramaninezhad, M.; Shahrak, M.N.; Balula, S.S. An Effective Magnetic Catalyst for Oxidative Desulfurization of Model and Real Fuels: Fe3O4/ZIF-8/TiO2. Microporous Mesoporous Mater. 2021, 317, 110992. [Google Scholar] [CrossRef]
- Julião, D.; Mirante, F.; Ribeiro, S.O.; Gomes, A.C.; Valença, R.; Ribeiro, J.C.; Pillinger, M.; de Castro, B.; Gonçalves, I.S.; Balula, S.S. Deep oxidative desulfurization of diesel fuels using homogeneous and SBA-15-supported peroxophosphotungstate catalysts. Fuel 2019, 241, 616–624. [Google Scholar] [CrossRef]
- Huang, P.; Liu, A.; Kang, L.; Zhu, M.; Dai, B. Heteropoly acid supported on sodium dodecyl benzene sulfonate modified layered double hydroxides as catalysts for oxidative desulfurization. New J. Chem. 2018, 42, 12830–12837. [Google Scholar] [CrossRef]
- Du, Y.; Yang, P.; Zhou, S.; Li, J.; Du, X.; Lei, J. Direct synthesis of ordered meso/macrostructured phosphotungstic acid/SiO2 by EISA method and its catalytic performance of fuel oil. Mater. Res. Bull. 2018, 97, 42–48. [Google Scholar] [CrossRef]



| Test | Results before Treatment | Results after Treatment |
|---|---|---|
| Specific gravity@ 15.6 °C | 0.826 | 0.809 |
| API gravity | 39.8 | 43.4 |
| Flashpoint °C | 67 | 68 |
| Pour point °C | −11 | −13 |
| Vis.@50 °C/Cst | 2.18 | 2.09 |
| %Sulfur content | 0.8104 | 0.057 |
| Distillation | ||
| Initial B.P °C | 171 | 180 |
| Vol. at %10 | 195 | 209 |
| Vol. at %50 | 256 | 252 |
| Vol. at %70 | 292 | 282 |
| Vol. at %90 | 336 | 320 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, B.S.; Hamasalih, L.O.; Hama Aziz, K.H.; Omer, K.M.; Shafiq, I. Oxidative Desulfurization of Real High-Sulfur Diesel Using Dicarboxylic Acid/H2O2 System. Processes 2022, 10, 2327. https://doi.org/10.3390/pr10112327
Ahmed BS, Hamasalih LO, Hama Aziz KH, Omer KM, Shafiq I. Oxidative Desulfurization of Real High-Sulfur Diesel Using Dicarboxylic Acid/H2O2 System. Processes. 2022; 10(11):2327. https://doi.org/10.3390/pr10112327
Chicago/Turabian StyleAhmed, Barham Sharif, Luqman Omar Hamasalih, Kosar Hikmat Hama Aziz, Khalid M. Omer, and Iqrash Shafiq. 2022. "Oxidative Desulfurization of Real High-Sulfur Diesel Using Dicarboxylic Acid/H2O2 System" Processes 10, no. 11: 2327. https://doi.org/10.3390/pr10112327
APA StyleAhmed, B. S., Hamasalih, L. O., Hama Aziz, K. H., Omer, K. M., & Shafiq, I. (2022). Oxidative Desulfurization of Real High-Sulfur Diesel Using Dicarboxylic Acid/H2O2 System. Processes, 10(11), 2327. https://doi.org/10.3390/pr10112327







