Reaction of Chloroacetyl-Modified Peptides with Mercaptoundecahydrododecaborate (BSH) Is Accelerated by Basic Amino Acid Residues in the Peptide
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Reactivity of BSH with Cl-3X
3.2. Reactivity of BSH with Cl-3R, Cl-2R and Cl-1R
3.3. Reactivity of BSH with Cl-Cn-3R
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Barth, R.F.; Vicente, M.G.H.; Harling, O.K.; Kiger, W.S., III.; Riley, K.J.; Binns, P.J.; Franz, M.; Wagner, F.M.; Suzuki, M.; Aihara, T.; et al. Current status of boron neutron capture therapy of high grade gliomas and recurrent head and neck cancer. Radiat. Oncol. 2012, 7, 146–166. [Google Scholar] [CrossRef] [PubMed]
- Kato, I.; Ono, K.; Sakurai, Y.; Ohmae, M.; Maruhashi, A.; Imahori, Y.; Kirihata, M.; Nakazawa, M.; Yura, Y. Effectiveness of BNCT for recurrent head and neck malignancies. Appl. Radiat. Isot. 2004, 61, 1069–1073. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Yang, Z.; Zhang, L.; Xie, L.; Wang, L.; Xu, H.; Josephson, L.; Liang, S.H.; Zhang, M.-R. Boron agents for neutron capture therapy. Coord. Chem. Rev. 2020, 405, 213139–213158. [Google Scholar] [CrossRef]
- Barth, R.F.; Mi, P.; Yang, W. Boron delivery agents for neutron capture therapy of cancer. Cancer Commun. 2018, 38, 1–15. [Google Scholar] [CrossRef]
- Lamba, M.; Goswami, A.; Bandyopadhyay, A. A periodic development of BPA and BSH based derivatives in boron neutron capture therapy (BNCT). Chem. Commun. 2021, 57, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Michiue, H.; Sakurai, Y.; Kondo, N.; Kitamatsu, M.; Bin, F.; Nakajima, K.; Hirota, Y.; Kawabata, S.; Nishiki, T.; Ohmori, I.; et al. The acceleration of boron neutron capture therapy using multi-linked mercaptoundecahydrododecaborate (BSH) fused cell-penetrating peptide. Biomaterials 2014, 35, 3396–3405. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, Y.; Michiue, H.; Kitamatsu, M.; Hayashi, Y.; Takenaka, F.; Nishiki, T.; Matsui, H. Tumor-specific delivery of BSH-3R for boron neutron capture therapy and positron emission tomography imaging in a mouse brain tumor model. Biomaterials 2015, 56, 10–17. [Google Scholar] [CrossRef]
- Nakase, I.; Katayama, M.; Hattori, Y.; Ishimura, M.; Inaura, S.; Fujiwara, D.; Takatani-Nakase, T.; Fujii, I.; Futaki, S.; Kirihata, M. Intracellular target delivery of cell-penetrating peptide-conjugated dodecaborate for boron neutron capture therapy (BNCT). Chem. Commun. 2019, 55, 13955–13958. [Google Scholar] [CrossRef]
- Kitamatsu, M.; Nakamura-Tachibana, A.; Ishikawa, Y.; Michiue, H. Improvement of water solubility of mercaptoundecahydrododecaborate (BSH)-peptides by conjugating with ethylene glycol linker and interaction with cyclodextrin. Processes 2020, 9, 167. [Google Scholar] [CrossRef]
- Kimura, S.; Masunaga, S.; Harada, T.; Kawamura, Y.; Ueda, S.; Okuda, K.; Nagasawa, H. Synthesis and evaluation of cyclic RGD-boron cluster conjugates to develop tumor-selective boron carriers for boron neutron capture therapy. Bioorg. Med. Chem. 2011, 19, 1721–1728. [Google Scholar] [CrossRef]
- Ol’shevskaya, V.A.; Alpatova, V.M.; Radchenko, A.S.; Ramonova, A.A.; Petrova, A.S.; Tatarskiy, V.V.; Zaitsev, A.V.; Kononova, E.G.; Ikonnikov, N.S.; Kostyukov, A.A.; et al. β-Maleimide substituted meso-arylporphyrins: Synthesis, transformations, physico-chemical and antitumor properties. Dyes Pigm. 2019, 171, 107760–107773. [Google Scholar] [CrossRef]
- Chen, J.; Yang, Q.; Liu, M.; Lin, M.; Wang, T.; Zhang, Z.; Zhong, X.; Guo, N.; Lu, Y.; Xu, J.; et al. Remarkable boron delivery of iRGD-modified polymeric nanoparticles for boron neutron capture therapy. Int. J. Nanomed. 2019, 14, 8161–8177. [Google Scholar] [CrossRef] [PubMed]
- Nakase, I.; Aoki, A.; Sakai, Y.; Hirase, S.; Ishimura, M.; Takatani-Nakase, T.; Hattori, Y.; Kirihata, M. Antibody-based receptor targeting using an Fc-binding peptide-dodecaborate conjugate and macropinocytosis induction for boron neutron capture therapy. ACS Omega 2020, 5, 22731–22738. [Google Scholar] [CrossRef] [PubMed]
- Ol’shevskaya, V.A.; Alpatova, V.M.; Makarenkov, A.V.; Kononova, E.G.; Smol’yakov, A.F.; Peregudov, A.S.; Rys, E.G. Synthesis of maleimide-functionalized carboranes and their utility in Michael addition reactions. New J. Chem. 2021, 45, 12159–12167. [Google Scholar] [CrossRef]
- Smith, S.W. Chiral toxicology: It’s the same thing…only different. Toxicol. Sci. 2009, 110, 4–30. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Ueno, U.; Lee, J.D.; Ban, H.S.; Justus, E.; Fan, P.; Gabel, D. Synthesis of dodecaborate-conjugated cholesterols for efficient boron delivery in neutron capture therapy. Tetrahedron Lett. 2007, 48, 3151–3154. [Google Scholar] [CrossRef]
- Justus, E.; Awad, D.; Hohnholt, M.; Schaffran, T.; Edwards, K.; Karlsson, G.; Damian, L.; Gabel, D. Synthesis, liposomal preparation, and in vitro toxicity of two novel dodecaborate cluster lipids for boron neutron capture therapy. Bioconjugate Chem. 2007, 18, 1287–1293. [Google Scholar] [CrossRef]
- Lee, J.D.; Ueno, M.; Miyajima, Y.; Nakamura, H. Synthesis of boron cluster lipids: Closo-dodecaborate as an alternative hydrophilic function of boronated liposomes for neutron capture therapy. Org. Lett. 2007, 9, 323–326. [Google Scholar] [CrossRef]
- El-Zaria, M.E.; Nakamura, H. New strategy for synthesis of mercaptoundecahydrododecaborate derivatives via click chemistry: Possible boron carriers and visualization in cells for neutron capture therapy. Inorg. Chem. 2009, 48, 11896–11902. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Sivaev, I.B.; Petrovskii, P.V.; Bregadze, V.I. Synthesis of monosubstituted functional derivatives of carboranes from 1-mercapto-ortho-carborane: 1-HOOC(CH2)nS-1,2-C2B10H11 and [7-HOOC(CH2)nS-7,8-C2B9H11]- (n = 1–4). Dalton Trans. 2010, 39, 1817–1822. [Google Scholar] [CrossRef]
- Kusaka, S.; Hattori, Y.; Uehara, K.; Asano, T.; Tanimori, S.; Kirihata, M. Synthesis of optically active dodecaborate-containing L-amino acids for BNCT. Appl. Radiat. Isot. 2011, 69, 1768–1770. [Google Scholar] [CrossRef]
- Hattori, Y.; Kusaka, S.; Mukumoto, M.; Uehara, K.; Asano, T.; Suzuki, M.; Masunaga, S.; Ono, K.; Tanimori, S.; Kirihata, M. Biological evaluation of dodecaborate-containing L-amino acids for boron neutron capture therapy. J. Med. Chem. 2012, 55, 6980–6984. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Ueda, N.; Ban, H.S.; Ueno, M.; Tachikawa, S. Design and synthesis of fluorescence-labeled closo-dodecaborate lipid: Its liposome formation and in vivo imaging targeting of tumors for boron neutron capture therapy. Org. Biomol. Chem. 2012, 10, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Asano, R.; Nagami, A.; Fukumoto, Y.; Miura, K.; Yazama, F.; Ito, H.; Sakata, I.; Tai, A. Synthesis and biological evaluation of new BSH-conjugated chlorin derivatives as agents for both photodynamic therapy and boron neutron capture therapy of cancer. J. Photochem. Photobiol. B Biol. 2014, 140, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Genady, A.R.; Ioppolo, J.A.; Azaam, M.M.; El-Zaria, M.E. New functionalized mercaptoundecahydrododecaborate derivatives for potential application in boron neutron capture therapy: Synthesis, characterization and dynamic visualization in cells. Eur. J. Med. Chem. 2015, 93, 574–583. [Google Scholar] [CrossRef]
- Worm, D.J.; Els-Heindl, S.; Kellert, M.; Kuhnert, R.; Saretz, S.; Koebberling, J.; Riedl, B.; Hey-Hawkins, E.; Beck-Sickinger, A.G. A stable meta-carborane enables the generation of boron-rich peptide agonists targeting the ghrelin receptor. J. Pept. Sci. 2018, 24, e3119. [Google Scholar] [CrossRef]
- Mochizuki, M.; Sato, S.; Asatyas, S.; Leśnikowski, Z.J.; Hayashi, T.; Nakamura, H. Raman cell imaging with boron cluster molecules conjugated with biomolecules. RSC Adv. 2019, 9, 23973–23978. [Google Scholar] [CrossRef]
- Worm, D.J.; Hoppenz, P.; Els-Heindl, S.; Kellert, M.; Kuhnert, R.; Saretz, S.; Köbberling, J.; Riedl, B.; Hey-Hawkins, E.; Beck-Sickinger, A.G. Selective neuropeptide Y conjugates with maximized carborane loading as promising boron delivery agents for boron neutron capture therapy. J. Med. Chem. 2020, 63, 2358–2371. [Google Scholar] [CrossRef]
- Takeuchi, K.; Hattori, Y.; Kawabata, S.; Futamura, G.; Hiramatsu, R.; Wanibuchi, M.; Tanaka, H.; Masunaga, S.; Ono, K.; Miyatake, S.; et al. Synthesis and evaluation of dodecaboranethiol containing kojic acid (KA-BSH) as a novel agent for boron neutron capture therapy. Cells 2020, 9, 1551. [Google Scholar] [CrossRef]
- Kubasov, A.S.; Turyshev, E.S.; Novikov, I.V.; Gurova, O.M.; Starodubets, P.A.; Golubev, A.V.; Zhizhin, K.Y.; Kuznetsov, N.T. Theoretical and experimental comparison of the reactivity of the sulfanyl-closo-decaborate and sulfanyl-closo-dodecaborate anions and their mono-S-substituted derivatives. Polyhedron 2021, 206, 115347–115356. [Google Scholar] [CrossRef]
- Woods, M.; Sherry, A.D. An improved and versatile synthetic route to 6,7:13,14-dibenzo-1,8,4,11-dioxadiazacyclotetradecane. Inorg. Chim. Acta. 2003, 351, 395–398. [Google Scholar] [CrossRef]
- Kawamura, A.; Münzel, M.; Kojima, T.; Yapp, C.; Bhushan, B.; Goto, Y.; Tumber, A.; Katoh, T.; King, O.N.F.; Passioura, T.; et al. Highly selective inhibition of histone demethylases by de novo macrocyclic peptides. Nat. Commun. 2017, 8, 14773–14782. [Google Scholar] [CrossRef] [PubMed]

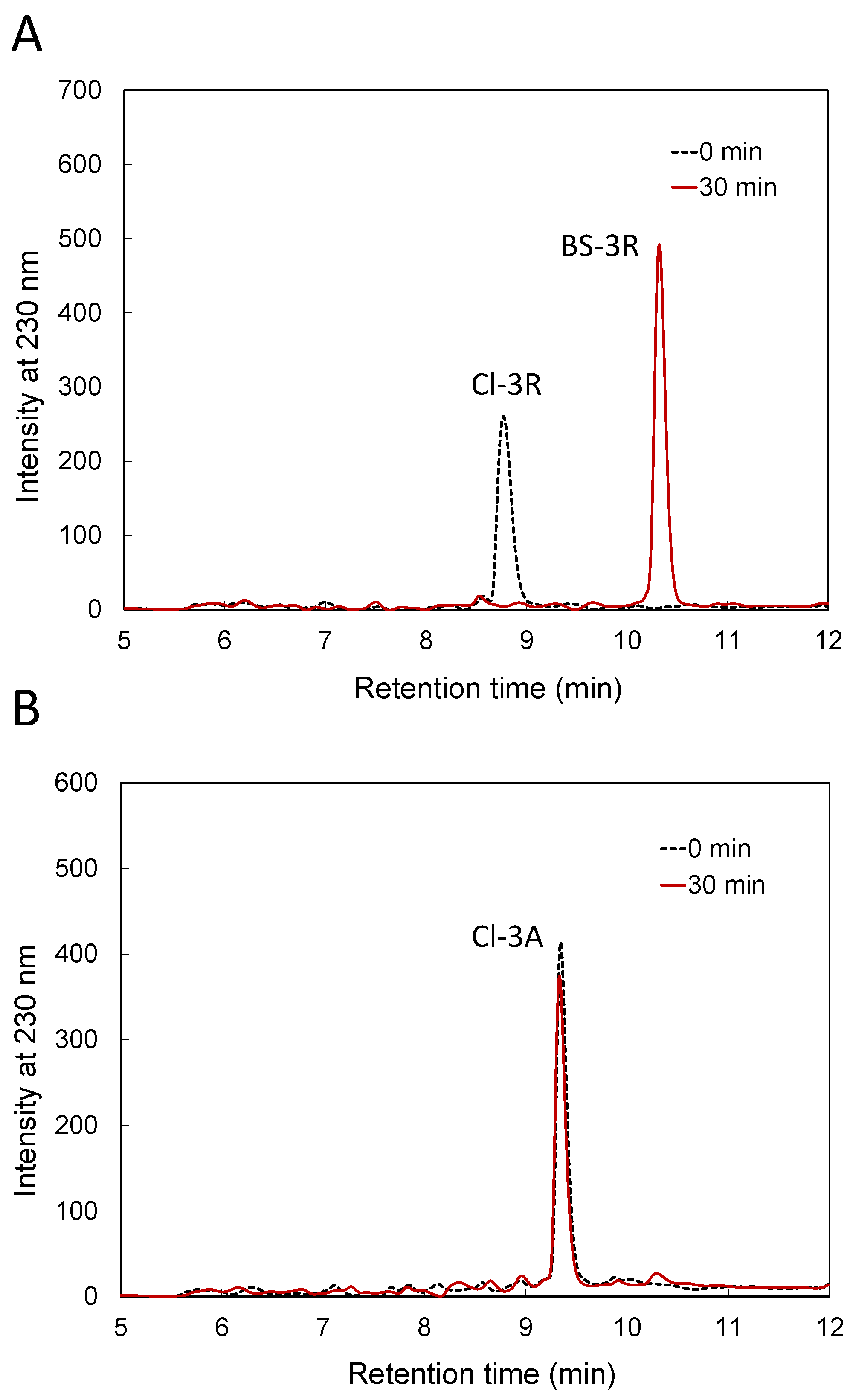

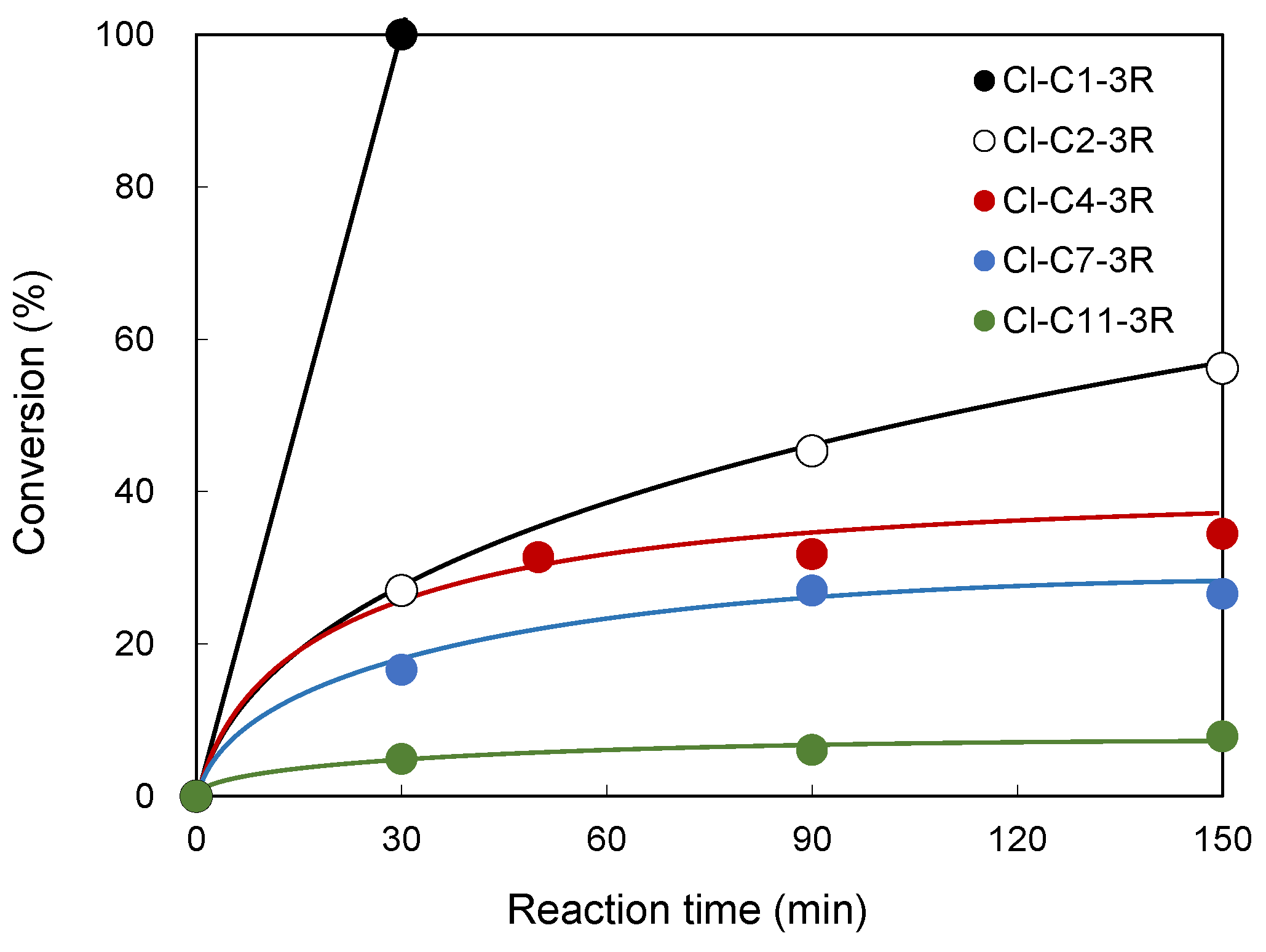
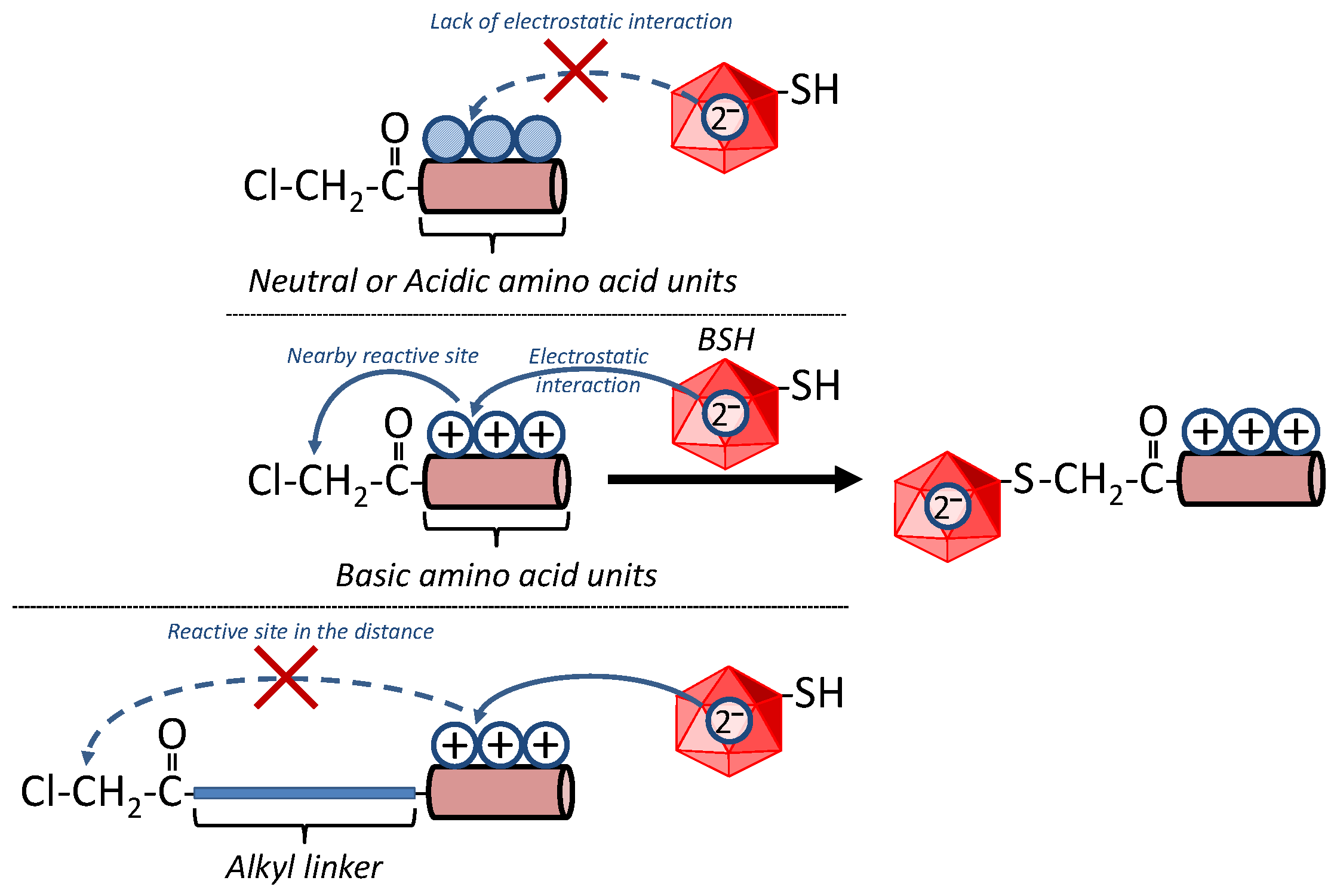
| Tripeptides for Which the Reaction Proceeded | Tripeptides for Which the Reaction Did Not Proceed |
|---|---|
| BS-3X | BSH/Cl-3X |
| X = His, Lys, Arg | X = Ala, Asp, Glu, Phe, Gly, Ile, Leu, Asn, Gln, Ser, Thr, Val, Trp, Tyr |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitamatsu, M.; Inoue, K.; Yamagata, N.; Michiue, H. Reaction of Chloroacetyl-Modified Peptides with Mercaptoundecahydrododecaborate (BSH) Is Accelerated by Basic Amino Acid Residues in the Peptide. Processes 2022, 10, 2200. https://doi.org/10.3390/pr10112200
Kitamatsu M, Inoue K, Yamagata N, Michiue H. Reaction of Chloroacetyl-Modified Peptides with Mercaptoundecahydrododecaborate (BSH) Is Accelerated by Basic Amino Acid Residues in the Peptide. Processes. 2022; 10(11):2200. https://doi.org/10.3390/pr10112200
Chicago/Turabian StyleKitamatsu, Mizuki, Ken Inoue, Naoki Yamagata, and Hiroyuki Michiue. 2022. "Reaction of Chloroacetyl-Modified Peptides with Mercaptoundecahydrododecaborate (BSH) Is Accelerated by Basic Amino Acid Residues in the Peptide" Processes 10, no. 11: 2200. https://doi.org/10.3390/pr10112200
APA StyleKitamatsu, M., Inoue, K., Yamagata, N., & Michiue, H. (2022). Reaction of Chloroacetyl-Modified Peptides with Mercaptoundecahydrododecaborate (BSH) Is Accelerated by Basic Amino Acid Residues in the Peptide. Processes, 10(11), 2200. https://doi.org/10.3390/pr10112200






