Effect of Surface Modification by Oxygen-Enriched Chemicals on the Surface Properties of Pine Bark Biochars
Abstract
:1. Introduction
2. Materials and Methods
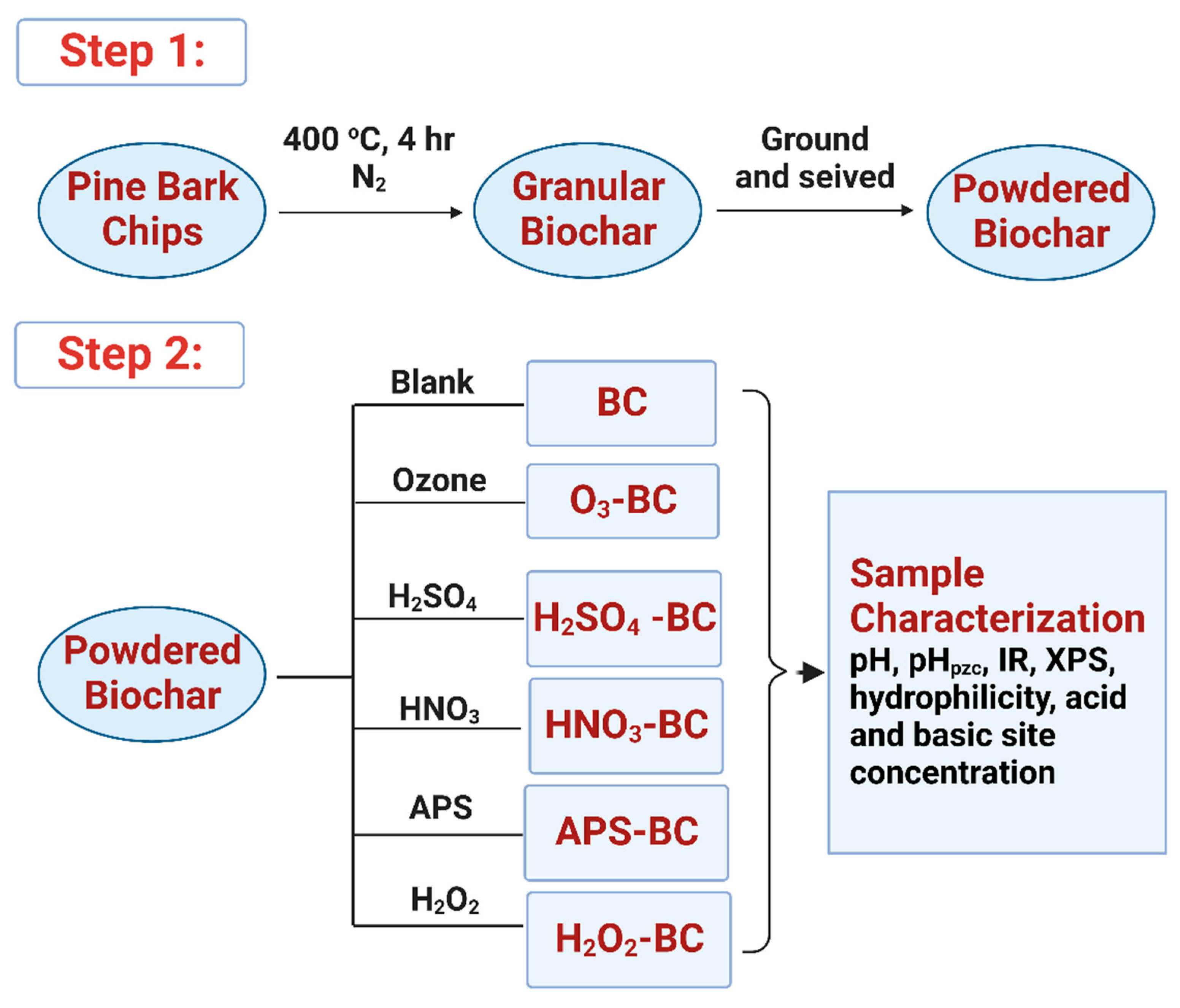
2.1. Sample Preparation
2.2. Sample Characterization
2.2.1. Biochar pH
2.2.2. Point of Zero Charges (pHpzc) in Biochar Samples
2.2.3. Hydrophilicity of Biochar Samples
2.2.4. Concentration of Acidic and Basic Sites in Biochar
2.2.5. Infrared (IR) Spectrum Analysis
2.2.6. X-ray Photoelectron Spectroscopy (XPS) Analysis
3. Results
3.1. pH Values of Biochar
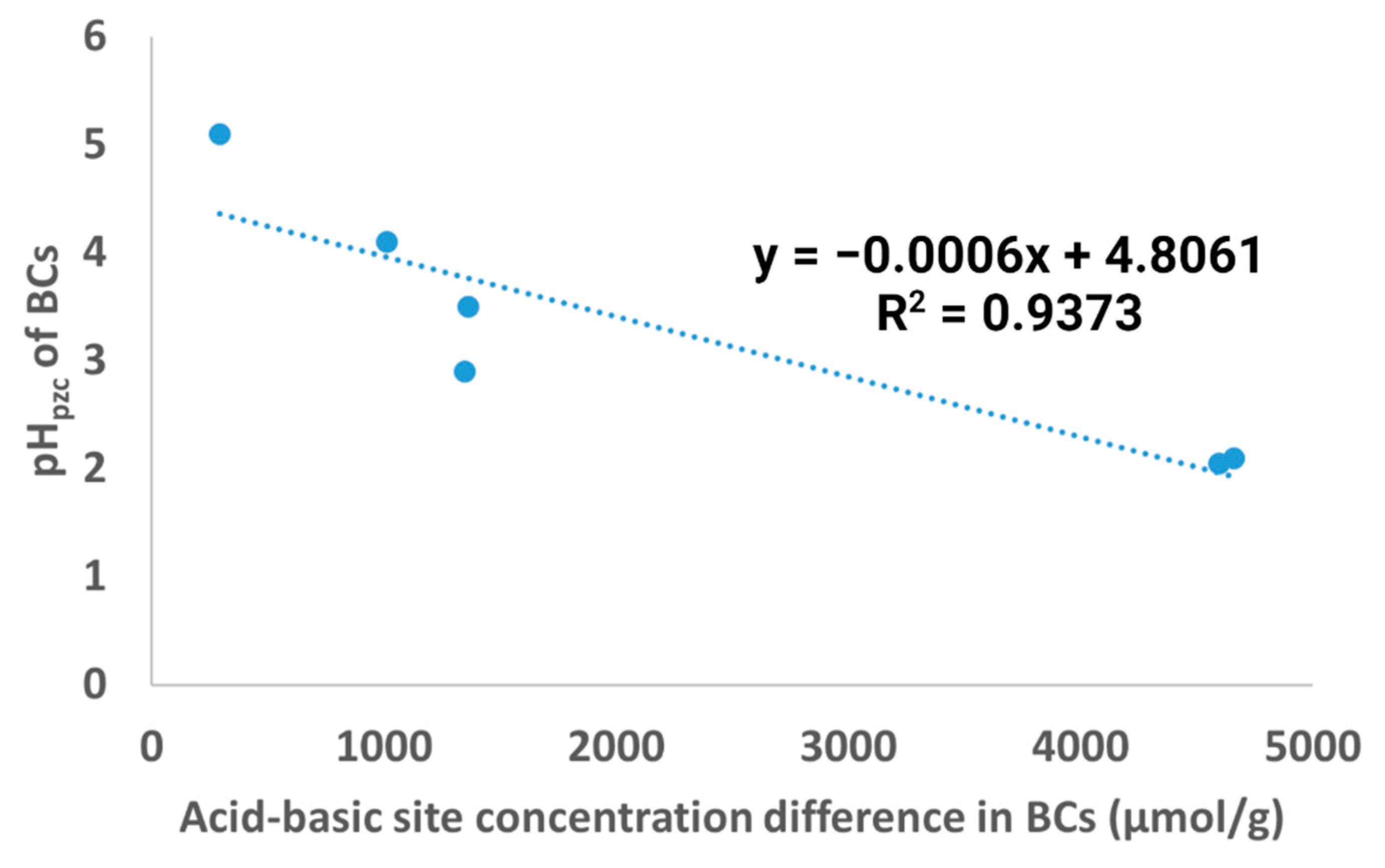
3.2. pHpzc of Biochar
3.3. Hydrophobicity Index of Biochar
3.4. Concentration of Acidic and Basic Sites in Biochar
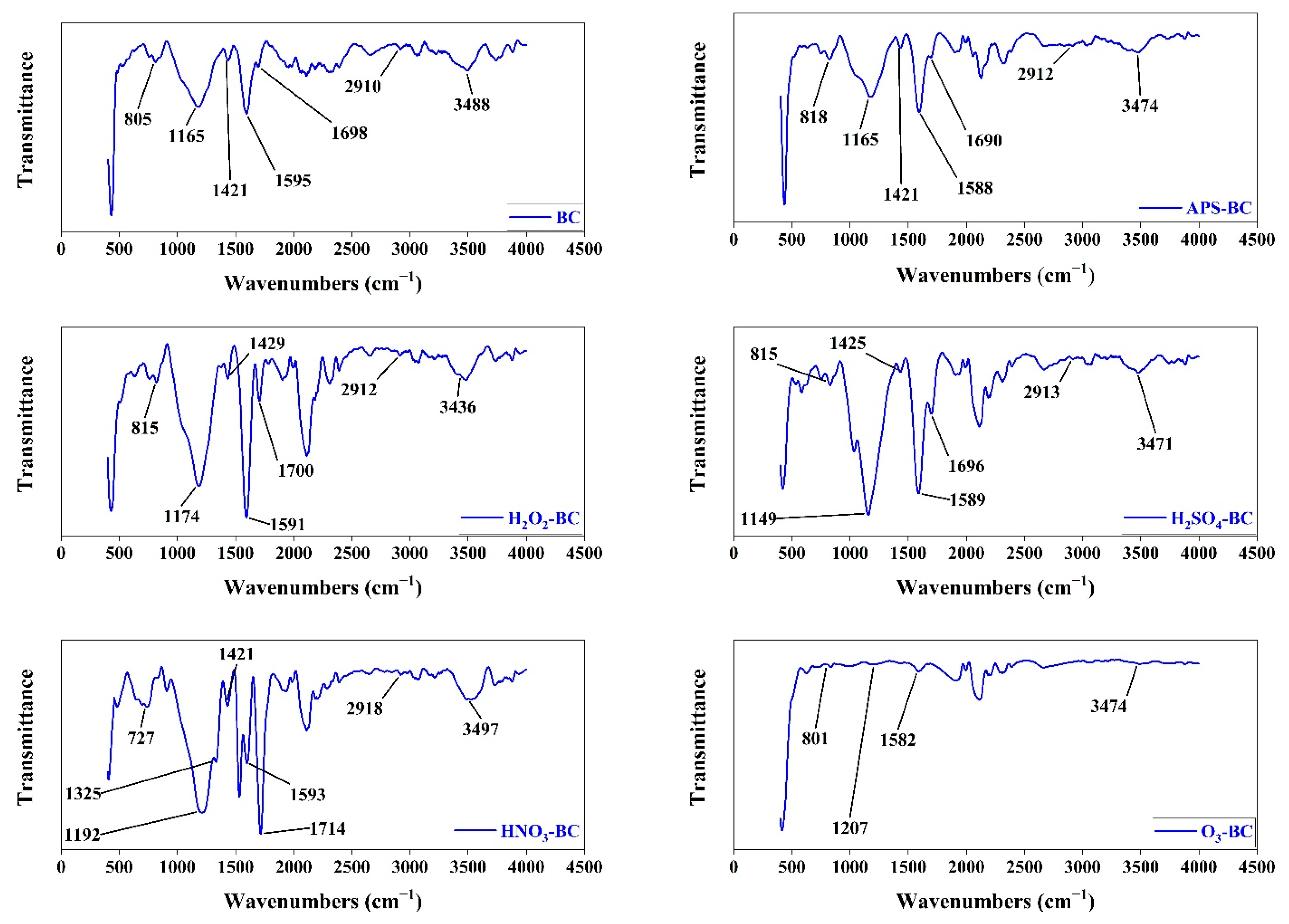
3.5. Infrared Spectra of Biochar
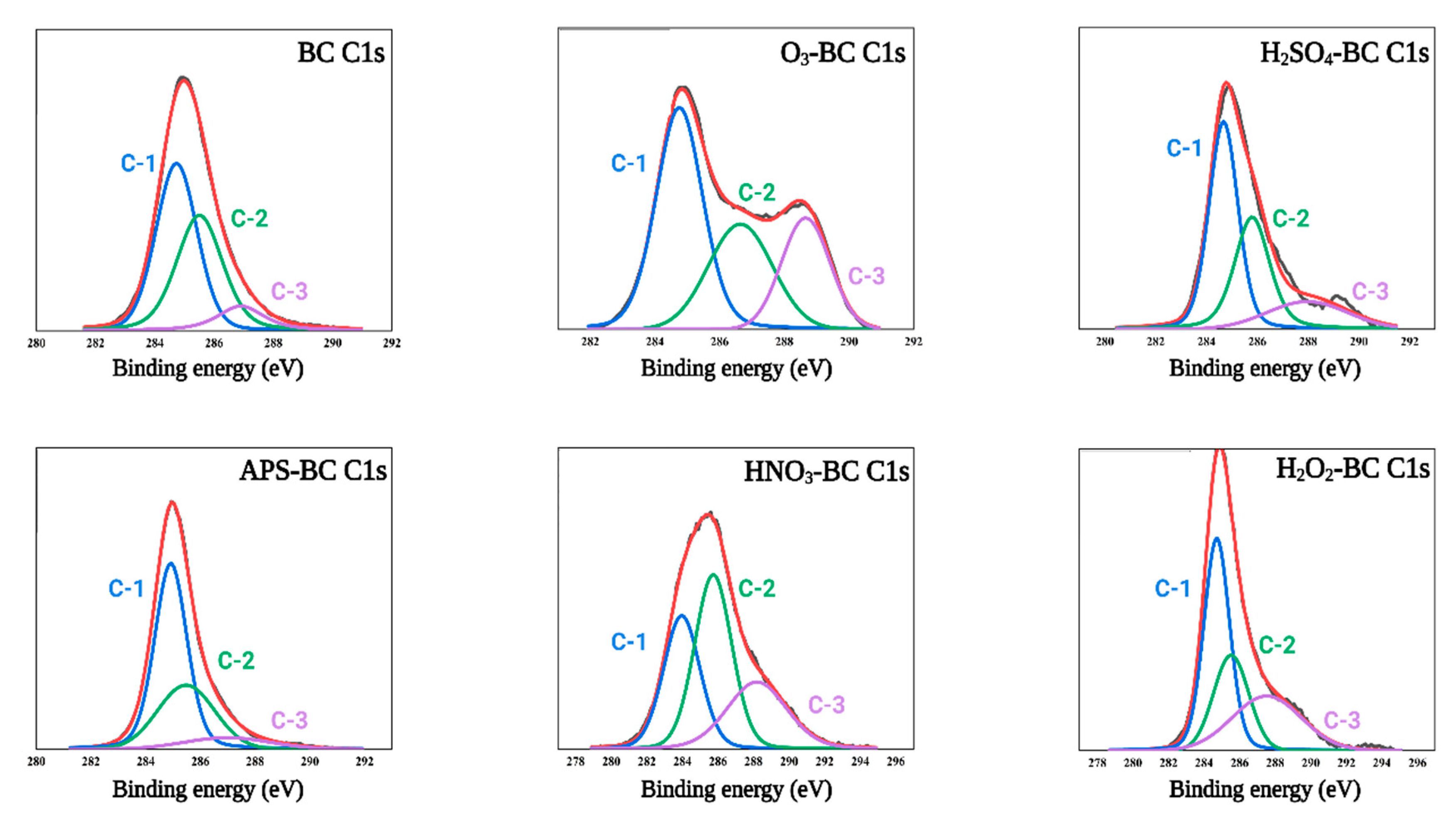
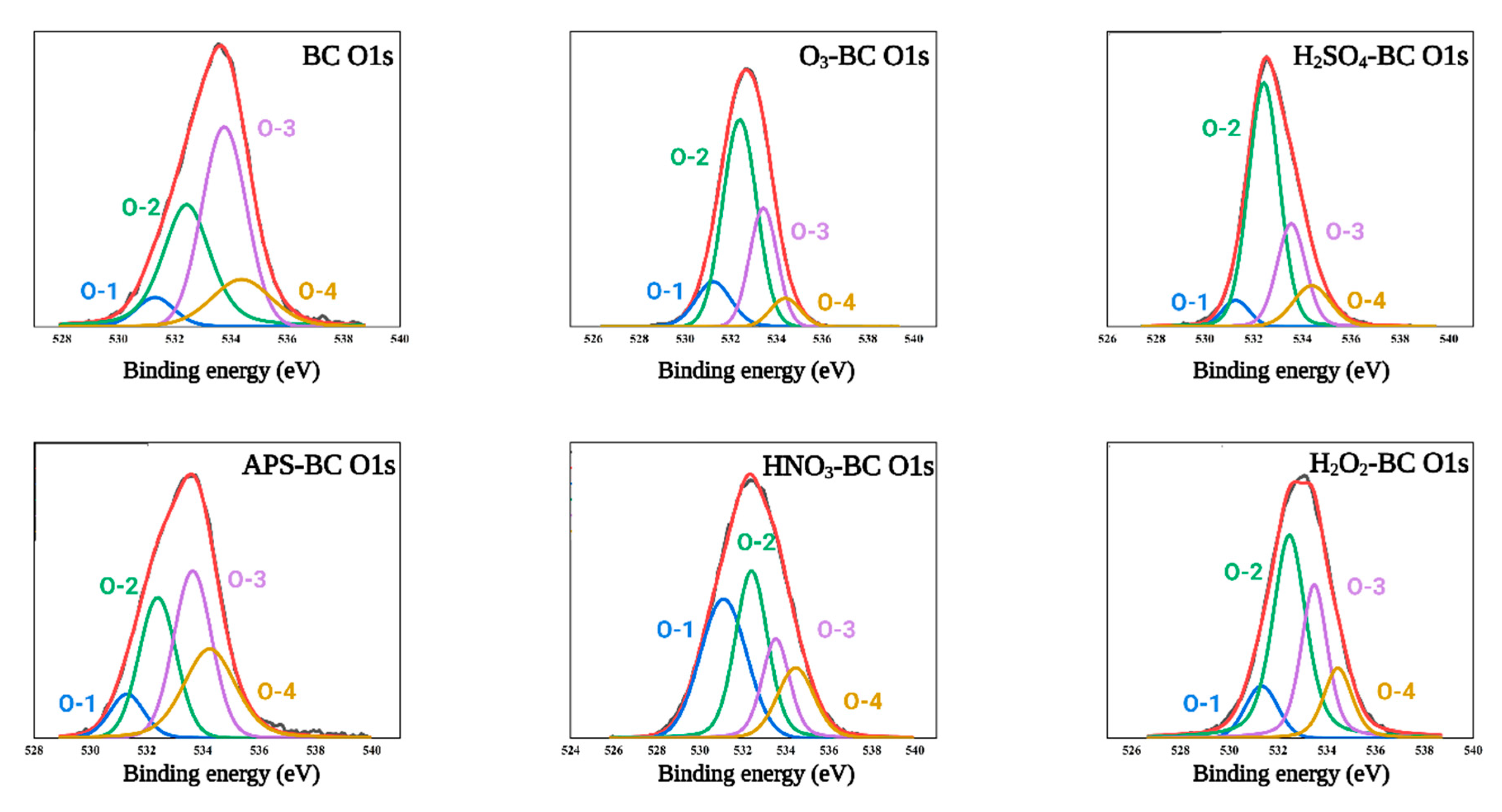
3.6. XPS Spectra of Biochar
4. Discussion
4.1. pH Values of Biochar
4.2. pHpzc of Biochar
4.3. Hydrophobicity Index of Biochar
4.4. Concentration of Acidic and Basic Sites in Biochar
4.5. Infrared Spectra of Biochar
4.6. XPS Spectra of Biochar
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koop, S.H.A.; Van Leeuwen, C.J. The challenges of water, waste and climate change in cities. Environ. Dev. Sustain. 2017, 19, 385–418. [Google Scholar] [CrossRef] [Green Version]
- von Braun, J. Bioeconomy—The global trend and its implications for sustainability and food security. Glob. Food Secur. 2018, 19, 81–83. [Google Scholar] [CrossRef]
- Fund, C.; El-Chichakli, B.; Patermann, C.; Dieckhoff, P. Bioeconomy Policy (Part II). Synopsis of National Strategies around World; German Bioeconomy Council: Berlin, Germany, 2018. [Google Scholar]
- Oni, B.A.; Oziegbe, O.; Olawole, O.O. Significance of biochar application to the environment and economy. Ann. Agric. Sci. 2019, 64, 222–236. [Google Scholar] [CrossRef]
- Wan, Z.; Sun, Y.; Tsang, D.C.W.; Khan, E.; Yip, A.C.K.; Ng, Y.H.; Rinklebe, J.; Ok, Y.S. Customised fabrication of nitrogen-doped biochar for environmental and energy applications. Chem. Eng. J. 2020, 401, 126136. [Google Scholar] [CrossRef]
- Enaime, G.; Baçaoui, A.; Yaacoubi, A.; Lübken, M. Biochar for Wastewater Treatment—Conversion Technologies and Applications. Appl. Sci. 2020, 10, 3492. [Google Scholar] [CrossRef]
- Kasera, N.; Kolar, P.; Hall, S.G. Nitrogen-doped biochars as adsorbents for mitigation of heavy metals and organics from water: A review. Biochar 2022, 4, 17. [Google Scholar] [CrossRef]
- Tang, D.; Lu, L.; Luo, Z.; Yang, B.; Ke, J.; Lei, W.; Zhen, H.; Zhuang, Y.; Sun, J.; Chen, K.; et al. Heteroatom-Doped Hierarchically Porous Biochar for Supercapacitor Application and Phenol Pollutant Remediation. Nanomaterials 2022, 12, 2586. [Google Scholar] [CrossRef]
- Cheng, B.-H.; Zeng, R.J.; Jiang, H. Recent developments of post-modification of biochar for electrochemical energy storage. Bioresour. Technol. 2017, 246, 224–233. [Google Scholar] [CrossRef]
- Cheng, F.; Li, X. Preparation and Application of Biochar-Based Catalysts for Biofuel Production. Catalysts 2018, 8, 346. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Atiyeh, H.K.; Li, M.; Chen, Y. Biochar facilitated bioprocessing and biorefinery for productions of biofuel and chemicals: A review. Bioresour. Technol. 2020, 295, 122252. [Google Scholar] [CrossRef]
- Shafawi, A.N.; Mohamed, A.R.; Lahijani, P.; Mohammadi, M. Recent advances in developing engineered biochar for CO2 capture: An insight into the biochar modification approaches. J. Environ. Chem. Eng. 2021, 9, 106869. [Google Scholar] [CrossRef]
- Choudhury, A.; Lansing, S. Adsorption of hydrogen sulfide in biogas using a novel iron-impregnated biochar scrubbing system. J. Environ. Chem. Eng. 2021, 9, 104837. [Google Scholar] [CrossRef]
- Kamali, M.; Sweygers, N.; Al-Salem, S.; Appels, L.; Aminabhavi, T.M.; Dewil, R. Biochar for soil applications-sustainability aspects, challenges and future prospects. Chem. Eng. J. 2022, 428, 131189. [Google Scholar] [CrossRef]
- Wu, W.; Yang, M.; Feng, Q.; McGrouther, K.; Wang, H.; Lu, H.; Chen, Y. Chemical characterization of rice straw-derived biochar for soil amendment. Biomass Bioenergy 2012, 47, 268–276. [Google Scholar] [CrossRef]
- Graves, C.; Kolar, P.; Shah, S.; Grimes, J.; Sharara, M. Can Biochar Improve the Sustainability of Animal Production? Appl. Sci. 2022, 12, 5042. [Google Scholar] [CrossRef]
- Joseph, S.; Pow, D.; Dawson, K.; Mitchell, D.R.G.; Rawal, A.; Hook, J.; Taherymoosavi, S.; Van Zwieten, L.; Rust, J.; Donne, S.; et al. Feeding Biochar to Cows: An Innovative Solution for Improving Soil Fertility and Farm Productivity. Pedosphere 2015, 25, 666–679. [Google Scholar] [CrossRef]
- Ouyang, D.; Chen, Y.; Yan, J.; Qian, L.; Han, L.; Chen, M. Activation mechanism of peroxymonosulfate by biochar for catalytic degradation of 1,4-dioxane: Important role of biochar defect structures. Chem. Eng. J. 2019, 370, 614–624. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, D.; Zhang, R.; Ding, Y.; Ren, Z.; Fu, M.; Cao, X.; Zeng, G. Singlet oxygen-dominated activation of peroxymonosulfate by passion fruit shell derived biochar for catalytic degradation of tetracycline through a non-radical oxidation pathway. J. Hazard. Mater. 2021, 419, 126495. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Tsang, D.C.W.; Guo, B.; Yang, J.; Shen, Z.; Hou, D.; Ok, Y.S.; Poon, C.S. Biochar as green additives in cement-based composites with carbon dioxide curing. J. Clean. Prod. 2020, 258, 120678. [Google Scholar] [CrossRef]
- Qin, Y.; Pang, X.; Tan, K.; Bao, T. Evaluation of pervious concrete performance with pulverized biochar as cement replacement. Cem. Concr. Compos. 2021, 119, 104022. [Google Scholar] [CrossRef]
- Liu, W.-J.; Jiang, H.; Yu, H.-Q. Development of Biochar-Based Functional Materials: Toward a Sustainable Platform Carbon Material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Sun, H.; Kang, J.; Wang, Y.; Indrawirawan, S.; Wang, S. Insights into Heterogeneous Catalysis of Persulfate Activation on Dimensional-Structured Nanocarbons. ACS Catal. 2015, 5, 4629–4636. [Google Scholar] [CrossRef]
- Uchimiya, M.; Chang, S.C.; Klasson, K.T. Screening biochars for heavy metal retention in soil: Role of oxygen functional groups. J. Hazard. Mater. 2011, 190, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Lyu, H.; Tang, J.; Wang, K.; Sun, H. Effects of ball milling on the photochemistry of biochar: Enrofloxacin degradation and possible mechanisms. Chem. Eng. J. 2020, 384, 123311. [Google Scholar] [CrossRef]
- Langley, L.A.; Fairbrother, D.H. Effect of wet chemical treatments on the distribution of surface oxides on carbonaceous materials. Carbon N.Y. 2007, 45, 47–54. [Google Scholar] [CrossRef]
- Kasera, N.; Hall, S.; Kolar, P. Effect of surface modification by nitrogen-containing chemicals on morphology and surface characteristics of N-doped pine bark biochars. J. Environ. Chem. Eng. 2021, 9, 105161. [Google Scholar] [CrossRef]
- Augoustides, V.; Kasera, N.; Kolar, P. Chemical characterization data of raw Loblolly pine bark nuggets. Chem. Data Collect. 2021, 33, 100727. [Google Scholar] [CrossRef]
- Zhu, Y.; Kolar, P. Adsorptive removal of p-cresol using coconut shell-activated char. J. Environ. Chem. Eng. 2014, 2, 2050–2058. [Google Scholar] [CrossRef]
- Usevičiūtė, L.; Baltrėnaitė, E. Methods for Determining Lignocellulosic Biochar Wettability. Waste Biomass Valorization 2020, 11, 4457–4468. [Google Scholar] [CrossRef]
- Gomes, H.T.; Miranda, S.M.; Sampaio, M.J.; Silva, A.M.T.; Faria, J.L. Activated carbons treated with sulphuric acid: Catalysts for catalytic wet peroxide oxidation. Catal. Today 2010, 151, 153–158. [Google Scholar] [CrossRef]
- Yin, W.; Zhang, W.; Zhao, C.; Xu, J. Evaluation of Removal Efficiency of Ni(II) and 2,4-DCP Using in Situ Nitrogen-Doped Biochar Modified with Aquatic Animal Waste. ACS Omega 2019, 4, 19366–19374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.; Hong, Z.-N.; Shi, R.-Y.; Cui, J.-Q.; Lai, H.-W.; Lu, H.-L.; Xu, R.-K. The effects of H2O2- and HNO3/H2SO4-modified biochars on the resistance of acid paddy soil to acidification. Environ. Pollut. 2022, 293, 118588. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xu, S.; Yue, Q.; Ortaboy, S.; Gao, B.; Sun, Y. Synthesis and characterization of heteroatom-enriched biochar from keratin-based and algous-based wastes. Adv. Powder Technol. 2016, 27, 1280–1286. [Google Scholar] [CrossRef]
- Chen, W.; Fang, Y.; Li, K.; Chen, Z.; Xia, M.; Gong, M.; Chen, Y.; Yang, H.; Tu, X.; Chen, H. Bamboo wastes catalytic pyrolysis with N-doped biochar catalyst for phenols products. Appl. Energy 2020, 260, 114242. [Google Scholar] [CrossRef]
- Chen, H.; Han, X.; Liu, Y. Gaseous Hydrogen Sulfide Removal Using Macroalgae Biochars Modified Synergistically by H2SO4/H2O2. Chem. Eng. Technol. 2021, 44, 698–709. [Google Scholar] [CrossRef]
- Pap, S.; Bezanovic, V.; Radonic, J.; Babic, A.; Saric, S.; Adamovic, D.; Turk Sekulic, M. Synthesis of highly-efficient functionalized biochars from fruit industry waste biomass for the removal of chromium and lead. J. Mol. Liq. 2018, 268, 315–325. [Google Scholar] [CrossRef]
- Hong, N.; Cheng, Q.; Goonetilleke, A.; Bandala, E.R.; Liu, A. Assessing the effect of surface hydrophobicity/hydrophilicity on pollutant leaching potential of biochar in water treatment. J. Ind. Eng. Chem. 2020, 89, 222–232. [Google Scholar] [CrossRef]
- Amin, F.R.; Huang, Y.; He, Y.; Zhang, R.; Liu, G.; Chen, C. Biochar applications and modern techniques for characterization. Clean Technol. Environ. Policy 2016, 18, 1457–1473. [Google Scholar] [CrossRef]

| Ethanol Percent | Index of Hydrophobicity |
|---|---|
| 0 | 0—Very hydrophilic |
| 3 | 1—Hydrophilic |
| 5 | 2—Slightly hydrophilic |
| 11 | 3—Slightly hydrophobic |
| 13 | 4—Moderate hydrophobic |
| 18 | 5—Strongly hydrophobic |
| 24 | 6—Very strongly hydrophobic |
| 36 | 7—Extremely Hydrophobic |
| Samples | pH | pHpzc | Hydrophobicity Index |
|---|---|---|---|
| BC | 6.4 | 5.1 | 2 |
| H2SO4-BC | 1.88 | 2.3 | 0 |
| O3-BC | 1.99 | 3.9 | 0–1 |
| HNO3-BC | 2.43 | 2.05 | 1–2 |
| H2O2-BC | 2.59 | 2.08 | 3 |
| APS-BC | 2.63 | 4.1 | 6 |
| Samples | Acidic Sites (µmol/g) | Basic Sites (µmol/g) | Difference (µmol/g) |
|---|---|---|---|
| BC | 917.84 | 625.08 | 292.76 |
| H2SO4-BC | 4969.90 | 307.37 | 4662.53 |
| O3-BC | 1646.27 | 297.51 | 1348.76 |
| HNO3-BC | 4946.04 | 350.98 | 4595.06 |
| H2O2-BC | 1800.70 | 437.76 | 1362.94 |
| APS-BC | 1442.41 | 428.57 | 1013.84 |
| Samples | C1s% | O1s% |
|---|---|---|
| BC | 86.8 | 13.4 |
| H2SO4-BC | 44.5 | 44.5 |
| O3-BC | 57.8 | 42.2 |
| HNO3-BC | 60.4 | 33.8 |
| H2O2-BC | 69.5 | 30.5 |
| APS-BC | 85.4 | 14.6 |
| Samples | C-1% | C-2% | C-3% |
|---|---|---|---|
| BC | 50.8 | 40.32 | 9.58 |
| H2SO4-BC | 49.16 | 34.20 | 16.62 |
| O3-BC | 47.92 | 28.93 | 23.14 |
| HNO3-BC | 31.73 | 41.64 | 26.61 |
| H2O2-BC | 45.16 | 25.84 | 28.98 |
| APS-BC | 58.96 | 32.38 | 8.64 |
| Samples | O-1% | O-2% | O-3% | O-4% |
|---|---|---|---|---|
| BC | 6.16 | 33.20 | 45.37 | 15.25 |
| H2SO4-BC | 5.34 | 59.20 | 23.36 | 12.08 |
| O3-BC | 13.23 | 54.72 | 25.47 | 6.56 |
| HNO3-BC | 35.24 | 32.57 | 16.90 | 15.26 |
| H2O2-BC | 10.17 | 47.91 | 28.22 | 13.67 |
| APS-BC | 8.77 | 28.44 | 34.97 | 27.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasera, N.; Augoustides, V.; Kolar, P.; Hall, S.G.; Vicente, B. Effect of Surface Modification by Oxygen-Enriched Chemicals on the Surface Properties of Pine Bark Biochars. Processes 2022, 10, 2136. https://doi.org/10.3390/pr10102136
Kasera N, Augoustides V, Kolar P, Hall SG, Vicente B. Effect of Surface Modification by Oxygen-Enriched Chemicals on the Surface Properties of Pine Bark Biochars. Processes. 2022; 10(10):2136. https://doi.org/10.3390/pr10102136
Chicago/Turabian StyleKasera, Nitesh, Victoria Augoustides, Praveen Kolar, Steven G. Hall, and Billie Vicente. 2022. "Effect of Surface Modification by Oxygen-Enriched Chemicals on the Surface Properties of Pine Bark Biochars" Processes 10, no. 10: 2136. https://doi.org/10.3390/pr10102136
APA StyleKasera, N., Augoustides, V., Kolar, P., Hall, S. G., & Vicente, B. (2022). Effect of Surface Modification by Oxygen-Enriched Chemicals on the Surface Properties of Pine Bark Biochars. Processes, 10(10), 2136. https://doi.org/10.3390/pr10102136







