Investigation of Glyphosate Removal from Aqueous Solutions Using Fenton-like System Based on Calcium Peroxide
Abstract
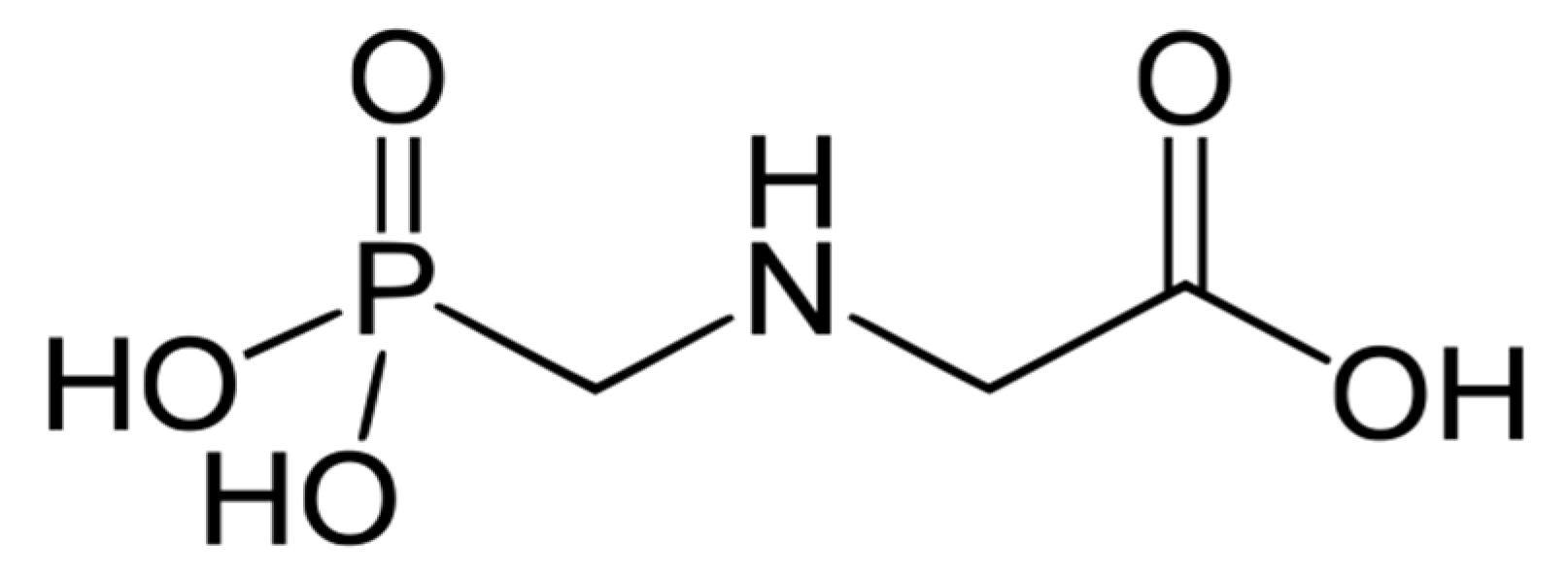
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents and Instruments
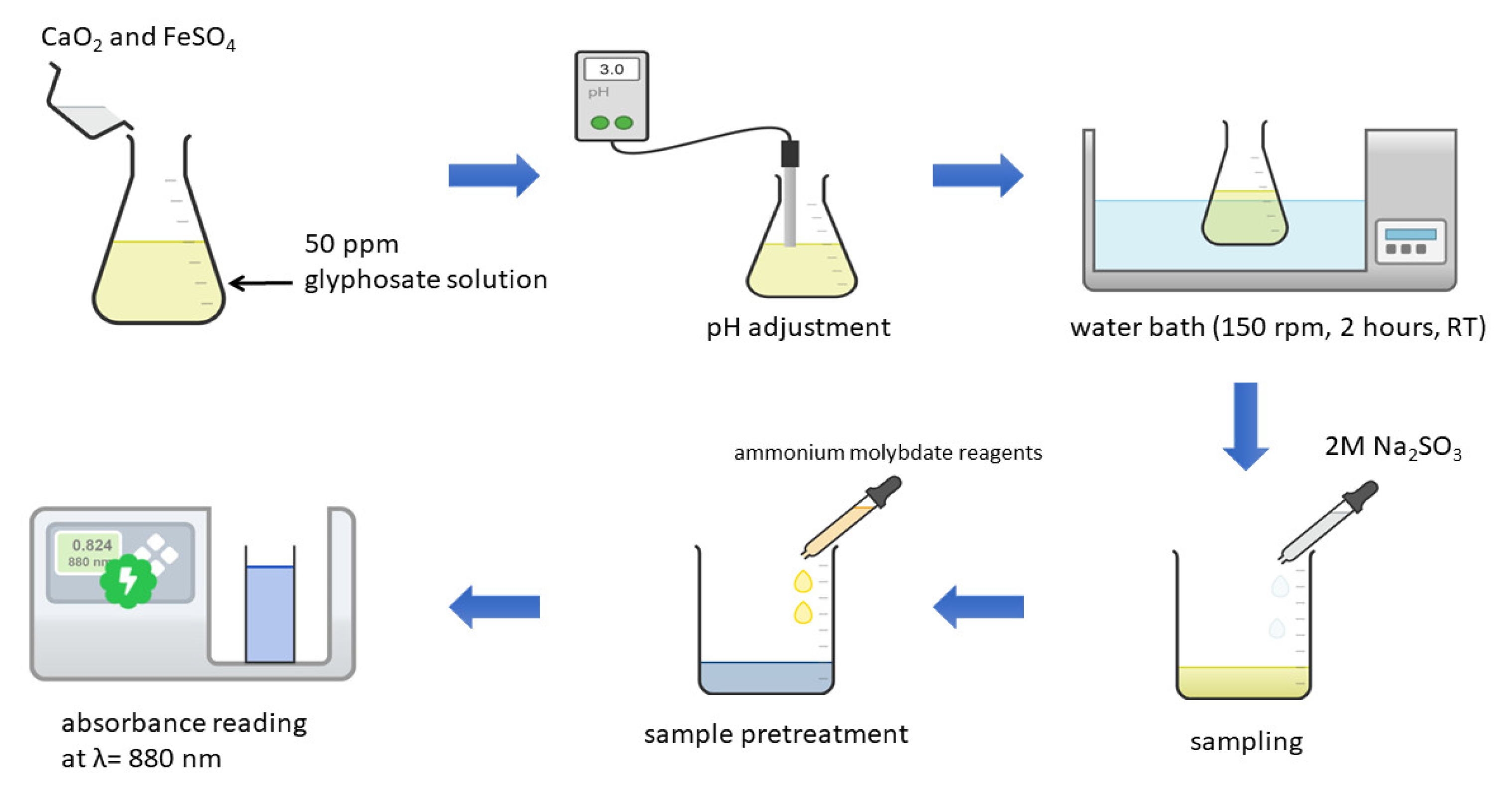
2.2. Methods
2.2.1. Effect of pH
2.2.2. Effect of the Molar Ratio of Ca2+:Fe2+
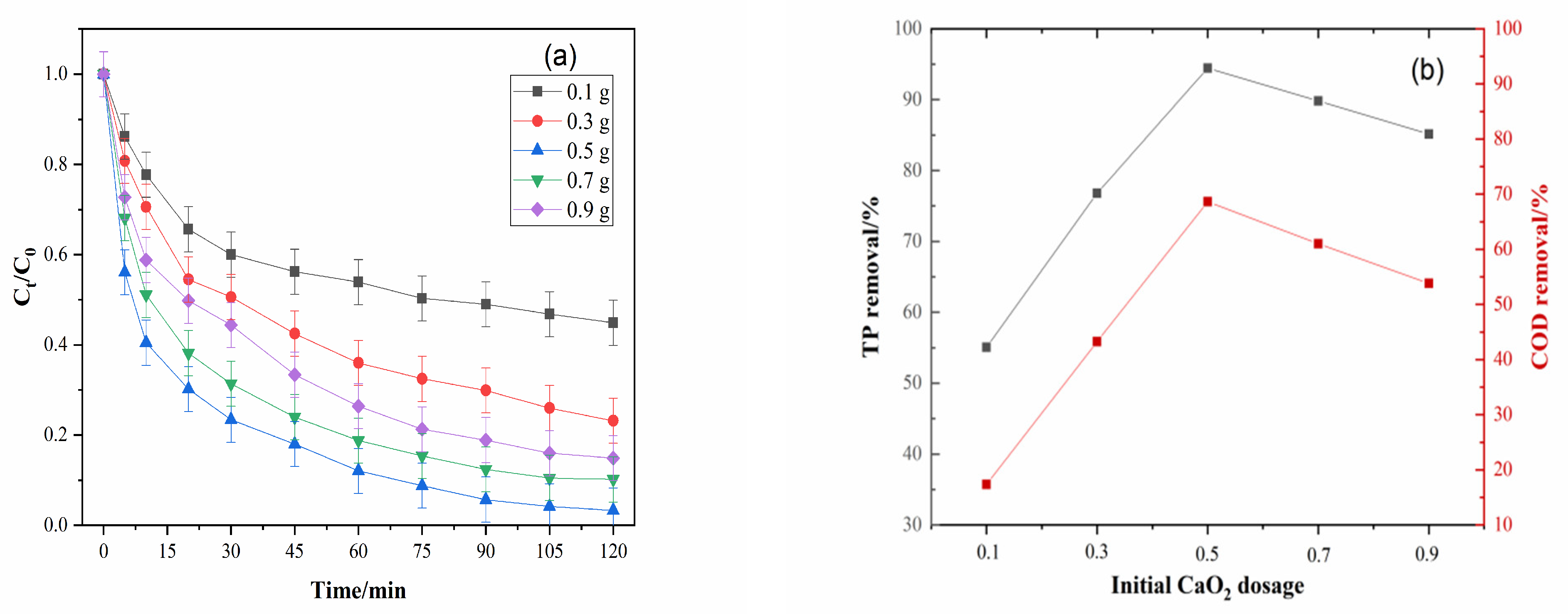
2.2.3. Effect of Initial CaO2 Dosage
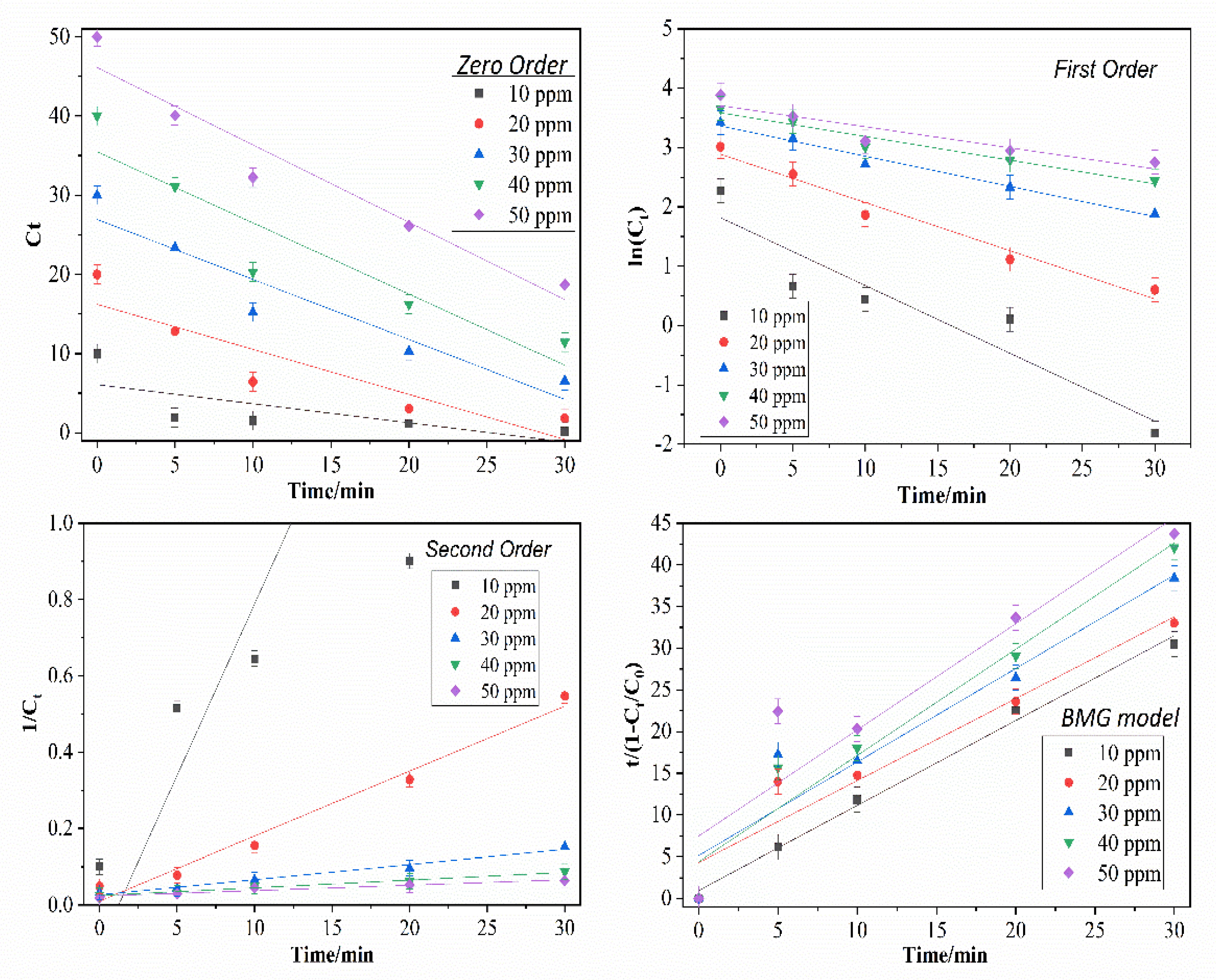
2.2.4. Effect of the Initial Glyphosate Concentration
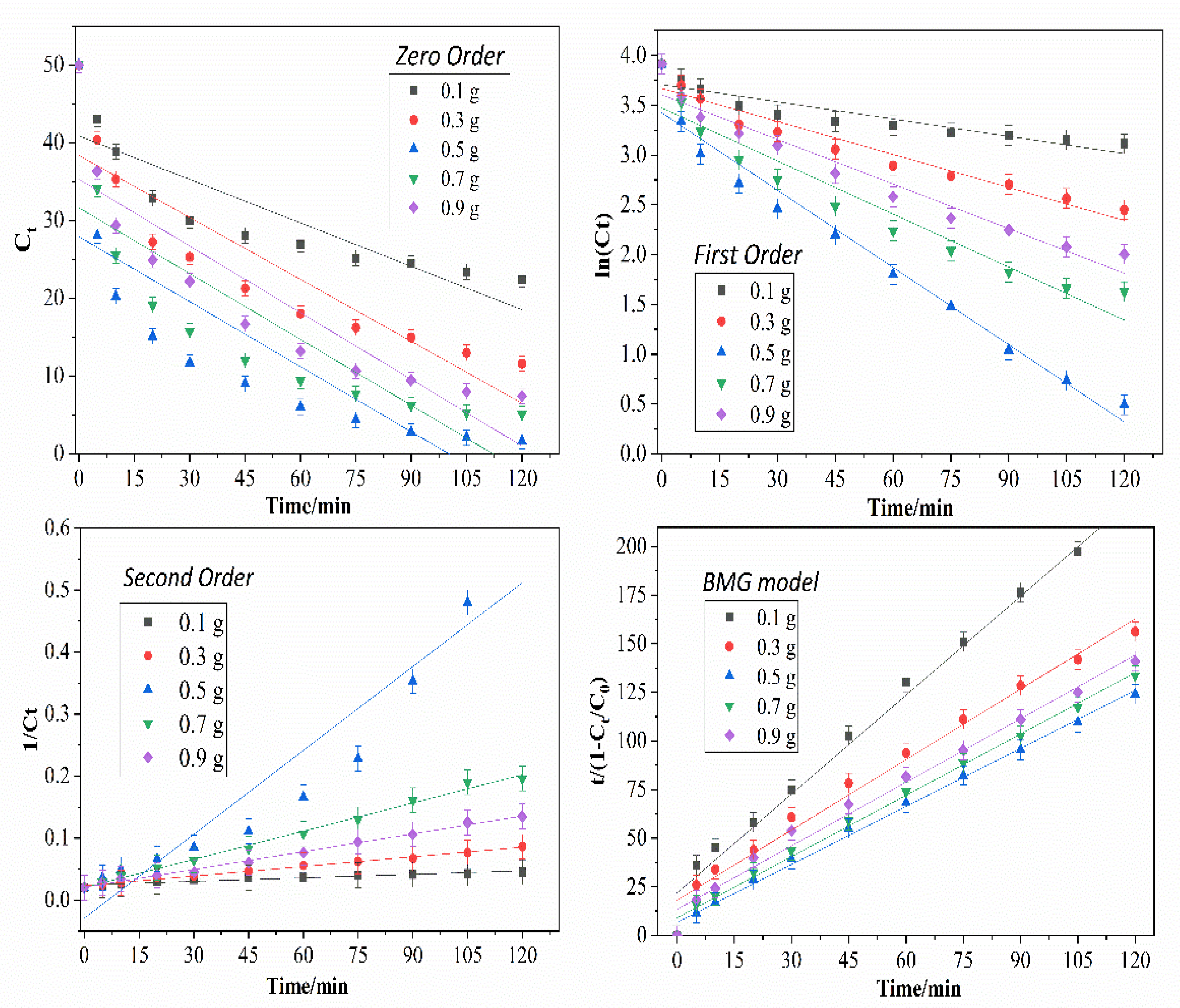
2.2.5. Kinetics Study
3. Results
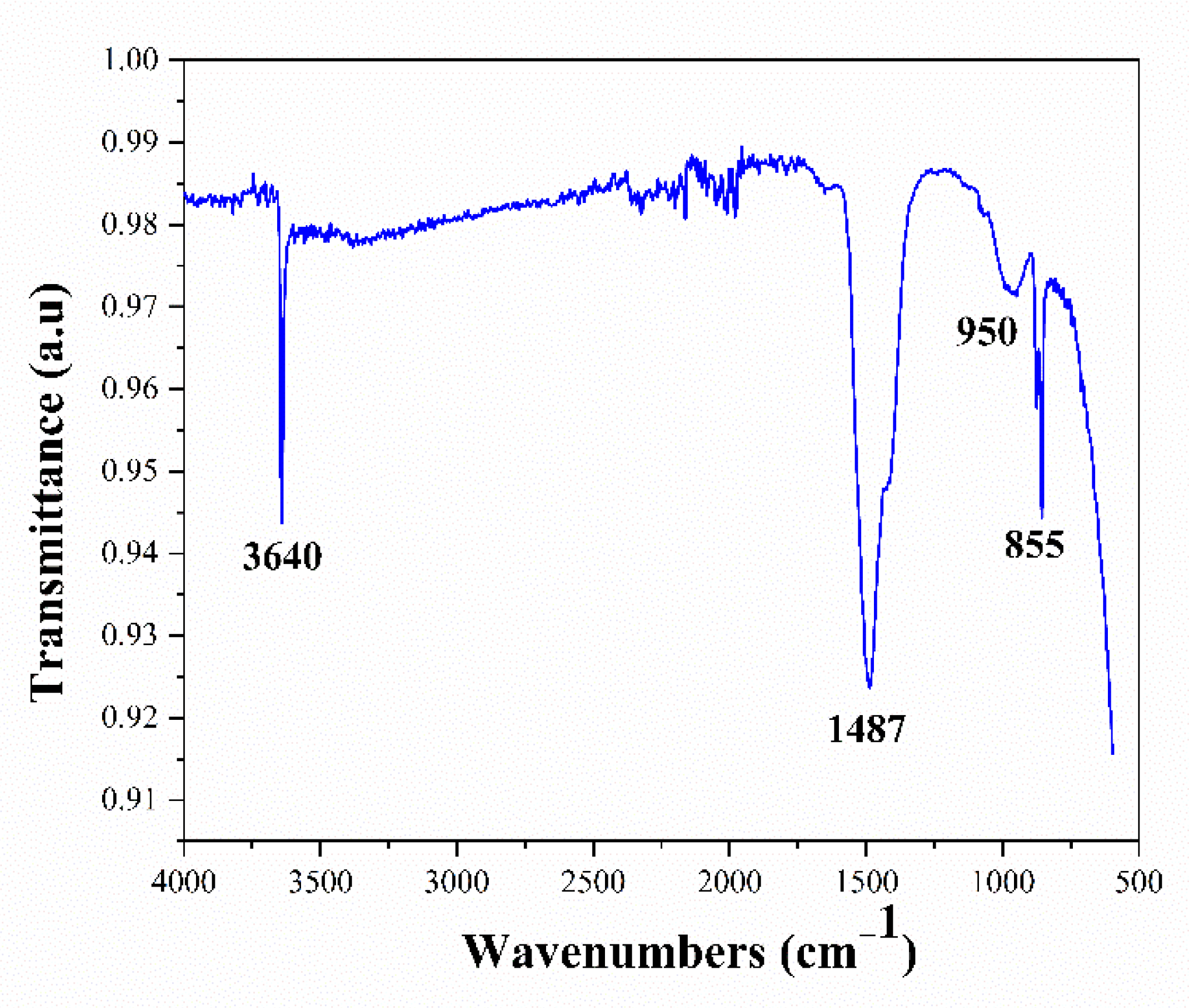
3.1. Characterization
3.2. Effect of pH
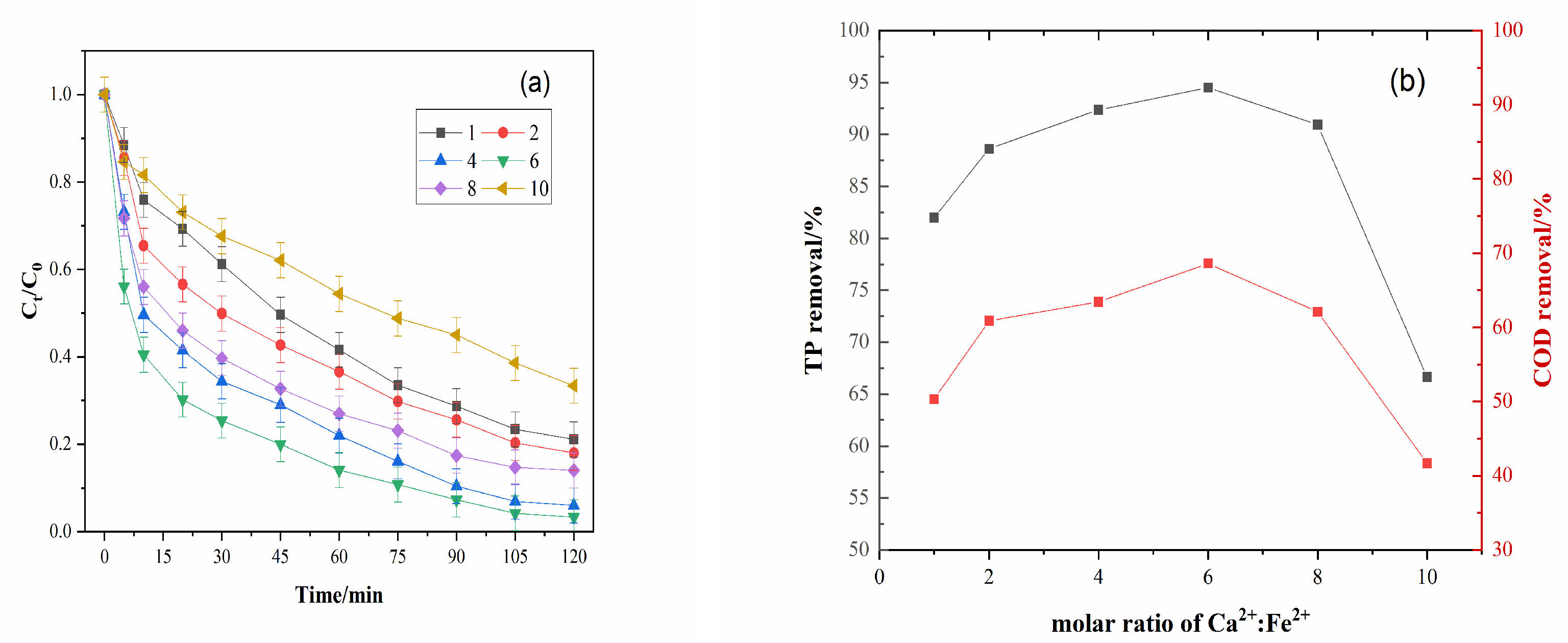
3.3. Effect of the Molar Ratio of Ca2+:Fe2+
3.4. Effect of Initial CaO2 Dosage
3.5. Effect of the Initial Glyphosate Concentration
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Landrigan, P.J.; Fuller, R.; Fisher, S.; Suk, W.A.; Sly, P.; Chiles, T.C.; Bose-O’Reilly, S. Pollution and Children’s Health. Sci. Total Environ. 2019, 650, 2389–2394. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Nauman, S.; Ghafari, M.; Parnianifard, A.; Gomes, A.; Gomes, C. Treatment of Wastewater for Agricultural Applications in Regions of Water Scarcity. Biointerface Res. Appl. Chem. 2021, 12, 6336–6360. [Google Scholar] [CrossRef]
- Kukurina, O.; Elemesova, Z.; Syskina, A. Mineralization of Organophosphorous Pesticides by Electro-generated Oxidants. Procedia Chem. 2014, 10, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Meftaul, I.M.; Venkateswarlu, K.; Annamalai, P.; Parven, A.; Megharaj, M. Glyphosate Use in Urban Landscape Soils: Fate, Distribution, and Potential Human and Environmental Health Risks. J. Environ. Manag. 2021, 292, 112786. [Google Scholar] [CrossRef]
- Wu, D.; Zhu, Z.-Q.; Tang, H.-X.; Shi, Z.-E.; Kang, J.; Liu, Q.; Qi, J. Efficacy-Shaping Nanomedicine by Loading Calcium Peroxide into Tumor Microenvironment-Responsive Nanoparticles for the Antitumor Therapy of Prostate Cancer. Theranostics 2020, 10, 9808–9829. [Google Scholar] [CrossRef]
- Saghafinia, M.S.; Emadian, S.M.; Vossoughi, M. Performances Evaluation of Photo-Fenton Process and Sonolysis for the Treatment of Penicillin G Formulation Effluent. Procedia Environ. Sci. 2011, 8, 202–208. [Google Scholar] [CrossRef] [Green Version]
- Acosta-Cortés, A.G.; Martinez-Ledezma, C.; López-Chuken, U.J.; Kaushik, G.; Nimesh, S.; Villarreal-Chiu, J.F. Polyphosphate recovery by a native Bacillus cereus strain as a direct effect of glyphosate uptake. ISME J. 2019, 13, 1497–1505. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Kumar, V.; Singh, J. Kinetic Study of the Biodegradation of Glyphosate by Indigenous Soil Bacterial Isolates in Presence of Humic Acid, Fe(III) And Cu(II) Ions. J. Environ. Chem. Eng. 2019, 7, 103098. [Google Scholar] [CrossRef]
- Zhan, H.; Feng, Y.; Fan, X.; Chen, S. Recent Advances in Glyphosate Biodegradation. Appl. Microbiol. Biotechnol. 2018, 102, 5033–5043. [Google Scholar] [CrossRef]
- Liao, H.; Tan, B.; Ke, M.; Li, Z.B.; Lu, J. Pretreatment of Glyphosate Wastewater with Fenton Reagent. Technol. Chem. Ind. 2009, 38, 48–50. [Google Scholar]
- Feng, D.; Soric, A.; Boutin, O. Treatment Technologies and Degradation Pathways of Glyphosate: A Critical Review. Sci. Total Environ. 2020, 742, 140559. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Review of Iron-Free Fenton-like Systems for Activating H2O2 in Advanced Oxidation Processes; Elsevier B.V.: Amsterdam, The Netherlands, 2014; Volume 275. [Google Scholar] [CrossRef]
- Balci, B.; Oturan, M.A.; Oturan, N.; Sirés, I. Decontamination of Aqueous Glyphosate, (Aminomethyl)phosphonic Acid, and Glufosinate Solutions by Electro-Fenton-like Process with Mn2+ as the Catalyst. J. Agric. Food Chem. 2009, 57, 4888–4894. [Google Scholar] [CrossRef]
- De Souza, D.R.; Trovó, A.G.; Filho, N.R.A.; Silva, M.A.A.; Machado, A.E.H. Degradation of the Commercial Herbicide Glyphosate by Photo-Fenton Process: Evaluation of Kinetic Parameters and Toxicity. J. Braz. Chem. Soc. 2013, 24, 1451–1460. [Google Scholar] [CrossRef]
- Sidney Santana, C.; Nicodemos Ramos, M.D.; Vieira Velloso, C.C.; Aguiar, A. Kinetic Evaluation of Dye Decolorization by Fenton Processes in the Presence of 3-Hydroxyanthranilic Acid. Int. J. Environ. Res. Public Health 2019, 16, 1602. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Gu, X.; Lu, S.; Brusseau, M.L.; Xu, M.; Fu, X.; Qiu, Z.; Sui, Q. Application of Ascorbic Acid to Enhance Trichloroethene Degradation by Fe(III)-Activated Calcium Peroxide. Chem. Eng. J. 2017, 325, 188–198. [Google Scholar] [CrossRef]
- Xue, Y.; Rajic, L.; Chen, L.; Lyu, S.; Alshawabkeh, A.N. Electrolytic Control of Hydrogen Peroxide Release from Calcium Peroxide in Aqueous Solution. Electrochem. Commun. 2018, 93, 81–85. [Google Scholar] [CrossRef]
- Li, F.; Shean, T.; Choong, Y.; Soltani, S.; Chuah, L.; Nurul, S.; Jamil, A. Kinetic Study of Fenton-Like Degradation of Methylene Blue in Aqueous Solution Using Calcium Peroxide. Pertanika J. Sci. Technol. 2022, 30, 1087–1102. [Google Scholar] [CrossRef]
- Lu, X.G.; Lin, P.; Jie, Q.W. Study of Pretreatment of Textile Wastewater Using CaO2/H+/Fe2+ Advanced Fenton-Like Oxidation Technology. Appl. Mech. Mater. 2014, 675–677, 568–573. [Google Scholar] [CrossRef]
- Xue, Y.; Lu, S.; Fu, X.; Sharma, V.K.; Mendoza-Sanchez, I.; Qiu, Z.; Sui, Q. Simultaneous Removal of Benzene, Toluene, Ethylbenzene and Xylene (BTEX) By Cao2 Based Fenton System: Enhanced Degradation by Chelating Agents. Chem. Eng. J. 2017, 331, 255–264. [Google Scholar] [CrossRef]
- Domaradzki, M.; Kaniewska, J.; Korpal, W. Oxygen Fertilizers in Technology of Plant Seeds. Influence of CaO2 Additive on the Quality of Pelleted Seeds. Chemik 2012, 66, 464–466. [Google Scholar]
- Tran, M.H.; Nguyen, H.C.; Le, T.S.; Dang, V.A.D.; Cao, T.H.; Le, C.K.; Dang, T.-D. Degradation of Glyphosate Herbicide by an Electro-Fenton Process Using Carbon Felt Cathode. Environ. Technol. 2019, 42, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Madan, S.S.; Wasewar, K.L. Synthesis of CaO2 Nanoparticles for Environmental Remediation. Energy Environ. 2017, 80, 69–76. [Google Scholar] [CrossRef]
- Yuan, D.; Zhang, C.; Tang, S.; Li, X.; Tang, J.; Rao, Y.; Wang, Z.; Zhang, Q. Enhancing CaO2 Fenton-Like Process by Fe(II)-Oxalic Acid Complexation for Organic Wastewater Treatment. Water Res. 2019, 163, 114861. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Wu, P.-S.; Chung, Y.-C. Coupled Biological and Photo-Fenton Pretreatment System for the Removal of Di-(2-Ethylhexyl) Phthalate (DEHP) from Water. Bioresour. Technol. 2009, 100, 4531–4534. [Google Scholar] [CrossRef]
- Lan, H.; He, W.; Wang, A.; Liu, R.; Liu, H.; Qu, J.; Huang, C. An Activated Carbon Fiber Cathode for the Degradation of Glyphosate in Aqueous Solutions by the Electro-Fenton Mode: Optimal Operational Conditions and the Deposition of Iron on Cathode on Electrode Reusability. Water Res. 2016, 105, 575–582. [Google Scholar] [CrossRef]
- Barreto, F.; Santana, C.S.; Aguiar, A. Behavior of Dihydroxybenzenes and Gallic Acid on the Fenton-Based Decolorization of Dyes. Desalination Water Treat. 2016, 57, 431–439. [Google Scholar] [CrossRef]
- Xue, Y.; Sui, Q.; Brusseau, M.L.; Zhou, W.; Qiu, Z.; Lyu, S. Insight into CaO2-Based Fenton and Fenton-Like Systems: Strategy for CaO2-Based Oxidation of Organic Contaminants. Chem. Eng. J. 2018, 361, 919–928. [Google Scholar] [CrossRef]





| Order | Equation Applied | Linear Form by Integration |
|---|---|---|
| Zero-order | ||
| First-order | ||
| Second-order | ||
| BMG model |
| Sample | m(CaO2)/g | V(KMnO4)/mL | Purity/% | Mean |
|---|---|---|---|---|
| 1 | 0.0571 | 10.10 | 63.75 | 63.30% |
| 2 | 0.0587 | 10.25 | 62.95 | |
| 3 | 0.0545 | 9.55 | 63.15 |
| Zero-Order | First-Order | Second-Order | BMG Model | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| pH | Removal (%) | k0 | R2 | k1 | R2 | k2 | R2 | 1/m | 1/b | R2 |
| 2.0 | 83.10 | 0.3108 | 0.9407 | 0.0138 | 0.9876 | 7.2772 | 0.9199 | 0.0327 | 0.9467 | 0.9502 |
| 2.5 | 91.68 | 0.3263 | 0.8843 | 0.0180 | 0.9725 | 0.0014 | 0.7979 | 0.0431 | 1.0185 | 0.9726 |
| 3.0 | 94.50 | 0.3139 | 0.7792 | 0.0250 | 0.9567 | 0.0026 | 0.8790 | 0.0837 | 1.0053 | 0.9919 |
| 3.5 | 92.32 | 0.3449 | 0.8894 | 0.0198 | 0.9549 | 0.0019 | 0.6753 | 0.0465 | 1.0256 | 0.9774 |
| 4.0 | 69.69 | 0.2703 | 0.9819 | 0.0093 | 0.9858 | 3.5069 | 0.9513 | 0.0191 | 0.8627 | 0.9148 |
| Average R2 | 0.8951 | 0.9715 | 0.8447 | 0.9614 | ||||||
| Zero-Order | First-Order | Second-Order | BMG Model | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ratio | Removal (%) | k0 | R2 | k1 | R2 | k2 | R2 | 1/m | 1/b | R2 |
| 1 | 81.99 | 0.3095 | 0.9245 | 0.0128 | 0.9926 | 6.1462 | 0.9794 | 0.0308 | 0.9489 | 0.9610 |
| 2 | 88.60 | 0.2924 | 0.8480 | 0.0132 | 0.9715 | 7.1500 | 0.9768 | 0.0475 | 0.9078 | 0.9827 |
| 4 | 92.36 | 0.3037 | 0.7645 | 0.0217 | 0.9764 | 0.0024 | 0.8959 | 0.0843 | 1.0025 | 0.9922 |
| 6 | 94.50 | 0.2766 | 0.6518 | 0.0250 | 0.9687 | 0.0043 | 0.8556 | 0.1406 | 1.0005 | 0.9970 |
| 8 | 90.94 | 0.2792 | 0.7626 | 0.0150 | 0.9493 | 0.0010 | 0.9825 | 0.0824 | 0.9152 | 0.9936 |
| 10 | 66.62 | 0.2411 | 0.9399 | 0.0082 | 0.9836 | 2.9442 | 0.9736 | 0.0275 | 0.7322 | 0.9576 |
| Average R2 | 0.8152 | 0.9737 | 0.9440 | 0.9807 | ||||||
| Zero-Order | First-Order | Second-Order | BMG Model | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Initial CaO2 Dosage/g | Removal (%) | k0 | R2 | k1 | R2 | k2 | R2 | 1/m | 1/b | R2 |
| 0.1 | 55.08 | 0.1862 | 0.7638 | 0.0128 | 0.9926 | 1.9050 | 0.9197 | 0.0457 | 0.5901 | 0.9932 |
| 0.3 | 76.81 | 0.2656 | 0.8116 | 0.0132 | 0.9715 | 5.1894 | 0.9928 | 0.0553 | 0.8291 | 0.9904 |
| 0.5 | 94.50 | 0.2788 | 0.6425 | 0.0217 | 0.9764 | 0.0045 | 0.9146 | 0.1493 | 1.0058 | 0.9977 |
| 0.7 | 89.82 | 0.2828 | 0.6966 | 0.0205 | 0.9687 | 0.0015 | 0.9940 | 0.1106 | 0.8693 | 0.9970 |
| 0.9 | 85.15 | 0.2858 | 0.7873 | 0.0150 | 0.9493 | 9.5876 | 0.9953 | 0.0747 | 0.8404 | 0.9926 |
| Average R2 | 0.7404 | 0.9717 | 0.9633 | 0.9942 | ||||||
| Zero-Order | First-Order | Second-Order | BMG Model | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Initial Glyphosate Concentration/ppm | Removal (%) | k0 | R2 | k1 | R2 | k2 | R2 | 1/m | 1/b | R2 |
| 10 | 99.67 | 0.2407 | 0.5264 | 0.1142 | 0.8862 | 0.0899 | 0.8229 | 0.5905 | 0.9817 | 0.9969 |
| 20 | 96.35 | 0.5687 | 0.8190 | 0.0813 | 0.9720 | 0.0170 | 0.9788 | 0.0530 | 1.0172 | 0.9641 |
| 30 | 86.88 | 0.7571 | 0.9070 | 0.0509 | 0.9842 | 0.0039 | 0.9812 | 0.0321 | 0.8913 | 0.9553 |
| 40 | 77.07 | 0.8981 | 0.8714 | 0.0399 | 0.9569 | 0.0020 | 0.9840 | 0.0499 | 0.7845 | 0.9769 |
| 50 | 74.10 | 0.9768 | 0.9374 | 0.0355 | 0.8701 | 0.0014 | 0.9426 | 0.0168 | 0.7851 | 0.9357 |
| Average R2 | 0.8122 | 0.9338 | 0.9419 | 0.9658 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Choong, T.S.Y.; Soltani, S.; Chuah Abdullah, L.; Md. Jamil, S.N.A.; Amerhaider Nuar, N.N. Investigation of Glyphosate Removal from Aqueous Solutions Using Fenton-like System Based on Calcium Peroxide. Processes 2022, 10, 2045. https://doi.org/10.3390/pr10102045
Li F, Choong TSY, Soltani S, Chuah Abdullah L, Md. Jamil SNA, Amerhaider Nuar NN. Investigation of Glyphosate Removal from Aqueous Solutions Using Fenton-like System Based on Calcium Peroxide. Processes. 2022; 10(10):2045. https://doi.org/10.3390/pr10102045
Chicago/Turabian StyleLi, Fan, Thomas Shean Yaw Choong, Soroush Soltani, Luqman Chuah Abdullah, Siti Nurul Ain Md. Jamil, and Nurul Nazihah Amerhaider Nuar. 2022. "Investigation of Glyphosate Removal from Aqueous Solutions Using Fenton-like System Based on Calcium Peroxide" Processes 10, no. 10: 2045. https://doi.org/10.3390/pr10102045
APA StyleLi, F., Choong, T. S. Y., Soltani, S., Chuah Abdullah, L., Md. Jamil, S. N. A., & Amerhaider Nuar, N. N. (2022). Investigation of Glyphosate Removal from Aqueous Solutions Using Fenton-like System Based on Calcium Peroxide. Processes, 10(10), 2045. https://doi.org/10.3390/pr10102045








