Bioassay-Guided Characterization, Antioxidant, Anti-Melanogenic and Anti-Photoaging Activities of Pueraria thunbergiana L. Leaf Extracts in Human Epidermal Keratinocytes (HaCaT) Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
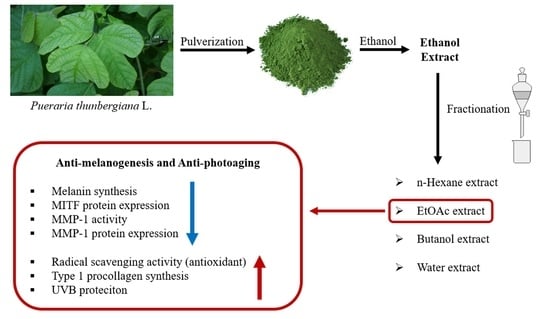
2.2. Preparation of P. thunbergiana L. Extract and Production of Different Fractionations
2.3. Total Phenolic Content (TPC)
2.4. Total Flavonoid Content (TFC)
2.5. Antioxidant Activity Evaluation
2.6. Cell Culture
2.7. Cell Viability Assay
2.8. Measurement of Melanin Content
2.9. UVB Protection Assay
2.10. Measurement of Type 1 Pro-Collagen Synthesis
2.11. Measurement of MMP-1 Inhibitory Activity
2.12. Western Blot Analysis
2.13. High-Performance Liquid Chromatography Analysis
2.14. Statistical Analyses
3. Results
3.1. Total Phenolic and Flavonoid Content of P. thunbergiana L. Extracts and Fractions
3.2. Antioxidant Activity of P. thunbergiana L. Extracts and Fractions
3.3. Inhibitory Effect of P. thunbergiana L. Extracts and Fractions on Melanogenesis in B16F10 Cells
3.4. Inhibitory Effect of P. thunbergiana L. Fractions on MITF Expression in B16F10 Cells
3.5. Effect of P. thunbergiana L. EtOAc Fraction on UVB-Induced Damage in HaCaT Cells
3.6. Effect of P. thunbergiana L. EtOAc Fraction on Anti-Photoaging in HaCaT Cells
3.7. Inhibitory Effect of P. thunbergiana L. Fractions on MMP-1 Expression in HaCaT Cells
3.8. High-Performance Liquid Chromatography (HPLC) Analysis of P. thunbergiana L. EtOAc Fraction
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Oh, J.Y.; Kim, Y.S.; Lee, H.G.; Lee, J.S.; Jeon, Y.J. Anti-photoaging and anti-melanogenesis effects of fucoidan isolated from Hizikia fusiforme and its underlying mechanisms. Mar. Drugs 2020, 18, 427. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, H.; Waditee-Sirisattha, R. Antioxidative, anti-inflammatory, and anti-aging properties of mycosporine-like Amino Acids: Molecular and cellular mechanisms in the protection of skin-aging. Mar. Drugs 2019, 17, 222. [Google Scholar] [CrossRef] [Green Version]
- Pangestuti, R.; Siahaan, E.A.; Kim, S.K. Photoprotective substances derived from Marine Algae. Mar. Drugs 2018, 16, 399. [Google Scholar] [CrossRef] [Green Version]
- Perez Davo, A.; Truchuelo, M.T.; Vitale, M.; Gonzalez-Castro, J. Efficacy of an antiaging treatment against environmental factors: Deschampsia antarctica extract and high-tolerance retinoids combination. J. Clin. Aesthetic Dermatol. 2019, 12, E65–E70. [Google Scholar]
- Gao, W.; Lin, P.; Hwang, E.; Wang, Y.; Yan, Z.; Ngo, H.T.T.; Yi, T.H. Pterocarpus santalinus L. regulated ultraviolet B irradiation-induced procollagen reduction and matrix metalloproteinases expression through activation of TGF-β/Smad and inhibition of the MAPK/AP-1 pathway in normal human dermal fibroblasts. Photochem. Photobiol. 2018, 94, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.Y.; Kim, G.W.; Kang, S.W.; Cho, H.S.; Kim, E.J.; Park, K.M. Anti-aging and anti-melanogenesis effect of Cimicifuge dahurica, Coptis chinensis, Phellodendri amurense and Magnol obovata extracts mixture. J. Soc. Cosmet. Sci. Korea 2017, 43, 1–10. [Google Scholar]
- Papaccio, F.; D’Arino, A.; Caputo, S.; Bellei, B. Focus on the Contribution of Oxidative Stress in Skin Aging. Antioxidants 2022, 11, 1121. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.H.; Shin, D.W. The Molecular Mechanism of Polyphenols with Anti-Aging Activity in Aged Human Dermal Fibroblasts. Molecules 2022, 27, 4351. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, H.; Zheng, Y.; Zheng, L.; Wang, X.; Luo, Z.; Tang, J.; Lin, L.; Du, Z.; Dong, C. The effects and mechanism of collagen peptide and elastin peptide on skin aging induced by D-galactose combined with ultraviolet radiation. J. Photochem. Photobiol. B: Biol. 2020, 210, 111964. [Google Scholar] [CrossRef]
- Shin, J.W.; Kwon, S.H.; Choi, J.Y.; Na, J.I.; Huh, C.H.; Choi, H.R.; Park, K.C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef] [Green Version]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in Skin Health, Repair, and Disease: A Comprehensive Review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, D.J.; Jung, J.C.; Choi, Y.M.; Ryu, H.Y.; Lee, S.; Davis, B.A. Wheat Extract Oil (WEO) Attenuates UVB-Induced Photoaging via Collagen Synthesis in Human Keratinocytes and Hairless Mice. Nutrients 2020, 12, 300. [Google Scholar] [CrossRef] [PubMed]
- Opperman, L.; De Kock, M.; Klaasen, J.; Rahiman, F. Tyrosinase and melanogenesis inhibition by indigenous African plants: A Review. Cosmetics 2020, 7, 60. [Google Scholar] [CrossRef]
- Han, H.J.; Park, S.K.; Kang, J.Y.; Kim, J.M.; Yoo, S.K.; Heo, H.J. Anti-melanogenic effect of ethanolic extract of Sorghum bicolor on IBMX–induced melanogenesis in B16/F10 melanoma cells. Nutrients 2020, 12, 832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.S.; Wu, W.C.; Lin, S.Y.; Hou, W.C. Glycine hydroxamate inhibits tyrosinase activity and melanin contents through downregulating cAMP/PKA signaling pathways. Amino Acids 2015, 47, 617–625. [Google Scholar] [CrossRef]
- Boo, Y.C. p-Coumaric Acid as An Active Ingredient in Cosmetics: A Review Focusing on its Antimelanogenic Effects. Antioxidants 2019, 8, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Bino, S.; Duval, C.; Bernerd, F. Clinical and biological characterization of skin pigmentation diversity and its consequences on UV impact. Int. J. Mol. Sci. 2018, 19, 2668. [Google Scholar] [CrossRef] [Green Version]
- Cadet, J.; Douki, T. Formation of UV-induced DNA damage contributing to skin cancer development. Photochem. Photobiol. Sci. 2018, 17, 1816–1841. [Google Scholar] [CrossRef]
- Passeron, T.; Krutmann, M.L.; Andersen, R.; Katta, C.C.; Zouboulis, C.C. Clinical and biological impact of the exposome on the skin. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 4–25. [Google Scholar] [CrossRef]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [Green Version]
- Sim, Y.Y.; Tan, C.P.; Cheong, L.Z.; Nyam, K.L. Hibiscus cannabinus L. leaf and seed in cosmetic formulation: An integrated approach as antioxidant and melanogenesis inhibitor. Sustain. Mater. Technol. 2022, 33, e00457. [Google Scholar] [CrossRef]
- Kim, D.E.; Chang, B.Y.; Ham, S.O.; Kim, Y.C.; Kim, S.Y. Neobavaisoflavone inhibits melanogenesis through the regulation of Akt/GSK-3β and MEK/ERK pathways in B16F10 cells and a reconstructed human 3D skin model. Molecules 2020, 25, 2683. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Liu, X.; Khan, A.; Wan, S.; Guo, X.; Xue, J.; Fan, R. miRNA-183∼96∼182 regulates melanogenesis, cell proliferation and migration in B16 cells. Acta Histochem. 2020, 122, 151508. [Google Scholar] [CrossRef]
- Miyasaka, K.; Manse, Y.; Yoneda, A.; Takeda, S.; Shimizu, N.; Yamada, W.; Morikawa, T.; Shimoda, H. Anti-melanogenic effects of glucosylceramides and elasticamide derived from rice oil by-products in melanoma cells, melanocytes, and human skin. J. Food Biochem. 2022, 46, e14353. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, E.L.; Becker, A.L.; Indra, A.K. NRF2 and Key Transcriptional Targets in Melanoma Redox Manipulation. Cancers 2022, 14, 1531. [Google Scholar] [CrossRef]
- Moon, H.R.; Jo, S.Y.; Kim, H.T.; Lee, W.J.; Won, C.H.; Lee, M.W.; Choi, J.H.; Chang, S.E. Loratadine, an H1 Antihistamine, Inhibits Melanogenesis in Human Melanocytes. BioMed Res. Int. 2019, 2019, 8. [Google Scholar] [CrossRef] [Green Version]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Niu, Z.; Wang, X.; Lu, X.; Sun, J.; Carpena, M. The Nutritional and Bioactive Components, Potential Health Function and Comprehensive Utilization of Pomegranate: A Review. Food Rev. Int. 2022, 1–27. [Google Scholar] [CrossRef]
- Niu, C.; Aisa, H.A. Upregulation of melanogenesis and tyrosinase activity: Potential agents for vitiligo. Molecules 2017, 22, 1303. [Google Scholar] [CrossRef] [Green Version]
- Draelos, Z.D. Skin lightening preparations and the hydroquinone controversy. Dermatol. Ther. 2007, 20, 308–313. [Google Scholar] [CrossRef]
- Kanthaliya, B.; Joshhi, A.; Meena, S.; Arora, J. Biology and Biotechnological Strategies for Conservation Management of Pueraria tuberosa, a Traditionally Established Medicinal Liana. Med. Plants 2021, 28, 693–719. [Google Scholar]
- Ulbricht, C.; Costa, D.; Dam, C.; D’Auria, D.; Giese, N.; Isaac, R.; Leblanc, Y.; Rusie, E.; Weissner, W.; Windsor, R.C. An evidence-based systematic review of kudzu (Pueraria lobata) by the natural standard research collaboration. J. Diet. Suppl. 2015, 12, 36–104. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.W.; Lee, C.; Kim, I.H.; Kim, Y.T. Anti-inflammatory effects of total isoflavones from Pueraria lobata on cerebral ischemia in rats. Molecules 2013, 18, 10404–10412. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.F.; Koon, C.M.; Cheung, D.W.S.; Lam, M.Y.; Leung, P.C.; Lau, C.B.S.; Fung, K.P. The anti-hypertensive effect of Danshen (Salvia miltiorrhiza) and Gegen (Pueraria lobata) formula in rats and its underlying mechanisms of vasorelaxation. J. Ethnopharmacol. 2011, 137, 1366–1372. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Yang, Y.; Yang, J.; Chai, H.; Li, Y.; Jia, Z.; Wang, Z. Tectoridin, an isoflavone glycoside from the flower of Pueraria lobata, prevents acute ethanol-induced liver steatosis in mice. Toxicology 2010, 276, 64–72. [Google Scholar] [CrossRef]
- Yasuda, T.; Endo, M.; Kon-no, T.; Kato, T.; Mitsuzuka, M.; Ohsawa, K. Antipyretic, analgesic and muscle relaxant activities of Pueraria isoflavonoids and their metabolites from Pueraria lobata Ohwi-a traditional chinese drug. Biol. Pharm. Bull. 2005, 28, 1224–1228. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, T.; Hosono, T.; Matsushita, Y.; Kawashima, K.; Someya, M.; Nakajima, Y.; Narui, K.; Hibi, Y.; Ishizaki, M.; Kinjo, J.; et al. Pharmacological studies on Puerariae Flos. IV: Effects of Pueraria thomsonii dried flower extracts on blood ethanol and acetaldehyde levels in humans. Int. J. Clin. Pharmacol. Res. 2002, 22, 23–28. [Google Scholar]
- Park, K.H.; Gu, D.R.; Jin, S.H.; Yoon, C.S.; Ko, W.; Kim, Y.C.; Lee, S.H. Pueraria lobate Inhibits RANKL-mediated Osteoclastogenesis via downregulation of CREB/PGC1β/c–Fos/ NFATc1 signaling. Am. J. Chin. Med. 2017, 45, 1725–1744. [Google Scholar] [CrossRef]
- Chang, B.Y.; Lee, D.S.; Lee, J.K.; Kim, Y.C.; Cho, H.K.; Kim, S.Y. Protective activity of kudzu (Pueraria thunbergiana) vine on chemically induced hepatotoxicity: In vitro and in vivo studies. BMC Complement. Altern. Med. 2015, 16, 39. [Google Scholar] [CrossRef] [Green Version]
- Han, E.; Chang, B.; Kim, D.; Cho, H.; Kim, S. Melanogenesis inhibitory effect of aerial part of Pueraria thunbergiana in vitro and in vivo. Arch. Dermatol. Res. 2015, 307, 57–72. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Zheng, N.; He, Q.; Li, R.; Zhang, K.; Liang, T. Puerarin, isolated from Pueraria lobata (Willd.), protects against hepatotoxicity via specific inhibition of the TGF-β1/Smad signaling pathway, thereby leading to anti-fibrotic effect. Phytomedicine 2013, 20, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Tang, H.; Yu, F.; Michihara, S.; Uzawa, Y.; Zaima, N.; Moriyama, T.; Kawamura, Y. Kudzu (Pueraria lobata) vine ethanol extracts improve ovariectomy-induced bone loss in female mice. J. Agric. Food Chem. 2011, 59, 13230–13237. [Google Scholar] [CrossRef] [PubMed]
- Folin, O.; Denis, W. On phosphotungstic-phosphomolybdic compounds as color reagents. J. Biol. 1912, 12, 239–243. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Zheng, L.; Zhao, M.; Xiao, C.; Zhao, Q.; Su, G. Practical problems when using ABTS assay to assess the radical-scavenging activity of peptides Importance of controlling reaction pH and time. Food Chem. 2016, 192, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Hosoi, J.; Abe, E.; Suda, T.; Kuroki, T. Regulation of Melanin Synthesis of B16 Mouse Melanoma Cells by 1α, 25-Dihydroxyvitamin D3 and Retinoic Acid. Cancer Res. 1985, 45, 1474–1478. [Google Scholar]
- Wang, Y.S.; Cho, J.G.; Hwang, E.S.; Yang, J.E.; Gao, W.; Fang, M.Z.; Zheng, S.D.; Yi, T.H. Enhancement of Protective Effects of Radix Scutellariae on UVB-induced Photo Damage in Human HaCaT Keratinocytes. Appiled Biochem. Biotechnol. 2018, 184, 1073–1093. [Google Scholar] [CrossRef]
- Jeong, D.E.; Lee, Y.; Lee, S.J.V. Western Blot Analysis of C. elegans Proteins. In Methods in Molecular Biology; Huang, L., Ed.; Humana Press: New York, NY, USA, 2018; pp. 213–225. [Google Scholar]
- Son, E.; Yoon, J.M.; An, B.J.; Lee, Y.M.; Cha, J.; Chi, G.Y.; Kim, D.S. Comparison among activities and isoflavonoids from Pueraria thunbergiana aerial parts and root. Molecules 2019, 24, 912. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.J.; Lee, A.K.; Park, Y.J.; Lee, S.; Kang, D.; Jung, Y.S.; Chung, H.Y.; Moon, H.R. (2E,5E)-2,5-Bis (3-hydroxy-4-methoxybenzylidene) cyclopentanone exerts anti-melanogenesis and anti-wrinkle activities in B16F10 melanoma and Hs27 fibroblast cells. Molecules 2018, 23, 1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maddodi, N.; Jayanthy, A.; Setaluri, V. Shining light on skin pigmentation: The darker and the brighter side of effects of UV radiation. Photochem. Photobiol. 2012, 88, 1075–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, P.; Hu, Y.; He, L. Regulation of melanocyte pivotal transcription factor MITF by some other transcription factors. Mol. Cell. Biochem. 2011, 354, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, C.W.; Kim, E.K.; Lee, S.J.; Park, N.H.; Kim, H.S.; Kim, H.K.; Char, K.H.; Jang, Y.P.; Kim, J.W. Inhibition effect of Gynura procumbens extract on UV-B-induced matrix-metalloproteinase expression in human dermal fibroblasts. J. Ethnopharmacol. 2011, 137, 427–433. [Google Scholar] [CrossRef]
- Inomata, S.; Takada, K.; Tsunenaga, M.; Fukuda, M.; Matsunaga, Y.; Amano, S.; Kobayashi, K.; Nishiyama, T.; Kohno, Y. Possible involvement of gelatinases in basement membrane damage and wrinkle formation in chronically ultraviolet B-exposed hairless mouse. J. Investig. Dermatol. 2003, 120, 128–134. [Google Scholar] [CrossRef]
- Kim, D.Y.; Won, K.J.; Hwang, D.I.; Yoon, S.W.; Lee, S.J.; Park, J.H.; Yoon, M.S.; Kim, B.K.; Lee, H.M. Potential skin regeneration activity and chemical composition of absolute from Pueraria thunbergiana flower. Nat. Prod. Commun. 2015, 10, 2009–2012. [Google Scholar]
- Park, E.K.; Shin, J.; Bae, E.A.; Lee, Y.C.; Kim, D.H. Intestinal bacteria activate estrogenic effect of main constituents puerarin and daidzin of Pueraria thunbergiana. Biol. Pharm. Bull. 2006, 29, 2432–2435. [Google Scholar] [CrossRef] [Green Version]
- Min, S.W.; Park, Y.J.; Kim, D.H. Kakkalide and its metabolite irisolidone ameliorate carrageenan-induced inflammation in mice by inhibiting NF-κB pathway. Inflammation 2011, 34, 344–351. [Google Scholar] [CrossRef]
- Cherdshewasart, W.; Sutjit, W.; Pulcharoen, K.; Chulasiri, M. The mutagenic and antimutagenic effects of the traditional phytoestrogen-rich herbs, Pueraria mirifica and Pueraria lobata. Braz. J. Med. Biol. Res. 2009, 42, 816–823. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.E.; Bae, E.A.; Lee, Y.C.; Ma, J.Y.; Kim, D.H. Estrogenic effect of main components kakkalide and tectoridin of Puerariae flos and their metabolites. Biol. Pharm. Bull. 2006, 29, 1202–1206. [Google Scholar] [CrossRef] [Green Version]
- Park, K.Y.; Jung, G.O.; Choi, J.; Lee, K.T.; Park, H.J. Potent antimutagenic and their anti-lipid peroxidative effect of kaikasaponin iii and tectorigenin from the flower of Pueraria thunbergiana. Arch. Pharmacal Res. 2002, 25, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.T.; Sohn, I.C.; Kim, Y.K.; Choi, J.H.; Choi, J.W.; Park, H.J.; Itoh, Y.; Miyamoto, K. Tectorigenin, an isoflavone of Pueraria thunbergiana Benth., induces differentiation and apoptosis in human promyelocytic leukemia HL-60 cells. Biol. Pharmceutical Bull. 2001, 24, 1117–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.T.; Sohn, I.C.; Kim, D.H.; Choi, J.W.; Kwon, S.H.; Park, H.J. Hypoglycemic and hypolipidemic effects of tectorigenin and kaikasaponin III in the streptozotocin-induced diabetic rat and their antioxidant activity in vitro. Arch. Pharmacal Res. 2000, 23, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.; Kaltia, S.; Wähälä, K. The phase transfer catalysed synthesis of isoflavone-O-glucosides. J. Chem. Soc. Perkin Trans. 1998, 1, 2481–2484. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, Y.; Wang, T.; Qi, J.; Liu, X. Enzyme-site blocking combined with optimization of molecular docking for efficient discovery of potential tyrosinase specific inhibitors from Puerariae lobatae radix. Molecules 2018, 23, 2612. [Google Scholar] [CrossRef]








| Sample | Solvent Extract/Fraction | Extraction Yield (%) | * TPC | * TFC |
|---|---|---|---|---|
| µg GAE/g | µg CE/g | |||
| Extracts | Aqueous solution | 18 | 23 ± 0.00 b | 101 ± 0.00 b |
| 30% EtOH | 16 | 28 ± 0.02 e | 122 ± 0.80 e | |
| 50% EtOH | 8 | 27 ± 0.03 d | 111 ± 0.22 d | |
| 70% EtOH | 12 | 24 ± 0.02 c | 103 ± 0.67 c | |
| 90% EtOH | 7 | 22 ± 0.03 a | 77 ± 0.44 a | |
| Fractions | n-Hexane | 4.5 | 16 ± 0.02 a | 76 ± 0.22 a |
| EtOAc | 1.4 | 29 ± 0.00 d | 113 ± 0.00 d | |
| Butanol | 3.6 | 23 ± 0.03 c | 88 ± 0.22 c | |
| Aqueous solution | 8.9 | 17 ± 0.07 b | 86 ± 0.89 b |
| Sample | Solvent Extract/Fraction | EC50 for DPPH (μg/mL) | EC50 for ABTS (μg/mL) |
|---|---|---|---|
| Extracts | Aqueous solution | 124 ± 0.67 e | 62.5 ± 0.84 b |
| 30% EtOH | 88.1 ± 1.34 c | 54.2 ± 0.57 a | |
| 50% EtOH | 73.4 ± 0.91 b | 57.2 ± 0.8 a | |
| 70% EtOH | 94.3 ± 1.2 d | 63.5 ± 1.03 b | |
| 90% EtOH | 166.1 ± 5.23 f | 85 ± 0.24 c | |
| ASA | 11.4 ± 0.77 a | - | |
| Fractions | n-Hexane | 290.4 ± 0.85 d | 79.6 ± 5.45 c |
| EtOAc | 195.4 ± 1.17 b | 49.4 ± 1.18 a | |
| Butanol | 199.8 ± 1.13 b | 67.6 ± 0.43 b | |
| Aqueous solution | 270.1 ± 1.2 c | 77.1 ± 1.16 c | |
| ASA | 9.1 ± 0.9 a | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.J.; Shin, S.Y.; Song, N.R.; Kim, S.; Sun, S.O.; Park, K.M. Bioassay-Guided Characterization, Antioxidant, Anti-Melanogenic and Anti-Photoaging Activities of Pueraria thunbergiana L. Leaf Extracts in Human Epidermal Keratinocytes (HaCaT) Cells. Processes 2022, 10, 2156. https://doi.org/10.3390/pr10102156
Kim MJ, Shin SY, Song NR, Kim S, Sun SO, Park KM. Bioassay-Guided Characterization, Antioxidant, Anti-Melanogenic and Anti-Photoaging Activities of Pueraria thunbergiana L. Leaf Extracts in Human Epidermal Keratinocytes (HaCaT) Cells. Processes. 2022; 10(10):2156. https://doi.org/10.3390/pr10102156
Chicago/Turabian StyleKim, Min Jeong, Seo Yeon Shin, Nu Ri Song, Sunoh Kim, Sang Ouk Sun, and Kyung Mok Park. 2022. "Bioassay-Guided Characterization, Antioxidant, Anti-Melanogenic and Anti-Photoaging Activities of Pueraria thunbergiana L. Leaf Extracts in Human Epidermal Keratinocytes (HaCaT) Cells" Processes 10, no. 10: 2156. https://doi.org/10.3390/pr10102156
APA StyleKim, M. J., Shin, S. Y., Song, N. R., Kim, S., Sun, S. O., & Park, K. M. (2022). Bioassay-Guided Characterization, Antioxidant, Anti-Melanogenic and Anti-Photoaging Activities of Pueraria thunbergiana L. Leaf Extracts in Human Epidermal Keratinocytes (HaCaT) Cells. Processes, 10(10), 2156. https://doi.org/10.3390/pr10102156