Abstract
Saudi Arabia is one of the major producers of date (Phoenix dactylifera) fruit. Date fruit flesh is considered a healthy food due to the presence of natural antioxidants. Green and innovative supercritical fluid (SFE, 52.5 °C temperature, 27.50 MPa pressure, 5 mL CO2/min flow rate) and subcritical (SubCO2, 250 extraction cycles, 29 °C temperature, 6.8 MPa, 12 h, ethanol solvent) extraction techniques were used to produce flesh extracts from four Saudi date fruits (Sukari (SKFE), Ambara (AMFE), Majdool (MJFE) and Sagai (SGFE)), and extracts prepared using 6 h Soxhlet extraction at 70 °C for 16 h using n-hexane as solvent, were taken as control. SFE produced the highest (p < 0.05) extract yields, whereas the SubCO2 method recovered significantly higher (p < 0.05) amounts of phytochemicals. Total phenolics (186.37–447.31 mg GAE/100 g), total flavonoids (82.12–215.28 mg QE/100 g), total anthocyanins (0.41–1.34 mg/100 g), and total carotenoid (1.24–2.85 mg BCE/100 g) were quantified in all the flesh extracts. The biological properties evaluation showed that flesh extracts had high antioxidant (17.79–45.08 µg AAE/mL), antiradical (191.36–34.66 µg/mL DPPH IC50), ferric-reducing (2.18–5.01 mmol TE/100 g) and ABTS-scavenging (444.75–883.96 µmol TE/100 g) activities. SubCO2 was the best technique and Majdool the best date variety, in terms of both phytochemicals and biological properties.
1. Introduction
For a long time, the date palm (Phoenix dactylifera L.) has been recognized as one of the most valuable fruiting plants in the Arabian Peninsula [1,2]. Saudi Arabia ranks among the top date fruit producers with continuously increasing exports [3]. Date fruits are excellent dietary sources of energy and fibre and are sometimes considered one of the staple foods of the region. Different date palm products, such as fruits, pollens, leaves, seeds and trunks, are considered to have medicinal properties [4]. The date fruit can be defined as a fleshy pericarp containing one seed [5]. The date fruit is rich in carbohydrates (mainly sucrose, glucose and fructose), vitamins (especially vitamin B complex), fiber, minerals, proteins and many other natural products and phytochemicals, including phenolics, carotenoids, phytosterols and flavonoids. These can be used in the promotion and protection of human health due to their antioxidant and anti-inflammatory properties [6]. In general, date fruits are a good source of minerals and amino acids, and they have been used for the treatment of chronic illnesses and diseases [7]. It has been reported that the consumption of date fruit is associated with a reduction of high blood pressure and oxidative stress. Furthermore, date fruits also aid in the treatment of health problems such as diabetes, cancer and atherosclerosis [8]. The presence of phytochemicals such as anthocyanins, flavonoids, tannins, phenolic compounds and water-soluble vitamins adds additional health benefits to these fruits, many of which still need to be explored [6,9].
The nutraceutical industry is one of the important future industries which may target the recovery, isolation and further utilization of various health-promoting components from various types of fruits, vegetables and agri-products. The extraction of phytochemicals from plant materials is an important area of research, as their utilization and biological/functional properties depend on extraction yield and quality of the extract and selectivity of the method [10]. The generally used solvent-based methods are nonselective, although in many cases they have high gravimetric yields [11]. In general, studies investigating date fruit as a source of nutrients and phytochemicals have involved the application of conventionally used organic solvents such as methanol [5,9]. There is a scarcity of reports involving the use of innovative and green techniques to study functional compounds and their properties in date fruit flesh. Supercritical fluid extraction (SFE) using supercritical CO2 is a green and innovative method for the recovery of natural antioxidants for food and pharmaceutical uses, as regulatory requirements discourage use of toxic organic solvents [12]. CO2, being environment friendly, safe, nontoxic, noncarcinogenic, nonflammable and cheap, is often used as a supercritical fluid, although the initial costs of SFE setup are quite high. CO2 can be used for a wide range of chemical and biochemical extraction processes [11]. Application of subcritical CO2 in subcritical CO2 (SubCO2) extraction is also increasing, as the apparatus used in this method is generally identical to the Soxhlet method and may be less costly in comparison to SFE systems [13,14]. In both states, the selectivity of CO2 during extraction processes can controlled or adjusted through temperature, cosolvent and pressure to obtain extract fractions, particularly rich in specific phytochemicals [11,12,13,14,15]. These two techniques are also generally referred to as green methods as they work at lower temperatures and the extracts obtained are considered safe for food applications [11,13,14].
The objectives of the present study included the application of conventional (Soxhlet) and green/innovative (SFE and SubCO2) extraction methods for the recovery of different phytochemicals, such as total phenolic compounds, total flavonoids, total anthocyanins and total carotenoids from fruit samples of four (Sukari date flesh, Ambara date flesh, Majdool date flesh and Sagai date flesh) different cultivars of date grown in Saudi Arabia. The SFE process was optimized using response surface methodology and regression analysis techniques. The biological activities of these flesh extracts were also studied using different in vitro antioxidant activity assays.
2. Materials and Methods
2.1. Date Flesh Sample Preparation
Sukari, Ambara, Majdool and Sagai date fruit (Figure 1), cultivated in Saudi Arabia and harvested during date season, were obtained from a date fruit market in Riyadh, Saudi Arabia during 2020. Date flesh was manually removed from the fruit and dried under vacuum at 50 °C. The dried flesh was made into a powder form using a blender (Panasonic, Shah Alam, Malaysia). Analytical grade chemicals from Sigma-Aldrich (St. Louis, MO, USA) were used in this work.

Figure 1.
Date Fruits from Four Date Cultivars grown in Saudi Arabia.
2.2. Moisture Content Determination
A halogen moisture analyzer-HB43 (Mettler-Toledo GmbH, Giessen, Germany) was used for the determination of moisture in date flesh samples following a Ca 2d- 25 AOAC official method [16]. The instrument was fixed at 105 °C, followed by sample evaluation. Date flesh sample (5 g) was placed on an aluminum plate in the sample handler for 5 min, followed by increase of its temperature with the help of halogen light. The samples’ moisture percentage was stored in an automatic recorder.
2.3. Date Flesh Antioxidants’ Extraction
2.3.1. Conventional Extraction
The conventional method employed to extract date flesh (25 g grounded sample) bioactive compounds (25 g) was carried out in Whatman® cellulose extraction thimbles (28 × 100 mm) and using n-hexane (250 mL) as solvent in a Soxhlet system. The extraction was carried out for 10–12 h at 70 °C. The dried extract was obtained by evaporating the n-hexane (containing extract) at 50 °C using a rotary evaporator (Eyela, Tokyo Rikakikai Co., Tokyo, Japan). The extracts were stored in airtight, light-proof containers at −20 °C. The calculation of the extract yield was based on following equation:
2.3.2. Supercritical Fluid CO2 Extraction (SFE)
The SFE unit (Jasco Corporation, Tokyo, Japan) used in this study consisted of two pumps: a CO2 pressurizing pump and cosolvent recovery pump. The cosolvent used was 30% ethanol and the CO2 cylinder pump was also equipped with a cooling system, whereas the pump head was cooled using a mixture (50:50) of ethylene glycol and deionized water. The process variables included extraction temperature (X1) (35–70 °C) CO2 pressure (X2) (15–40 MPa), and CO2 flow rate (X3) (0.5–5 mL/min). The extraction trials were based on a central composite rotatable experimental design [11,13,17]. A total of at least 18 trials were carried out for optimization of the extraction. In each run, a 20 g sample was placed in the extraction vessel and CO2 was pressurized into the vessel to carry out bioactive compounds’ extraction at different combinations of process variables. The extracts were collected at backpressure regulator in a Schott bottle followed by vacuum drying. The percentage yields of the extracts were estimated according to Equation (1). Extract samples thus prepared were packaged in light-proof bottles, sealed to prevent air penetration, and stored at −20 °C. In order to optimize the SFE extraction process for the extract yield, a predictive model was designed as follows:
The response variable Y denoted the extract yield. b0 is the regression coefficient for the offset term. The regression coefficients were also denoted using b2 and b3 (linear terms); b11, b22 and b33 (quadratic terms) and b12, b13 and b23 (interaction terms). The process variables of SFE temperature, pressure and CO2 flow rate were represented by X1, X2 and X3 in the above model, respectively.
2.3.3. Subcritical CO2 Extraction (SubCO2)
The extraction of bioactive compounds from date flesh was also carried out using SubCO2 methods, as reported earlier [14,18,19]. The extraction process was accomplished by treating 150 g powdered date flesh with 75 mL 95% absolute alcohol (cosolvent) followed by placement of the sample in the extraction vessels. The extraction cycles (250 cycles), pressure (6.8 MPa) and temperatures at heating (29 °C) and cooling controls were set and achieved, followed by the flow of the liquid CO2 (6 kg) into the extraction vessel. The extract and remaining raw sample after extraction were recovered through system depressurization; the extract was dried under reduced pressure and stored at −20 °C. The extract yield was calculated according to Equation (1).
2.4. Date Flesh Bioactive Compounds
2.4.1. Analysis of Total Phenolic Contents (TPC)
The TPC determination was based on the Folin–Ciocalteu (FC) method [20]. A 10 mg vacuum-dried extract, obtained from three different techniques, was dissolved in 100 mL methanol. A sample of 200 µL from the extract in methanol, or a standard solution (gallic acid in methanol) having different concentrations, was added to 400 μL of FC reagent, followed by the addition of deionized water to make a total volume in test tube as 4.6 mL. One mL of 10% sodium carbonate was mixed with the contents of test tubes after 10 min, followed by further room temperature incubation for 2 h. The absorbance values were recorded at 765 nm using a spectrophotometer (Shimadzu, Kyoto, Japan). TPC results were expressed as mg gallic acid equivalent (GAE) per 100 g of date flesh.
2.4.2. Analysis of Total Flavonoids Contents (TFC)
The TFC were evaluated using a colorimetric method [21]. A sample of 1 mL of extract (prepared in TPC method) was taken in a test tube sodium nitrite (5%, 0.3 mL), and aluminum chloride (10%, 0.3 mL) solutions were added. The contents of reaction mixture were kept at room temperature for 5 min, after which 2 mL of sodium hydroxide (1 M) was added and the volume of the contents increased to 10 mL using deionized water. The absorbance values were recorded at 510 nm, and quercetin was used was used as a standard compound. The results for TFC were presented as mg of quercetin equivalent (QE)/100 g of date flesh.
2.4.3. Analysis of Total Anthocyanins
The evaluation of total anthocyanin [22] in date flesh extract samples was carried out by taking 1 mL of extract sample (from TPC method) and mixing it with 10 mL of 5% ethanol. The samples were then centrifuged at 1800× g for 5 min and 200 μL of the supernatant was mixed with 3.8 mL of HCl (1 M). The contents were then incubated for 3 h at room temperature. The absorbance (A) values were recorded at 520 nm using HCl as a blank. The standard used was malvidin-3-glucoside, which was dissolved in methanol and prepared following the same procedure as that used for the extract sample. Absorbance values (B) of standard solution were recorded. The total anthocyanins were presented as mg/100 g of date flesh and initially calculated according to the following formula:
2.4.4. Analysis of Total Carotenoids
A previously described method [23], in which β-carotene was used as a standard, was used for the determination of total carotenoids in date flesh extracts. The dried extract was mixed in 500 µL of 5% NaCl by mixing at vortex, followed by centrifugation at 3000× g for 10 min. n-hexane was used to dilute the samples (supernatants) before the measurement of absorbance values at 460 nm. Different concentrations of the standard were used to obtain a standard curve. The total carotenoids were quantified from the standard curve and sample absorbance values as mg of β-carotene equivalent (BCE) per 100 g of date flesh.
2.5. Biological Properties of Date Flesh Extracts
2.5.1. Phosphomolybdenum Complex Method for the Antioxidant Activity
A previously described phosphomolybdenum complex (PC) method [24] was used for the estimation of the antioxidant activity of date flesh extracts obtained using different techniques. A 100 µg sample of date flesh extract from each technique was solubilized in 1 mL of methanol. From this solution, a 400 µL sample of this solution was mixed with 4 mL of PC solution that contained 0.6 M sulphuric acid, 2 mM sodium phosphate and 4 mM ammonium molybdate. The blank consisted of 4 mL PC solution and of reagent solution and 400 µL of methanol. Blank and sample tubes were capped and placed in water bath to incubate at 95 °C for 1 h. Followed by cooling under tap water, samples were measured for absorbance at 695 nm. The ascorbic acid calibration curve was prepared following the above procedure for comparison and measurement of antioxidant activity.
2.5.2. Free Radical Scavenging Activity
The radical scavenging activity of date flesh extracts was measured using 1-diphenyl-2-picrylhydrazyl (DPPH) method [25]. Briefly, 1 mL sample (1 mg of extract of sample was dissolved in 100 mL methanol) was made to react with 2 mL of DPPH solution (prepared mixing 1 mg of DPPH reagent in 100 mL of methanol). After 5 min incubation at room temperature, the absorbance values of reaction mixture were estimated at 517 nm. The radical scavenging activities were expressed as IC50 values, where a lower value reflected higher ability of the extract to scavenge DPPH free radicals.
2.5.3. Ferric Reducing Antioxidant Power (FRAP)
A method described by Benzie and Szeto [26] was used for the evaluation of FRAP of date flesh extracts, after some modifications. The FRAP reagent used in this assay consisted of acetate buffer (300 mM and pH 3.6); ferric chloride hexahydrate (20 mM in water) and TPTZ (10 mM in 40 mM HCl). Date flesh extract was dissolved in 3 mL of FRAP solution, making the concentration of the extract 0.02–1 mg/mL. Once incubated at room temperature for 30 min, the absorbance values of the reaction mixture were recorded at 593 nm. The trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) was employed as a standard for calibration curve preparation. The FRAP activity was expressed as millimole trolox equivalent (mmolTE)/100 g.
2.5.4. Cation Radical Scavenging Assay
A 2, 2- azinobis, 3- ethylbenzothiazoline, 6- sulfonic acid diammonium salt (ABTS) was used to measure the cation radical scavenging activity of date flesh extracts. A previously explained method [27] was used for this assay, in which ABTS (7 mM) was reacted with potassium persulfate (2.45 mM) under dark conditions for 12–16 h at ambient temperatures. Diluted mixture (ethanol for dilution) was evaluated for absorbance using a spectrophotometer and a value of 0.700 ± 0.01 at 734 nm was achieved for the mixture. Samples from extracts (10–100 µg/mL ethanol) were mixed with 3 mL of ABTS reagent and incubated at 23 °C for 6 min, after which the absorbance values were recorded. Trolox was used for constructing the calibration curve and the results were expressed as μmol TE/100 g.
2.6. Statistical Analyses
The analytical and extraction methods were properly replicated (at least triplicates) and the data were expressed as means ± SD. Regression analysis and analysis of variance (ANOVA) methods were applied to the obtained data using a statistical analysis system (SAS, version 9.4) software and the significance was defined as p < 0.05.
3. Results and Discussion
3.1. Date Flesh Extraction Using Supercritical Fluid Technique, Optimization and Modelling of the Method
Date flesh samples from four different date fruit verities, namely Sukari, Ambara, Majdool and Sagai (as shown in Figure 1), were estimated for moisture contents. These were found to be 21.71 ± 0.97, 28.35 ± 1.29, 28.42 ± 1.02 and 26.93 ± 1.10%, respectively. Considering the possibility that SFE, using CO2 as supercritical fluid, could yield extracts of superior quality with higher bioactive contents from date fruit flesh in comparison to other conventional methods [11], an experiment involving 18 different sets of process variables (temperature (X1), pressure (X2) and CO2 flow rate (X3)) was designed (Table 1). The extract yield was selected as response variable (Y). The extracts from flesh of Sukari, Ambara, Majdool and Sagai dates were denoted as SKFE, AMFE, MJFE and SGFE, respectively. A similar approach was previously followed and reported on using date seed from the same varieties [18].

Table 1.
Date flesh extraction experimental design for supercritical fluid extraction (SFE).
SFE process from different date varieties were invariably affected by the selected process parameters in terms of the total extract yield. In the case of SKFE, the regression analyses of the collected data revealed that all SFE parameters had significant effects (p < 0.05) on the yield of SKFE and the total model was mainly affected by linear and quadratic terms. The total model was highly significant (p = 0.001), and a full model can be presented using Equation (4). The R2 value for this model was 0.9291.
Preparation of AMFE using SFE method was significantly (p < 0.05) affected by the temperature of the extraction, whereas pressure and fluid flow rate seemed to be nonsignificant. The total model was significant (p < 0.05); however, only quadratic terms showed significant effects. A full model for AMFE can be presented using Equation (5) with R2 value of 0.9458.
MJFE was prepared from Majdool fruit flesh using the designed experiment. Temperature and CO2 flow rate were significant (p < 0.05) process variables. The linear and quadratic interaction during regression analysis revealed significant impact. The total model (R2 value: 0.9419), as presented using Equation (6), was also significant (p < 0.05).
The SFE model (Equation (7)) for extract yield (SGFE) can be presented using the following equation (R2 value: 0.9350), and it was observed that, like the majority of other date flesh extract data, the linear and quadratic terms demonstrated a significant effect on the response variable, i.e., the yield of the extract. Temperature seemed to be the most significant (p = 0.0002) process variable during SFE for SGFE.
In general, temperature and pressure were the most important process variables during SFE using CO2, in addition to various other factors (time, cosolvent, CO2 flow rate, etc.) that could have affected the extraction of natural antioxidants from different types of plant material [14,18,25]. During the extraction of carotenoids and other bioactive compounds, temperature and pressure were observed to be the main variables affecting the extract yield [28]. The relationship of SFE pressure and temperature for the extract yields from flesh of four different date fruits can be presented by response surface plots (Figure 2) obtained using regression analysis data and according to the models presented in Equations (4)–(7). As can be observed from data in Table 1 and Figure 2, the yields of extracts first increased with increasing temperature at fixed pressure and the maximal yields were obtained at an SFE temperature of 52.50 °C and pressure of 27.50 MPs. Regarding CO2 flow rate, the highest extract yields were obtained when the flow rate was kept at 5 mL/min and this was the highest rate used among all 18 trials. We observed that higher temperatures (60–70 °C) did not seem to have positive effects on the extracts’ yields. The SFE extracts prepared during experimental run 18, demonstrating the maximal extract yields for all four types of date fruit flesh, were considered optimal in the current study. The high extract yields in run 18 indicate that the levels of process variables chosen in this run and their combinations proved the best to maximize extract yields from date flesh. However, as the process was optimized only for the global, it may be important that bioactive compound (phenolics, flavonoids, carotenoids etc.) yields be considered a response variable in place of extract yield.
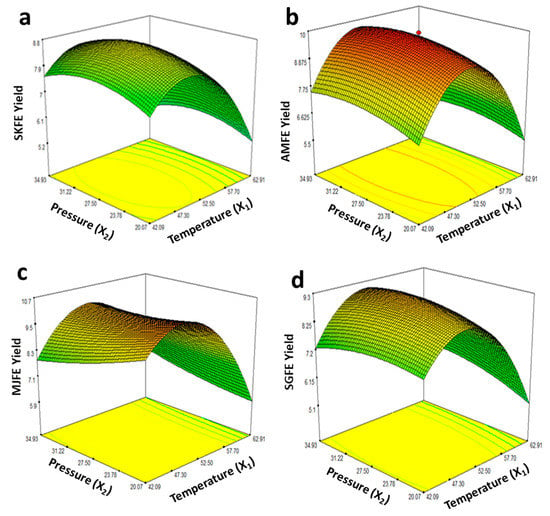
Figure 2.
Response surface graphs showing relationship of supercritical fluid (CO2) extraction (SFE) temperature and pressure (process variables) with extract yields (response variable) from Sukari (SKFE, (a)), Ambara (AMFE, (b)), Majdool (MJFE, (c)) and Sagai (SGFE (d)) flesh extracts.
Global extraction yield is an important response variable when it comes to optimization of an extraction process, as it generally directly relates with the yield of individual phytochemicals and natural antioxidants from plant matrices [29].
3.2. Compairson of Different Techniques for the Extraction Yields from Date Flesh
SFE is believed to yield extracts with greater purity and higher biological activity [28]. However, other techniques, such as subcritical CO2, can also be recommended [18]. The optimized extraction yields, as obtained during run 18 in Table 1, were compared with those obtained from two other techniques i.e., Soxhlet and subcritical CO2 (SubCO2), as optimized in other studies [18,19]. The results for extraction yields from three different techniques employed in the current study are presented in Table 2.

Table 2.
Date flesh yield (%) obtained using Soxhlet, subcritical CO2 (SubCO2) and supercritical fluid-CO2 (SFE) extraction methods.
It was observed that the extract yield obtained using SFE was significantly (p < 0.05) higher than the other two methods in each type of date flesh. Moreover, the extract yields of the SubCO2 method were significantly (p < 0.05) higher than those using Soxhlet method. In addition to the effects of extraction technique used, significant differences in extract yields were observed within date fruit cultivars within the same technique owing to the possibility of differences in fruit matrices and compositional variations. The beneficial effects of SubCO2 and SFE in the recovery of extracts from all the varieties were quite evident. In the present study, SubCO2 method, an advanced technique based on Soxhlet method using liquid CO2 and pure ethanol as cosolvent, significantly improved the solvating properties of CO2 at 29 °C [15,30]. The Soxhlet method, using n-hexane along with higher temperatures (70 °C), may be considered environmentally toxic and energy intensive [31]. In the present study, the high temperature method seemed to be less beneficial (0.44–2.17% yield) for extraction from date flesh, whereas it showed higher extraction yields (93.44–5.66%) from date seed in a previous study [18], proving the significance of plant matrix during extraction. Majdool flesh showed the lowest extract yields in comparison to the other three date flesh types during the Soxhlet and SubCO2 methods. However, Majdool extract yield was the highest when obtained using SFE, proving the importance of selection of the appropriate optimized method for each type of plant material. A general concept that higher temperatures positively affect extraction yields [22,32] was also contradicted here. We inferred that solubility properties of CO2, both in subcritical (2.75–4.19% yield) and supercritical (10.23–11.86% yield) states, showed profound improvements in date flesh extract recovery at lower temperatures in comparison to the Soxhlet method. Extraction pressure (SubCO2 and SFE systems) also showed positive effects on extraction yields. The application of high pressure during extraction of phytochemicals may cause cell disruptions and structural modification in the plant matrix, increasing the density of solvent and improve the CO2 solubility strength [11,17,18]. Other plant matrices, such as feijoa leaf [33] and date seed [18], also showed significant improvements in extract yields due to pressure and the use of CO2 as solvent, as compared to low pressure and organic solvent high temperature methods (Soxhlet). SubCO2 was previously used as pretreatment for enhanced recovery of sugars from date fruit flesh. It is expected to enhance the recovery of bioactive compounds from date flesh, despite the fact that the global extract yield from date flesh is considerably lower than SFE [30]. It was clear that the extraction yield of date flesh using the Soxhlet system was very low compared to green extraction methods (SFE, SubCO2). Furthermore, it makes use of toxic organic solvents, such as n-hexane in the current study, whereas ethanol was used as cosolvent or modifier in SFE and SubCO2 systems. Organic solvents (30% ethanol in SFE and 99.9% ethanol in SubCO2) were used as modifiers/cosolvents in current study. A modifier or cosolvent is used to improve the solubility power of CO2 for extraction of phytochemicals [13]. Ethanol is generally considered a nontoxic, food pharmaceutical-grade and environment friendly alcohol [11,32].
3.3. Phytochemicals and Their Contents in Date Flesh Extracts
Date flesh is the edible part of date fruit and contains sugars, essential and nonessential amino acids, dietary fiber, vitamins, minerals and phytochemicals, including phenolic antioxidants, tannin-based pigments, carotenoids and epicatechin oligomers [5,6]. In the current study, date flesh extracts were prepared using different techniques from four different date verities. The extracts were evaluated for total phenolic compounds (TPC), total flavonoids (TFC), total anthocyanins (TAC) and total carotenoids contents (TCC). The results for these quantitative analyses are presented in Table 3.

Table 3.
Antioxidants and bioactive compounds of date fruit flesh extracts from four date varieties subjected to different extraction methods.
3.3.1. Total Phenolics and Total Flavonoids
Among phytochemicals, phenolic compounds are dominant. They are considered non-nutrients, but are very important due to the health benefits associated with their consumption [34]. TPC of date flesh extracts obtained using three different techniques are presented in Table 1. As can be observed, date flesh is a good source of these natural antioxidants, and extraction methods significantly (p < 0.05) affected their contents in the extracts from the flesh of Saudi date fruit. TPC ranged between 186.37–447.32 mgGAE/100 g among 12 extract samples, where SubCO2 extracts showed higher contents than in extracts prepared using other methods. Significant (p < 0.05) differences in TPC of fruit from different varieties were also observed. Majdool date flesh seemed to be particularly rich in these compounds. Extraction methods seemed to considerably improve the recovery of these compounds from date flesh. SKFE, when prepared using the Soxhlet method, contained 198.24 mgGAE/100 g of TPC, which increased to 426.14 mgGAE/100 g when the SubCO2 method was applied for their recovery from date flesh matrix.
The second most abundant group of phytochemicals detected in date flesh extract was TFC. Similar to phenolics, flavonoids are considered as polyphenols with valuable biological properties that are beneficial for human health [35]. Significant (p < 0.05) difference were observed in TFC of date flesh extracts depending on the variety of fruit and the extraction methods used. Majdool seemed to be the richest source, and SubCO2 the best extraction method, in the current study. The minimum (85.83 mgQE/100 g) TFC were observed in SGFE, whereas those of MJFE were the highest (215.28 mgQE/100 g), among the studied samples.
Considering the TPC and TFC of the date flesh extracts, it could be inferred that the SubCO2 technique could be a good process for maximizing their recovery. Other modified processes, such as subcritical water extraction (SCWE), could also be used [36]. Application of SCWE was previously reported for the recovery of phenolic compounds from microwave pretreated date fruit pulp (MW-FP), which significantly improved their recovery in comparison to conventional methods [37]. Both SubCO2 and SFE techniques were found to be more effective for the recovery of phenolic and flavonoids compounds using less energy-extensive methods than Soxhlet. The process used lower extraction temperatures and can be considered a green method for obtaining phytochemical-rich extracts from date flesh [38,39,40].
3.3.2. Total Anthocyanins and Total Carotenoids
The total anthocyanin (TAC) and total carotenoids contents (TCC) in date fruit flesh extracts from three different extraction methods are shown in Table 3. The detected TAC in date fruit flesh were in the range of 0.41–1.38 mg/100 g. The recovery of TAC was significantly (p < 0.05) higher when SubCO2 and SFE techniques were used for their recovery. The varietal differences among TAC were also evident, with Ambara date flesh showing higher contents of anthocyanins. Among TCC results, the SubCO2 AMFE showed the highest contents (2.85 mgBCE/100 g), whereas the lowest TCC (1.24 mgBCE/100 g) was detected in Soxhlet MJFE. Similar to the TAC, TCC of SubCO2 and SFE extracts were significantly higher than those prepared using Soxhlet. Both anthocyanins and carotenoids are important phytochemicals with various reported health benefits. Anthocyanins, pigmented natural antioxidants, have been studied for their antioxidative, anti-inflammatory and cancer-preventive properties using different in vivo and in vitro methods [41,42]. Similar to anthocyanins, carotenoids are also colored compounds or natural pigments with reported health benefits [43]. The TAC and TCC reported in the present study were in accordance with other reports. Fard, Khasab, and Khalas date varieties were observed to contain 0.24−1.52 mg/100 g and 1.31−3.03 mg/100 g of TAC and TCC, respectively [44]. Babova et al. [45] used both supercritical and subcritical CO2 for the extraction of anthocyanins and other polyphenolic antioxidants from bilberry fruit. A combined process (SFE and SubCO2) was used to improve the selectivity and recovery of phytochemicals from bilberry. This approach resulted in improved extraction selectivity for anthocyanins, cyanidin-3-O-glucoside, and cyanidin-3-O-arabinoside. Other phenolic antioxidants, including delphinidin-3-O-glucoside, ellagic acid pentoside, feruloyl hexoside and several quercetin glycosides, were also selectively extracted.
3.4. Biological Activities of Date Flesh Extracts
The human body undergoes various degenerative phenomena as a result of free radicals. These free radicals are responsible for different deleterious oxidation reactions. Phytochemicals, such as those detected in date fruit flesh extracts, as well as certain vitamins, enzymes and amino acids, help in the eradication of such reactive species or help increase the human body’s immunity [46]. The research on in vitro estimation of the ability of different phytochemical and natural extracts to fight against oxidative processes is important. It was observed that extracts from the flesh of date fruit contained large amounts of phytochemicals, hence, their biological activities were determined using different in vitro assays, including phosphomolybdenum complex method (antioxidant), free radical scavenging activity (DPPH), ferric-reducing antioxidant power (FRAP), and ABTS cation radical-scavenging (ABTS). The results are summarized in Table 4. These assays are based on calorimetric principles and involve finding the abilities of phytochemicals to prevent the activities of the oxidation causing agents or reactive species such DPPH radicals, ferric and ABTS ions [46].

Table 4.
Biological properties and antioxidant potential of date flesh extracts obtained using different extraction techniques.
3.4.1. Antioxidant Activity Using Phosphomolybdenum Complex
The data presented in Table show that the antioxidant activity of date flesh extracts was significantly (p < 0.05) affected by the extraction technique used. The antioxidant activity ranged from 17.79 in Soxhlet-AMFE to 45.08 µgAAE/mL in SubCO2-MJFE. The SubCO2 method seemed to be the best for SKFE, MJFE and SGFE in terms of their antioxidant activity. The SFE method enhanced the antioxidant activities (35.56 µgAAE/mL) of AMFE in comparison to Soxhlet and SubCO2 methods, where it was 17.79 and 30.24 µgAAE/mL, respectively.
3.4.2. Scavenging Activity against DPPH Radicals
The ability of date flesh extracts to scavenge free DPPH radicals was expressed using IC50 values, i.e., the antioxidants’ quantity in the extract for 50% reduction in DPPH radicals. A lower IC50 value indicated that lesser amounts of antioxidants were needed in free radical scavenging, indicating their higher antiradical ability [18]. Both SubCO2 and SFE extracts had significantly higher ability to scavenge free radicals or lower IC50 values than Soxhlet extracts. The lowest IC50 value was in 34.66 µg/mL in SubCO2-MJFE followed by 40.18 µg/mL in SFE-MJFE. Under each extraction technique, MJFE showed better antiradical activity then other date flesh extracts obtained by the same method. Furthermore, the ability of date flesh extracts to scavenge DPPH radicals was superior than date seed extracts, as observed in a previous study [18], in which seed extracts had 109.69 µg/mL of IC50 value in SubCO2 extracts of Majdool seed and the highest IC50 value (353.83 µg/mL), or the weakest potential against DPPH free radicals, was observed in extracts of Sagai date seed. We may infer that both seed and flesh extracts of Majdool dates have good antioxidant and antiradical potential. MJFE also demonstrated the presence of higher amounts of phenolics and flavonoids (Table 3) and it is probable that these phytochemicals contributed to the higher antioxidant potential of these dates.
3.4.3. Reducing Ferric to Ferrous Ions
In the FRAP test, the antioxidants present in plant extracts can transfer an electron, which results in the reduction of ferric ions (Fe3+) to ferrous ions (Fe2+) [46]. The FRAP values of date flesh extracts (Table 4) ranged from 2.18 in Soxhlet-SGFE to 5.01 mmolTE/100 g in SubCO2-SKFE. The highest FRAP value (4.25 mmolTE/100 g) among SFE extracts was shown in the case of MJFE, whereas the FRAP potential of the same extract, when prepared using Soxhlet, was 2.93). Hence, significant (p < 0.05) improvements were observed when SFE and SubCO2 were used for extract preparation from date flesh. The FRAP values of flesh extracts of all other dates were also higher in SFE and SubCO2 extracts in comparison to their Soxhlet counterparts. The differences in FRAP values could be related to the date variety; however, extraction method seemed to play a major role. Similar results were obtained in previous studies where both extraction methods and plant variety seemed to affect the FRAP of the extracts [47,48].
3.4.4. Antiradical Activity against ABTS Cations
This test can be referred to as a mixed assay, as it involves the transfer of both electrons and a hydrogen atom from the main reagent, 2,2ʹ-Azinobis-(3-ethylbenzothiazoline-6-sulfonic acid (ABTS), during its reaction with antioxidants present in phytochemical-rich extracts [49,50]. The date flesh extracts prepared in the current study, using different extraction techniques and date varieties, showed substantial ABTS cations scavenging abilities. The SubCO2 and SFE extraction methods showed significance (p < 0.05) in the abilities of date flesh extracts to scavenge ABTS cations, when compared with Soxhlet extract. The lowest (444.75 µmolTE/100 g) activity was observed in Soxhlet-SGFE, whereas the highest (883.96 µmolTE/100 g) was observed in SubCO2 MJFE. MJFE showed higher ABTS cation scavenging activity in each extraction method, whereas SGFE showed the lowest values in each extraction category. SKFE also showed good ABTS potential among each extraction category. The use of innovative techniques for the extraction of phytochemicals can increase their recovery which, in turn, results in improvement of their biological potential. Bioactive compound quantities and antiradical activity against ABTS cations were reported to increase in date fruit extracts prepared using the subcritical water extraction method in another study [37].
The biological activities reported here have been previously studied for different fruit extracts. Generally, they are associated with the presence of polyphenolic antioxidants and other phytochemicals as is the case with bilberry fruit extracts prepared using SFE and SubCO2, where the antioxidant properties were closely associated with the presence of phytochemicals such as cyanidin-3-O-glucoside, delphinidin-3-O-glucoside, chlorogenic acid, caffeic acid derivatives, flavonoids, proanthocyanidins, ellagic acid and other phenolic acids [45]. These compounds have been reported to neutralize reactive oxygen species and inhibit their formation. Furthermore, these phytochemicals, and others (such as those reported here in SKFE, AMFE, MJFE and SGFE) may induce DPPH radical scavenging, ferric chelation and ABTS cation scavenging [51]. The phytochemicals present in fruit extracts are rich in phytochemicals which can impart anti-inflammatory effects, thereby preventing cell degeneration, offering treatment of diabetes, and suppressing cancer cell invasion [52,53,54]. In the current study, the SubCO2 extraction method seemed to be the best for extracting higher amounts of phytochemicals with better bioactivities. Majdool date, which is also known as Mejdool and Mahjool, seemed to have the highest contents of bioactive compounds, with significantly higher antioxidant effects than the remaining studied date varieties. Majdool is a globally-demanded date fruit due to its large size and soft fruit. Current studies have reported it as an excellent source of nutraceuticals [55]. The higher yields of phytochemicals and increased activities in extracts from date flesh using SFE and SubCO2 methods in comparison to Soxhlet (which may extract nonpolar compounds) might be a result of extraction of both polar and nonpolar compounds due to combined temperature and pressure effects (SFE and SubCO2 methods), as reported in other studies [56,57]. Further studies may be carried out for detailed analytical characterization of different bioactive compounds and any other toxic components, if present in the date flesh extracts, using chromatographic analytical techniques.
4. Conclusions
Date fruit is a good source of phytochemicals with valuable antioxidant properties, which may make it suitable for functional food and nutraceutical applications. Such applications could be made more effective and beneficial if these phytochemicals were recovered using safe and innovative extraction techniques. Date fruit flesh from four different date varieties was extracted in order to analyze their bioactive compounds using conventional and modern extraction processes—i.e., Soxhlet and supercritical/subcritical (SFE/SubCO2) techniques. The extraction yields from modern methods were found to be significantly higher than those from the conventional method. The SFE process was optimized using response surface optimization technique. It was observed that low temperature SFE (57.5 °C) and SubCO2 (29 °C) methods were more effective in the recovery of phenolics, flavonoids, anthocyanins and carotenoids from date flesh than high temperature (70 °C) Soxhlet methods, as the quantities of all these bioactive compounds were higher when pressure-assisted innovative/green methods were used. The functional qualitiesd of date flesh extracts were also assessed using in vitro procedures for biological/antioxidant properties, i.e., the phosphomolybdenum complex, DPPH, FRAP and ABTS methods. It was observed that date flesh extracts prepared using both SFE and SubCO2 methods were of significantly improved quality in terms of these properties. In addition to these beneficial effects of extraction methods, varietal influences on the qualitative and quantitative aspects of date flesh were also observed. Majdool date flesh extract was richer in bioactive compounds than Sukari, Ambara and Sagai date flesh extracts. As there is generally a correlation among bioactive compound contents and functional properties of the extracts, Majdool flesh extracts showed better antioxidant properties. Further studies based on utilization of these extracts in functional foods could be carried out. A more detailed analytical characterization of date flesh extracts using chromatographic procedures would also help in understanding the selectivity of the extraction techniques and the possible effects of individual compounds on the antioxidant properties of date flesh extracts.
Author Contributions
Conceptualization, methodology, resources, supervision, project administration, funding acquisition, writing-review and editing, K.G.; investigation, writing—original draft preparation, visualization, software, data curation, and validation, M.Z.I.S.; Data curation, resources, F.Y.A.-J.; formal analysis, E.E.B.; M.S.A.; A.K.A. and I.A.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, award number (15-AGR3527-02).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ahmed, I.A.; Ahmed, A.W.K.; Robinson, R.K. Chemical composition of date varieties as influenced by the stage repining. Food Chem. 1995, 54, 305–309. [Google Scholar] [CrossRef]
- Ghnimi, S.; Umer, S. Date fruit (Phoenix dactylifera L.): An underutilized food seeking industrial valorization. NFS J. 2017, 6, 1–10. [Google Scholar] [CrossRef]
- 25 February 2021/13, Rajab, 1442. Available online: https://saudigazette.com.sa (accessed on 1 October 2022).
- Abdullahi, M.H.; Garko, M. Medicinal value of date palm (Phoenix dactylifera L.). In Proceedings of the Agricultural Society of Nigeria Conference, Nsukka, Nigeria, 11–14 March 2012. [Google Scholar]
- Al-Farsi, M.A.; Lee, C.Y. Nutritional and functional properties of dates: A review. Crit. Rev. Food Sci. Nutr. 2008, 48, 877–887. [Google Scholar] [CrossRef]
- Al-Alawi, R.A.; Al-Mashiqri, J.H.; Al-Nadabi, J.S.M.; Al-Shihi, B.I.; Baqi, Y. Date palm tree (Phoenix dactylifera L.): Natural products and therapeutic options. Front. Plant Sci. 2017, 8, 845. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Khalid, N.; Khan, R.S.; Ahmed, H.; Ahmad, A. A review on chemistry and pharmacology of Ajwa date fruit and pit. Trends Food Sci. Tech. 2017, 63, 60–69. [Google Scholar] [CrossRef]
- Nasir, M.U.; Hussain, S.; Jabbar, S.; Rahid, F.; Khalid, N.; Mehmood, A. A review on the nutritional content, functional properties and medicinal potential of dates. Sci. Lett. 2014, 3, 17–22. [Google Scholar]
- Saafi, E.B.; El Arem, A.; Issaoui, M.; Hammami, M. Phenolic content and antioxidant activity of four date palm (Phoenix dactylifera L.) fruit varieties grown in Tunisia. Food Sci. Technol. 2009, 56, 2314–2319. [Google Scholar]
- Spigno, G.; Tramelli, L.; De-Faveri, D.M. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 2008, 81, 200–208. [Google Scholar] [CrossRef]
- Ghafoor, K.; Al-Juhaimi, F.Y.; Choi, Y.H. Supercritical fluid extraction of phenolic compounds and antioxidants from grape (Vitis labrusca B.) seeds. Plant Foods Hum. Nutr. 2012, 67, 407–414. [Google Scholar] [CrossRef]
- Babovic, N.; Djilas, S.; Jadranin, M.; Vajs, V.; Ivanovic, J.; Petrovic, S.; Zizovic, I. Supercritical carbon dioxide extraction of antioxidant fractions from selected Lamiaceae herbs and their antioxidant capacity. Innov. Food Sci. Emerg. Technol. 2010, 11, 98–107. [Google Scholar] [CrossRef]
- Ferdosh, S.; Sarker, M.Z.I.; Ab Rahman, N.N.N.; Akand, M.J.H.; Ghafoor, K.; Awang, M.B.; Ab Kadir, M.O. Supercritical carbon dioxide extraction of oil from Thunnus tonggol head by optimization of process parameters using response surface methodology. Kor. J. Chem. Eng. 2013, 30, 1466–1472. [Google Scholar] [CrossRef]
- Easmin, S.; Zaidul, I.S.M.; Ghafoor, K.; Sahena, F.; Juliana, M.J.; Jahurul, M.H.A.; AL-Juhaimi, F.Y.; Fauzi, M.B.; Alfi, K. Extraction of α-glucosidase inhibitory compounds from Phaleria macrocarpa fruit flesh using solvent, sonication, and subcritical carbon dioxide soxhlet methods. J. Food Biochem. 2017, 41, e12399. [Google Scholar] [CrossRef]
- Leitner, W. Green chemistry: Designed to dissolve. Nature 2000, 405, 129–130. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Method of Analysis, 5th ed.; Association of Official Agricultural Chemists: Washington, DC, USA, 1998. [Google Scholar]
- Ghafoor, K.; Park, J.; Choi, Y.H. Optimization of supercritical carbon dioxide extraction of bioactive compounds from grape peel (Vitis labrusca B.) by using response surface methodology. Innov. Food Sci. Emerg. Technol. 2010, 11, 485–490. [Google Scholar] [CrossRef]
- Ghafoor, K.; Sarker, M.Z.I.; Al-Juhaimi, F.Y.; Babiker, E.E.; Alkaltham, M.S.; Almubarak, A.K. Extraction and evaluation of bioactive compounds from date (Phoenix dactylifera) seed using supercritical and subcritical CO2 techniques. Foods 2022, 11, 1806. [Google Scholar] [CrossRef] [PubMed]
- Arumugham, T.; Rambabu, K.; Hasan, S.W.; Show, P.L.; Rinklebe, J.; Banat, F. Supercritical carbon dioxide extraction of plant phytochemicals for biological and environmental applications—A review. Chemosphere 2021, 271, 129525. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Biglari, F.; AlKarkhi, A.F.; Easa, A.M. Antioxidant activity and phenolic content of various date palm (Phoenix dactylifera) fruits from Iran. Food Chem. 2008, 107, 1636–1641. [Google Scholar] [CrossRef]
- Ghafoor, K.; Hui, T.; Choi, Y.H. Optimization of ultrasound-assisted extraction of total anthocyanins from grape peel. J. Food Biochem. 2011, 35, 735–746. [Google Scholar] [CrossRef]
- Ranjith, A.; Kumar, K.S.; Venugopalan, V.V.; Arumughan, C.; Sawhney, R.C.; Singh, V. Fatty acids, tocols, and carotenoids in pulp oil of three sea buckthorn species (Hippophae rhamnoides, H. salicifolia, and H. tibetana) grown in the Indian Himalayas. J. Am. Oil Chem. Soc. 2006, 83, 359–364. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Mbwambo, Z.H.; Chung, H.; Luyengi, L.; Gamez, E.J.; Mehta, R.G.; Pezzuto, J.M. Evaluation of the antioxidant potential of natural products. Comb. Chem. High Throughput Screen. 1998, 1, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Szeto, Y.T. Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay. J. Agric. Food Chem. 1999, 47, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Vafaei, N.; Rempel, C.B.; Scanlon, M.G.; Jones, P.J.H.; Eskin, M.N.A. Application of supercritical fluid extraction (SFE) of tocopherols and carotenoids (Hydrophobic Antioxidants) compared to non-SFE methods. Appl. Chem. 2022, 2, 68–92. [Google Scholar] [CrossRef]
- Kashaninejad, M.; Blanco, B.; Benito-Román, O.; Beltrán, S.; Mehdi Niknam, S.; Sanz, M.T. Maximizing the freeze-dried extract yield by considering the solvent retention index: Extraction kinetics and characterization of Moringa oleifera leaves extracts. Food Bioprod. Process. 2021, 130, 132–142. [Google Scholar] [CrossRef]
- Arumugham, T.; AlYammahi, J.; Rambabu, K.; Hassan, S.W.; Banat, F. Supercritical CO2 pretreatment of date fruit biomass for enhanced recovery of fruit sugars. Sustain. Energy Technol. Assess. 2022, 52, 102231. [Google Scholar] [CrossRef]
- Kumar, S.; Prasad, S.R.; Banerjee, R.; Agarwal, D.K.; Kulkarni, K.S.; Ramesh, K.V. Green solvents and technologies for oil extraction from oilseeds. Chem. Cent. J. 2017, 11, 9. [Google Scholar] [CrossRef]
- Ghafoor, K.; Choi, Y.H.; Jeon, J.Y.; Jo, I.H. Optimization of ultrasound-assisted extraction of phenolic compounds, antioxidants and anthocyanins from grape (Vitis vinifera) seeds. J. Agric. Food Chem. 2009, 57, 4988–4994. [Google Scholar] [CrossRef]
- Santos, P.H.; Kammers, J.C.; Silva, A.P.; Oliveira, J.V.; Hense, H. Antioxidant and antibacterial compounds from feijoa leaf extracts obtained by pressurized liquid extraction and supercritical fluid extraction. Food Chem. 2021, 344, 128620. [Google Scholar] [CrossRef]
- de la Rosa, L.A.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; Alvarez-Parrilla, E. Phenolic compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E.M., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 253–271. [Google Scholar]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Akram, M.; Al-Zuhair, S.; Elnajjar, E.; Munir, M.J. Subcritical water extraction of phenolics, antioxidants and dietary fibres from waste date pits. J. Environ. Chem. Eng. 2020, 8, 104490. [Google Scholar] [CrossRef]
- Rambabu, K.; AlYammahi, J.; Thanigaivelan, A.; Bharath, G.; Sivarajasekar, N.; Velu, S.; Banat, F. Sub-critical water extraction of reducing sugars and phenolic compounds from date palm fruit. Biomass Conv. Bioref. 2022. [Google Scholar] [CrossRef]
- Todd, R.; Baroutian, S. A techno-economic comparison of subcritical water, supercritical CO2 and organic solvent extraction of bioactives from grape marc. J Clean. Prod. 2017, 158, 349–358. [Google Scholar] [CrossRef]
- Mesquita, P.C.; Rodrigues, L.G.G.; Mazzutt, S.; da Silva, M.; Vitali, L.; Lanza, M. Intensified green-based extraction process as a circular economy approach to recover bioactive compounds from soursop seeds (Annona muricata L.). Food Chem. X 2021, 12, 100164. [Google Scholar] [CrossRef]
- Vinitha, U.G.; Sathasivam, R.; Muthuraman, M.S.; Park, S.U. Intensification of supercritical fluid in the extraction of flavonoids: A comprehensive review. Physiol. Mol. Plant Path. 2022, 118, 101815. [Google Scholar] [CrossRef]
- Mazza, G.; Kay, C.D. Bioactivity, absorption and metabolism of anthocyanins. In Recent Advances in Polyphenols Research; Daayf, F., Lattanzio, V., Eds.; Blackwell Publishing Ltd.: West Sussex, UK, 2008. [Google Scholar]
- Padayachee, A.; Netzel, G.; Netzel, M.; Day, L.; Zabaras, D.; Mikkelsen, D.; Gidley, M.J. Binding of polyphenols to plant cell wall analogues—Part 1: Anthocyanins. Food Chem. 2012, 134, 155–161. [Google Scholar] [CrossRef]
- Murillo, E.; Deli, J.; Nagy, V.; Molinar-Toribio, E.; Sandor, V.; Marton, K.; Agocs, A. Carotenoid profile of two capsorubin-rich tropical plants. J. Food Compos. Anal. 2021, 97, 103798. [Google Scholar] [CrossRef]
- Al-Farsi, M.; Alasalvar, C.; Morris, N.; Baron, M.; Shahidi, F. Comparison of antioxidant activity, anthocyanins, carotenoids, and phenolics of three native fresh and sun-dried date (Phoenix dactylifera L.) varieties grown in Oman. J. Agric. Food Chem. 2005, 53, 7592–7599. [Google Scholar] [CrossRef] [PubMed]
- Babova, O.; Occhipinti, A.; Capuzzo, A.; Maffei, M.E. Extraction of bilberry (Vaccinium myrtillus) antioxidants using supercritical/subcritical CO2 and ethanol as co-solvent. J. Supercrit. Fluids 2016, 107, 358–363. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Al Juhaimi, F.; Ozcan, M.M.; Adiamo, O.Q.; Alsawmahi, O.N.; Ghafoor, K.; Babiker, E.E. Effect of date varieties on physico-chemical properties, fatty acid composition, tocopherol contents, and phenolic compounds of some date seed and oils. J. Food Process. Preserv. 2018, 42, e13584. [Google Scholar] [CrossRef]
- Al Juhaimi, F.; Ghafoor, K.; Ozcan, M.M. Physical and chemical properties, antioxidant activity, total phenol and mineral profile of seeds of seven different date fruit (Phoenix dactylifera L.) varieties. Int. J. Food Sci. Nutr. 2012, 63, 84–89. [Google Scholar] [CrossRef]
- Hamzah, N.N.; Ferdosh, S.; Sarker, M.Z.I.; Ghafoor, K.; Yunus, K.; Chowdhury, A.J.K.; Bari, N.A.A. Biological activities and extraction technologies of Pheonix dactylifera: A review. Nat. Prod. J. 2019, 9, 3–13. [Google Scholar]
- Siddeeg, A.; AlKehayez, N.M.; Abu-Hiamed, H.A.; Al-Sanea, E.A.; AL-Farga, A.M. Mode of action and determination of antioxidant activity in the dietary sources: An overview. Saudi J. Biol. Sci. 2021, 28, 1633–1644. [Google Scholar] [CrossRef] [PubMed]
- Sak, K. Site-specific anticancer effects of dietary flavonoid quercetin. Nutr. Cancer 2014, 66, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.N.; Ryu, J.M.; Yun, S.P.; Jeon, J.H.; Park, S.S.; Oh, K.B.; Park, J.K.; Han, H.J. Delphinidin prevents hypoxia-induced mouse embryonic stem cell apoptosis through reduction of intracellular reactive oxygen species-mediated activation of JNK and NF-kappa B, and Akt inhibition. Apoptosis 2013, 18, 811–824. [Google Scholar] [CrossRef] [PubMed]
- Gharib, A.; Faezizadeh, Z.; Godarzee, M. Treatment of diabetes in the mouse model by delphinidin and cyanidin hydrochloride in free and liposomal forms. Planta Med. 2013, 79, 1599–1604. [Google Scholar] [CrossRef]
- Im, N.K.; Jang, W.J.; Jeong, C.H.; Jeong, G.S. Delphinidin suppresses PMA-induced MMP-9 expression by blocking the NF-kappa B activation through MAPK signaling pathways in MCF-7 human breast carcinoma cells. J. Med. Food 2014, 17, 855–861. [Google Scholar] [CrossRef]
- Sirisena, S.; Ng, K.; Ajlouni, S. The emerging Australian date palm industry: Date fruit nutritional and bioactive compounds and valuable processing by-products. Compr. Rev. Food Sci. Food Saf. 2015, 14, 813–823. [Google Scholar] [CrossRef]
- Wrona, O.; Rafińska, K.; Walczak-Skierska, J.; Możeński, C.; Buszewski, B. Extraction and determination of polar bioactive compounds from alfalfa (Medicago sativa L.) using supercritical techniques. Molecules 2019, 24, 4608. [Google Scholar] [CrossRef] [PubMed]
- Luong, D.; Sephton, M.A.; Watson, J.S. Subcritical water extraction of organic matter from sedimentary rocks. Anal. Chim. Acta 2015, 879, 48–57. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).