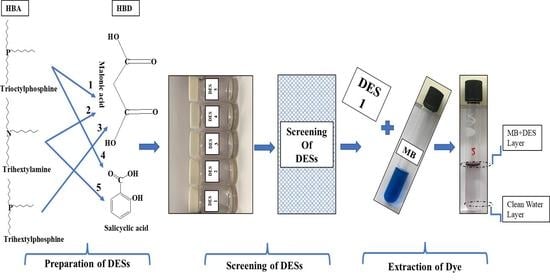
Efficient Extraction of Methylene Blue from Aqueous Solution Using Phosphine-Based Deep Eutectic Solvents with Carboxylic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. DES Synthesis
2.2. Instrumentation
3. Methods
3.1. MB Extraction
3.2. Single Parameter Optimizations
3.3. Cell Viability Assays
4. Results and Discussion
4.1. DES Selection
4.2. Effect of Initial pH
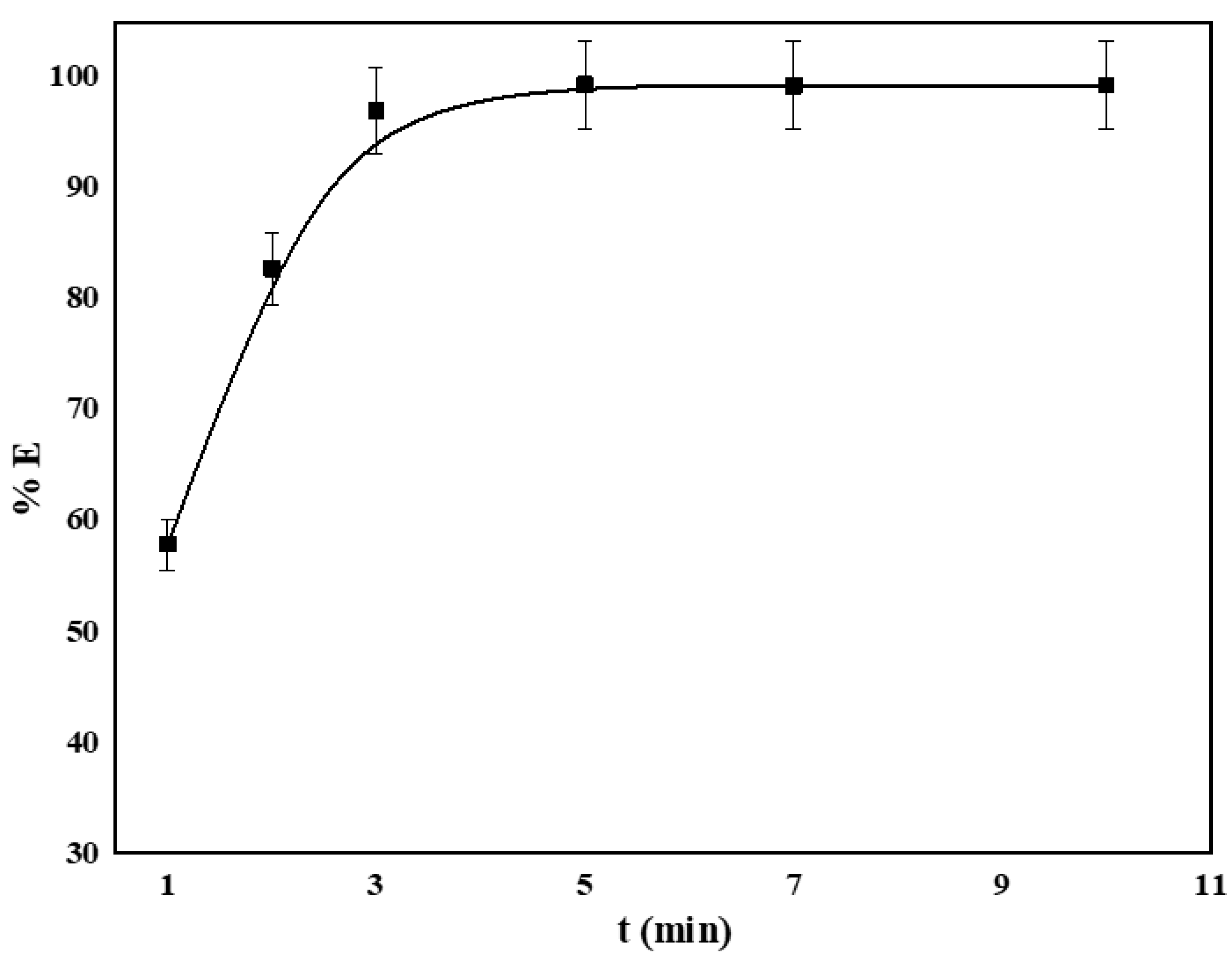
4.3. Effect of Vortex Mixing Time
4.4. Effect of Phase Ratio
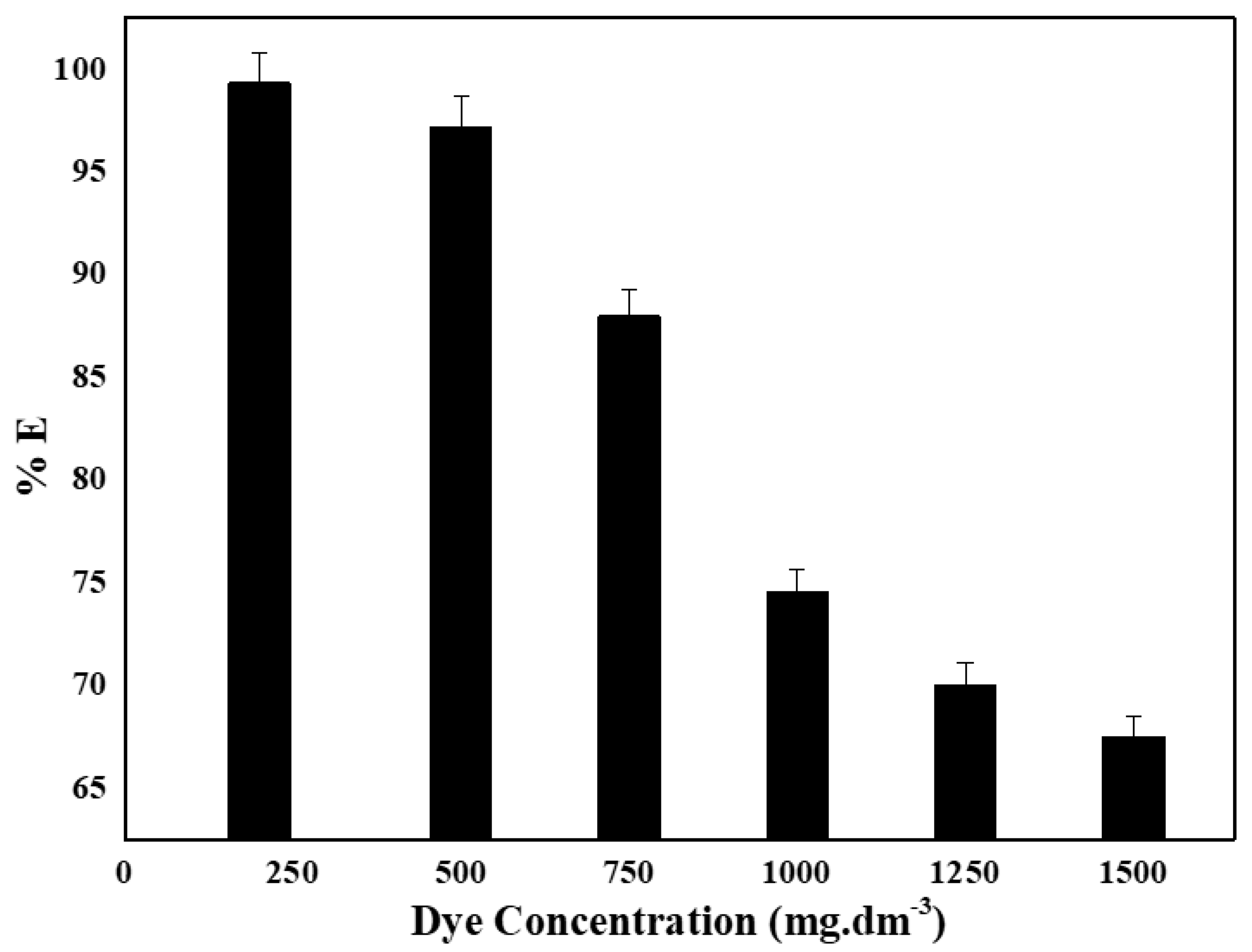
4.5. Effect of Initial MB Concentration
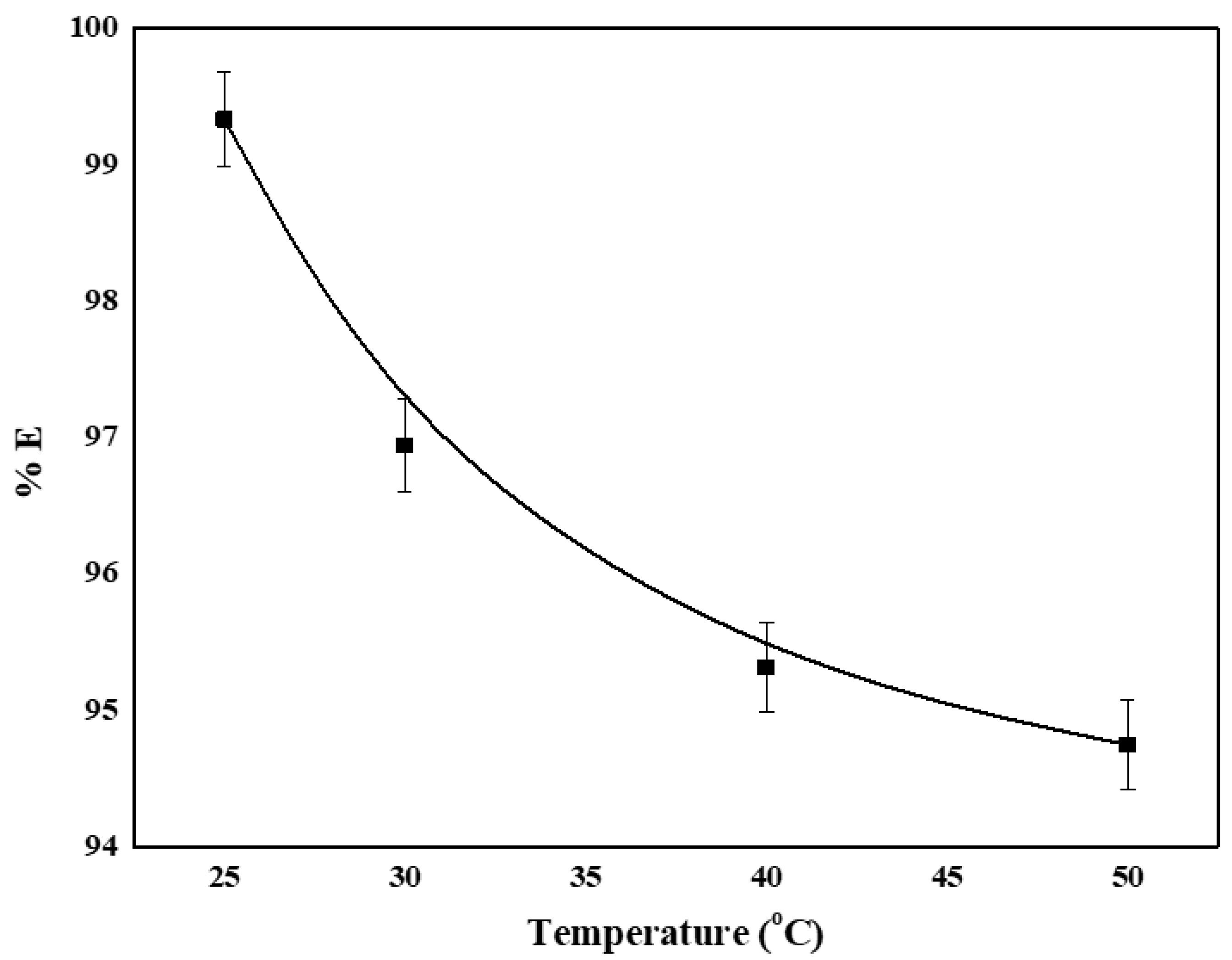
4.6. Effect of Temperature
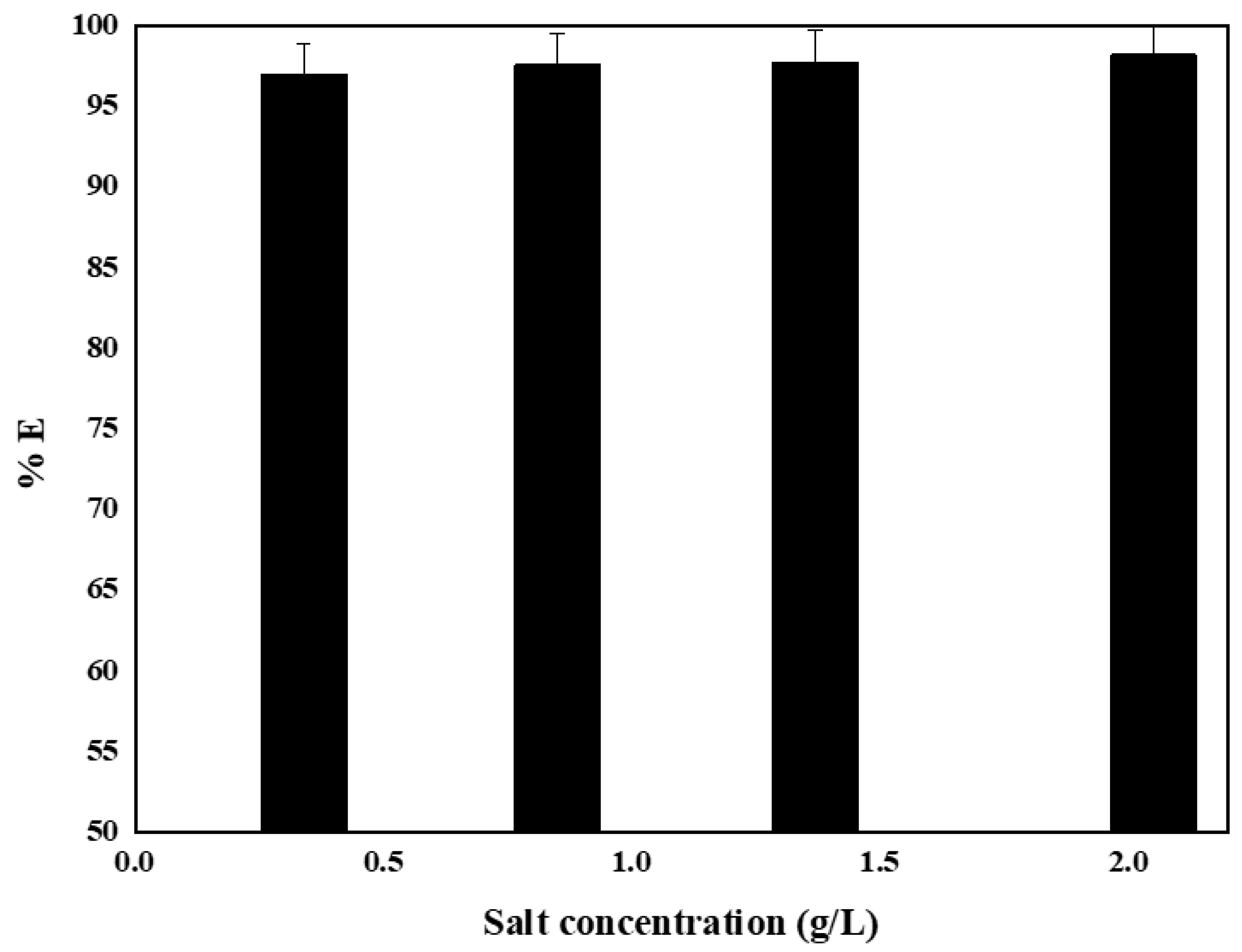
4.7. Effect of the Ionic Strength of Aqueous Solution
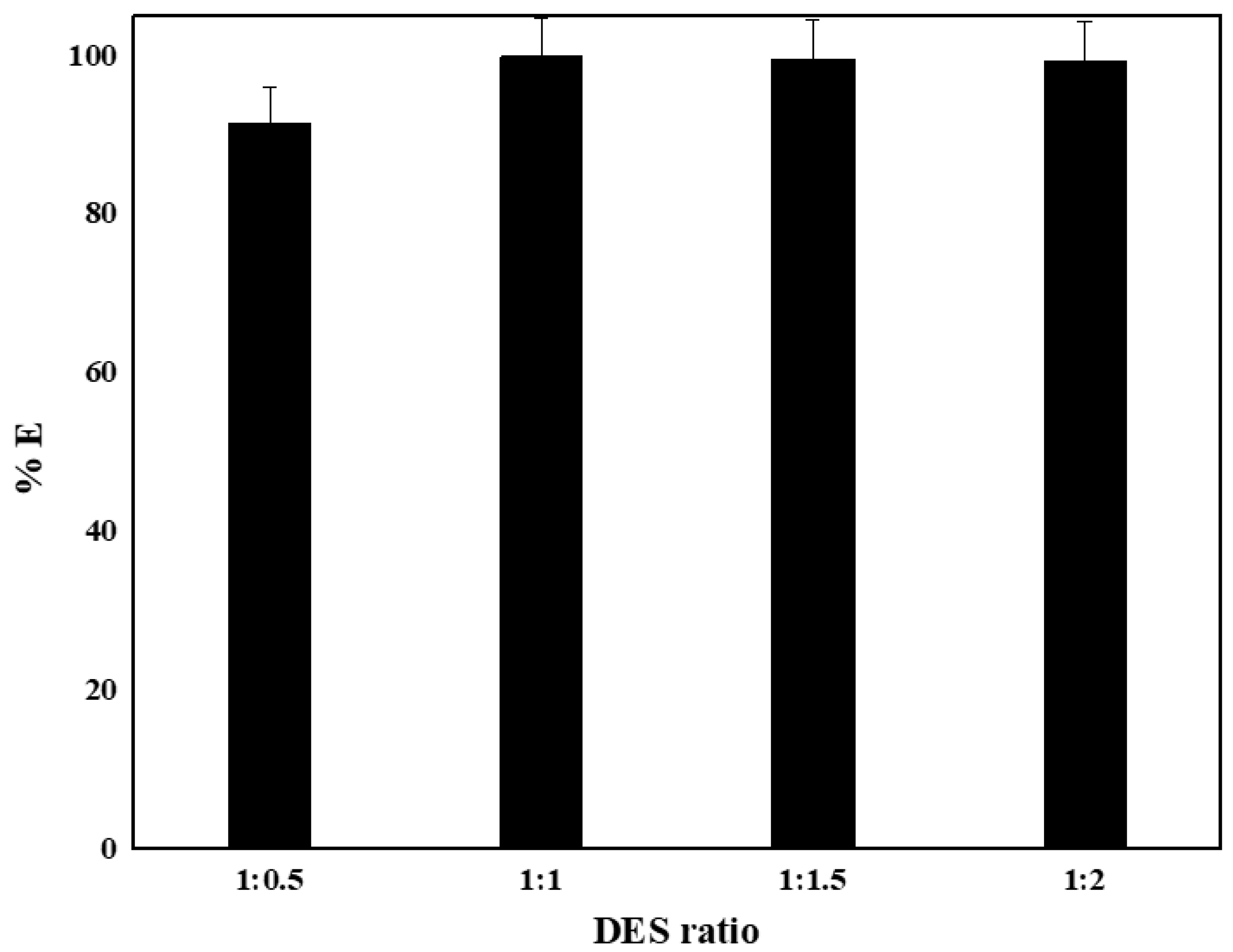
4.8. Effect of Molar Ratio
4.9. Thermodynamic study
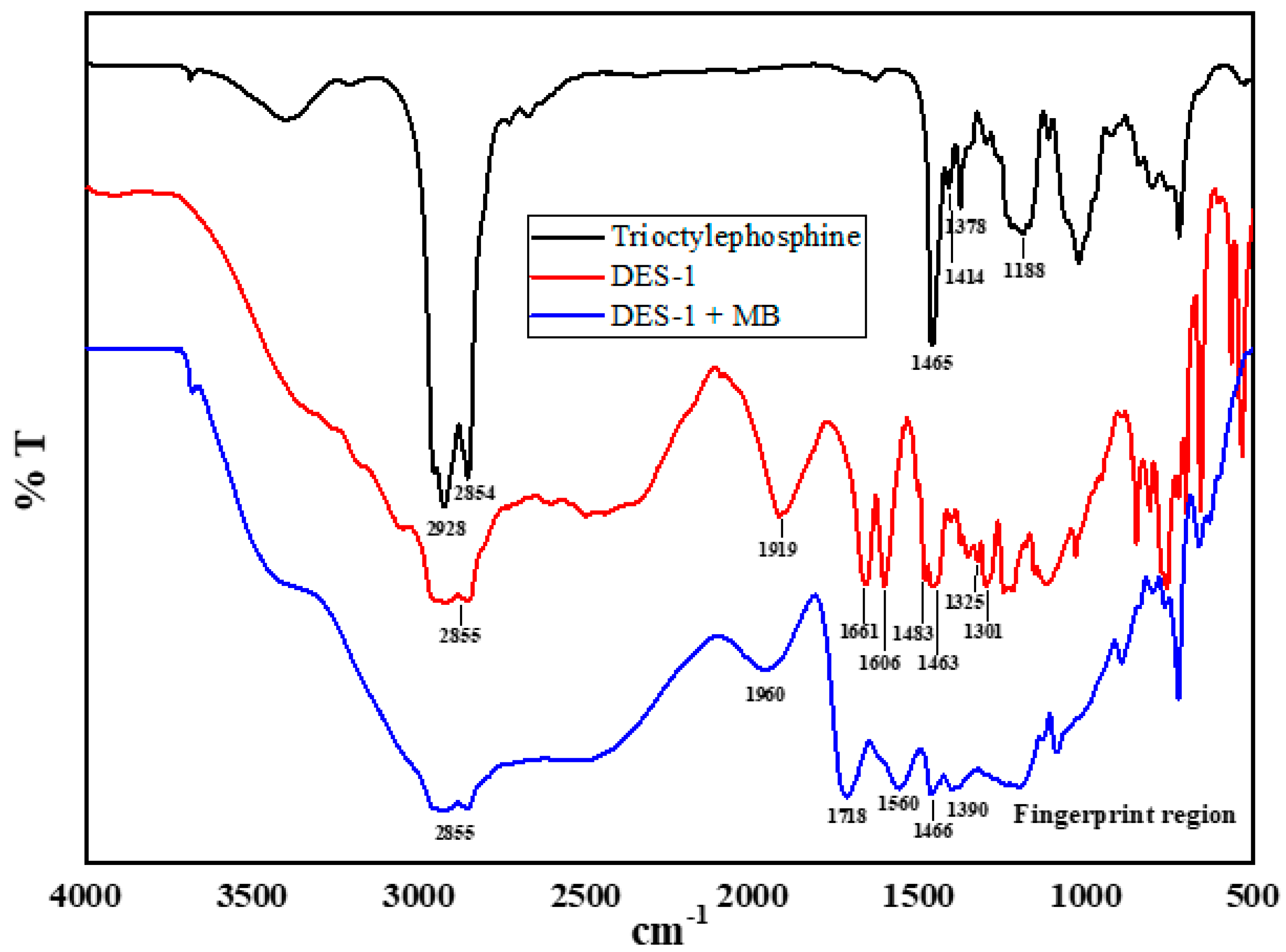
4.10. FTIR Spectra
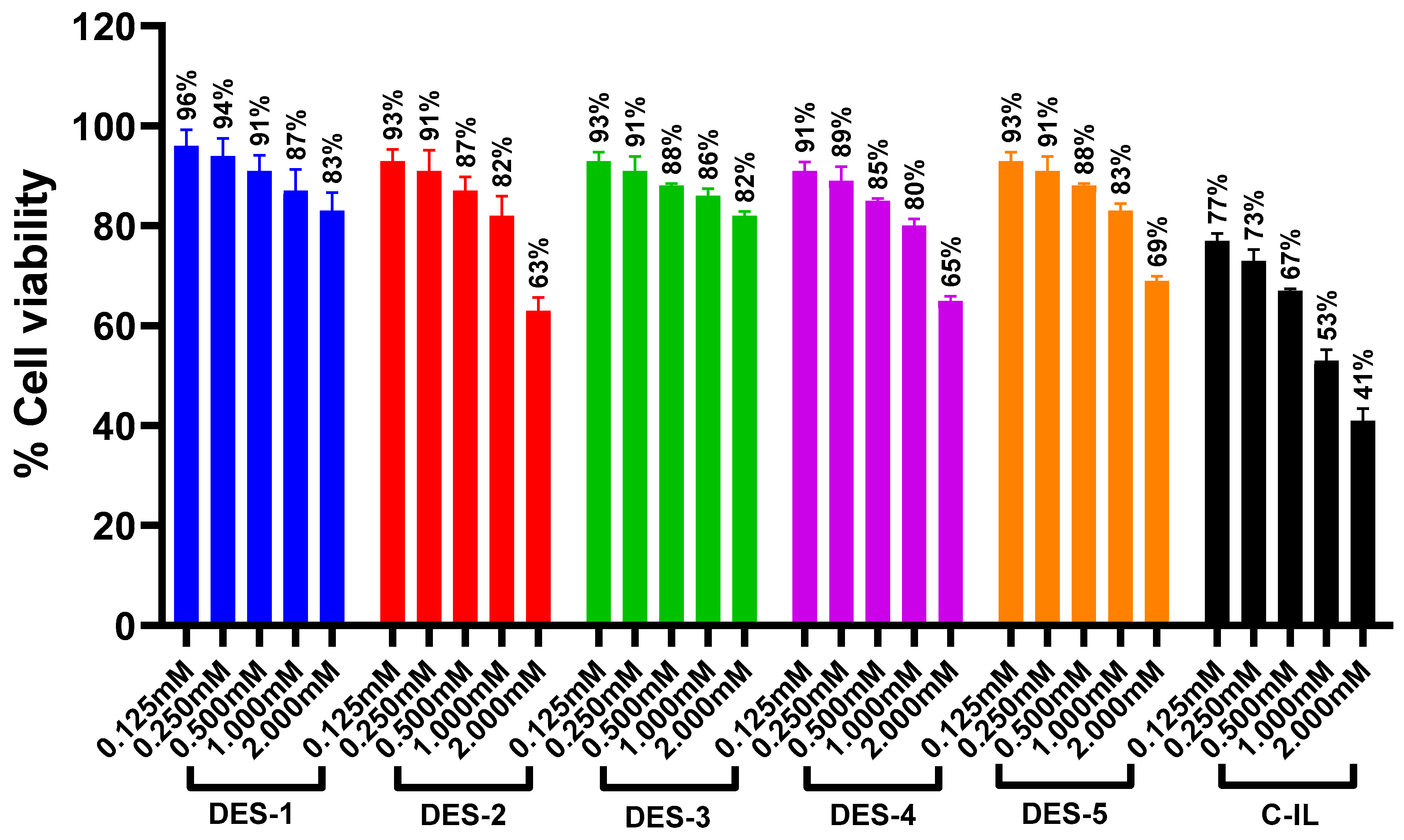
4.11. Cell Viability Assays
4.12. Comparison with Other DES and ILs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nasrullah, A.; Bhat, A.H.; Naeem, A.; Isa, M.H.; Danish, M. High Surface Area Mesoporous Activated Carbon-Alginate Beads for Efficient Removal of Methylene Blue. Int. J. Biol. Macromol. 2018, 107, 1792–1799. [Google Scholar] [CrossRef]
- Ghosh, D.; Bhattacharyya, K.G. Adsorption of Methylene Blue on Kaolinite. Appl. Clay Sci. 2002, 20, 295–300. [Google Scholar] [CrossRef]
- Nasrullah, A.; Saad, B.; Bhat, A.H.; Khan, A.S.; Danish, M.; Isa, M.H.; Naeem, A. Mangosteen Peel Waste as a Sustainable Precursor for High Surface Area Mesoporous Activated Carbon: Characterization and Application for Methylene Blue Removal. J. Clean. Prod. 2019, 211, 1190–1200. [Google Scholar] [CrossRef]
- Kaur, P.; Rajani, N.; Kumawat, P.; Singh, N.; Kushwaha, J.P. Performance and Mechanism of Dye Extraction from Aqueous Solution Using Synthesized Deep Eutectic Solvents. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 539, 85–91. [Google Scholar] [CrossRef]
- Yu, H.; Fugetsu, B. A Novel Adsorbent Obtained by Inserting Carbon Nanotubes into Cavities of Diatomite and Applications for Organic Dye Elimination from Contaminated Water. J. Hazard. Mater. 2010, 177, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Asfaram, A.; Ghaedi, M.; Hajati, S.; Goudarzi, A. Synthesis of Magnetic γ-Fe2O3-Based Nanomaterial for Ultrasonic Assisted Dyes Adsorption: Modeling and Optimization. Ultrason. Sonochem. 2016, 32, 418–431. [Google Scholar] [CrossRef]
- Zinadini, S.; Zinatizadeh, A.A.; Rahimi, M.; Vatanpour, V.; Zangeneh, H.; Beygzadeh, M. Novel High Flux Antifouling Nanofiltration Membranes for Dye Removal Containing Carboxymethyl Chitosan Coated Fe3O4 Nanoparticles. Desalination 2014, 349, 145–154. [Google Scholar] [CrossRef]
- Emamjomeh, M.M.; Sivakumar, M. Review of Pollutants Removed by Electrocoagulation and Electrocoagulation/Flotation Processes. J. Environ. Manag. 2009, 90, 1663–1679. [Google Scholar] [CrossRef]
- Son, G.; Lee, H. Methylene Blue Removal by Submerged Plasma Irradiation System in the Presence of Persulfate. Environ. Sci. Pollut. Res. 2016, 23, 15651–15656. [Google Scholar] [CrossRef]
- Liu, C.-H.; Wu, J.-S.; Chiu, H.-C.; Suen, S.-Y.; Chu, K.H. Removal of Anionic Reactive Dyes from Water Using Anion Exchange Membranes as Adsorbers. Water Res. 2007, 41, 1491–1500. [Google Scholar] [CrossRef]
- Kerkez-Kuyumcu, Ö.; Kibar, E.; Dayıoğlu, K.; Gedik, F.; Akın, A.N.; Özkara-Aydınoğlu, Ş. A Comparative Study for Removal of Different Dyes over M/TiO2 (M= Cu, Ni, Co, Fe, Mn and Cr) Photocatalysts under Visible Light Irradiation. J. Photochem. Photobiol. A Chem. 2015, 311, 176–185. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Chang, J.-S.; Govindwar, S.P. Bacterial Decolorization and Degradation of Azo Dyes: A Review. J. Taiwan Inst. Chem. Eng. 2011, 42, 138–157. [Google Scholar] [CrossRef]
- Khan, A.S.; Man, Z.; Arvina, A.; Bustam, M.A.; Nasrullah, A.; Ullah, Z.; Sarwono, A.; Muhammad, N. Dicationic Imidazolium Based Ionic Liquids: Synthesis and Properties. J. Mol. Liq. 2017, 227, 98–105. [Google Scholar] [CrossRef]
- Khan, A.S.; Nasrullah, A.; Ullah, Z.; Bhat, A.H.; Ghanem, O.B.; Muhammad, N.; Rashid, M.U.; Man, Z. Thermophysical Properties and Ecotoxicity of New Nitrile Functionalised Protic Ionic Liquids. J. Mol. Liq. 2018, 249, 583–590. [Google Scholar] [CrossRef]
- Sakina; Khan, A.S.; Nasrullah, A.; Ullah, F.; Muhammad, N.; Kubra, S.; Din, I.U.; Mutahir, Z. Effect of Imidazolium’s Ionic Liquids with Different Anions and Alkyl Chain Length on Phytotoxicity and Biochemical Analysis of Maize Seedling. J. Mol. Liq. 2021, 321, 114491. [Google Scholar] [CrossRef]
- AlOmar, M.K.; Alsaadi, M.A.; Hayyan, M.; Akib, S.; Hashim, M.A. Functionalization of CNTs Surface with Phosphonuim Based Deep Eutectic Solvents for Arsenic Removal from Water. Appl. Surf. Sci. 2016, 389, 216–226. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; Hayyan, A.; Al-Saadi, M.A.; AlNashef, I.M.; Mirghani, M.E.S.; Saheed, O.K. Are Deep Eutectic Solvents Benign or Toxic? Chemosphere 2013, 90, 2193–2195. [Google Scholar] [CrossRef]
- Tome, L.I.N.; Baiao, V.; da Silva, W.; Brett, C.M.A. Deep Eutectic Solvents for the Production and Application of New Materials. Appl. Mater. Today 2018, 10, 30–50. [Google Scholar] [CrossRef]
- Zhang, Q.; Vigier, K.D.O.; Royer, S.; Jerome, F. Deep Eutectic Solvents: Syntheses, Properties and Applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef]
- Mehrabi, N.; Abdul Haq, U.F.; Reza, M.T.; Aich, N. Using Deep Eutectic Solvent for Conjugation of Fe3O4 Nanoparticles onto Graphene Oxide for Water Pollutant Removal. 2020. Available online: https://chemrxiv.org/engage/chemrxiv/article-details/60c7499c337d6c1d2de27748 (accessed on 12 September 2022).
- Fu, L.; Hu, X.; Yu, S.; Guo, Y.; Liu, H.; Zhang, W.; Lou, Y.; Li, D.; Yu, Q. Sustainable Wastewater Treatment by Deep Eutectic Solvents and Natural Silk for Radioactive Iodine Capture. Water Sci. Technol. 2019, 80, 1683–1691. [Google Scholar] [CrossRef]
- Almustafa, G.; Sulaiman, R.; Kumar, M.; Adeyemi, I.; Arafat, H.A.; AlNashef, I. Boron Extraction from Aqueous Medium Using Novel Hydrophobic Deep Eutectic Solvents. Chem. Eng. J. 2020, 395, 125173. [Google Scholar] [CrossRef]
- Shi, Y.; Xiong, D.; Zhao, Y.; Li, T.; Zhang, K.; Fan, J. Highly Efficient Extraction/Separation of Cr (VI) by a New Family of Hydrophobic Deep Eutectic Solvents. Chemosphere 2020, 241, 125082. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Xu, P.; Wang, Y. Aqueous Biphasic Systems Formed by Hydrophilic and Hydrophobic Deep Eutectic Solvents for the Partitioning of Dyes. Talanta 2020, 213, 120839. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Tang, S.; Yang, Y. Emulsification Liquid–Liquid Micro-Extraction Based on Natural Deep Eutectic Solvent for (Triarylmethane) Dyes Determination. Chem. Pap. 2020, 74, 3617–3626. [Google Scholar] [CrossRef]
- Ahmadi, R.; Kazemi, G.; Ramezani, A.M.; Safavi, A. Shaker-Assisted Liquid-Liquid Microextraction of Methylene Blue Using Deep Eutectic Solvent Followed by Back-Extraction and Spectrophotometric Determination. Microchem. J. 2019, 145, 501–507. [Google Scholar] [CrossRef]
- Stepnowski, P.; Skladanowski, A.C.; Ludwiczak, A.; Laczyńska, E. Evaluating the Cytotoxicity of Ionic Liquids Using Human Cell Line HeLa. Hum. Exp. Toxicol. 2004, 23, 513–517. [Google Scholar] [CrossRef]
- Akbar, N.; Khan, N.A.; Sagathevan, K.; Iqbal, M.; Tawab, A.; Siddiqui, R. Gut Bacteria of Cuora Amboinensis (Turtle) Produce Broad-Spectrum Antibacterial Molecules. Sci. Rep. 2019, 9, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Laidler, K.J.; Meiser, J.H.; Sanctuary, B.C.; McCann, M.P. Chemical Education Today-Book & Media Reviews-Physical Chemistry CD. J. Chem. Educ. 2003, 80, 489–490. [Google Scholar]
- Ramachandran, S.; Sathishkumar, M.; Kothurkar, N.K.; Senthilkumar, R. Synthesis and Characterization of Graphene Quantum Dots/Cobalt Ferrite Nanocomposite. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Bengaluru, India, 17–19 August 2017; IOP Publishing: Bristol, UK, 2018; Volume 310, p. 12139. [Google Scholar]
- Okram, G.S.; Singh, J.; Kaurav, N.; Lalla, N.P. Trioctylphosphine as Self-Assembly Inducer. Faraday Discuss. 2015, 181, 211–223. [Google Scholar] [CrossRef] [Green Version]
- Grumezescu, A.M. Inorganic Frameworks as Smart Nanomedicines; William Andrew: Norwich, NY, USA, 2018; ISBN 0128136626. [Google Scholar]
- Smith, B.C. The Carbonyl Group, Part I: Introduction. Spectroscopy 2017, 32, 31–36. [Google Scholar]
- Tang, R.; Dai, C.; Li, C.; Liu, W.; Gao, S.; Wang, C. Removal of Methylene Blue from Aqueous Solution Using Agricultural Residue Walnut Shell: Equilibrium, Kinetic, and Thermodynamic Studies. J. Chem. 2017, 2017, 8404965. [Google Scholar] [CrossRef] [Green Version]
- López-García, J.; Lehocký, M.; Humpolíček, P.; Sáha, P. HaCaT Keratinocytes Response on Antimicrobial Atelocollagen Substrates: Extent of Cytotoxicity, Cell Viability and Proliferation. J. Funct. Biomater. 2014, 5, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Sajadiyeh, E.; Tabatabaee, M.; Seifati, S.; Derikvend, Z. The Study of Cytotoxicity of an Imidazolium Based Ionic Liquid on MCF-7 Cell Line. J. Chem. Heal. Risks 2021, 11, 85–89. [Google Scholar]
- Radošević, K.; Železnjak, J.; Cvjetko Bubalo, M.; Radojčić Redovniković, I.; Slivac, I.; Gaurina Srček, V. Comparative in Vitro Study of Cholinium-Based Ionic Liquids and Deep Eutectic Solvents toward Fish Cell Line. Ecotoxicol. Environ. Saf. 2016, 131, 30–36. [Google Scholar] [CrossRef]
- McLaughlin, M.; Gilea, M.A.; Earle, M.J.; Seddon, K.R.; Gilmore, B.F.; Kelly, S.A. Characterization of Ionic Liquid Cytotoxicity Mechanisms in Human Keratinocytes Compared with Conventional Biocides. Chemosphere 2021, 270, 129432. [Google Scholar] [CrossRef] [PubMed]
- Cvjetko, M.; Radošević, K.; Tomica, A.; Slivac, I.; Vorkapić-Furač, J.; Gaurina Srček, V. Cytotoxic Effects of Imidazolium Ionic Liquids on Fish and Human Cell Lines. Arh. Hig. Rada Toksikol. 2012, 63, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Kermanioryani, M.; Mutalib, M.I.A.; Gonfa, G.; El-Harbawi, M.; Mazlan, F.A.; Lethesh, K.C.; Leveque, J.-M. Using Tunability of Ionic Liquids to Remove Methylene Blue from Aqueous Solution. J. Environ. Chem. Eng. 2016, 4, 2327–2332. [Google Scholar] [CrossRef]
- Talbi, Z.; Haddou, B.; Ghouas, H.; Kameche, M.; Derriche, Z.; Gourdon, C. Cationic Dye Removal from Aqueous Solutions Using Ionic Liquid and Nonionic Surfactant-Ionic Liquid Systems: A Comparative Study Based upon Experimental Design. Chem. Eng. Commun. 2014, 201, 41–52. [Google Scholar] [CrossRef]




| HBA | HBD | Molar Ratio HBA:HBD | Abbreviation | Property |
|---|---|---|---|---|
| Trioctylphosphine | Salicylic acid | 1:0.5, 1:1, 1:1.5, 1:2 | DES-1 | Hydrophilic |
| Trioctylphosphine | Malonic acid | 1:1 | DES-2 | Hydrophilic |
| Trioctylamine | Salicylic acid | 1:1 | DES-3 | Hydrophilic |
| Trioctylamine | Malonic acid | 1:1 | DES-4 | Hydrophilic |
| Trihexylamine | Salicylic acid | 1:1 | DES-5 | Hydrophilic |
| pH | 1.0 | 3.0 | 5.0 | 6.5 | 8.0 | 10.0 |
|---|---|---|---|---|---|---|
| PDES-1 | 5.02 | 86.3 | 120 | 1140 | 365 | 357 |
| T (K) | ΔG° (kJ mol−1) | ΔH° (kJ mol−1) | ΔS° (J K−1 mol−1) |
|---|---|---|---|
| 298 | −12.39 | −6.40 | 20.0 |
| 303 | −8.71 | −17.9 | −3.0 |
| 313 | −7.84 | −116 | −35.0 |
| 323 | −7.76 | −541 | −165 |
| Method | Extraction Solvent | Full Name | Abbreviations | Extraction Efficiency (%) | References |
|---|---|---|---|---|---|
| L-L extraction | IL | 1-hexyl-2-phenyl-imidazolium bis(trifluoromethanesulfonyl) imide | [HPhIm][NTf2] | 97.9 | [40] |
| 1-benzyl-3-methyl-imidazolium bis(trifluoromethanesulfonyl) imide | [BnMIm][NTf2] | 94.5 | |||
| 1-butyl-3-methyl-imidazolium bis(trifluoromethanesulfonyl) imide | [BMIm][NTf2] | 91.5 | |||
| 1-hexyl-3-methyl-imidazolium bis(trifluoromethanesulfonyl) imide | [HMIm][NTf2] | 90.0 | |||
| 1-octyl-3-methyl-imidazolium bis(trifluoromethanesulfonyl) imide | [OMIm][NTf2] | 85.0 | |||
| Aqueous two-phase system | DES | Choline chloride–glycerol | ChCl-G | >95.0 | [26] |
| L-L Extraction | IL | 1-butyl-3-methylimidazolium hexafluorophosphate | [C4mim]PF6 | 91.5 | [41] |
| L-L Extraction | DES | Trioctylphosphine–salicylic acid | DES-1 | 99.3 | This study |
| Trioctylphosphine–malonic acid | DES-2 | 44.0 | |||
| Trioctylamine–salicylic acid | DES-3 | 29.9 | |||
| Trioctylamine–malonic acid | DES-4 | 17.8 | |||
| Trihexylamine–salicylic acid | DES-5 | 6.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, M.F.; Khan, A.S.; Akbar, N.; Ibrahim, T.H.; Khamis, M.I.; Jumean, F.H.; Siddiqui, R.; Khan, N.A.; Yasir, N. Efficient Extraction of Methylene Blue from Aqueous Solution Using Phosphine-Based Deep Eutectic Solvents with Carboxylic Acid. Processes 2022, 10, 2152. https://doi.org/10.3390/pr10102152
Hassan MF, Khan AS, Akbar N, Ibrahim TH, Khamis MI, Jumean FH, Siddiqui R, Khan NA, Yasir N. Efficient Extraction of Methylene Blue from Aqueous Solution Using Phosphine-Based Deep Eutectic Solvents with Carboxylic Acid. Processes. 2022; 10(10):2152. https://doi.org/10.3390/pr10102152
Chicago/Turabian StyleHassan, Muhammad Faheem, Amir Sada Khan, Noor Akbar, Taleb Hassan Ibrahim, Mustafa I. Khamis, Fawwaz H. Jumean, Ruqaiyyah Siddiqui, Naveed Ahmed Khan, and Nihal Yasir. 2022. "Efficient Extraction of Methylene Blue from Aqueous Solution Using Phosphine-Based Deep Eutectic Solvents with Carboxylic Acid" Processes 10, no. 10: 2152. https://doi.org/10.3390/pr10102152
APA StyleHassan, M. F., Khan, A. S., Akbar, N., Ibrahim, T. H., Khamis, M. I., Jumean, F. H., Siddiqui, R., Khan, N. A., & Yasir, N. (2022). Efficient Extraction of Methylene Blue from Aqueous Solution Using Phosphine-Based Deep Eutectic Solvents with Carboxylic Acid. Processes, 10(10), 2152. https://doi.org/10.3390/pr10102152