Tertiary Wastewater Treatment Technologies: A Review of Technical, Economic, and Life Cycle Aspects
Abstract
1. Introduction
2. Tertiary Wastewater Treatment Technologies
2.1. Chlorination
2.2. Ultraviolet Irradiation
2.3. Membrane Filtration
2.4. Constructed Wetlands
2.5. Microalgae
2.6. Ozonation
2.7. Photo-Fenton
3. Benchmarking of Tertiary Wastewater Treatment Technologies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AO52 | acid orange 52 |
| AOPs | advance oxidation processes |
| ARB | anaerobic resistant bacteria |
| ARG | antibiotic resistance genes |
| BOD | biochemical oxygen demand |
| BR | biofilm reactor |
| COD | chemical oxygen demand |
| CW | constructed wetlands |
| EC | emerging contaminants |
| GHG | greenhouse gases |
| GO | graphene oxide |
| HFCW | horizontal flow constructed wetlands |
| HRAP | high-rate algal pond |
| HRP | high-rate pond |
| HSSF | horizontal subsurface flow |
| LCC | life cycle costing |
| MF | microfiltration |
| MFCs | microbial fuel cells |
| MWCNTs | multi-walled carbon nanotubes |
| NF | nanofiltration |
| PBR | photobioreactor |
| PVDF | polyvinylidene fluoride |
| RO | reverse osmosis |
| SBR | sequencing batch reactor |
| SPF | solar photo-Fenton |
| TOC | total organic carbon |
| UF | ultrafiltration |
| UV | ultraviolet |
| VFCW | vertical flow constructed wetlands |
Appendix A
| Process Information | Technical | Economic (Cost EUR/m3) | Life Cycle (kg CO2 eq./m3) | Ref. | ||
|---|---|---|---|---|---|---|
| Microbial (Log Reduction) | Nutrients (% Reduction) | Pharmaceuticals (% Reduction) | ||||
| Sulfamethoxazole 220 ng/L | 73 | [10] | ||||
| Ciprofloxacin 153 ng/L | 66 | [10] | ||||
| Norfloxacin 92 ng/L | 50 | [10] | ||||
| Tetracycline 86 ng/L | 39 | [10] | ||||
| Trimethoprim 155 ng/L | 65 | [10] | ||||
| Erythromycin 273 ng/L | 43 | [10] | ||||
| Diclofenac 40 μg/L | 97 | [72] | ||||
| Ibuprofen 40 μg/L | 0 | [72] | ||||
| Clofibric acid 40 μg/L | 5 | [72] | ||||
| Naproxen 40 μg/L | 11 | [72] | ||||
| Gemfibrozil 40 μg/L | 45 | [72] | ||||
| Mefenamic acid 40 μg/L | 12 | [72] | ||||
| E. coli 3.7 Log CFU/100 mL | 2.5 | [8] | ||||
| E. coli 4.34 Log CFU/100 mL | 2.57 | [9] | ||||
| Enterococci 3.46 Log CFU/100 mL | 1.18 | [9] | ||||
| Fecal coliforms 4.57 Log CFU/100 mL | 2.34 | [9] | ||||
| F-specific coliphage 2.33 Log CFU/100 mL | 0.71 | [9] | ||||
| Somatic coliphage 3.92 Log CFU/100 mL | 1.68 | [9] | ||||
| Adenovirus 0.97 Log CFU/100 mL | 0.81 | [9] | ||||
| Norovirus 0.74 Log CFU/100 mL | 0.74 | [9] | ||||
| Coliforms 4 Log CFU/100 mL | 4 | [55] | ||||
| Antib. Resist. Genes 6 Log | 1.97 | [13] | ||||
| E. coli 7 Log CFU/mL | 5 | [57] | ||||
| 0.0003 | [11] | |||||
| 0.005 | [12] | |||||
| 0.006 | [13] | |||||
| 0.046 | [73] | |||||
| 0.007 | [15] | |||||
| 0.004 | [14] | |||||
| Process Information | Technical | Economic (Cost EUR/m3) | Life Cycle (kg CO2 eq./m3) | Ref. | ||
|---|---|---|---|---|---|---|
| Microbial (Log Reduction) | Nutrients (% Reduction) | Pharmaceuticals (% Reduction) | ||||
| E. coli 5 × 105 CFU/100 mL | 4 | [74] | ||||
| E. coli 7.7 Log × 10 CFU/L | 3.82 | [9] | ||||
| Enterococci 8.56 Log × 10 CFU/L | 3.38 | [9] | ||||
| Fecal coliforms 8.26 Log × 10 CFU/L | 3.89 | [9] | ||||
| F-specific coliphage 6.4 Log × 10 CFU/L | 1.17 | [9] | ||||
| Somatic coliphage 7.36 Log × 10 CFU/L | 2.98 | [9] | ||||
| Adenovirus 2.73 Log gc/L | 0.24 | [9] | ||||
| Coliforms 5 Log CFU/mL | 4 | [55] | ||||
| Antib. Resist. Genes 6 Log | 1 | [13] | ||||
| Antib. Resist. Genes 5 Log copies/L | 2.5 | [20] | ||||
| E. coli 2 × 107 CFU/mL | 5.1 | [57] | ||||
| Sulfamethoxazole 250 ng/L | 100 | [75] | ||||
| Trimethoprim 90 ng/L | 100 | [75] | ||||
| Erythromycin 200 ng/L | 100 | [75] | ||||
| Acetaminophen 0.1 mM, caffeine 0.12 mM, antipyrine 0.05 mM, doxycycline 0.03 mM, ketorolac 0.05 mM | 100 | [76] | ||||
| Atrazine diuron, alachlor, pentachlorophenol 1 mg/L | 72 | [76] | ||||
| Boldenone 6.57 μM | 98 | [76] | ||||
| BPA 60 μM | 22 | [76] | ||||
| Butylparaben 8 × 10−5 M | 97 | [76] | ||||
| Carbamazepine 3 μΜ | 52 | [76] | ||||
| Chlorfenvinphos | 91 | [76] | ||||
| Ciprofloxacin | 99 | [76] | ||||
| Chloromycetin 10 mg/L | 80 | [76] | ||||
| Clofibric acid 10 mg/L | 98 | [76] | ||||
| Cyclophosphamide 10 μg/L | 28 | [76] | ||||
| Cytarabine 10 mg/L | 10 | [76] | ||||
| Diatrizoate 50 μM | 97 | [76] | ||||
| Diclofenac 20 mg/L | 74 | [76] | ||||
| Diphenhydramine 5 μM | 26 | [76] | ||||
| Doxycycline 5 × 10−5 M | 27 | [76] | ||||
| E1 20 mg/L | 69 | [76] | ||||
| E2 20 mg/L | 59 | [76] | ||||
| EE2 | 37 | [76] | ||||
| Hydrochlorothiazide 1 μM | 59 | [76] | ||||
| Ibuprofen 10−4 M | 74 | [76] | ||||
| Iopromide | 53 | [76] | ||||
| Iohexol 3 μΜ | 12 | [76] | ||||
| Irinotecan 10 µg/L | 18 | [76] | ||||
| Isoproturon 1 mg/L | 12 | [76] | ||||
| Ketoprofen 50 µM | 99 | [76] | ||||
| Mefenamic acid 5.5 Log M | 56 | [76] | ||||
| Melatonin 20 mg/L | 32 | [76] | ||||
| Metoprolol 5 × 10−4 M | 69 | [76] | ||||
| Metronidazole 6 μM | 55 | [76] | ||||
| Naproxen 3 μM | 65 | [76] | ||||
| NDMA 1 mM | 100 | [76] | ||||
| Norfloxacin 5 × 10−5 M | 55 | [76] | ||||
| Oxtetracycline | 93 | [76] | ||||
| Phenazone 5 μM | 96 | [76] | ||||
| Phenytion 5 μM | 88 | [76] | ||||
| Primidone 50 µM | 9 | [76] | ||||
| Propranolol 100 mg/L | 61 | [76] | ||||
| Sulfadimethoxine 3.2 mM | 99 | [76] | ||||
| Sulfamethoxazole 10 mg/L | 83 | [76] | ||||
| Tamoxifen 10 µg/L | 43 | [76] | ||||
| TCE 8.14 × 10−3 mol/L | 95 | [76] | ||||
| Tibetene 0.03 mM | 87 | [76] | ||||
| Bezafibrate 112 ng/L | 0 | [19] | ||||
| Metformin 1736 ng/L | 27 | [19] | ||||
| Carbamazepine 333 ng/L | 48 | [19] | ||||
| Gabapentin 1508 ng/L | 0 | [19] | ||||
| Diclofenac 925 ng/L | 96 | [19] | ||||
| Ketoprofen 40 ng/L | 97 | [19] | ||||
| Naproxen 372 ng/L | 70 | [19] | ||||
| Primidone 65 ng/L | 3 | [19] | ||||
| Atenolol 320 ng/L | 0 | [19] | ||||
| Metoprolol 255 ng/L | 0 | [19] | ||||
| Ciprofloxacin 72 ng/L | 56 | [19] | ||||
| Clarithromycin 187 ng/L | 10 | [19] | ||||
| Sulfamethoxazole 355 ng/L | 3 | [19] | ||||
| Trimethoprim 31 ng/L | 0 | [19] | ||||
| Iohexol 4313 ng/L | 16 | [19] | ||||
| Iomeprol 5806 ng/L | 0 | [19] | ||||
| Benzotriazole 6736 ng/L | 18 | [19] | ||||
| Atrazin 25 ng/L | 58 | [19] | ||||
| Isoproturon 4 ng/L | 0 | [19] | ||||
| Mecoprop 365 ng/L | 0 | [19] | ||||
| Terbutryn 23 ng/L | 39 | [19] | ||||
| 0.00001 | [11] | |||||
| 0.00644 | [13] | |||||
| 0.0063 | [13] | |||||
| 0.013 | [73] | |||||
| 0.026 | [15] | |||||
| 0.22 | [77] | |||||
| Process Information | Technical Aspect | Economic Aspect (Cost EUR/m3) | Life Cycle (kg CO2 eq./m3) | Ref. | ||
|---|---|---|---|---|---|---|
| Microbial (Log Reduction) | Nutrients (% Reduction) | Pharmaceuticals (% Reduction) | ||||
| UF enterococcus 1.87 × 105 CFU/100 mL | 5 | [6] | ||||
| UF other coliforms 5.05 × 104 CFU/100 mL | 2 | [6] | ||||
| UF N 3.62 mg/L | 10 | [6] | ||||
| UF P 1.86 mg/L | 9 | [6] | ||||
| UF K 16.15 mg/L | 0 | [6] | ||||
| NF-90 2 μg/L | 73 | [78] | ||||
| NF-200 neutral PhACs 65 μg/L | 70 | [78] | ||||
| NF-200 ionic PhACs 65 μg/L | 94 | [78] | ||||
| NF-90 neutral 65 μg/L | 97 | [78] | ||||
| NF-90 ionic 65 μg/L | 99 | [78] | ||||
| NF-90 65 μg/L | 73 | [78] | ||||
| UF 2000 0.5 mg/L | 70 | [78] | ||||
| UF-NF90 750 μg/L | 50 | [78] | ||||
| NF 270 RO 2 μg/L | 95 | [78] | ||||
| NF 150-RO 100 ng/L | 95 | [78] | ||||
| NF 200 100 ng/L | 80 | [78] | ||||
| UF 8000-NF 600 10 ng/L | 60 | [78] | ||||
| UF 8000 10 ng/L | 30 | [78] | ||||
| NF 90-RO 10 mg/L | 99 | [78] | ||||
| NF 200 21 ng/L | 100 | [78] | ||||
| NF 270 10 mg L | 60 | [78] | ||||
| NF270 800 μg/L | 58 | [78] | ||||
| NF90 750 μg/L | 97 | [78] | ||||
| RO 0.55 mg/L | 100 | [78] | ||||
| NF90 10 mg/L | 90 | [78] | ||||
| NF270 10 mg/L | 61 | [78] | ||||
| NF90 0.5 mg/L | 98 | [78] | ||||
| NF270 0.5 mg/L | 71 | [78] | ||||
| RO 0.5 mg/L | 89 | [78] | ||||
| NF90 5400 μg/L | 77 | [78] | ||||
| NF270 5400 μg/L | 58 | [78] | ||||
| RO 5400 μg/L | 93 | [78] | ||||
| UF-Atenolol 778 ng/L | 0 | [22] | ||||
| UF-Bezafibrate 208 ng/L | 21 | [22] | ||||
| UF-Caffeine 17,725 ng/L | 0 | [22] | ||||
| UF-Fenofibric acid 139 ng/L | 0 | [22] | ||||
| UF-Furosemide 1302 ng/L | 17 | [22] | ||||
| UF-Gemfibrozil 18,504 ng/L | 71 | [22] | ||||
| UF-Hydrochlorothiazide 16,628 ng/L | 90 | [22] | ||||
| UF-Ibuprofen 2514 ng/L | 1 | [22] | ||||
| UF-4-AAA 7364 ng/L | 0 | [22] | ||||
| UF-Naproxen 2672 ng/L | 12 | [22] | ||||
| UF-Nicotine 10,954 ng/L | 63 | [22] | ||||
| UF-Ofloxacin 94 ng/L | 0 | [22] | ||||
| RO-Atenolol 1044 ng/L | 100 | [22] | ||||
| RO-Bezafibrate 164 ng/L | 100 | [22] | ||||
| RO-Caffeine 6288 ng/L | 99 | [22] | ||||
| RO-Fenofibric acid 194 ng/L | 100 | [22] | ||||
| RO-Furosemide 811 ng/L | 100 | [22] | ||||
| RO-Gemfibrozil 1035 ng/L | 99 | [22] | ||||
| RO-Hydrochlorothiazide 239 ng/L | 95 | [22] | ||||
| RO-Ibuprofen 574 ng/L | 97 | [22] | ||||
| RO-4-AAA 4472 ng/L | 99 | [22] | ||||
| RO-Naproxen 2583 ng/L | 98 | [22] | ||||
| RO-Nicotine 75 ng/L | 76 | [22] | ||||
| RO-Ofloxacin 87 ng/L | 95 | [22] | ||||
| Including RO | 0.46 | [27] | ||||
| FO-NF | 0.96 | [27] | ||||
| UF-RO | 0.4 | [27] | ||||
| UF | 0.45 | [27] | ||||
| UF-RO | 0.8 | [27] | ||||
| UF-RO | 2.32 | [29] | ||||
| NF | 0.2 | [28] | ||||
| UF | 0.25 | [77] | ||||
| UF | 0.40 | [73] | ||||
| MF-RO | 0.89 | [73] | ||||
| UF-RO | 0.91 | [73] | ||||
| Process Information | Technical Aspect | Economic Aspect (Cost EUR/m3) | Life Cycle (kg CO2 eq./m3) | Ref. | ||
|---|---|---|---|---|---|---|
| Microbial (Log Reduction) | Nutrients (% Reduction) | Pharmaceuticals (% Reduction) | ||||
| 16 s rDNA, intI1, and tet genes | 1.78 | [34] | ||||
| 14 antibiotic resistance genes | 0.50 | [36] | ||||
| ARGs 8–9 Log of copies/mL | 0.49 | [40] | ||||
| N 23.4 mg/L | 5 | [31] | ||||
| P 0.2 mg/L | 0 | [31] | ||||
| N 29.3 mg/L | 35 | [31] | ||||
| P 0.2 mg/L | 50 | [31] | ||||
| N 17.3 mg/L | 39 | [31] | ||||
| P 0.2 mg/L | 50 | [31] | ||||
| N 1.39 mg/L | 66 | [33] | ||||
| P 3 mg/L | 46 | [33] | ||||
| N 84.4 mg/L | 63 | [34] | ||||
| P 28.2 mg/L | 92 | [34] | ||||
| N 35 mg/L | 46 | [40] | ||||
| N 72 mg/L | 99 | [79] | ||||
| P 11.7 mg/L | 97 | [79] | ||||
| 65 pharmaceuticals 4.3 μg/L | 64 | [31] | ||||
| 55 pharmaceuticals 300 ng/L | 43 | [31] | ||||
| 53 pharmaceuticals | 50 | [31] | ||||
| 56 pharmaceuticals 190 ng/L | 32 | [31] | ||||
| 6 pharmaceuticals 7.6–150 μg/L | 93 | [80] | ||||
| Antibiotics 300 ng/L | 58 | [40] | ||||
| Pharmaceuticals 50–200 ng/L | 59 | [79] | ||||
| 1.224 | [45] | |||||
| 0.729 | [45] | |||||
| 0.4 | [49] | |||||
| 0.129 | [73] | |||||
| 0.432 | [42] | |||||
| 0.646 | [42] | |||||
| 0.911 | [42] | |||||
| 0.5 | [42] | |||||
| 0.26 | [47] | |||||
| 0.7 | [49] | |||||
| Process Information | Technical | Economic (Cost EUR/m3) | Life Cycle (kg CO2 eq./m3) | Ref. | ||
|---|---|---|---|---|---|---|
| Microbial (Log Reduction) | Nutrients (% Reduction) | Pharmaceuticals (% Reduction) | ||||
| N 40 mg/L | 55 | [81] | ||||
| P 80 mg/L | 15 | [81] | ||||
| N 52 mg/L | 82 | [82] | ||||
| P 8.5 mg/L | 95 | [82] | ||||
| N 18 mg/L | 100 | [83] | ||||
| P 1.4 mg/L | 84 | [83] | ||||
| N 46 mg/L | 94 | [51] | ||||
| P 5.5 mg/L | 95 | [51] | ||||
| Metronidazole 5 μM | 100 | [84] | ||||
| Florfenicol 46 mg/L | 97 | [84] | ||||
| Enrofloxacin 1 mg/L | 23 | [84] | ||||
| Tetracycline 100 μg/L | 99 | [84] | ||||
| Methyl parathion 20 mg/L | 80 | [84] | ||||
| Trimethoprim, Sulfamethoxazole, Triclosan 1.6 ng/L, 360 ng/L, 8 ng/L | 44 | [84] | ||||
| 7-amino cephalosporanic acid 100 mg/L | 70 | [84] | ||||
| Cefradine 100 mg/L | 94 | [84] | ||||
| β-estradiol | 93 | [84] | ||||
| 17 α-estradiol, 17 β-estradiol, Estrone, Estriol 5 μg/L | 90 | [84] | ||||
| Sulfathiazole, Sulfapyridine, Sulfamethazine, Sulfamethoxazole, Tetracycline, Oxytetracycline 200 μg/L | 47 | [84] | ||||
| Norfloxacin mg/L | 37 | [84] | ||||
| 0.42 | [49] | |||||
| 0.162 | [85] | |||||
| 0.6 | [49] | |||||
| 0.336 | [86] | |||||
| Process Information | Technical | Economic (Cost EUR/m3) | Life Cycle (kg CO2 eq./m3) | Ref. | ||
|---|---|---|---|---|---|---|
| Microbial (Log Reduction) | Nutrients (% Reduction) | Pharmaceuticals (% Reduction) | ||||
| Coliforms 4 Log CFU/100 mL | 4 | [55] | ||||
| Coliforms 5 Log MPN/100 mL | 2.5 | [56] | ||||
| Coliforms 5 Log MPN/100 mL | 2.2 | [56] | ||||
| Coliforms 7 Log MPN/100 mL | 4.8 | [56] | ||||
| E. coli 7.3 Log CFU/mL | 5.3 | [57] | ||||
| E. coli 4 Log CFU/mL | 2.2 | [58] | ||||
| Salmonella 2.9 Log CFU/mL | 2.2 | [58] | ||||
| Enterococcus 3 Log CFU/mL | 2.2 | [58] | ||||
| Carbamazepine | 75 | [59] | ||||
| Alachlor | 20 | [59] | ||||
| Bisphenol A | 60 | [59] | ||||
| Atrazine | 5 | [59] | ||||
| Pentachlorophenol | 35 | [59] | ||||
| 17-α thinylestradiol | 80 | [59] | ||||
| Carbamazepine 1 μg/L | 100 | [60] | ||||
| Naproxen 1 μg/L | 100 | [60] | ||||
| Beclomethasone 1 μg/L | 70 | [60] | ||||
| Memantine 1 μg/L | 80 | [60] | ||||
| 0.03 | [61] | |||||
| 0.03 | [61] | |||||
| 0.025 | [87] | |||||
| 0.25 | [88] | |||||
| 0.3 | [28] | |||||
| 0.3 | [89] | |||||
| Process Information | Technical | Economic (Cost EUR/m3) | Life Cycle (kg CO2 eq./m3) | Ref. | ||
|---|---|---|---|---|---|---|
| Microbial (Log Reduction) | Nutrients (% Reduction) | Pharmaceuticals (% Reduction) | ||||
| S. aureus 6 Log CFU/mL | 6 | [64] | ||||
| MRSA ATCC 29213 6 Log CFU/mL | 6 | [64] | ||||
| E. coli 6 Log CFU/mL | 6 | [64] | ||||
| K. pneumoniae 6 Log CFU/mL | 6 | [64] | ||||
| MSSA 1112 6 Log CFU/mL | 6 | [64] | ||||
| MSSA 1112 RifR 6 Log CFU/mL | 6 | [64] | ||||
| MSSA 1112 CipR 6 Log CFU/mL | 6 | [64] | ||||
| MSSA 133 6 Log CFU/mL | 6 | [64] | ||||
| MSSA 133 CipR 6 Log CFU/mL | 6 | [64] | ||||
| MRSA PC1 6 Log CFU/mL | 6 | [64] | ||||
| VISA PC# 6 Log CFU/mL | 6 | [64] | ||||
| E. coli 4 Log CFU/mL | 1 | [58] | ||||
| Salmonella 2.9 Log CFU/mL | 2 | [58] | ||||
| Enterococcus 3 Log CFU/mL | 0 | [58] | ||||
| Sulfamethazine 50 mg/L | 100 | [62] | ||||
| Amoxicillin 50 mg/L | 100 | [63] | ||||
| Bezafibrate 112 ng/L | 0 | [19] | ||||
| Gemfibrozil 9 ng/L | 96 | [19] | ||||
| Metformin 1736 ng/L | 63 | [19] | ||||
| Carbamazepine 333 ng/L | 94 | [19] | ||||
| Gabapentin 1508 ng/L | 77 | [19] | ||||
| Diclofenac 925 ng/L | 100 | [19] | ||||
| Ketoprofen 40 ng/L | 97 | [19] | ||||
| Naproxen372 ng/L | 97 | [19] | ||||
| Primidone 65 ng/L | 77 | [19] | ||||
| Atenolol 320 ng/L | 87 | [19] | ||||
| Metoprolol 255 ng/L | 90 | [19] | ||||
| Ciprofloxacin 72 ng/L | 61 | [19] | ||||
| Clarithromycin 187 ng/L | 76 | [19] | ||||
| Sulfamethoxazole 355 ng/L | 82 | [19] | ||||
| Trimethoprim 31 ng/L | 88 | [19] | ||||
| Iohexol 4313 ng/L | 94 | [19] | ||||
| Iomeprol 5806 ng/L | 87 | [19] | ||||
| Benzotriazole 6736 ng/L | 95 | [19] | ||||
| Atrazin 25 ng/L | 82 | [19] | ||||
| Isoproturon 4 ng/L | 32 | [19] | ||||
| Mecoprop 365 ng/L | 93 | [19] | ||||
| Terbutryn 23 ng/L | 83 | [19] | ||||
| Ofloxacin 110 μg/L | 100 | [65] | ||||
| Carbamazepine130 μg/L | 96 | [65] | ||||
| Flumequine 145 μg/L | 98 | [65] | ||||
| Ibuprofen 130 μg/L | 95 | [65] | ||||
| Sulfamethoxazole 140 μg/L | 90 | [65] | ||||
| 0.25 | [90] | |||||
| 0.56 | [90] | |||||
| 0.331 | [68] | |||||
| 0.554 | [69] | |||||
| 0.762 | [71] | |||||
References
- Adam, D. How far will global population rise? Researchers can’t agree. Nature 2021, 597, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Deaver, J.A.; Kerr, C.A.; Popat, S.C. Primary sludge-based blackwater favors electrical current over methane production in microbial electrochemical cells. J. Water Process Eng. 2022, 47, 102848. [Google Scholar] [CrossRef]
- Wallin, J.; Knutsson, J.; Karpouzoglou, T. A multi-criteria analysis of building level graywater reuse for personal hygiene. Resour. Conserv. Recycl. Adv. 2021, 12, 200054. [Google Scholar] [CrossRef]
- Saliu, T.D.; Ali, J.; Ololade, I.A.; Oladoja, N.A. Preparation and characterization of a decentralized modular yellow water nutrient recovery system. J. Environ. Manag. 2020, 276, 111345. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.-H. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef]
- Zagklis, D.; Katrivesis, F.K.; Sygouni, V.; Tsarouchi, L.; Tsigkou, K.; Kornaros, M.; Paraskeva, C.A. Recovery of Water from Secondary Effluent through Pilot Scale Ultrafiltration Membranes: Implementation at Patras’ Wastewater Treatment Plant. Membranes 2021, 11, 663. [Google Scholar] [CrossRef]
- Albolafio, S.; Marín, A.; Allende, A.; García, F.; Simón-Andreu, P.J.; Soler, M.A.; Gil, M.I. Strategies for mitigating chlorinated disinfection byproducts in wastewater treatment plants. Chemosphere 2022, 288, 132583. [Google Scholar] [CrossRef]
- Tombini Decol, L.; López-Gálvez, F.; Truchado, P.; Tondo, E.C.; Gil, M.I.; Allende, A. Suitability of chlorine dioxide as a tertiary treatment for municipal wastewater and use of reclaimed water for overhead irrigation of baby lettuce. Food Control 2019, 96, 186–193. [Google Scholar] [CrossRef]
- Francy, D.S.; Stelzer, E.A.; Bushon, R.N.; Brady, A.M.G.; Williston, A.G.; Riddell, K.R.; Borchardt, M.A.; Spencer, S.K.; Gellner, T.M. Comparative effectiveness of membrane bioreactors, conventional secondary treatment, and chlorine and UV disinfection to remove microorganisms from municipal wastewaters. Water Res. 2012, 46, 4164–4178. [Google Scholar] [CrossRef]
- Li, B.; Zhang, T. Mass flows and removal of antibiotics in two municipal wastewater treatment plants. Chemosphere 2011, 83, 1284–1289. [Google Scholar] [CrossRef]
- Tak, S.; Kumar, A. Chlorination disinfection by-products and comparative cost analysis of chlorination and UV disinfection in sewage treatment plants: Indian scenario. Environ. Sci. Pollut. Res. 2017, 24, 26269–26278. [Google Scholar] [CrossRef]
- Gallego Valero, L.; Moral Pajares, E.; Román Sánchez, I.M.; Sánchez Pérez, J.A. Analysis of Environmental Taxes to Finance Wastewater Treatment in Spain: An Opportunity for Regeneration? Water 2018, 10, 226. [Google Scholar] [CrossRef]
- Zhuang, Y.; Ren, H.; Geng, J.; Zhang, Y.; Zhang, Y.; Ding, L.; Xu, K. Inactivation of antibiotic resistance genes in municipal wastewater by chlorination, ultraviolet, and ozonation disinfection. Environ. Sci. Pollut. Res. 2015, 22, 7037–7044. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.; Mellor, J. Comparative life cycle assessment of four commonly used point-of-use water treatment technologies. J. Water Sanit. Hyg. Dev. 2020, 10, 862–873. [Google Scholar] [CrossRef]
- Pasqualino, J.C.; Meneses, M.; Castells, F. Life Cycle Assessment of Urban Wastewater Reclamation and Reuse Alternatives. J. Ind. Ecol. 2011, 15, 49–63. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhuang, Y.; Geng, J.; Ren, H.; Xu, K.; Ding, L. Reduction of antibiotic resistance genes in municipal wastewater effluent by advanced oxidation processes. Sci. Total Environ. 2016, 550, 184–191. [Google Scholar] [CrossRef]
- Qiao, R.-P.; Li, N.; Qi, X.-H.; Wang, Q.-S.; Zhuang, Y.-Y. Degradation of microcystin-RR by UV radiation in the presence of hydrogen peroxide. Toxicon 2005, 45, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Chin, A.; Bérubé, P.R. Removal of disinfection by-product precursors with ozone-UV advanced oxidation process. Water Res. 2005, 39, 2136–2144. [Google Scholar] [CrossRef] [PubMed]
- De la Cruz, N.; Esquius, L.; Grandjean, D.; Magnet, A.; Tungler, A.; de Alencastro, L.F.; Pulgarín, C. Degradation of emergent contaminants by UV, UV/H2O2 and neutral photo-Fenton at pilot scale in a domestic wastewater treatment plant. Water Res. 2013, 47, 5836–5845. [Google Scholar] [CrossRef]
- Guo, M.-T.; Yuan, Q.-B.; Yang, J. Ultraviolet reduction of erythromycin and tetracycline resistant heterotrophic bacteria and their resistance genes in municipal wastewater. Chemosphere 2013, 93, 2864–2868. [Google Scholar] [CrossRef]
- Zagklis, D.P.; Papageorgiou, C.S.; Paraskeva, C.A. 18—Valorization of phenolic extracts from Olea europaea L. by membrane operations. In Membrane Engineering in the Circular Economy: Renewable Sources Valorization in Energy and Downstream Processing in Agro-Food Industry; Iulianelli, A., Cassano, A., Conidi, C., Petrotos, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 495–524. ISBN 978-0-323-85253-1. [Google Scholar]
- Urtiaga, A.M.; Pérez, G.; Ibáñez, R.; Ortiz, I. Removal of pharmaceuticals from a WWTP secondary effluent by ultrafiltration/reverse osmosis followed by electrochemical oxidation of the RO concentrate. Desalination 2013, 331, 26–34. [Google Scholar] [CrossRef]
- Cheng, H.; Hong, P.-Y. Removal of Antibiotic-Resistant Bacteria and Antibiotic Resistance Genes Affected by Varying Degrees of Fouling on Anaerobic Microfiltration Membranes. Environ. Sci. Technol. 2017, 51, 12200–12209. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Boo, C.; Guo, N.; Wang, S.; Elimelech, M.; Wang, Y. Photocatalytic Reactive Ultrafiltration Membrane for Removal of Antibiotic Resistant Bacteria and Antibiotic Resistance Genes from Wastewater Effluent. Environ. Sci. Technol. 2018, 52, 8666–8673. [Google Scholar] [CrossRef] [PubMed]
- Dolar, D.; Ignjatić Zokić, T.; Košutić, K.; Ašperger, D.; Mutavdžić Pavlović, D. RO/NF membrane treatment of veterinary pharmaceutical wastewater: Comparison of results obtained on a laboratory and a pilot scale. Environ. Sci. Pollut. Res. 2012, 19, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.C.; Teoh, Y.X.; Teow, Y.H.; Mohammad, A.W. Life cycle assessment (LCA) of electrically-enhanced POME filtration: Environmental impacts of conductive-membrane formulation and process operating parameters. J. Environ. Manag. 2021, 277, 111434. [Google Scholar] [CrossRef]
- Pérez, G.; Gómez, P.; Ortiz, I.; Urtiaga, A. Techno-economic assessment of a membrane-based wastewater reclamation process. Desalination 2022, 522, 115409. [Google Scholar] [CrossRef]
- Zepon Tarpani, R.R.; Azapagic, A. Life cycle environmental impacts of advanced wastewater treatment techniques for removal of pharmaceuticals and personal care products (PPCPs). J. Environ. Manag. 2018, 215, 258–272. [Google Scholar] [CrossRef]
- Teow, Y.H.; Chong, M.T.; Ho, K.C.; Mohammad, A.W. Comparative environmental impact evaluation using life cycle assessment approach: A case study of integrated membrane-filtration system for the treatment of aerobically-digested palm oil mill effluent. Sustain. Environ. Res. 2021, 31, 15. [Google Scholar] [CrossRef]
- Vymazal, J. Constructed Wetlands for Wastewater Treatment. Water 2010, 2, 530–549. [Google Scholar] [CrossRef]
- Breitholtz, M.; Näslund, M.; Stråe, D.; Borg, H.; Grabic, R.; Fick, J. An evaluation of free water surface wetlands as tertiary sewage water treatment of micro-pollutants. Ecotoxicol. Environ. Saf. 2012, 78, 63–71. [Google Scholar] [CrossRef]
- Adrados, B.; Sánchez, O.; Arias, C.A.; Becares, E.; Garrido, L.; Mas, J.; Brix, H.; Morató, J. Microbial communities from different types of natural wastewater treatment systems: Vertical and horizontal flow constructed wetlands and biofilters. Water Res. 2014, 55, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Younger, P.L.; Henderson, R. Synergistic wetland treatment of sewage and mine water: Pollutant removal performance of the first full-scale system. Water Res. 2014, 55, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, C.; Li, K.; Su, J.; Zhu, G.; Liu, L. Performance of vertical up-flow constructed wetlands on swine wastewater containing tetracyclines and tet genes. Water Res. 2015, 70, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Tran, N.H.; Yin, T.; He, Y.; Gin, K.Y.-H. Removal of selected PPCPs, EDCs, and antibiotic resistance genes in landfill leachate by a full-scale constructed wetlands system. Water Res. 2017, 121, 46–60. [Google Scholar] [CrossRef]
- Fang, H.; Zhang, Q.; Nie, X.; Chen, B.; Xiao, Y.; Zhou, Q.; Liao, W.; Liang, X. Occurrence and elimination of antibiotic resistance genes in a long-term operation integrated surface flow constructed wetland. Chemosphere 2017, 173, 99–106. [Google Scholar] [CrossRef]
- Chen, J.; Ying, G.-G.; Wei, X.-D.; Liu, Y.-S.; Liu, S.-S.; Hu, L.-X.; He, L.-Y.; Chen, Z.-F.; Chen, F.-R.; Yang, Y.-Q. Removal of antibiotics and antibiotic resistance genes from domestic sewage by constructed wetlands: Effect of flow configuration and plant species. Sci. Total Environ. 2016, 571, 974–982. [Google Scholar] [CrossRef]
- Liu, L.; Liu, C.; Zheng, J.; Huang, X.; Wang, Z.; Liu, Y.; Zhu, G. Elimination of veterinary antibiotics and antibiotic resistance genes from swine wastewater in the vertical flow constructed wetlands. Chemosphere 2013, 91, 1088–1093. [Google Scholar] [CrossRef]
- Nõlvak, H.; Truu, M.; Tiirik, K.; Oopkaup, K.; Sildvee, T.; Kaasik, A.; Mander, Ü.; Truu, J. Dynamics of antibiotic resistance genes and their relationships with system treatment efficiency in a horizontal subsurface flow constructed wetland. Sci. Total Environ. 2013, 461–462, 636–644. [Google Scholar] [CrossRef]
- Chen, J.; Wei, X.-D.; Liu, Y.-S.; Ying, G.-G.; Liu, S.-S.; He, L.-Y.; Su, H.-C.; Hu, L.-X.; Chen, F.-R.; Yang, Y.-Q. Removal of antibiotics and antibiotic resistance genes from domestic sewage by constructed wetlands: Optimization of wetland substrates and hydraulic loading. Sci. Total Environ. 2016, 565, 240–248. [Google Scholar] [CrossRef]
- Huang, X.; Zheng, J.; Liu, C.; Liu, L.; Liu, Y.; Fan, H. Removal of antibiotics and resistance genes from swine wastewater using vertical flow constructed wetlands: Effect of hydraulic flow direction and substrate type. Chem. Eng. J. 2017, 308, 692–699. [Google Scholar] [CrossRef]
- Casas Ledón, Y.; Rivas, A.; López, D.; Vidal, G. Life-cycle greenhouse gas emissions assessment and extended exergy accounting of a horizontal-flow constructed wetland for municipal wastewater treatment: A case study in Chile. Ecol. Indic. 2017, 74, 130–139. [Google Scholar] [CrossRef]
- Corbella, C.; Puigagut, J.; Garfí, M. Life cycle assessment of constructed wetland systems for wastewater treatment coupled with microbial fuel cells. Sci. Total Environ. 2017, 584–585, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Flores, L.; García, J.; Pena, R.; Garfí, M. Constructed wetlands for winery wastewater treatment: A comparative Life Cycle Assessment. Sci. Total Environ. 2019, 659, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Resende, J.D.; Nolasco, M.A.; Pacca, S.A. Life cycle assessment and costing of wastewater treatment systems coupled to constructed wetlands. Resour. Conserv. Recycl. 2019, 148, 170–177. [Google Scholar] [CrossRef]
- Lakho, F.H.; Qureshi, A.; Igodt, W.; Le, H.Q.; Depuydt, V.; Rousseau, D.P.L.; Van Hulle, S.W.H. Life cycle assessment of two decentralized water treatment systems combining a constructed wetland and a membrane based drinking water production system. Resour. Conserv. Recycl. 2022, 178, 106104. [Google Scholar] [CrossRef]
- Pan, T.; Zhu, X.-D.; Ye, Y.-P. Estimate of life-cycle greenhouse gas emissions from a vertical subsurface flow constructed wetland and conventional wastewater treatment plants: A case study in China. Ecol. Eng. 2011, 37, 248–254. [Google Scholar] [CrossRef]
- Lutterbeck, C.A.; Kist, L.T.; Lopez, D.R.; Zerwes, F.V.; Machado, Ê.L. Life cycle assessment of integrated wastewater treatment systems with constructed wetlands in rural areas. J. Clean. Prod. 2017, 148, 527–536. [Google Scholar] [CrossRef]
- Garfí, M.; Flores, L.; Ferrer, I. Life Cycle Assessment of wastewater treatment systems for small communities: Activated sludge, constructed wetlands and high rate algal ponds. J. Clean. Prod. 2017, 161, 211–219. [Google Scholar] [CrossRef]
- Magalhães, I.B.; Ferreira, J.; de Castro, J.S.; de Assis, L.R.; Calijuri, M.L. Agro-industrial wastewater-grown microalgae: A techno-environmental assessment of open and closed systems. Sci. Total Environ. 2022, 834, 155282. [Google Scholar] [CrossRef]
- Silambarasan, S.; Logeswari, P.; Sivaramakrishnan, R.; Incharoensakdi, A.; Cornejo, P.; Kamaraj, B.; Chi, N.T.L. Removal of nutrients from domestic wastewater by microalgae coupled to lipid augmentation for biodiesel production and influence of deoiled algal biomass as biofertilizer for Solanum lycopersicum cultivation. Chemosphere 2021, 268, 129323. [Google Scholar] [CrossRef]
- Marangon, B.B.; Calijuri, M.L.; de Siqueira Castro, J.; Assemany, P.P. A life cycle assessment of energy recovery using briquette from wastewater grown microalgae biomass. J. Environ. Manag. 2021, 285, 112171. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Luo, Y.; Yuan, X. Life cycle and techno-economic assessment of source-separated wastewater-integrated microalgae biofuel production plant: A nutrient organization approach. Bioresour. Technol. 2022, 344, 126230. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, H. Catalytic ozonation for water and wastewater treatment: Recent advances and perspective. Sci. Total Environ. 2020, 704, 135249. [Google Scholar] [CrossRef] [PubMed]
- Lamba, M.; Ahammad, S.Z. Performance comparison of secondary and tertiary treatment systems for treating antibiotic resistance. Water Res. 2017, 127, 172–182. [Google Scholar] [CrossRef]
- Nasuhoglu, D.; Isazadeh, S.; Westlund, P.; Neamatallah, S.; Yargeau, V. Chemical, microbial and toxicological assessment of wastewater treatment plant effluents during disinfection by ozonation. Chem. Eng. J. 2018, 346, 466–476. [Google Scholar] [CrossRef]
- Shi, Q.; Chen, Z.; Liu, H.; Lu, Y.; Li, K.; Shi, Y.; Mao, Y.; Hu, H.-Y. Efficient synergistic disinfection by ozone, ultraviolet irradiation and chlorine in secondary effluents. Sci. Total Environ. 2021, 758, 143641. [Google Scholar] [CrossRef]
- Maniakova, G.; Salmerón, I.; Nahim-Granados, S.; Malato, S.; Oller, I.; Rizzo, L.; Polo-López, M.I. Sunlight advanced oxidation processes vs ozonation for wastewater disinfection and safe reclamation. Sci. Total Environ. 2021, 787, 147531. [Google Scholar] [CrossRef]
- Liu, Z.; Chys, M.; Yang, Y.; Demeestere, K.; Van Hulle, S. Oxidation of Trace Organic Contaminants (TrOCs) in Wastewater Effluent with Different Ozone-Based AOPs: Comparison of Ozone Exposure and •OH Formation. Ind. Eng. Chem. Res. 2019, 58, 8896–8902. [Google Scholar] [CrossRef]
- Antoniou, M.G.; Hey, G.; Rodríguez Vega, S.; Spiliotopoulou, A.; Fick, J.; Tysklind, M.; la Cour Jansen, J.; Andersen, H.R. Required ozone doses for removing pharmaceuticals from wastewater effluents. Sci. Total Environ. 2013, 456–457, 42–49. [Google Scholar] [CrossRef]
- Chys, M.; Audenaert, W.T.M.; Stapel, H.; Ried, A.; Wieland, A.; Weemaes, M.; Van Langenhove, H.; Nopens, I.; Demeestere, K.; Van Hulle, S.W.H. Techno-economic assessment of surrogate-based real-time control and monitoring of secondary effluent ozonation at pilot scale. Chem. Eng. J. 2018, 352, 431–440. [Google Scholar] [CrossRef]
- Pérez-Moya, M.; Graells, M.; Castells, G.; Amigó, J.; Ortega, E.; Buhigas, G.; Pérez, L.M.; Mansilla, H.D. Characterization of the degradation performance of the sulfamethazine antibiotic by photo-Fenton process. Water Res. 2010, 44, 2533–2540. [Google Scholar] [CrossRef]
- Trovó, A.G.; Pupo Nogueira, R.F.; Agüera, A.; Fernandez-Alba, A.R.; Malato, S. Degradation of the antibiotic amoxicillin by photo-Fenton process—Chemical and toxicological assessment. Water Res. 2011, 45, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Giannakis, S.; Le, T.-T.M.; Entenza, J.M.; Pulgarin, C. Solar photo-Fenton disinfection of 11 antibiotic-resistant bacteria (ARB) and elimination of representative AR genes. Evidence that antibiotic resistance does not imply resistance to oxidative treatment. Water Res. 2018, 143, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Miralles-Cuevas, S.; Oller, I.; Ruiz Aguirre, A.; Sánchez Pérez, J.A.; Malato Rodríguez, S. Removal of pharmaceuticals at microg L−1 by combined nanofiltration and mild solar photo-Fenton. Chem. Eng. J. 2014, 239, 68–74. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M. Combined photo-Fenton–SBR process for antibiotic wastewater treatment. J. Hazard. Mater. 2011, 192, 1418–1426. [Google Scholar] [CrossRef]
- Rodríguez, R.; Espada, J.J.; Pariente, M.I.; Melero, J.A.; Martínez, F.; Molina, R. Comparative life cycle assessment (LCA) study of heterogeneous and homogenous Fenton processes for the treatment of pharmaceutical wastewater. J. Clean. Prod. 2016, 124, 21–29. [Google Scholar] [CrossRef]
- Pesqueira, J.F.J.R.; Pereira, M.F.R.; Silva, A.M.T. A life cycle assessment of solar-based treatments (H2O2, TiO2 photocatalysis, circumneutral photo-Fenton) for the removal of organic micropollutants. Sci. Total Environ. 2021, 761, 143258. [Google Scholar] [CrossRef]
- Gallego-Schmid, A.; Tarpani, R.R.Z.; Miralles-Cuevas, S.; Cabrera-Reina, A.; Malato, S.; Azapagic, A. Environmental assessment of solar photo-Fenton processes in combination with nanofiltration for the removal of micro-contaminants from real wastewaters. Sci. Total Environ. 2019, 650, 2210–2220. [Google Scholar] [CrossRef]
- Foteinis, S.; Monteagudo, J.M.; Durán, A.; Chatzisymeon, E. Environmental sustainability of the solar photo-Fenton process for wastewater treatment and pharmaceuticals mineralization at semi-industrial scale. Sci. Total Environ. 2018, 612, 605–612. [Google Scholar] [CrossRef]
- Belalcázar-Saldarriaga, A.; Prato-Garcia, D.; Vasquez-Medrano, R. Photo-Fenton processes in raceway reactors: Technical, economic, and environmental implications during treatment of colored wastewaters. J. Clean. Prod. 2018, 182, 818–829. [Google Scholar] [CrossRef]
- Hey, G.; Ledin, A.; la Cour Jansen, J.; Andersen, H.R. Removal of pharmaceuticals in biologically treated wastewater by chlorine dioxide or peracetic acid. Environ. Technol. 2012, 33, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Akhoundi, A.; Nazif, S. Life-cycle assessment of tertiary treatment technologies to treat secondary municipal wastewater for reuse in agricultural irrigation, artificial recharge of groundwater, and industrial usages. J. Environ. Eng. 2020, 146, 4020031. [Google Scholar] [CrossRef]
- Licciardello, F.; Milani, M.; Consoli, S.; Pappalardo, N.; Barbagallo, S.; Cirelli, G. Wastewater tertiary treatment options to match reuse standards in agriculture. Agric. Water Manag. 2018, 210, 232–242. [Google Scholar] [CrossRef]
- Kim, I.; Yamashita, N.; Tanaka, H. Performance of UV and UV/H2O2 processes for the removal of pharmaceuticals detected in secondary effluent of a sewage treatment plant in Japan. J. Hazard. Mater. 2009, 166, 1134–1140. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, H.; Cicek, N. Treatment of Organic Micropollutants in Water and Wastewater by UV-Based Processes: A Literature Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1443–1476. [Google Scholar] [CrossRef]
- Carré, E.; Beigbeder, J.; Jauzein, V.; Junqua, G.; Lopez-Ferber, M. Life cycle assessment case study: Tertiary treatment process options for wastewater reuse. Integr. Environ. Assess. Manag. 2017, 13, 1113–1121. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; van Hullebusch, E.D.; Cretin, M.; Esposito, G.; Oturan, M.A. Coupling of membrane filtration and advanced oxidation processes for removal of pharmaceutical residues: A critical review. Sep. Purif. Technol. 2015, 156, 891–914. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.-S.; Su, H.-C.; Ying, G.-G.; Liu, F.; Liu, S.-S.; He, L.-Y.; Chen, Z.-F.; Yang, Y.-Q.; Chen, F.-R. Removal of antibiotics and antibiotic resistance genes in rural wastewater by an integrated constructed wetland. Environ. Sci. Pollut. Res. 2015, 22, 1794–1803. [Google Scholar] [CrossRef]
- Cardinal, P.; Anderson, J.C.; Carlson, J.C.; Low, J.E.; Challis, J.K.; Beattie, S.A.; Bartel, C.N.; Elliott, A.D.; Montero, O.F.; Lokesh, S.; et al. Macrophytes may not contribute significantly to removal of nutrients, pharmaceuticals, and antibiotic resistance in model surface constructed wetlands. Sci. Total Environ. 2014, 482–483, 294–304. [Google Scholar] [CrossRef]
- Kong, Q.; Li, L.; Martinez, B.; Chen, P.; Ruan, R. Culture of Microalgae Chlamydomonas reinhardtii in Wastewater for Biomass Feedstock Production. Appl. Biochem. Biotechnol. 2009, 160, 9. [Google Scholar] [CrossRef]
- Ansari, F.A.; Ravindran, B.; Gupta, S.K.; Nasr, M.; Rawat, I.; Bux, F. Techno-economic estimation of wastewater phycoremediation and environmental benefits using Scenedesmus obliquus microalgae. J. Environ. Manag. 2019, 240, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Salama, E.-S.; Abou-Shanab, R.A.I.; Kim, J.R.; Lee, S.; Kim, S.-H.; Oh, S.-E.; Kim, H.-C.; Roh, H.-S.; Jeon, B.-H. The effects of salinity on the growth and biochemical properties of Chlamydomonas mexicana GU732420 cultivated in municipal wastewater. Environ. Technol. 2014, 35, 1491–1498. [Google Scholar] [CrossRef] [PubMed]
- Hena, S.; Gutierrez, L.; Croué, J.-P. Removal of pharmaceutical and personal care products (PPCPs) from wastewater using microalgae: A review. J. Hazard. Mater. 2021, 403, 124041. [Google Scholar] [CrossRef] [PubMed]
- Chaudry, S. Integrating Microalgae Cultivation with Wastewater Treatment: A Peek into Economics. Appl. Biochem. Biotechnol. 2021, 193, 3395–3406. [Google Scholar] [CrossRef]
- De Schneider, R.C.S.; de Moura Lima, M.; Hoeltz, M.; de Farias Neves, F.; John, D.K.; de Azevedo, A. Life cycle assessment of microalgae production in a raceway pond with alternative culture media. Algal Res. 2018, 32, 280–292. [Google Scholar] [CrossRef]
- Igos, E.; Mailler, R.; Guillossou, R.; Rocher, V.; Gasperi, J. Life cycle assessment of powder and micro-grain activated carbon in a fluidized bed to remove micropollutants from wastewater and their comparison with ozonation. J. Clean. Prod. 2021, 287, 125067. [Google Scholar] [CrossRef]
- Arzate, S.; Pfister, S.; Oberschelp, C.; Sánchez-Pérez, J.A. Environmental impacts of an advanced oxidation process as tertiary treatment in a wastewater treatment plant. Sci. Total Environ. 2019, 694, 133572. [Google Scholar] [CrossRef]
- Muñoz, I.; Rodríguez, A.; Rosal, R.; Fernández-Alba, A.R. Life Cycle Assessment of urban wastewater reuse with ozonation as tertiary treatment: A focus on toxicity-related impacts. Sci. Total Environ. 2009, 407, 1245–1256. [Google Scholar] [CrossRef]
- Sánchez Pérez, J.A.; Arzate, S.; Soriano-Molina, P.; García Sánchez, J.L.; Casas López, J.L.; Plaza-Bolaños, P. Neutral or acidic pH for the removal of contaminants of emerging concern in wastewater by solar photo-Fenton? A techno-economic assessment of continuous raceway pond reactors. Sci. Total Environ. 2020, 736, 139681. [Google Scholar] [CrossRef]

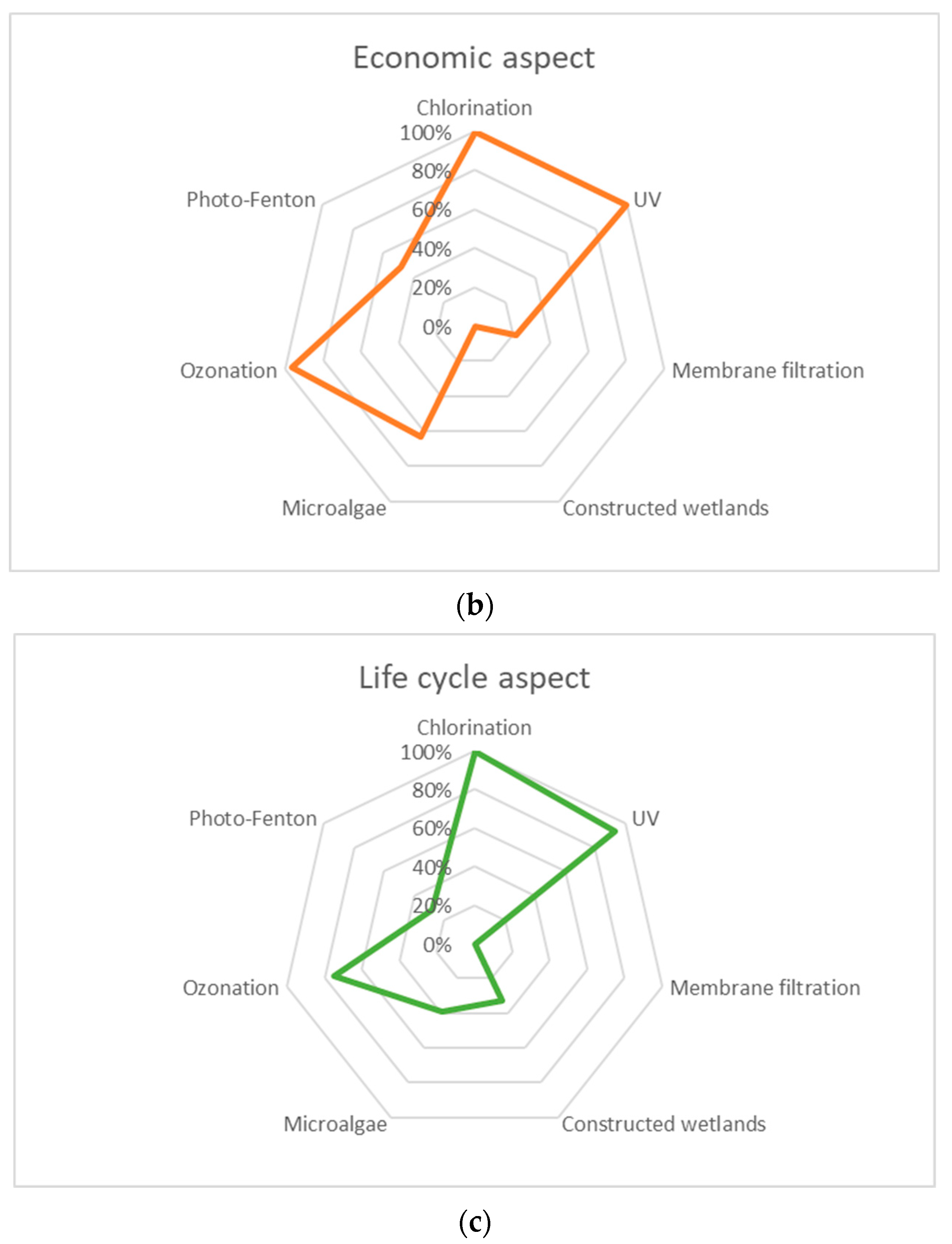

| Category | Treatment Method | Technical | Economic (Cost EUR/m3) | Life Cycle (kg CO2 eq. m−3) | ||
|---|---|---|---|---|---|---|
| Microbial (Log Reduction) | Nutrients (% Reduction) | Pharmaceuticals (% Reduction) | ||||
| Physicochemical | Chlorination | 2.14 | 0 | 42 | 0.004 | 0.040 |
| UV | 2.92 | 0 | 53 | 0.004 | 0.086 | |
| Membrane filtration | 3.50 | 6 | 70 | 0.614 | 0.754 | |
| Biological | Constructed wetlands | 0.87 | 53 | 57 | 0.784 | 0.511 |
| Microalgae | 0.00 | 77 | 73 | 0.291 | 0.468 | |
| Advanced oxidation | Ozonation | 3.18 | 0 | 63 | 0.030 | 0.219 |
| Photo-Fenton | 4.93 | 0 | 84 | 0.405 | 0.549 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zagklis, D.P.; Bampos, G. Tertiary Wastewater Treatment Technologies: A Review of Technical, Economic, and Life Cycle Aspects. Processes 2022, 10, 2304. https://doi.org/10.3390/pr10112304
Zagklis DP, Bampos G. Tertiary Wastewater Treatment Technologies: A Review of Technical, Economic, and Life Cycle Aspects. Processes. 2022; 10(11):2304. https://doi.org/10.3390/pr10112304
Chicago/Turabian StyleZagklis, Dimitris P., and Georgios Bampos. 2022. "Tertiary Wastewater Treatment Technologies: A Review of Technical, Economic, and Life Cycle Aspects" Processes 10, no. 11: 2304. https://doi.org/10.3390/pr10112304
APA StyleZagklis, D. P., & Bampos, G. (2022). Tertiary Wastewater Treatment Technologies: A Review of Technical, Economic, and Life Cycle Aspects. Processes, 10(11), 2304. https://doi.org/10.3390/pr10112304







