Comparative Evaluation of Pyrolysis and Hydrothermal Liquefaction for Obtaining Biofuel from a Sustainable Consortium of Microalgae Arthrospira platensis with Heterotrophic Bacteria
Abstract
1. Introduction
2. Materials and Methods
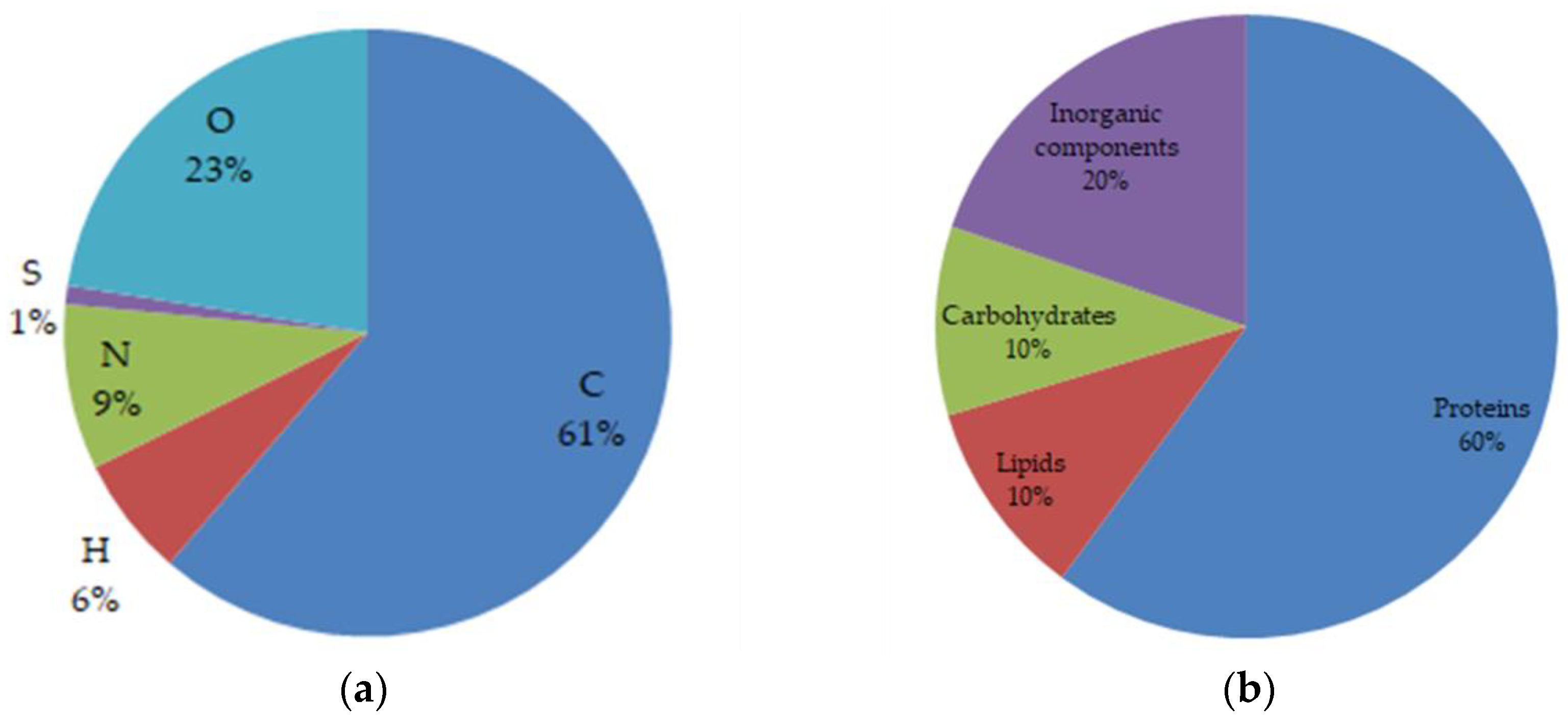
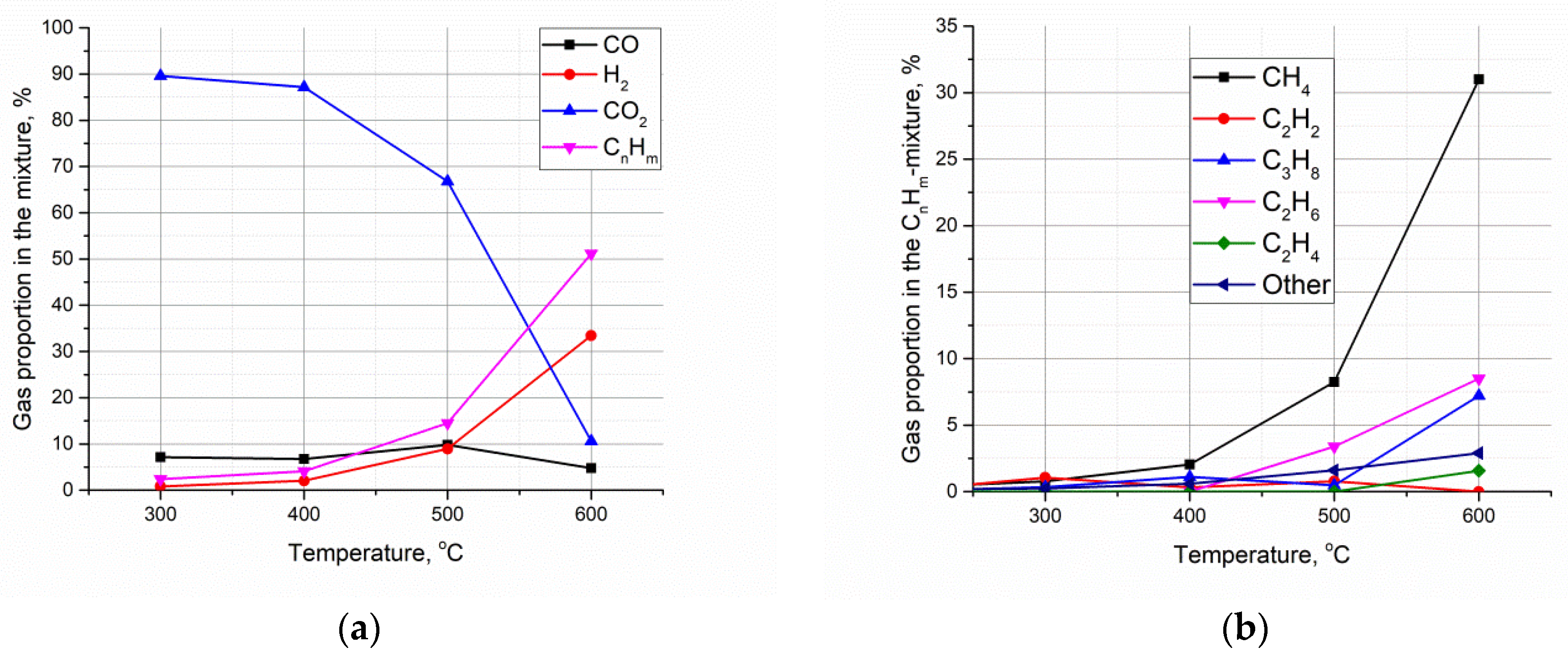
3. Results and Discussion
- -
- Non-condensable pyrolysis gases in the amount of 76.7 wt.% of the original dry weight of the sample;
- -
- Pyrolysis liquid (bio-oil)—about 21.9 wt.%.;
- -
- Residual semi-coke (biochar)—about 27 wt.% of the original dry weight of the sample.
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Our Climate Target. Shell [Electronic Resource] Official Site. Available online: https://www.shell.com/energy-and-innovation/the-energy-future/our-climate-target/_jcr_content/par/relatedtopics.stream/1660578577521/3e2b80bcac2d053636ad41890ac7eebd33aadf8a/shell-our-climate-target-ax.pdf (accessed on 25 October 2022).
- Zhu, X.G.; Long, S.P.; Ort, D.R. What is the maximum efficiency with which photosynthesis can convert solar energy into biomass? Curr. Opin. Biotechnol. 2008, 19, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Hielmann, S.M.; Davis, H.T.; Jader, L.R.; Lefebvre, P.A.; Sadowsky, M.J.; Schendel, F.J.; von Keitz, M.G.; Valentas, K.J. Hydrothermal carbonization of microalgae. Biom. Bioen. 2010, 34, 875–882. [Google Scholar] [CrossRef]
- Vlaskin, M.S.; Grigorenko, A.V.; Chernova, N.I.; Kiseleva, S.V.; Kumar, V. Bio-oil production by hydrothermal liquefaction of microalgae biomass. Altern. Energy Ecolog. 2018, 22–24, 23–33. [Google Scholar] [CrossRef]
- Gua, X.; Yu, L.; Pang, N.; Martinez-Fernandez, J.S.; Fu, X.; Chen, S. Comparative techno-economic analysis of algal biofuel production via hydrothermal liquefaction: One stage versus two stages. Appl. Energy 2020, 259, 114115. [Google Scholar] [CrossRef]
- Su, Y.; Song, K.; Zhang, P.; Su, Y.; Cheng, J.; Chen, X. Progress of microalgae biofuel’s commercialization. Ren. Sustain. Energy Rev. 2017, 74, 402–411. [Google Scholar] [CrossRef]
- Han, T.; Lu, H.F.; Ma, S.S.; Zhang, Y.H.; Liu, Z.D.; Duan, N. Progress in microalgae cultivation photobioreactors and applications in wastewater treatment: A review. Int. J. Agric. Biol. Eng. 2017, 10, 1–29. [Google Scholar]
- García-González, M.; Fernández, J.M.; Canavate, J.P.; Anguis, V. Conditions for open-air outdoor culture of Dunaliella salina in Southern Spain. J. Appl. Phycol. 2003, 15, 177–184. [Google Scholar] [CrossRef]
- Muller, E.E.L.; Sheik, A.R.; Wilmes, P. Lipid-based biofuel production from wastewater. Curr. Opin. Biotechn. 2014, 30, 9–16. [Google Scholar] [CrossRef]
- Chernova, N.I.; Kiseleva, S.V. Microalgae biofuels: Induction of lipid synthesis for biodiesel production and biomass residues into hydrogen conversion. Int. J. Hydr. Energy 2017, 42, 2861–2867. [Google Scholar] [CrossRef]
- Zhang, B.; Li, W.; Guo, Y.; Zhang, Z.; Shi, W.; Cui, F.; Lens, P.N.; Tay, J.H. Microalgal-bacterial consortia: From interspecies interactions to biotechnological applications. Ren. Sustain. Energy Rev. 2020, 118, 109563. [Google Scholar] [CrossRef]
- Raslavičius, L.; Semenov, V.G.; Chernova, N.I.; Keršys, A.; Kopeyka, A.K. Producing transportation fuels from algae: In search of synergy. Ren. Sustain Energy Rev. 2014, 40, 133–142. [Google Scholar] [CrossRef]
- Gouveia, L.; Oliveira, A.C. Microalgae as a raw material for biofuels production. J. Ind. Microbiol. Biotechnol. 2009, 36, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Raikova, S.; Smith-Baedorf, H.; Bransgrove, R.; Barlow, O.; Santomauro, F.; Wagner, J.L.; Allen, M.J.; Bryan, C.G.; Sapsford, D.; Chuck, C.J. Assessing hydrothermal liquefaction for the production of bio-oil and enhanced metal recovery from microalgae cultivated on acid mine drainage. Fuel Process. Technol. 2016, 142, 219–227. [Google Scholar] [CrossRef]
- Chernova, N.I.; Kiseleva, S.V.; Vlaskin, M.S.; Rafikova, Y.Y. Renewable energy technologies: Enlargement of biofuels list and co-products from microalgae. MATEC Web Conf. 2017, 112, 10010. [Google Scholar] [CrossRef]
- Chernova, N.I.; Kiseleva, S.V.; Popel, O.S. Efficiency of the biodiesel production from microalgae. Therm. Eng. 2014, 61, 399–405. [Google Scholar] [CrossRef]
- Vlaskin, M.S.; Chernova, N.I.; Kiseleva, S.V.; Popel, O.S.; Zhuk, A.Z. Hydrothermal liquefaction of microalgae to produce biofuels: State of the art and future prospects. Therm. Eng. 2017, 64, 627–636. [Google Scholar] [CrossRef]
- Zarrouk, C. Contribution a L’etuded’unecyanophycee: Influence de Diverse Facteurs Physiques et Chimiquessur la Croissance et la Photosynthese de Spirulina Maxima (Setch et Gardner) Geitler. Ph.D. Thesis, University of Paris, Paris, France, 1966. [Google Scholar]
- Posadas, E.; Alcantara, C.; Garcí-Encina, P.A.; Gouveia, L.; Guieysse, B.; Norvill, Z.; Acién, F.G.; Markou, G.; Congestri, R.; Koreiviene, J.; et al. Microalgae cultivation in wastewater. In Microalgae-Based Biofuels and Bioproducts: From Feedstock Cultivation to End-Products; Elsevier: Amsterdam, The Netherlands, 2017; Chapter 3; pp. 67–92. [Google Scholar]
- Dawson, R.M.C.; Elliott, D.C.; Elliott, W.H.; Jones, K.M. Data for Biochemical Research, 3rd ed.; Oxford Science Publications: Oxford, UK, 1986; p. 580. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Kosov, V.; Kosov, V.; Sinelschikov, V.; Zaichenko, V. High-calorific gas mixtures produced by pyrolysis of wood and peat. In Proceedings of the XVII European Biomass Conference and Exhibition 2009, Hamburg, Germany, 29 June–3 July 2009; pp. 1085–1088. [Google Scholar]
- Vlaskin, M.S.; Grigorenko, A.V.; Chernova, N.I.; Kiseleva, S.V.; Kumar, V.; Pruthi, V. Bio-oil production by hydrothermal liquefaction of wet biomass of microalgae in a plant with heat recovery. SSRN Electron. J. 2018, 11, 1–11. [Google Scholar] [CrossRef]
- Gururani, P.; Bhatnagar, P.; Bisht, B.; Kumar Jaiswal, K.; Kumar, V.; Kumar, S.; Vlaskin, M.S.; Grigorenko, A.V.; Rindin, K.G. Recent advances and viability in sustainable thermochemical conversion of sludge to bio-fuel production. Fuel 2022, 316, 123351. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chernova, N.I.; Grigorenko, A.V.; Kiseleva, S.V.; Larina, O.M.; Kumar, V.; Vlaskin, M.S. Comparative Evaluation of Pyrolysis and Hydrothermal Liquefaction for Obtaining Biofuel from a Sustainable Consortium of Microalgae Arthrospira platensis with Heterotrophic Bacteria. Processes 2022, 10, 2202. https://doi.org/10.3390/pr10112202
Chernova NI, Grigorenko AV, Kiseleva SV, Larina OM, Kumar V, Vlaskin MS. Comparative Evaluation of Pyrolysis and Hydrothermal Liquefaction for Obtaining Biofuel from a Sustainable Consortium of Microalgae Arthrospira platensis with Heterotrophic Bacteria. Processes. 2022; 10(11):2202. https://doi.org/10.3390/pr10112202
Chicago/Turabian StyleChernova, Nadezhda I., Anatolii V. Grigorenko, Sophia V. Kiseleva, Olga M. Larina, Vinod Kumar, and Mikhail S. Vlaskin. 2022. "Comparative Evaluation of Pyrolysis and Hydrothermal Liquefaction for Obtaining Biofuel from a Sustainable Consortium of Microalgae Arthrospira platensis with Heterotrophic Bacteria" Processes 10, no. 11: 2202. https://doi.org/10.3390/pr10112202
APA StyleChernova, N. I., Grigorenko, A. V., Kiseleva, S. V., Larina, O. M., Kumar, V., & Vlaskin, M. S. (2022). Comparative Evaluation of Pyrolysis and Hydrothermal Liquefaction for Obtaining Biofuel from a Sustainable Consortium of Microalgae Arthrospira platensis with Heterotrophic Bacteria. Processes, 10(11), 2202. https://doi.org/10.3390/pr10112202





