Abstract
This paper investigates the differences in timelines involved in Lean Six Sigma (LSS) project deployment in a regulated industry versus in an unregulated one. Two case studies utilising Lean Six Sigma methods—in order to compare the transfer of manual manufacturing lines within a medical device and electronics manufacturing site—are discussed and utilised. This research aims to show the effects of regulatory procedures on LSS project implementation and timelines. This study particularly highlights how a regulatory environment can be a barrier, or bottleneck, to project management, continuous improvement, and engineering changes in the MedTech or medical device manufacturing industry. The results of this study represent an important first step towards a full understanding of the influence of regulations on operations in medical devices and, by extension, on pharmaceutical manufacturing industries on a global scale. The research limitations are that the data collected were from two specific case study comparisons alone.
Keywords:
continuous improvement; medical device; MedTech; Lean Six Sigma; ISO 13485; 21CFR 820; validation 1. Introduction
Medical device manufacturers play an important role in public health and the global economy. Further, the manufacturing of medical devices is very tightly controlled by regulatory authorities [1]. The medical device industry is one of the most regulated industries; it is regulated by laws that govern the safety and performance of devices across their lifetime, in their pre- and post-market lifecycle. Regulated manufacturing industries operate under government laws and regulations, while unregulated manufacturing industries do not operate under government rules and laws [2]. As a result, implementing continuous improvement methods such as Lean Six Sigma (LSS) in regulated manufacturing industries differs from unregulated manufacturing industries. One of the differences entails the requirement for the validation and submission of the desired changes to authorities for verification [3]. For instance, regulated industries, such as pharmaceuticals and medical device manufacturers, operate under the U.S. Food and Drug Administration’s (FDA) or, in Europe, the European Medicines Agency’s (EMA) regulations, guidelines, or legislation as relevant [4]. Therefore, before any significant changes can be made in any organisation, the FDA or EMA must approve it for the safety of the employees and customers [5]. In addition, regulated bodies control the level to which processes are validated; these controls are not present in unregulated industries, allowing for relatively quick process changes. As a result, implementing LSS in regulated manufacturing industries takes longer than in unregulated industries as they need to contend with various validation activities, which are not required to the same level in unregulated industries [3,6,7].
The cost of implementing LSS in regulated industries ranks higher than in unregulated manufacturing industries, as the validation activities require significant resources [8]. In addition, since the government or state bodies govern regulated manufacturing industries, implementing new changes involves numerous processes [9]. On the other hand, in regard to unregulated manufacturing industries, the implementation of new operational strategies does not require evaluation from a notified body; thus, the expenses of implementing a new change are significantly reduced [6,7,10].
The effective implementation rate of LSS in regulated industries is lower than in unregulated industries [11]. However, the emphasis is on “effective.” Moreover, the literature can differ, but recent studies have shown that more than 95% of companies in Pharma and Medtech have some type of Lean, Six sigma, or LSS program [6,7]. Due to the numerous processes (i.e., regulatory) encountered before a change is implemented, the interval between implementing a new change is widened. On the other hand, a series of new changes may be witnessed in unregulated manufacturing industries due to the fact that implementing a new change does not go through a strict process. Unregulated industries rely on the management, support teams, and operators to suggest new changes; therefore, many changes may be suggested quickly [12]. There has been an increase in the recall of medical devices from 2006 to 2010 [13] and from 2015 to 2019 [14]. These increases occur, ironically, while regulatory bodies increase the regulatory burden on medical device manufacturers to protect patients in response to recalls. Continuous improvement programs can face many challenges in the medical device and pharmaceutical industries, which are highly regulated in order to ensure patient and customer safety [6,15]. Due to the regulated nature of the industries, manufacturing, and other, changes must be approved by regulatory authorities, such as by the FDA and EMA, for example [15]. Indeed, one of many quality standards for medical device quality management system (QMS) implementation does not focus on continuous improvement as ISO 9001 [16] does; instead, in regard to ISO 13485, it is risk focused [6,17]. Medical device companies are so focused on compliance—and for good reason, as they wish to protect the patient and maintain their market authorisation—but little time is, therefore, left to remove the sources of waste [18].
Process improvement teams can follow the standard DMAIC method and implement change, or even test improvements, in a live manufacturing environment that is within an unregulated industry [7]. In a regulated environment, the define, measure, and analyse phases are critically important as there is only one opportunity to improve, and it must be correct the first time as any error will result in a corrective and preventative action report (CAPA) being raised [6]. The required time investment of the validation process prevents process improvement in a regulated environment as the extra burden of regulation consumes resources [6,7]. While it is clear that LSS has been proven to reduce cost and increase quality in many industries [19], few published examples of the barriers found in implementing LSS in a regulated industry are found in the literature [3].
The scope of this study is to compare an LSS project implementation in an electronics company versus in a MedTech company—with the specific scope being focused on the timelines to implement. This study aims to evaluate the issues surrounding the implementation of LSS in a regulated environment by utilising a practical example of an LSS project implementation. The aim is to ascertain where time is lost in project execution due to overcoming or complying with regulations. A better understanding of LSS implementation issues in regulated industries could help planning projects and perhaps mitigate against delays by providing contingencies. A case study on implementing a LSS project in an Irish medical device company manufacturing line will be utilised in order to ascertain the barriers and enablers to LSS deployment in a regulated environment. The LSS implementation in a medical device company will then be compared to implementing a similar LSS project in an unregulated industry in order to ascertain the differences through a real-world example.
The research questions are:
- What effect can a regulatory environment have on the implementation of a LSS project as opposed to a similar project in a regulated environment?
- What are the barriers to implementing Lean Six Sigma projects in regulated environments?
The remainder of the paper is as follows: Section 2 describes the literature, followed by the research methodology in Section 3. Next, the results are explicated in Section 4, followed by a discussion and consideration of the implications in Section 5. Finally, the conclusion, limitations, and scope for future research are elucidated in Section 6.
2. Literature Review
In the European Union and globally, medical devices are stringently regulated by laws that govern the safety and performance of the devices throughout their lifetime, both pre- and post-market. Medical devices range from Band-Aids to sophisticated lifesaving products, such as pacemakers and implantable pacemakers [20]. The classification of medical devices dictates the level of the regulatory pre- and post-market requirements. The higher classification of a device the higher the risk and, therefore, the more stringent the regulatory controls required [3]. Within the U.S. regulatory system, for example, devices classified as Class I are deemed low risk and are not subject to the most stringent regulatory controls [20]. On the other hand, class II devices are in a higher class than Class I and thus require more regulatory controls in order to ensure the device’s safety and effectiveness. Lastly, Class III devices are usually the highest risk devices and are therefore subject to the most stringent regulatory control and pre-marketing approvals, e.g., replacement heart valves [21].
2.1. Manufacturers Embracing Lean Six Sigma
When different organisations implement LSS, it frequently appears that they have only been added in unstructured design; further, a small number of tools are in their toolbox via training [22]. The implementation does not always succeed as expected. LSS can present challenges or be affected by specific challenges [6]. Studies suggest that regulated industries lag behind other industries due to the regulatory burden [15,23]. The FDA has also noticed the lack of innovative process improvement and has started its journey to enable manufacturers to improve processes [24,25].
According to Pavlovic and Bozanic [15], manufacturing industries have utilised Lean Six Sigma to mitigate production cost increases and improve productivity and quality by reducing defects and variation. The concepts of Lean Six Sigma can be utilised in regulated industries in order to mitigate the process of defect and loss. However, processes are validated and can only be reviewed, changed, or updated with revalidation [26]. Change in the concept of fundamental regulation can allow improvement processes to run smoothly [27].
The MedTech industry has complained that the FDA rules hinder them from successfully applying the improvement process for a long time [27,28]. The FDA found that a predominant focus on compliance was a key one over and above quality and CI, with an industry focus that was on meeting regulatory requirements (or “compliance”) rather than adopting the best quality practices [29]. Low investment in automation and digitalisation by the Medtech industry was not promoting continuous improvement in order to enable better processes and more responsive learning [30]. It was stated that serious adverse event reports, related to medical device use, had outpaced industry growth by 8% per annum since 2001 [29]. From 2011 to 2018, the FDA launched the Case for Quality and worked with the Device Innovation Consortium in order to trial the Capability Maturity Model Integration (CMMI) system, commonly used in the U.S. government for software development [31]. The CMMI promotes more focus on operational excellence rather than over-emphasising compliance. The CMMI process allows for relaxation in the regulations around the frequency of compliance audits and promotes more focus on process improvement. An example of the literature reviews and barriers to LSS that have been identified are outlined in Table 1.

Table 1.
Summary of the literature in relation to barriers to LSS methods as well as including literature with a focus on LSS in Medtech.
2.2. Barriers and Enablers for CI Methodologies
In two studies completed in 2021, which surveyed people working at various levels and functions in the pharmaceutical and Medtech industries, it was found that 45% perceived that a highly regulated environment was a barrier to continuous improvement. The respondents cited that, in Medtech and Pharma industries, regulatory approvals and submissions, fear of validation activity, and compliance versus quality culture were barriers to continuous improvement. However, over 95% of those surveyed worked in organisations with LSS programs and utilised continuous improvement tools [6,7]. This study shows that, contrary to the previously cited literature, while LSS is being advocated, utilised, and promoted in heavily regulated industries, there is also agreement that heavy regulations are a barrier to implementation. Respondents cited the fear of extra validation activity in both studies as a barrier to continuous improvement. A study by Byrne, McDermott, and Noonan in 2021 highlighted a contextual example in a regulated industry LSS project where the most appropriate corrective action to fix issues with breaking tablets was not taken, as it would mean revalidation of the problematic process under study and, therefore, incurring more time in order to obtain approval from the regulatory authorities [3].
2.3. Research Gap
While there has been much written about LSS application in automotive manufacturing, in particular, there is very little literature on LSS in the Medtech industry [6,7]. Studies have suggested that LSS is, when applied in highly regulated industries such as Medtech, not as effectively applied as elsewhere [3,4,6,7]. In fact, LSS project actions and selection are based upon, and prioritised, on the project’s impact on a company’s regulatory compliance. However, despite the suggestion that regulated barriers deter effective CI and LSS actions—there has been no study undertaken to particularly demonstrate how long LSS projects can take in a regulated environment.
3. Methodology
A systematic literature review (SLR) was used to help answer the research question in conjunction with a case study approach. The flow chart for the SLR methodology is, as follows, in Figure 1. The flow chart outlines the five stages of the review as well as the inclusion/exclusion criteria. Once the researchers had reduced and screened the articles that they felt best matched the research themes, these articles were then grouped by themes related to the barriers and delays that were faced when applying LSS in Medtech, Pharma, and other regulated type environments.
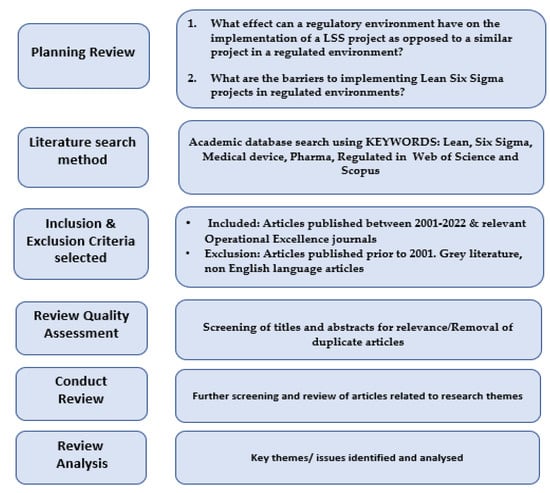
Figure 1.
SLR flowchart method.
Due to this research project being conducted on the real-world application of a LSS project, it was felt that the best approach to utilise was that of a ‘Case Study’ research approach. A case study lends itself to observing and quantifying, using qualitative and quantitative methods that require the researcher to fully engage with the research project, thereby allowing a more comprehensive understanding of the investigated problems [41].
The case study approach is very applicable to this case when there is a scarce body of knowledge on the subject [42], as there is in the case of the literature and practical examples of LSS related to the Medical Device industry [43,44]. Furthermore, case study examples further aid in understanding the dynamics present within the particular t context for the purposes of a better understanding of the phenomenon [45].
In this instance—based on comparing similar type line implementations projects utilising LSS, which had not been studied previously—the case study approach is most suitable if the phenomenon under investigation is unique, critical, and revelatory [44,45].
In summary, this research was undertaken in two manufacturing organisations. One manufacturing organisation acts as a medical device manufacturer, and the other is an electronics manufacturer. The organisations involved started their LSS journeys within the last 5–8 years. The organisations have requested to remain anonymous.
The case study data have been collected primarily due to two LSS black belt projects completed by authors as mentioned above, one in an unregulated electronics manufacturer and the other in a heavily regulated medical device manufacturer. Both projects were of similar size and scope, as such they would be ideal to provide a real-world example of the differences between LSS project timelines in a regulated versus unregulated environment. While the methodological choices made in this study are sound, there is a margin of error in any conclusion due to the lack of similar practical case study comparisons in implementing LSS projects in regulated industries. However, the projects picked to compare are identical in scope, size, and project goals (Table 2). In order to ensure the LSS projects picked for comparison were as similar as possible, a number of attributes were used. First, customer approval was a requirement. Both case study organisations needed to meet in order to compare the time involved in gaining approval. The size of the transfer was the second attribute selected. The transfer size involved in moving from Thailand to Ireland in the electronics industry was comparable to the time involved in gaining regulatory approval to move from one manufacturing building or site to another; as such, the two case studies were deemed comparable. Finally, both projects were line transfers, as such they are similar in project type and scale. Regarding the project complexity, both projects were deemed “copy exact” or, in other words, moving from A to B line transfers.

Table 2.
Attributes of case study organisations listed in order to demonstrate similarities in projects under review.
4. Results
To provide evidence of the time delay caused by validation and other regulatory requirements of a regulated industry, such as in a medical device manufacturer, the improvement of a medical device manufacturing line is compared to a manual assembly line of relatively equal complexity in the electronics industry. While an exact copy would not be possible, the chosen examples have a similar level of manual and automated tasks, as well as types of steps. With some margin of error, the only difference between these two cases is that one occurs under the rigour of tight regulation, while the other is only bound by more simple quality management system regulations, such as ISO 9001.
4.1. Case Study 1—An Unregulated Industry Example of a Manual Assembly Line Project
In the first case study, where Lean Six Sigma was applied in an unregulated manual assembly, the capability of the process was increased. The project involved moving the manufacturing process for enterprise storage servers from a low-cost country, Thailand, to a high-cost country, Ireland. As in any move from a low-cost to a high-cost country, the challenge was to improve the process and adjust the processing costs to those of the higher-cost country [46,47].
The product was produced by a contract manufacturer and required all changes to be approved by the customer (i.e., the primary manufacturer) before implementation and to ensure that quality controls compliant with ISO 9001 were also in place. In addition, all QMS documents and procedures were updated, and an engineering build was completed to prove the process.
There was no validation of the process change since the case study was conducted in an unregulated environment. Transferring from Thailand to Ireland did not require consultations with regulatory authorities as the manufactured products’ market authorisation was not affected (this is because the product was not highly regulated).
They were incorporating the Lean Six Sigma DMAIC methods in an unregulated environment, showing that, in this first case study, the process is straightforward and not time-consuming. For example, it only took two weeks to define the problem. The following measure phase took a further two weeks, while the analysis phase took three weeks. The analysis phase determined layout review, process capability, and review of current process times. The next improvement phase took eight weeks and involved line balancing, work instruction revisions, and layout improvements. The last phase is the control phase, where failures were actioned via Pareto analysis, process capability was measured, and statistical process control was implemented. After incorporating Lean Six Sigma methods in order to improve and transition the unregulated manual assembly, a pre-project headcount of five was maintained post-implementation. As a result, productivity and output increased by twenty-five per cent. The Lean Six Sigma methodology ensured that the customer’s goals were met while maintaining the project’s commercial success.
In summary, there was no requirement for validation and qualification, allowing the project to be completed in under six months from team formation to ramping up to total production in Ireland. Table 3 outlines the project phases, the LSS tools utilised for the project’s duration, and any particular project delays.

Table 3.
Unregulated electronics industry example.
LSS was used in order to achieve the customer goal, while ensuring the project’s commercial success by meeting a new demand with the same resources. In addition, lean tools were used to improve the process, while Six Sigma was used to measure its capability and track its progress through to completion.
4.2. Case Study 2—Regulated Manual Assembly
A Lean Six Sigma project at a medical device company contrasts with the previously discussed project; further, this project took nine months to complete due to the additional time and resources required for the implementation, notably in the validation and regulatory approval activities. The project required the relocation of a medical device manufacturing line to a cleanroom in a nearby building. Due to the nature of the business, with the medical device company being a contract manufacturer, no changes can be made to the process without the customer’s written approval and standard validation activities being carried out [46]. The requirement for change approvals and other regulatory requirements related to the change in manufacturing location is outlined in ISO 13485 [47]. Due to the extended duration of the improvement phase and as the process was to be transferred to a new building, a new line design was created. Technically the process transfer was to be a “copy exact” transfer, but with the LSS methods used in order to identify line enhancements and optimisation.
4.3. Key Project Stages
As changes are required in the process, the first step, in any change, is to detail how the change will happen. A plan was drawn up, and as this was conducted at a contract manufacturer, the plan must be sent to the customer for approval per legal and contractual agreements. Under various regulatory jurisdictions, including Europe, the regulations ensure robust quality agreements are executed with the contract manufacturers. Some of the items in these quality agreements include a quality management system, availability of records, validations, and change control [48]. To gain market authorisation in Europe, manufacturers of medical devices must certify to ISO 13485:2015, which has requirements in regard to the quality management system that is used by the device manufacturers [6].
The customer will then assess the changes with their notified body and their regulatory department in order to see if they can be accepted, or if further testing or regulatory approval is required. Regulatory approval was required as the process changed address (i.e., moved to a different location). This approval was a simple submission required by the regulator and took 60 days, including preparation and follow-up work. Part of this new design required procuring new equipment (similar to the unregulated project). However, this new equipment must undergo a validation process.
Under ISO 13485:2015, section 7.5.6 states that “the organisation shall validate any processes for production and service provision where the resulting output cannot be or is not verified by subsequent monitoring or measurement and, as a consequence, deficiencies become apparent only after the product is in use or the service has been delivered” [49]. Similarly, the USA’s FDA requirements relating to validation are outlined in 21CFR Part 820 Section 820.75 subsection (a), which states:
“Where the results of a process cannot be fully verified by subsequent inspection and test, the process shall be validated with a high degree of assurance and approved according to established procedures. The validation activities and results, including the date and signature of the individual(s) approving the validation and where appropriate the major equipment validated, shall be documented” [50].
Even though the new equipment is identical to the current equipment, it must still go through the equipment installation qualification (EIQ) and equipment operational qualification (EOQ) processes. Installation qualification (I.Q.) verifies that an instrument—or unit of equipment being qualified (as well as its sub-systems and any ancillary systems)—has been installed and configured according to the manufacturer’s specifications or installation checklist [43]. The EIQ is a process of detailing all relevant information about the equipment and completing safety, quality, microbiological, and calibration checks in order to ensure the equipment will not cause harm to the devices being produced or any devices in the vicinity of the equipment. The EIQ, once complete, then needs to be approved by all operations departments before the EOQ can begin. EIQ will generally take 25 working days once the equipment is onsite from start to finish. This process subsequently took 40 working days.
The EOQ serves as the operational check and challenges everything in the EIQ to ensure the equipment will function correctly over time. During this testing, the equipment is typically used to produce devices that will be scrapped in order to ensure the equipment performs as it should. Again, the EOQ must be approved by all operations departments before proceeding, and this process typically takes five working days to complete.
While detailed and time-consuming, the validation process does not take up the bulk of the time; rather, it is the paperwork associated with the validation. First, all process documentation must be updated as the process now contains new equipment in a new location. To complete this task, all documentation was reviewed, and references to equipment and their location were changed where necessary. This change included reviewing and updating manufacturing procedures, as well as the device manufacturing record, and also the master validation plan for the product. This process took 30 days as each document must be approved by all departments and the customer, as well as be approved via the internal document control processes. This left all documents ready for implementation; however, the project was held up on the approval of the process qualification stage.
To ensure the change in equipment and location had no impact on the process, the process qualification (P.Q.) must be repeated despite having been completed when the initial process in the previous location had been set up. This required building batches of devices using different batches of raw material and at different settings within the qualified range throughout the process and ensuring as high a yield as possible. Additionally, the validation engineers had to ensure that no defects escaped the process. In total, the P.Q. stage took 20 days, including the time required to complete the adapted design of the P.Q.
By customer request and to ensure greater confidence in the process, the customer requested additional key performance and monitoring metrics stay in place post move for six weeks in order to ensure the process performance did not drop.
The breakdown of the tools used, duration of the phases, and the delays encountered are outlined in Table 4.

Table 4.
Regulated medical device industry example.
Table 5 shows the time differences between the two projects, the most notable difference being the improvement phase. The validation activity was performed as part of the improvement phase leading to a much extended improve phase of 22 weeks. It should be noted that the second case study example involved the transfer of product manufacturers from one country to another (as in the first case study) and not a transfer of 0.1 KM to a different building, where even more regulatory submissions and approval of changes would have been involved. Even with the “local” and non-country transfer, the regulated project took 20 weeks or 5 months longer, representing an increase of a 55% time difference.

Table 5.
Differences between lines implementation in the regulated projects versus the unregulated project.
5. Discussion
Lean Six Sigma methodology contains some benefits in saving money, improving competencies and performances, and achieving a better customer relationship, thus achieving an almost perfect performance at business levels, including in the medical device industry [51]. In addition, this method can be effectively used in an unregulated environment without regulatory procedures, which would hinder its application.
Both case studies involved in this research are different, whereby the first case study involves the application of Lean Six Sigma in an unregulated environment, and the second case study applies Lean Six Sigma in a regulated environment. The results of the Lean Six Sigma application in both cases studies are outlined in the results section, as well as the specific regulatory milestones required for completing the project. The data for the regulated environment project demonstrate that regulations were deemed a delay in completing the project or perhaps a barrier in regard to a seamless transitioning of changes [RQ1]. The LSS tools utilised in both projects were similar, but it should be noted that the way in which the different tools can be applied may affect project deployment success [36]. However, the case studies did demonstrate that an increased 55% time difference, or 20 week difference, in the delivery of both projects is sizeable in terms of time, resources, and cost effect on the customers.
These findings and evidence align with the many literature studies concerning the impact of regulations on MedTech and pharmaceutical manufacturers [3,6,7].
Managerial Implications
This research aims to prove and show evidence that regulatory milestones have many implications for LSS projects in Medtech organisations, as changes identified in such organisations may affect manufacturers’ market authorisations and require regulatory submissions and approvals to, thus, be avoided [6,7]. The case study has shown and explained, in detail, the effect regulations and the validation process has on LSS implementation in highly regulated industries, as well as the barriers encountered while implementing these projects [RQ2]. These findings also demonstrate why compliance over quality may stifle operational excellence in Medtech [29,31,40]. Validation typically accounts for 30–55% of the total project budget in terms of time and cost and requires highly skilled engineers in order to analyse the system, author and execute the test scripts, and, finally, document the results per regulatory requirements [52].
Deploying a new approach in the first case study was easier due to the fact that the project was in the electronics industry, eliminating cumbersome regulatory requirements that would otherwise lower the swiftness of the implementation of the improvements. As seen from case study 1, we can deduce that the Lean Six Sigma method is more compatible with the unregulated environment than the regulated environment [3,6,7].
The authors understand that this is the very first research that utilised examples of manufacturing line setups in order to demonstrate how regulation impacts these environments. As such, the research was limited in what it could derive from the published literature in order to substantiate the results.
6. Conclusions
Lean Six Sigma is used to improve both unregulated and regulated companies’ performance, as well as to reduce production and development costs. It also increases the profit and improvement of customer satisfaction by the improving of productivity and in keeping supply available. This study shows that the application of Lean Six Sigma can be easily achieved in an unregulated environment, as can be seen in the first case study. This is because there are no consultations and regulations to be followed, even when changing from one country to another. For example, the organisation’s move from Thailand to Ireland was accessible due to a lack of regulation. At the same time, in the second case study, shifting production from one building to another will take much more time due to the multiple consultations and regulations that must be adhered to. The only way to improve regulated manufacturing processes is to develop a regulator-backed LSS methodology for regulated industries. Integrated within this should be a streamlined revalidation process for the affected implementation of process improvements, particularly where the improvements increase yield and quality, thereby reducing the potential for patient harm. Within the academic community, this study is one of the first studies demonstrating the timeline differences between LSS projects within a regulated environment and a non-regulated one. Moreover, it should aid further study, research, and understanding of LSS in regulated environments.
Author Contributions
Conceptualization, V.M. and O.M; methodology, O.M., A.T., M.S. and A.R.; formal analysis, O.M., V.M. and A.T; writing—original draft preparation, V.M., O.M., M.S., A.T. and A.R.; writing—review and editing, O.M., M.S., A.T. and A.R.; supervision, V.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Granlund, T.; Mikkonen, T.; Stirbu, V. On Medical Device Software C.E. Compliance and Conformity Assessment. In Proceedings of the 2020 IEEE International Conference on Software Architecture Companion (ICSA-C), Salvador, Brazil, 16–20 March 2020; pp. 185–191. [Google Scholar]
- Awasthi, K.; Yayavaram, S.; George, R.; Sastry, T. Classification for regulated industries: A new index. IIMB Manag. Rev. 2019, 31, 309–315. [Google Scholar] [CrossRef]
- Byrne, B.; McDermott, O.; Noonan, J. Applying Lean Six Sigma Methodology to a Pharmaceutical Manufacturing Facility: A Case Study. Processes 2021, 9, 550. [Google Scholar] [CrossRef]
- Trubetskaya, A.; Manto, D.; McDermott, O. A Review of Lean Adoption in the Irish MedTech Industry. Processes 2022, 10, 391. [Google Scholar] [CrossRef]
- Llamas, M. Food and Drug Administration (FDA). 2021. Available online: https://www.drugwatch.com/fda/ (accessed on 3 September 2021).
- McDermott, O.; Antony, J.; Sony, M.; Healy, T. Critical failure factors for continuous improvement methodologies in the Irish MedTech industry. TQM J. 2022, 34, 18–38. [Google Scholar] [CrossRef]
- McDermott, O.; Antony, J.; Sony, M.; Daly, S. Barriers and Enablers for Continuous Improvement Methodologies within the Irish Pharmaceutical Industry. Processes 2021, 10, 73. [Google Scholar] [CrossRef]
- Torbeck, L.D. Pharmaceutical and Medical Device Validation by Experimental Design; CRC Press: New York, NY, USA, 2007. [Google Scholar]
- Blind, K.; Petersen, S.S.; Riillo, C.A. The impact of standards and regulation on innovation in uncertain markets. Res. Policy 2017, 46, 249–264. [Google Scholar] [CrossRef]
- Kitching, J.; Hart, M.; Wilson, N. Burden or benefit? Regulation as a dynamic influence on small business performance. Int. Small Bus. J. Res. Entrep. 2015, 33, 130–147. [Google Scholar] [CrossRef]
- Tappen, R.M.; Wolf, D.G.; Rahemi, Z.; Engstrom, G.; Rojido, C.; Shutes, J.M.; Ouslander, J.G. Barriers and Facilitators to Implementing a Change Initiative in Long-Term Care Utilising the INTERACT™ Quality Improvement Program. Health Care Manag. 2017, 36, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Clarke, T. Ethics, Values and Corporate Governance. BBVA, Values and Ethics for the 21st Century; BBVA: Madrid, Spain, 2021; pp. 513–558. [Google Scholar]
- Heneghan, C.; Thompson, M.; Billingsley, M.; Cohen, D. Medical-device recalls in the UK and the device-regulation process: Retrospective review of safety notices and alerts. BMJ Open 2011, 1, e000155. [Google Scholar] [CrossRef]
- Vajapey, S.P.; Mengnai, L. Medical Device Recalls in Orthopedics: Recent Trends and Areas for Improvement. J. Arthroplast. 2020, 35, 2259–2266. [Google Scholar] [CrossRef]
- Pavlović, K.; Božanić, V. Lean and Six Sigma Concepts—Application in Pharmaceutical Industry. In Proceedings of the 4th International Quality Conference, Kragujevac, Serbia, 19 May 2010; pp. 259–268. [Google Scholar]
- ISO 9001:2015; Quality Management Systems and Requirements. International Organisation for Standardisation: Geneva, Switzerland, 2015.
- Troschinetz, A. ISO 13485: Medical Devices and Risk Management. Quality 2010, 49, 44. [Google Scholar]
- McDermott, O.; Antony, J.; Sony, M.; Looby, E. A critical evaluation and measurement of organisational readiness and adoption for continuous improvement within a medical device manufacturer. Int. J. Manag. Sci. Eng. Manag. 2022, 1–11. [Google Scholar] [CrossRef]
- Womack, J.P.; Jones, D.T.; Roos, D. The Machine That Changed the World; Simon and Schuster: New York, NY, USA, 1990. [Google Scholar]
- Food and Drug Administration. CDRH Classify Your Medical Device. Available online: https://www.fda.gov/medical-devices/overview-device-regulation/classify-your-medical-device (accessed on 15 August 2021).
- Martin, J.L.; Norris, B.J.; Murphy, E.; Crowe, J.A. Medical device development: The challenge for ergonomics. Appl. Ergon. 2008, 39, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Alsyouf, I.; Kumar, U.; Al-Ashi, L.; Al-Hammadi, M. Improving baggage flow in the baggage handling system at a UAE-based airline using lean Six Sigma tools. Qual. Eng. 2018, 30, 432–452. [Google Scholar] [CrossRef]
- Shanley, A. Reinventing Lean Six Sigma for the Pharmaceutical Industry. Pharm. Technol. 2017, 41, 76–80. [Google Scholar]
- Food and Drug Administration. Guidance for Industry, PAT-A Framework for Innovative Pharmaceutical Development, Manufacturing and Quality Assurance. 2004. Available online: https://www.fda.gov/media/71012/download (accessed on 15 September 2022).
- Bush, L. FDA Lowers Barriers to Process Improvement. PharmaTech 2005, 29. [Google Scholar]
- Alkunsol, W.H.; Sharabati, A.-A.A.; AlSalhi, N.A.; El-Tamimi, H.S. Lean Six Sigma effect on Jordanian pharmaceutical industry’s performance. Int. J. Lean Six Sigma 2019, 10, 23–43. [Google Scholar] [CrossRef]
- Zhong, H.; Chan, G.; Hu, Y.; Hu, H.; Ouyang, D. A Comprehensive Map of FDA-Approved Pharmaceutical Products. Pharmaceutics 2018, 10, 63. [Google Scholar] [CrossRef]
- Drozda, K.; Pacanowski, M.A.; Grimstein, C.; Zineh, I. Pharmacogenetic Labeling of FDA-Approved Drugs: A regulatory Retrospective. JACC Basic Transl. Sci. 2018, 3, 545–549. [Google Scholar] [CrossRef]
- FDA. CDRH Understanding Barriers to Medical Device Quality; FDA: Silver Spring, MD, USA, 2010.
- Speer, J. FDA Case for Quality Program: What, Why and How? Available online: https://www.greenlight.guru/blog/fda-case-for-quality-program-what-why-and-how (accessed on 13 August 2021).
- FDA. CDRH Case for Quality; FDA: Silver Spring, MD, USA, 2020.
- Singh, M.; Rathi, R. Investigation and modeling of lean six sigma barriers in small and medium-sized industries using hybrid ISM-SEM approach. Int. J. Lean Six Sigma 2021, 12, 1115–1145. [Google Scholar] [CrossRef]
- Antony, J.; Sony, M.; McDermott, O.; Swarnakar, V.; Galli, B.; Doulatabadi, M.; Kaul, R. An empirical study into the reasons for failure of sustaining operational excellence initiatives in organisations. TQM J. 2022. ahead-of-print. [Google Scholar] [CrossRef]
- Albliwi, S.; Antony, J.; Lim, S.A.H.; Van Der Wiele, T. Critical failure factors of Lean Six Sigma: A systematic literature review. Int. J. Qual. Reliab. Manag. 2014, 31, 1012–1030. [Google Scholar] [CrossRef]
- Antony, J.; Krishan, N.; Cullen, D.; Kumar, M. Lean Six Sigma for higher education institutions (HEIs): Challenges, barriers, success factors, tools/techniques. Int. J. Product. Perform. Manag. 2012, 61, 940–948. [Google Scholar] [CrossRef]
- Singh, M.; Rathi, R.; Antony, J.; Garza-Reyes, J.A. A toolset for complex decision-making in analyse phase of Lean Six Sigma project: A case validation. Int. J. Lean Six Sigma 2022. ahead-of-print. [Google Scholar] [CrossRef]
- Boylan, B.; McDermott, O.; Kinahan, N. Manufacturing Control System Development for an In Vitro Diagnostic Product Platform. Processes 2021, 9, 975. [Google Scholar] [CrossRef]
- Duggan, J.; Cormican, K.; McDermott, O. Lean implementation: Analysis of individual-level factors in a biopharmaceutical organisation. Int. J. Lean Six Sigma 2022. ahead-of-print. [Google Scholar] [CrossRef]
- Brown, A.; Eatock, J.; Dixon, D.; Meenan, B.J.; Anderson, J. Quality and continuous improvement in medical device manufacturing. TQM J. 2008, 20, 541–555. [Google Scholar] [CrossRef]
- Iyede, R.; Fallon, E.F.; Donnellan, P. An exploration of the extent of Lean Six Sigma implementation in the West of Ireland. Int. J. Lean Six Sigma 2018, 9, 444–462. [Google Scholar] [CrossRef]
- Tracy, S.J. Qualitative Research Methods: Collecting Evidence, Crafting Analysis, Creating Impact; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Yin, R. Case Study Research Design and Methods, 5th ed.; SAGE: Thousand Oaks, CA, USA, 2016; Available online: https://cjpe.journalhosting.ucalgary.ca/cjpe/index.php/cjpe/article/view/257 (accessed on 15 September 2022).
- Eisenhardt, K.M.; Graebner, M.E. Theory Building from Cases: Opportunities and Challenges. Acad. Manag. J. 2007, 50, 25–32. [Google Scholar] [CrossRef]
- Dubé, L.; Paré, G. Rigor in Information Systems Positivist Case Research: Current Practices, Trends, and Recommendations. MIS Q. 2003, 27, 597–636. [Google Scholar] [CrossRef]
- Hadid, W. Lean service, business strategy and ABC and their impact on firm performance. Prod. Plan. Control 2019, 30, 1203–1217. [Google Scholar] [CrossRef]
- Durivage, M. The Role of The Contract Manufacturer Under the EU MDR & IVDR. Available online: https://www.meddeviceonline.com/doc/the-role-of-the-contract-manufacturer-under-the-eu-mdr-and-ivdr-0001 (accessed on 8 September 2022).
- Slattery, O.; Trubetskaya, A.; Moore, S.; McDermott, O. A Review of Lean Methodology Application and Its Integration in Medical Device New Product Introduction Processes. Processes 2022, 10, 2005. [Google Scholar] [CrossRef]
- Schmitt, S. How a Team Led by Medtronic Quality Experts Plans to Stand Up to A Monster Called CAPA—And Make It Cool. Available online: https://medtech.pharmaintelligence.informa.com/MT124933/How-A-Team-Led-By-Medtronic-Quality-Experts-Plans-To-Stand-Up-To-A-Monster-Called-CAPA--And-Make-It-Cool (accessed on 15 August 2021).
- ISO 13485—Medical Devices. Available online: https://www.iso.org/iso-13485-medical-devices.html (accessed on 30 June 2021).
- FDA. CDRH CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=820&showFR=1&subpartNode=21:8.0.1.1.12.3 (accessed on 16 September 2021).
- Sim, C.L.; Chuah, F.; Sin, K.Y.; Lim, Y.J. The moderating role of Lean Six Sigma practices on quality management practices and quality performance in medical device manufacturing industry. TQM J. 2022. ahead-of-print. [Google Scholar] [CrossRef]
- McCarthy, D.; McMorrow, D.; O’Dowd, N.P.; McCarthy, C.T.; Hinchy, E.P. A Model-Based Approach to Automated Validation and Generation of PLC Code for Manufacturing Equipment in Regulated Environments. Appl. Sci. 2022, 12, 7506. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).