Abstract
An environmentally friendly, biobased film was prepared from cellulose and lignin extracted from oil palm dried long fiber (DLF). DLF crude cellulose was first extracted from this lignocellulosic biomass by an alkaline pretreatment process at an elevated temperature (5.75 wt% NaOH, 200 °C, and 1 h), before it was carboxymethylated to obtain carboxymethyl cellulose (CMC). CMC is highly soluble in water, whereas lignin was precipitated out of the filtrate of the alkaline pretreatment process by adding acid to pH 2 (50 wt% H2SO4). The lignin/CMC films were synthesized at varying lignin concentrations of 0.25%, 0.5%, and 1% (w/v) in ethanolic solution with 0.25% (v/v) of glycerol; a neat CMC film was also prepared as a control. These lignin/CMC films were evaluated and compared for their morphological, physical, chemical, and thermal characteristics. The films displayed a brownish physical appearance, which was attributed to the natural color of lignin. The successful incorporation of lignin in the films was confirmed with the characteristic spectral bands of lignin in the mid-IR range (4000–400 cm−1). When measured with UV-vis spectrometer, the lignin/CMC films showed more enhanced UV blocking properties than the neat CMC film. The CMC films incorporated with lignin also showed slightly improved hydrophobicity and thermal stability. However, due to the low compatibility of lignin with CMC, the distribution of the lignin/CMC film was observed to be inhomogeneous in SEM images. Nonetheless, the addition of lignin to CMC in synthesizing biobased films is promising, potentially providing better properties that can be useful as biodegradable material.
1. Introduction
It is just over a century since the production of plastics from fossil fuels. The use of plastic materials has rapidly risen in a broad range of industries, including packaging, manufacturing, building and construction, electronics, aerospace, and transportation, transforming the modern age to such an extreme that life without plastic is now unimaginable. Global plastic production increased by 13.9% between 2015 and 2020 to reach a staggering amount of 367 million metric tons [1]. To put this in context, this is roughly equivalent to the mass of two-thirds of the world’s population, and the production of plastics is expected to accelerate in the coming years and double by 2050. Today, single-use plastics account for 40% of the plastics annually produced [2]. Many of these products, such as plastic bags and packaging materials, have a lifespan of mere minutes to hours, yet they lead to serious environmental waste pollution when they are discarded. The major impact of plastic waste on the environment is that it takes a long time, up to years, before they decompose. Pressing concerns surrounding plastic waste has called for efforts to seek alternative sustainable and biodegradable materials. To tackle issues related to oil-based plastic pollution, a lot of attention has been drawn to the research and development of so-called ‘biopolymer’ with analogous characteristics, but at a lower cost. In this respect, films and coatings from alginate, chitosan, starch, cellulose derivatives, and gelatine have successfully been developed for applications, especially in the food packaging industry [3]. Among all, biopolymers derived from sugar, starch, and cellulose are appearing as promising substitutes to oil-based plastics, as these raw materials are abundant, viable, eco-friendly, non-toxic, biodegradable, and biocompatible [4].
In our study, biofilms are synthesized from carboxymethylcellulose (CMC) and lignin obtained from oil palm dried long fibers, an agro-industrial waste in the palm oil extraction mill process. The fibers are sourced in Malaysia, which produces great quantities of waste lignocellulosic biomass every year from oil palm cultivation [4]. Our work aims at recycling this waste into economically useful products. CMC is an anionic, water-soluble derivative of natural cellulose polymers abundant in oil palm dried long fiber, and is composed of derivatized glucose joined via β-(1, 4) glycosidic linkages. Typically, CMC is made by first dispersing cellulose in an alkali medium, followed by treatment with chloroacetic acid to substitute the hydroxyl groups of glucose at the C-2, C-3, or C-6 positions [5], of which substitution slightly dominates at the C-2 position [6]. The degree of substitution (DS) is considered a significant property of CMC, particularly for its solubility in water, and the highest theoretical DS of CMC is considered as 3. It is reported that commercially available CMC has a DS value of 0.4 to 1.5 [6]. In the past, CMC has been synthesized from a variety of natural cellulose sources, such as asparagus stalk ends [7], sago waste [8], durian rinds [9], cotton waste [10], corn husks [11], and grapefruit peels [12], to name a few. The biofilm created from CMC generally exhibits many worthwhile attributes, such as biodegradability, ease of production, and stability of the cross-linked matrix suitable for packaging purposes, compared to many other biopolymers [13]. Thus, CMC can be versatile and applied as thickener, binder, stabilizer, and dispersing agent in the food, paper, pharmaceutical, cosmetics, and textile industries [14]. Lignin, on the other hand, is the second most abundant biopolymer in nature, after cellulose [15]. It is a polyphenolic material, and one of the main components in the plant cell wall, which is made up of the three basic building blocks; i.e., p-coumaryl alcohol (H), coniferyl alcohol (G), and sinapyl alcohol (S) [16]. Over the years, research into lignin value-added products has shown potential in numerous applications due to its anti-UV, anti-oxidant, and anti-bacterial properties. In this study, lignin from oil palm waste, extracted via a simple pathway, was incorporated into the CMC films to demonstrate enhanced properties that would play a role as a lucrative substitute in the current plastic packaging industry.
The current study describes the synthesis pathway to obtain lignin/CMC films from oil palm waste; for the first time utilizing the waste lignocellulosic materials from bio-based Mono Ethylene Glycol pilot plants at Petronas Research Sdn Bhd, Selangor. This was conducted using the latest specific in-house pretreatment conditions, that have not been reported elsewhere, followed by the characterization of the films to compare their properties with common packaging polymers, such as polyethylene (PE), and other biodegradable polymers, such as polybutylene adipate terephthalate (PBAT) and polylactic acid (PLA). By varying the amount of lignin in the film with a neat CMC film as the control, the benefits of incorporating lignin into the films were analyzed and discussed. This experimental study is significant in the sense that the synthesis routes are modified and improved from standard practices in the literature, to obtain the optimum yields that are robust enough to accommodate quality variation on the DLF feedstock. Most importantly, this work made use of oil palm waste, found in abundance in Malaysia, which differentiated this study from others using different sources for lignin and cellulose components, as their properties, and subsequently, the biofilms’ properties, are heavily reliant on the origin of the biomass. Additionally, this work presented more exhaustive analytical findings than a similar previous study of lignin films synthesis from oil palm waste by Haqiqi et al. [17].
2. Materials and Methods
Subsequent alkaline/acid pretreatment for cellulose and lignin extraction
Oil palm dried long fiber (DLF), hydrothermally pre-processed to be free from dirt, was obtained from local mills at Panching, Kuantan in Malaysia (FGV Holdings Berhad). The DLF was ground and sieved to <1 mm. Subsequently, it was dried in an oven at 105 °C for >24 h to remove moisture content. It was then subjected to alkaline pre-treatment with diluted NaOH solution, with the in-house specific conditions developed for DLF pretreatment pilot plant at Petronas Research Sdn Bhd, modified from Law et al. [18]. Briefly, the DLF was treated with 5.75 wt% of NaOH solution in a reactor, at a ratio of 1:9 (w/w). The reaction was conducted at 200 °C for a duration of 60 min, with stirring at 500 rpm, after which the mixture was cooled down to room temperature. Next, the DLF pulp residue was filtered, washed several times with distilled water to a neutral pH, dried in an oven at 80 °C until a constant mass was obtained, and designated as crude cellulose. The purity of the crude cellulose obtained is presented in Section 3.1.
As for the filtrate, lignin extraction was carried out by acidifying the filtrate, generally known as the black liquor, following the steps described by Hidayat et al., with slight modification [19]. Briefly, the pH of the black liquor was lowered to pH 6 by adding 50 wt% H2SO4. At this pH, the black liquor started showing a change in color; from black to brown. This marked the first observation of lignin precipitation. After half an hour, another 50 wt% of H2SO4 was added to the brown liquor to bring the pH down to 2.0–2.5. The solution was stirred until homogeneous, using a magnetic stirrer at 1000 rpm for 20 min. It was then set aside overnight for precipitation of acid-insoluble lignin to take place. Like the crude cellulose, the lignin obtained was filtered, rinsed with distilled water, and dried in an oven before characterization and further processing into film. The two-stage acid precipitation implemented here allows formation of lignin of high purity, as the lignin particles are allowed to grow at pH 6 to a larger size before further precipitation at a lower pH, where the proportion of impurities is the highest [20].
Bleaching of crude cellulose
The obtained crude cellulose, which is represented by holocellulose (α-cellulose and hemicellulose), was bleached using the chlorination method, modified from Shui et al. [21]. Briefly, the crude cellulose, distilled water (DI), acetic acid, and hydrogen peroxide (H2O2) were added to a 250 mL Erlenmeyer flask at a mass ratio of 5:160:1:2. For example, in this study, 5 g of crude cellulose was mixed with 160 g DI water, 1 g acetic acid, and 2 g H2O2. The flask was sealed with aluminum foil and then heated in an oil bath at 70 °C for an hour, under stirring at 200 rpm. At every one-hour interval, a fresh batch of acetic acid and H2O2 mixture, at the same mass previously applied (1 g and 2 g, respectively), was added to the sample. It usually took 6 to 8 h of bleaching, after which the sample was left without further addition of acetic acid and H2O2 in the 70 °C oil bath overnight. The sample was later cooled down to room temperature before it was filtered and rinsed with acetone to completely remove the residual acetic acid in the solid residue. H2O2 was adopted in this study to replace sodium chlorite, commonly used in the acid chlorite method, due to the serious environmental and health hazards associated with sodium chlorite [22].
Preparation of α-cellulose
α-cellulose was obtained in a continuous process from the previous procedures, adopted from Rowell et al. [23]. An amount of 2 g of the dried bleached holocellulose previously obtained was placed in a 250 mL Erlenmeyer flask, treated with 10 mL of 17.5 wt% NaOH solution at room temperature, and stirred well at 200 rpm for 5 min. After that, another 5 mL of the 17.5 wt% was added to the mixture. This step was repeated at every 5 min interval (3–4 times) until all the holocellulose was soaked in NaOH, after which the mixture was set aside for half an hour. An amount of 33 mL distilled water was added to the mixture and left to stand for an hour. The mixture was filtered and the residue was washed with 100 mL of 8.3 wt% NaOH, and then rinsed with distilled water several times. Lastly, the residue was washed with 15 mL of 10 wt% acetic acid, and then rinsed with >250 mL distilled water until neutral pH was obtained (all the acetic acid was completely removed). The obtained residue was dried in an oven at 105 °C and the final dried specimen was the α-cellulose, which was used to synthesize carboxymethyl cellulose, CMC.
Synthesis of carboxymethyl cellulose (CMC)
CMC was produced by etherifying the hydroxyl groups of α-cellulose with chloroacetic acid in the presence of aqueous alkali, NaOH [22,24], summarized in the process flow chart, as follows (Figure 1):

Figure 1.
The process flow chart of CMC film synthesis.
- Alkalization: 1 g of α-cellulose was mixed with 1 g of NaOH (50 wt%) and 10 mL distilled water under constant stirring for 1.5 h.
- Etherification: The mixture from alkalization was added to a separately prepared mixture (which contained 1 g of 50 wt% NaOH, 1.17 g chloroacetic acid, and 35 mL ethanol (95–97 wt%)). The combined mixture was covered with aluminum foil and heated in an oil bath at 75 °C for 4 h, under stirring at 200 rpm.
- Neutralization and filtration: After cooling down to room temperature, the solution mixture was neutralized with 50 wt% hydrochloric acid and filtered, followed by being rinsed with ethanol at least 3 times.
- Drying: The residue was collected and dried in an oven at 105 °C for more than 4 h until a constant mass was obtained.
- CMC: The dried residue was collected as the CMC product, which was stored in a desiccator to reduce the amount of moisture when it was not used in the preparation of CMC film.
The average degree of substitution (DS) was determined by potentiometric titration, as described in ASTM D-1439 (2016) [25]. An amount of 2 g of CMC was mixed with 38 mL ethanol (95–97 wt%) and 2.5 mL 2 M nitric acid, under stirring conditions and heated to boiling for 20 min, to turn the water-soluble CMC into solid particles. The solid CMC was filtered and washed with ethanol, until all the acid had been removed (measured with pH probe), and dried. An amount of 1 g of the obtained CMC was then dissolved in 100 mL distilled water and 25 mL 0.5 M nitric acid, and boiled for 20 min. Prior to titration with 0.3 M hydrochloric acid (HCl), the mixture was allowed to cool to room temperature. A few drops of phenolphthalein were added to the mixture as an indicator of the end point; the first instance when the pink color turned colorless. The average DS was calculated using the following equation [16]:
where 162 is the molecular weight of the anhydrous glucose unit; 80 is the net increment in the anhydrous glucose unit for every substituted carboxymethyl group; is the total consumption volume of the acid 0.3 M HCl added; and is the mass of CMC.
Synthesis of lignin/CMC film
CMC film was synthesized by first dissolving 0.5 g of CMC in 25 mL of 50 vol% ethanolic solution, under stirring for approximately 2 h until no white powder was seen, after which 0.1 mL of glycerol was added to the solution and kept stirring for another hour. At the same time, lignin solution was prepared separately at various concentrations by dissolving 62.5 mg, 125 mg, and 250 mg of lignin in 25 mL of 50 vol% ethanolic solution, stirred overnight. The lignin/CMC solution for the synthesis of film was obtained by mixing the two solutions and sonicating for 20 min in the sonification bath to remove air bubbles, before they were subjected to heating at 80 °C for an hour. An amount of 20 mL of this solution was cast onto a petri dish (PyrexTM 3160-101) with a diameter of 100 mm. The petri dish (without cover) was later placed in an oven at 45 °C for the formation of the film. Lastly, the lignin/CMC films were evaluated for their physical, morphological, chemical, and thermal properties.
Characterization of lignin
Purity of the lignin. The purity of the lignin was estimated using UV/vis spectrophotometry (DR6000TM UV VIS Spectrophotometer, Hach, Malaysia), with reference to the method previously reported [26,27]. The acid insoluble lignin obtained was dissolved in 0.1 N NaOH and adjusted to a total lignin content of approximately 10%. An amount of 1 mL of the solution was extracted to be diluted with the same concentration of NaOH solution until the absorbance measured by UV/vis spectrophotometry at 280 nm was in the range of 0.3–0.8 a.u (with dilution factor of approximately 1:2500). An amount of 0.1 N NaOH solution was used as the blank reference in the measurement. The absorptivity was calculated from the Beer-Lambert law, which states that:
where is the absorptivity (L (g.cm)−1), is the absorbance (a.u.), is the concentration of the sample (g L−1), and is the pathlength of the measured cell (cm). The purity of the lignin was estimated by comparing the calculated with the literature value of 23.7 L (g.cm)−1 at 280 nm. Simultaneously, the purity of the lignin was also compared with the NREL procedure, assuming the lignin as the ‘biomass’ and obtaining the yield of the ash-free Klason lignin [28].
Molecular weight of lignin. Gel permeation chromatography (GPC) was a simple technique utilized to determine the molecular weight of lignin. Lignin samples (in triplicate) were prepared in 0.05 M LiBr in DMF solution at 1.5 mg mL−1, just prior to analysis. DMF with LiBr is a suitable solvent as a variety of lignin from different processing routes have been shown to readily dissolve in this solvent [29]. The solutions were filtered through a 0.45 m syringe filter before injecting into a GPC system (Viscotek Gpcmax Ve2001, Malvern Panalytical, Malvern, UK). Mw, Mz, Mn, and polydispersity were determined from the chromatogram obtained.
Characterization of lignin/CMC film
The UV and visible light absorption of the lignin/CMC films were measured with a UV/vis spectrophotometer (Hach DR6000TM) in the range of 210–800 nm. In addition, the FTIR measurements were carried out with a Cary 630 FTIR spectrometer coupled to an MCT detector (Agilent Technologies, USA) in attenuated total reflection (ATR) mode. The films were placed in close contact to the single reflection diamond IRE on a golden gate (PIKE Technologies, USA). The FTIR spectral data were acquired and recorded in the mid FTIR range from 4000 to 450 cm−1, at 8 cm−1 spectral resolution, and with 32 co-added scans. A new background was recorded before each measurement. A water vapor permeability (WVP) test was also carried out following the gravimetric determination standard ASTM E96 [30]. The films were cut into a small circular shape of 5 cm in diameter. Four measuring cups were filled with 10 mL of distilled water, leaving a small gap of air space (approx. 1 cm) between the films and the water. The cups were then sealed to prevent water vapor loss, except in the test sample, after which they were left in a humidity chamber kept at room conditions (25 °C and 75% RH). An initial weight was taken at the start of the experiment and then samples were periodically weighed over time at 1-h interval for 8 h. The slopes of the linear decrease in weight loss were used to calculate the water vapor transmission rate, which were used to calculate WVP, following the equation:
The water contact angle was measured by a sessile drop method using an optical tensiometer (Attension® Theta Lite, Biolin Scientific, Selangor, Malaysia) over a span of 10 s. The films were prepared into a small rectangular size of 2 cm × 3 cm for measurements, and a microsyringe was used to inject a drop of water on the horizontal surface of the films. Measurements were taken at 0.08 s-interval over a span of 10 s. Furthermore, microstructural analysis of the surfaces of the neat CMC and lignin/CMC films was investigated using a field emission-scanning electron microscope (FE-SEM, SU8000, Hitachi High-Technologies Corporation, Tokyo, Japan). The films were cut into a diameter of 1 cm and placed on the copper base and sputter coated with platinum (Q150R S Quorum Technologies Ltd., Lewes, UK). After coating with a thin layer of platinum, the films were photographed at a voltage of 5 kV and a magnification of 1000× and 60,000×. Lastly, the thermal stability of the neat CMC and lignin/CMC films were studied using thermogravimetric analysis (TGA) in an inert nitrogen medium to prevent combustion of the samples, from room temperature to 900 °C at a heating rate of 10 °C min−1 (Mettler Toledo Thermal Analysis, Columbus, OH, USA).
3. Results and Discussions
3.1. Properties of Pretreated DLF
The compositions of the DLF pulp after alkali extraction are similar to the results reported by Zawawi et al. [31] which used the NREL protocol for the determination of structural carbohydrates and lignin in biomass [32], with crude cellulose concentrations as high as 67 wt%. After acid extraction of the black liquor, the lignin obtained in our study has a purity as high as 97 wt%, and contains carbon, hydrogen, oxygen, and sulfur contents of 62–66 wt%, 4–9 wt%, 24–27 wt%, and <1.3 wt%, respectively. The ash content, after thorough washing with water, is found to be <1 wt%. Analyses of the lignin with GPC have shown that the lignin has a Mn, Mw, and Mz of 2558 Da, 2908 Da, and 3483 Da, respectively, with a polydispersity index of 1.13. The DS of CMC in this study was found to be 1.23. Interestingly, this value is higher compared to CMC from agro-cellulosic sources, such as water hyacinth (0.24–0.73), sago waste (0.33–0.82), sugar beet pulp (0.11–0.67), and durian husk (0.67), but lower than corn husk (2.41) and wheat straw (2.50) [33].
3.2. Properties of Lignin/CMC Films
3.2.1. Optical and UV Blocking Properties
Lignin extracted in the sequential acid/alkali treatment process displayed a brown color. When lignin was incorporated into the CMC films, this changed the color and the appearance of the films. Neat CMC was transparent, and the optical properties of neat CMC film were dependent on the DS, and it has been demonstrated that the transparency of the white color decreases with the decrease of DS. In addition to the DS, lignin concentration is another factor that plays a role in impacting the appearance of the films. The reduction in the transparency was seen in lignin/CMC films, which showed themselves in different shades of brown color, depending on the concentration of lignin used, as shown in Figure 2. This is due to the presence of chromophores in lignin, such as quinoids, catechols, aromatic ketones, stilbenes, and conjugated carbonyls with phenolics [34], which were formed and transformed in the lignin extraction and recovery process. These chromophores have, to some extent, the unsaturated functional groups (conjugated carbonyl groups, aromatic rings, and carbon-carbon double bonds) that absorb the visible light (400–700 nm) to give lignin its distinctive brown color [35].
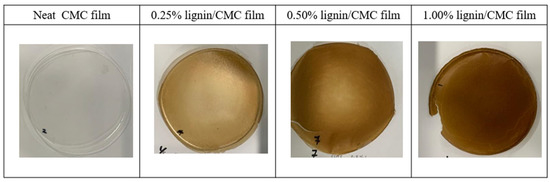
Figure 2.
The appearance and colour of the neat CMC film and lignin/CMC films.
In addition to the visual observation, the results of the UV/vis measurement are presented in Figure 3. Neat CMC film absorbed some UVC (200–279 nm), but allowed a majority of UVB (280–314 nm) and UVA (315–399 nm) to pass through. On the contrary, lignin/CMC was shown to absorb UVA and UVB, attributed to the UV-shielding property exhibited by lignin and substantiated by the enhanced absorption (or reduced transmittance) of UV wavelengths with the increased amount of lignin present in the films. The different chromophores that contributed to the enhanced absorbance in the 200–400 nm-UV range were described previously by Sadeghifar et al. [35]. In fact, UV-vis was established as an excellent system and process analytical tool to quickly quantify lignin by employing the wavelengths at 205 nm and 280 nm [36]. While the dark color of lignin might limit its application as a natural ingredient in certain areas, for instance, in dye-stuff dispersants, sunscreens, and also in packaging, where transparent materials are generally preferred; for products that are easily degraded from exposure to light, the UV-blocking lignin/CMC films could be an advantage to protect the products and preserve their quality. An example of the potential use of lignin/CMC film is the packaging of laver or fish oil, and many other food products rich in triglycerides, to shield the effect of sunlight, which has been shown to accelerate lipid oxidation and the degradation of antioxidants [37,38].
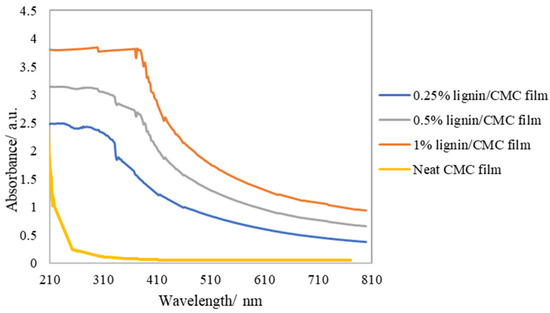
Figure 3.
UV/vis spectra of lignin/CMC films, recorded from 210–800 nm.
Numerous research efforts are underway, and methods have been developed to reduce the color of lignin, including one-step UV irradiation of lignin at 365 nm in tetrahydrofuran (THF) solvent [39]. Modifications to the extraction and fractionation process of lignin with methanol/water to get a low molecular weight fraction, which has a lighter color compared to its high molecular weight counterpart [40,41], and the bleaching aspect of lignin/CMC film, will be explored in future study.
3.2.2. FTIR Spectra Measurement
The FTIR spectra are shown in Figure 4. The spectral peaks at 1513 cm−1 and 1220 cm−1 were characteristic for the presence of lignin, which were assigned to the C=C aromatic skeletal vibrations stretching of the benzene ring and the −CO bond of syringyl and guaicyl units in lignin, respectively. These spectral bands were absent in neat CMC film, but these increased in absorbance and became more significant beyond 0.25 wt% lignin concentration. Additionally, the presence of glycerol was detected by the wavenumber at 920 cm−1, which corresponds to the symmetric (C–O–C) stretching vibrations bands [3]. The vibrational modes corresponding to the spectral bands at various wavenumbers are described in Table 1.
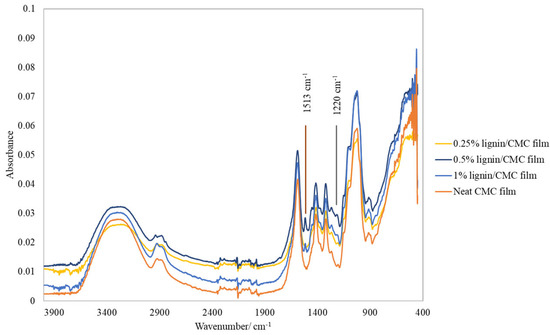
Figure 4.
FTIR spectra of lignin/CMC films, from 4000–400 cm−1.

Table 1.
FTIR spectral bands assignment for the lignin/CMC films, with comparison to literature values from [11,28,30,42,43].
3.2.3. Water Vapor Permeability (WVP) Test
The mean WVP (from triplicate measurements) of the neat CMC films was 7.23 × 10−11 g m−1 s−1 Pa−1, and a slight decrease in permeability was found for lignin/CMC films (7.21 × 10−11, 7.20 × 10−11, and 7.17 × 10−11 g m−1 s−1 Pa−1 for 0.25%, 0.5%, and 1% lignin, respectively). The values calculated were in the range of the WVPs reported in previous work on lignin-incorporated CMC films [17]. These results correlated to the hydrophilic nature of cellulose, whereby the abundance of hydroxy (−OH) groups present allowed the formation of hydrogen bonds with water molecules. On the contrary, lignin is hydrophobic in nature, as it is rich in aromatic units; thus, lignin functioned as a physical barrier to water vapor transmission in the film matrix, by forcing water vapor to diffuse around them through a more tortuous pathway [3]. The results were consistent to observations made when lignin was incorporated in nanofibrillated cellulosic film (NFC) [44] and another biopolymer matrix, such as polyvinyl alcohol-cellulose nanocrystals (PVA-CNC) [45] and chitosan [46]. WVP is an important evaluation standard to measure the performance of packaging materials for food, as it affects the shelf life of products. Indeed, for fresh food, WVP needs to be high to avoid dehydration. Whereas for bakery or delicatessen, it is the opposite as water permeation should be avoided [47,48]. When the WVP of lignin/CMC film is compared with other common biodegradable polymers used as packaging materials, as well as PE, and as shown in Figure 5, it is found to be slightly higher than polycaprolactone (PCL) and comparable to PBAT.
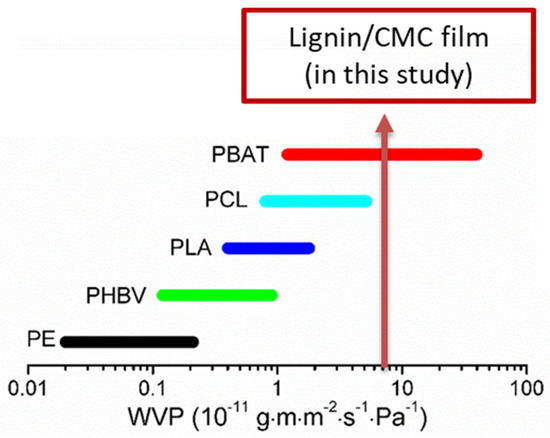
Figure 5.
Comparison of WVP values of lignin/CMC film with other biodegradable polymers (PE: polyethylene; PHBV: Poly(3−hydroxybutyrate−co−3−hydroxyvalerate); PLA: polylactic acid; PCL: polycaprolactone; and PBAT: Polybutylene adipate terephthalate) and with PE: polyethylene. Adapted with permission from [49]. Copyright 2018 American Chemical Society.
3.2.4. Contact Angle Measurement
The results of the contact angle measurements are shown in Figure 6. Lignin plays a role in increasing the contact angle of water on the CMC films, due to the hydrophobic nature of lignin, as explained in the previous section. This is evident from the highest initial contact angle recorded with the CMC film doped with 1% lignin at 80.7°. Generally, a film exhibiting a water contact angle greater than 65° is considered hydrophobic [50]. The observation was consistent with the hypothesis formulated by Maximova et al., that the adsorbed lignin is capable of weakening the adhesion of water to both nonporous and porous hydrophilic substrates [51]. Another factor that could be attributed to the higher water contact angle is the increased surface roughness of the lignin/CMC films, which were investigated with SEM microscopic technique and are described in a later section. For the other lignin/CMC films, the initial water contact angles are rather close to one another, in the range of 60–70°, which are comparable to some other common food packaging polymers such as PLA (68.8°) and PHBV (60.4°); but lower than low-density polyethylene (LDPE) (85.2°) [50]. Although the starting contact angles were similar, the neat CMC demonstrated a remarkably rapid deceleration of water contact angle of over 20° in just 10 s, indicating the high wettability and spreadability of this film. This result is strengthened by the fact that neat CMC exhibited a significant swelling upon absorption of water, which is undesirable, as it will result in a deterioration in the mechanical performance, depending on the extent of water-induced swelling. With the incorporation of lignin, the wetting property of the film could be manipulated, which is believed to be crucial for its application as packaging materials and to prolong its shelf life, especially in high moisture conditions. In spite of that, the interpretation of the contact angle values with surface properties still present a great challenge, since many experimental conditions, such as the size of the surface heterogeneities and asperities, surface cleanliness, and the resolution of measuring equipment and data interpretation, all play a role in affecting the outcomes. To make matters worse, it is rather difficult to distinguish the effects of each contributing factor due to their interrelation or overlapping effect [52].
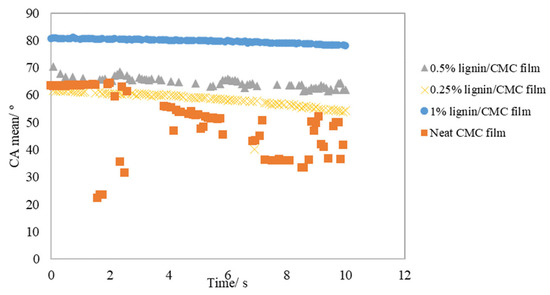
Figure 6.
Mean contact angle of lignin/CMC films plotted against time.
3.2.5. SEM Imaging
The surface roughness clearly increased when lignin was incorporated into the CMC film (Figure 7). The explanation for this observation is related to an evaporation-induced self-assembly process that leads to the formation of small aggregate structures [3].
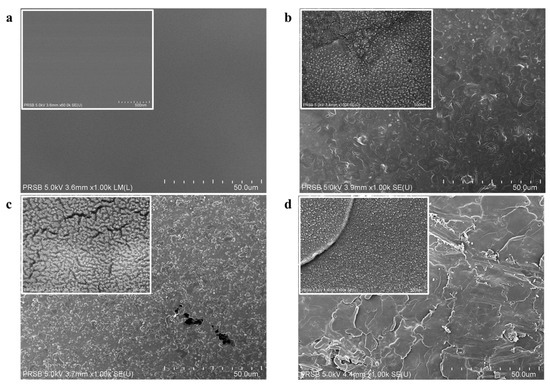
Figure 7.
SEM micrographs of the surface of (a) neat CMC film, (b) 0.25 wt% lignin/CMC film, (c) 0.5 wt% lignin/CMC film, and (d) 1 wt% lignin/CMC film. Regular images were taken at 1000× magnification, and the insert images were at 60,000× magnification.
3.2.6. Thermal Analysis
The decomposition behavior in terms of percentage mass loss versus temperature and the differential thermogravimetric (DTG) plot, are shown in Figure 8. The DTG plot shows a subtle broad peak representing initial mass loss below 169 °C, from the evaporation of the remnant moisture, ethanol solvent, and the glycerol additive; the latter reported previously to lose up to 81% of its mass in the range of 68–164 °C [53]. Other than that, significant large peaks were also seen between the temperature range of 250−320 °C, which largely corresponded to the pyrolysis of cellulose chains resulting from depolymerization, dehydration, and decomposition of glycosyl units [54]. Similar thermal behavior was obtained for all neat CMC film and lignin/CMC films. Unlike cellulose or CMC, lignin gradually lost its mass consistently with heating, as shown by Hornung et al. [55]. As a result, there was no detectable standout peaks for lignin in the thermograms. Furthermore, the thermal stability of the CMC film was not detected to improve in the presence of lignin, in spite of the hypothesis that the presence of the phenolic OH groups within the lignin structure and the aromatic char originating from the lignin at elevated temperature are responsible for enhancement of thermal stability of the lignin-incorporated polymer matrix [54]. In brief, the samples showed a three-step thermal degradation between: (i) 200–400 °C for organic volatiles with low molecular weight; (ii) 400–600 °C for decarboxylation of CMC; and (iii) 600–800 °C for residual organic fractions, with the degradation temperature highly dependent on the degree of substitution of the carboxymethyl groups along the cellulose backbone that will affect its crystallinity. The trend observed was consistent with the observation documented in other research work involving CMC film [56].
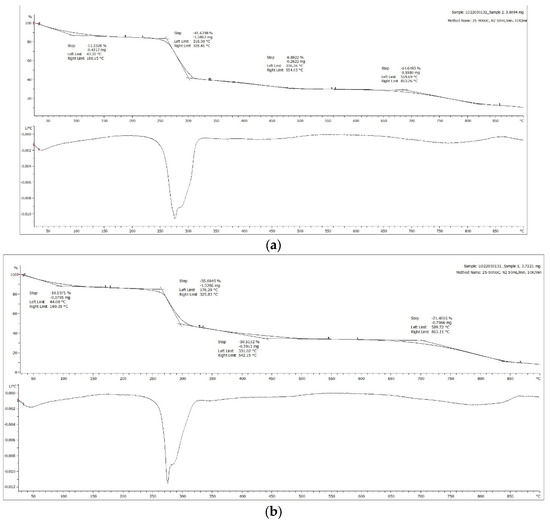
Figure 8.
TGA thermograms of (a) neat CMC film and (b) 1% lignin/CMC film. The top graphs show the mass loss, whilst the bottom graphs show the differential of the mass loss as a function of temperature.
4. Conclusions
A pathway for the synthesis of lignin/CMC films from oil palm waste was established in this work. Crude cellulose and lignin were successfully extracted using the sequential alkaline/acid pre-treatment and precipitation method. After that, crude cellulose was made into CMC film by carboxymethylation with NaOH in the presence of ethanol and glycerol, then lignin was incorporated into the CMC films at various concentrations. The successful incorporation of lignin in the film was confirmed by FTIR measurements, whereby the characteristic peaks of lignin were identified from the spectra obtained. The properties of the lignin/CMC films were investigated to demonstrate its potential as a green material for application in food packaging. The results demonstrated that lignin exhibits anti-UV properties and plays an important role in increasing the hydrophobicity and water vapor transmission rate, which would aid in prolonging the shelf life of food products. The source of the lignin was hypothesized to play a significant role in determining this outcome. Nonetheless, lignin/CMC films are not transparent and bleaching procedures need to be carried out if transparent packaging is desired. Another challenge is the difficulty in producing homogenous lignin/CMC with smooth surface when viewed under SEM; thus, future research on lignin modification is necessary to increase the miscibility of lignin and CMC. Despite the aforementioned limitations of the lignin/CMC films, owing to their environmentally friendly feedstock and high biodegradability, the lignin/CMC films are promising as a sustainable packaging material in the future.
Author Contributions
Conceptualization, C.-L.S. and J.B.O.; methodology, C.-L.S.; software, C.-L.S.; validation, C.-L.S. and J.B.O.; formal analysis, C.-L.S.; investigation, C.-L.S.; resources, C.-L.S.; data curation, C.-L.S.; writing—original draft preparation, C.-L.S.; writing—review and editing, J.B.O.; visualization, C.-L.S.; supervision, J.B.O.; project administration, J.B.O.; funding acquisition, J.B.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Acknowledgements to PRSB staff for technical support (SEM—Afidah Bt Sastro, Thermal analyses—Alia Bt Khalid, and Contact Angle—Nor Hafizah Bt Yasin).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Statista. Annual Production of Plastics Worldwide from 1950 to 2020. 2022. Available online: https://www.statista.com/statistics/282732/global-production-of-plastics-since-1950/ (accessed on 9 May 2022).
- Ritchie, H.; Roser, M. Plastic Pollution. Our World in Data. 2018. Available online: https://ourworldindata.org/plastic-pollution (accessed on 9 May 2022).
- Michelin, M.; Marques, A.M.; Pastrana, L.M.; Teixeira, J.A.; Cerqueira, M.A. Effect of organosolv lignin incorporation on physicochemical and antioxidant properties. J. Food Eng. 2020, 285, 110107. [Google Scholar] [CrossRef]
- Gupta, H.; Kumar, H.; Kumar, M.; Gehlaut, A.K.; Gaur, A.; Sachan, S.; Park, J.W. Synthesis of biodegradable films obtained from rice husk and sugarcane bagasse to be used as food packaging material. Environ. Eng. Res. 2020, 25, 506–514. [Google Scholar] [CrossRef]
- Kontogiorgos, V. Stabilisers. In Encyclopedia of Dairy Sciences, 3rd ed.; McSweeney, P.L.H., McNamara, J.P., Eds.; Academic Press: Oxford, UK, 2022; pp. 689–694. [Google Scholar]
- Rahman, M.S.; Mondal, H.M.I.; Yeasmin, M.S.; Sayeed, M.A.; Hossain, M.A.; Ahmed, M.B. Conversion of Lignocellulosic Corn Agro-Waste into Cellulose Derivative and Its Potential Application as Pharmaceutical Excipient. Processes 2020, 8, 711. [Google Scholar] [CrossRef]
- Klunklin, W.; Jantanasakulwong, K.; Phimolsiripol, Y.; Leksawasdi, N.; Seesuriyachan, P.; Chaiyaso, T.; Insomphun, C.; Phongthai, S.; Jantrawut, P.; Sommano, S.R.; et al. Synthesis, Characterization, and Application of Carboxymethyl Cellulose from Asparagus Stalk End. Polymers 2020, 13, 81. [Google Scholar] [CrossRef]
- Pushpamalar, V.; Langford, S.J.; Ahmad, M.; Lim, Y.Y. Optimization of reaction conditions for preparing carboxymethyl cellulose from sago waste. Carbohydr. Polym. 2006, 64, 312–318. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Luangkamin, S.; Tanprasert, K.; Suriyatem, R. Carboxymethyl cellulose film from durian rind. LWT Food Sci. Technol. 2012, 48, 52–58. [Google Scholar] [CrossRef]
- Haleem, N.; Arshad, M.; Shahid, M.; Tahir, M.A. Synthesis of carboxymethyl cellulose from waste of cotton ginning industry. Carbohydr. Polym. 2014, 113, 249–255. [Google Scholar] [CrossRef]
- Mondal, M.I.H.; Yeasmin, M.S.; Rahman, M.S. Preparation of food grade carboxymethyl cellulose from corn husk agrowaste. Int. J. Biol. Macromol. 2015, 79, 144–150. [Google Scholar] [CrossRef]
- Karataş, M.; Arslan, N. Flow behaviours of cellulose and carboxymethyl cellulose from grapefruit peel. Food Hydrocoll. 2016, 58, 235–245. [Google Scholar] [CrossRef]
- Nguyen, T.M.N.; Huu, D.T.; Vinh, H.T.; Thanh, T.N. Chemical Engineering Transactions Effects of Xanthan Gum, Carboxymethyl Cellulose, And Gum Arabic on The Properties Of Bean Powder-Based Biofilms. Chem. Eng. Trans. 2021, 89, 607–612. [Google Scholar]
- Sahin, U. An easy and accurate method for determining degree of substitution on carboxymethylated cotton fabric. Tekst. Konfeksiyon 2018, 28, 118–124. [Google Scholar]
- Suota, M.J.; da Silva, T.A.; Zawadzki, S.F.; Sassaki, G.L.; Hansel, F.A.; Paleologou, M.; Ramos, L.P. Chemical and structural characterization of hardwood and softwood LignoForce™ lignins. Ind. Crop. Prod. 2021, 173, 114138. [Google Scholar] [CrossRef]
- Katahira, R.; Elder, T.J.; Beckham, G.T. Chapter 1 A Brief Introduction to Lignin Structure. In Lignin Valorization: Emerging Approaches; The Royal Society of Chemistry: London, UK, 2018; pp. 1–20. [Google Scholar]
- Haqiqi, M.T.; Bankeeree, W.; Lotrakul, P.; Pattananuwat, P.; Punnapayak, H.; Ramadhan, R.; Kobayashi, T.; Amirta, R.; Prasongsuk, S. Antioxidant and UV-Blocking Properties of a Carboxymethyl Cellulose–Lignin Composite Film Produced from Oil Palm Empty Fruit Bunch. ACS Omega 2021, 6, 9653–9666. [Google Scholar] [CrossRef] [PubMed]
- Law, P.G.; Sebran, N.H.; Zawawi, A.Z.; Hussain, A.S. Optimization Study of Biomass Hydrogenation to Ethylene Glycol Using Response Surface Methodology. Processes 2020, 8, 588. [Google Scholar] [CrossRef]
- Hidayat, M.; Aqilah, N.A.; Winata, A. Pretreatment of Oil Palm Empty Fruit Bunch using Caustic Soda Solution for Lignin Isolation. J. Appl. Sci. Eng. 2022, 25, 1025–1030. [Google Scholar]
- Mousavioun, P.; Doherty, W.O.S. Chemical and thermal properties of fractionated bagasse soda lignin. Ind. Crops Prod. 2010, 31, 52–58. [Google Scholar] [CrossRef]
- Shui, T.; Feng, S.; Yuan, Z.; Kuboki, T.; Xu, C.C. Highly efficient organosolv fractionation of cornstalk into cellulose and lignin in organic acids. Bioresour. Technol. 2016, 218, 953–961. [Google Scholar] [CrossRef]
- Shui, T.; Feng, S.; Chen, G.; Li, A.; Yuan, Z.; Shui, H.; Kuboki, T.; Xu, C. Synthesis of sodium carboxymethyl cellulose using bleached crude cellulose fractionated from cornstalk. Biomass Bioenergy 2017, 105, 51–58. [Google Scholar] [CrossRef]
- Rowell, R.M. Handbook of Wood Chemistry and Wood Composites, 1st ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Othman, N.E.A.; Ismail, F.; Aziz, A.A.; Wahab, N.A. Preparation and Characterization of Palm-Based Sodium Carboxymethyl Cellulose for Application in Food Additive. University of Florida. 2021. Available online: https://biointerfaceresearch.com/wp-content/uploads/2021/02/20695837115.1305313063.pdf (accessed on 9 May 2022).
- Easson, M.; Villalpando, A.; Condon, B.A. Absorbent Properties of Carboxymethylated Fiber, Hydroentangled Nonwoven and Regenerated Cellulose: A Comparative Study. J. Eng. Fibers Fabr. 2017, 12, 408. [Google Scholar] [CrossRef]
- Dong, D. Kraft Lignin and Investigation of Pulping Effects on Pulp Yield, Lignin Molecular Mass and Lignin Content of Black Liquor with a Central Composite Pulping Design; University of Florida: Gainesville, FL, USA, 1993. [Google Scholar]
- Alen, R.; Hartus, T. UV spectrophotometric determination of lignin from alkaline pulping liquors. Cellul. Chem. Technol. 1988, 22, 613–618. [Google Scholar]
- Medina, J.D.C.; Woiciechowski, A.; Zandona Filho, A.; Noseda, M.D.; Kaur, B.S.; Soccol, C.R. Lignin preparation from oil palm empty fruit bunches by sequential acid/alkaline treatment—A biorefinery approach. Bioresour. Technol. 2015, 194, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Gaugler, E.C.; Radke, W.; Vogt, A.P.; Smith, D.A. Molar mass determination of lignins and characterization of their polymeric structures by multi-detector gel permeation chromatography. J. Anal. Sci. Technol. 2021, 12, 30. [Google Scholar] [CrossRef]
- Mishra, V.; Kumar, R. Graft copolymerization of Carboxymethylcellulose: An overview. Trends Carbohydr. Res. 2012, 4, 1–17. [Google Scholar]
- Zawawi, A.Z.; Gaik, L.P.; Sebran, N.H.; Othman, J.; Hussain, A.S. An optimisation study on biomass delignification process using alkaline wash. Biomass Convers. Biorefin. 2018, 8, 59–68. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of structural carbohydrates and lignin in biomass. In Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Ahmed, N. A novel optimization method for preparing carboxymethyl cellulose with higher yield from wheat straw. J. Chem. Biol. Phys. Sci. 2018, 8, 444–460. [Google Scholar]
- Zhang, H.; Fu, S.; Chen, Y. Basic understanding of the color distinction of lignin and the proper selection of lignin in color-depended utilizations. Int. J. Biol. Macromol. 2020, 147, 607–615. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Ragauskas, A. Lignin as a UV Light Blocker—A Review. Polymers 2020, 12, 1134. [Google Scholar] [CrossRef]
- Skulcova, A.; Majova, V.; Kohutova, M.; Grosik, M.; Sima, J.; Jablonsky, M. UV/Vis Spectrometry as a Quantification Tool for Lignin Solubilized in Deep Eutectic Solvents. BioResources 2017, 12, 2017. [Google Scholar] [CrossRef]
- Oh, S.; Lee, E.; Choe, E. Light effects on lipid oxidation, antioxidants, and pigments in dried laver (Porphyra) during storage. Food Sci. Biotechnol. 2014, 23, 701–709. [Google Scholar] [CrossRef]
- Ahmed, M.; Pickova, J.; Ahmad, T. Oxidation of Lipids in Foods. Sarhad J. Agric. 2016, 32, 230–238. [Google Scholar] [CrossRef]
- Wang, J.; Deng, Y.; Qian, Y.; Qiu, X.; Ren, Y.; Yang, D. Reduction of lignin color via one-step UV irradiation. Green Chem. 2016, 18, 695–699. [Google Scholar] [CrossRef]
- Zhang, H.; Bai, Y.; Yu, B.; Liu, X.; Chen, F. A practicable process for lignin color reduction: Fractionation of lignin using methanol/water as a solvent. Green Chem. 2017, 19, 5152–5162. [Google Scholar] [CrossRef]
- Lee, S.C.; Yoo, E.; Lee, S.H.; Won, K. Preparation and Application of Light-Colored Lignin Nanoparticles for Broad-Spectrum Sunscreens. Polymers 2020, 12, 699. [Google Scholar] [CrossRef] [PubMed]
- Riaz, T.; Zeeshan, R.; Zarif, F.; Ilyas, K.; Muhammad, N.; Safi, S.Z.; Rahim, A.; Rizvi, S.A.; Rehman, I.U. FTIR analysis of natural and synthetic collagen. Appl. Spectrosc. Rev. 2018, 53, 703–746. [Google Scholar] [CrossRef]
- Kubovský, I.; Kačíková, D.; Kačík, F. Structural Changes of Oak Wood Main Components Caused by Thermal Modification. Polymers 2020, 12, 12. [Google Scholar] [CrossRef]
- Wang, W.; Guo, T.; Sun, K.; Jin, Y.; Gu, F.; Xiao, H. Lignin Redistribution for Enhancing Barrier Properties of Cellulose-Based Materials. Polymers 2019, 11, 1929. [Google Scholar] [CrossRef]
- Yang, W.; Qi, G.; Kenny, J.M.; Puglia, D.; Ma, P. Effect of Cellulose Nanocrystals and Lignin Nanoparticles on Mechanical, Antioxidant and Water Vapour Barrier Properties of Glutaraldehyde Crosslinked PVA Films. Polymers 2020, 12, 1364. [Google Scholar] [CrossRef]
- Chen, L.; Tang, C.Y.; Ning, N.Y.; Wang, C.Y.; Fu, Q.; Zhang, Q. Preparation and Properties of Chitosan/Lignin Composite Films. Chin. J. Polym. Sci. 2009, 27, 739–746. [Google Scholar] [CrossRef]
- Wang, S.; Shen, Q.; Guo, C.; Guo, H. Comparative Study on Water Vapour Resistance of Poly(lactic acid) Films Prepared by Blending, Filling and Surface Deposit. Membranes 2021, 11, 915. [Google Scholar] [CrossRef]
- Siracusa, V. Food Packaging Permeability Behaviour: A Report. Int. J. Polym. Sci. 2012, 2012, 302029. [Google Scholar] [CrossRef]
- Li, J.; Lai, L.; Wu, L.; Severtson, S.J.; Wang, W.J. Enhancement of Water Vapor Barrier Properties of Biodegradable Poly(butylene adipate-co-terephthalate) Films with Highly Oriented Organomontmorillonite. ACS Sustain. Chem. Eng. 2018, 6, 6654–6662. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Hong, S.I. Wetting properties of biopolyester films prepared by thermo-compression method. Food Sci. Biotechnol. 2007, 16, 234–237. [Google Scholar]
- Maximova, N.; Österberg, M.; Laine, J.; Stenius, P. The wetting properties and morphology of lignin adsorbed on cellulose fibres and mica. Colloids Surf. A Physicochem. Eng. Asp. 2004, 239, 65–75. [Google Scholar] [CrossRef]
- Chau, T.T.; Bruckard, W.J.; Koh, P.T.L.; Nguyen, A.V. A review of factors that affect contact angle and implications for flotation practice. Adv. Colloid Interface Sci. 2009, 150, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Dou, B.; Dupont, V.; Williams, P.T.; Chen, H.; Ding, Y. Thermogravimetric kinetics of crude glycerol. Bioresour. Technol. 2009, 100, 2613–2620. [Google Scholar] [CrossRef] [PubMed]
- Parit, M.; Saha, P.; Davis, V.A.; Jiang, Z. Transparent and Homogenous Cellulose Nanocrystal/Lignin UV-Protection Films. ACS Omega 2018, 3, 10679–10691. [Google Scholar] [CrossRef] [PubMed]
- Hornung, A.; Stenzel, F.; Grunwald, J. Biochar—Just a Black Matter Is Not Enough. Biomass Convers. Biorefin. 2021, in press. [Google Scholar] [CrossRef]
- Moussa, I.; Khiari, R.; Moussa, A.; Belgacem, M.N.; Mhenni, M.F.; Belgacem, M.N.; Mhenni, M.F. Preparation and Characterization of Carboxymethyl Cellulose with a High Degree of Substitution from Agricultural Wastes. Fibers Polym. 2019, 20, 933–943. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).