Development and Performance Evaluation of Scale-Inhibiting Fracturing Fluid System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
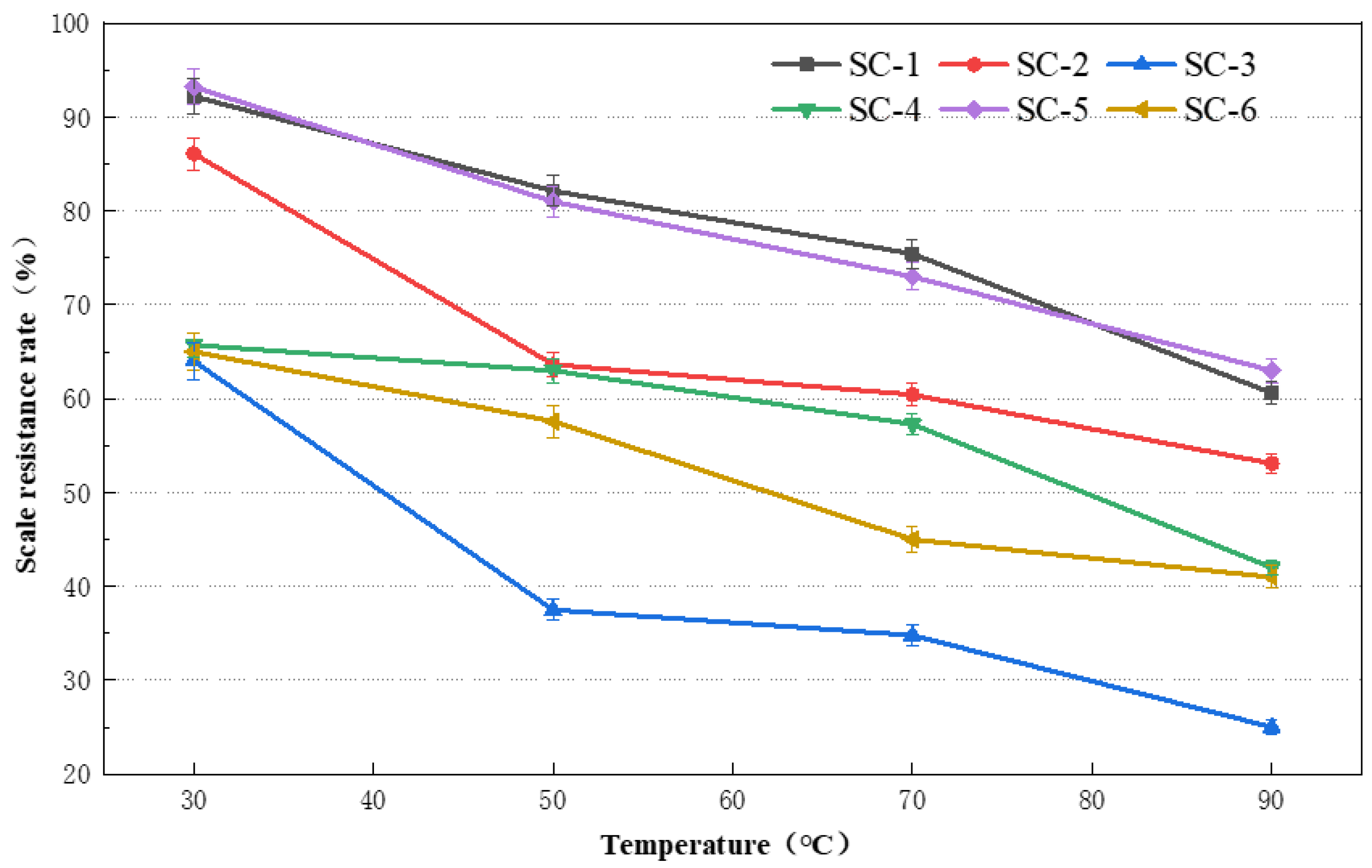
2.1.1. Effect of Temperature on CaSO4 Scale Inhibition
2.1.2. Effect of Temperature on CaCO3 Scale Inhibition
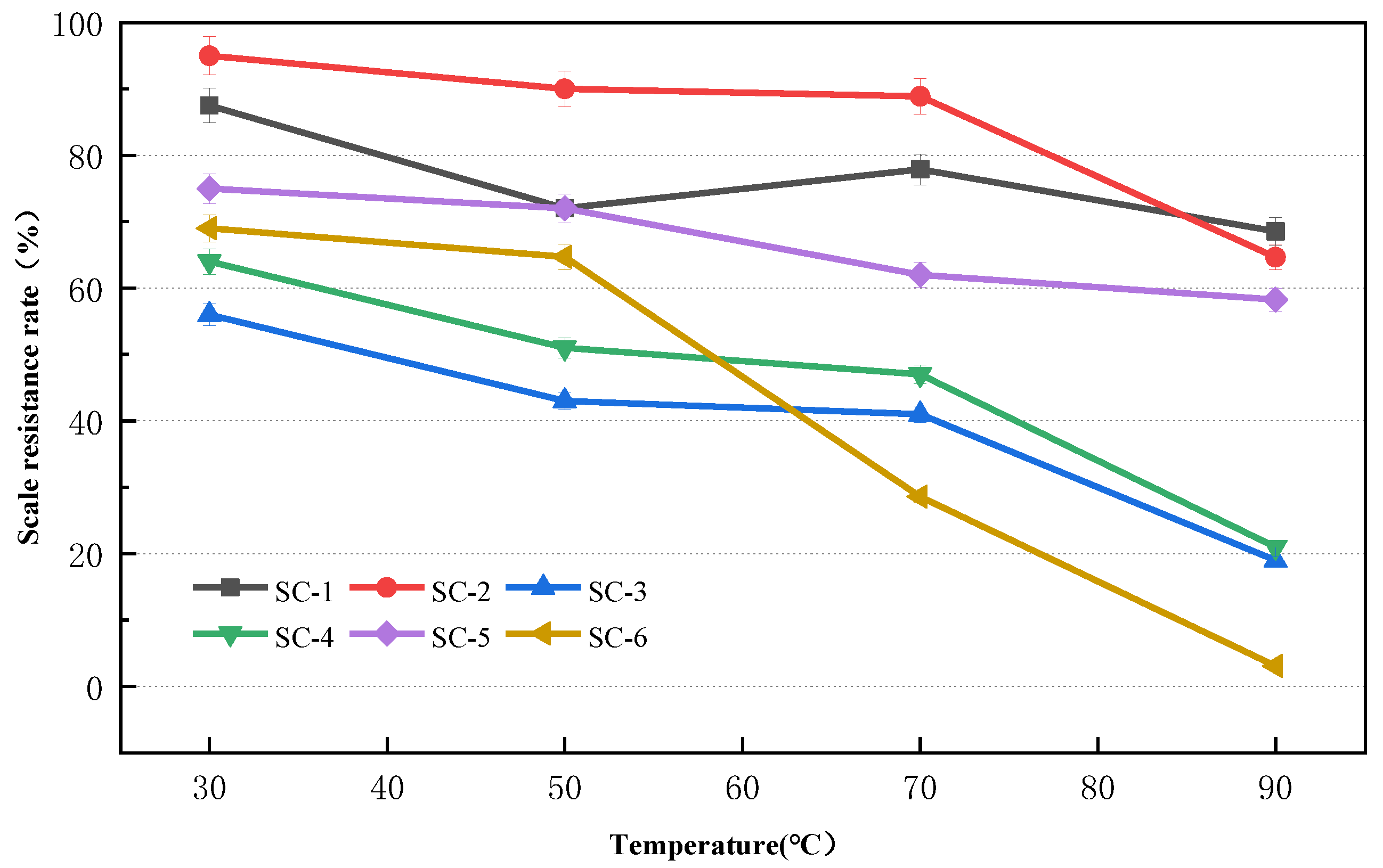
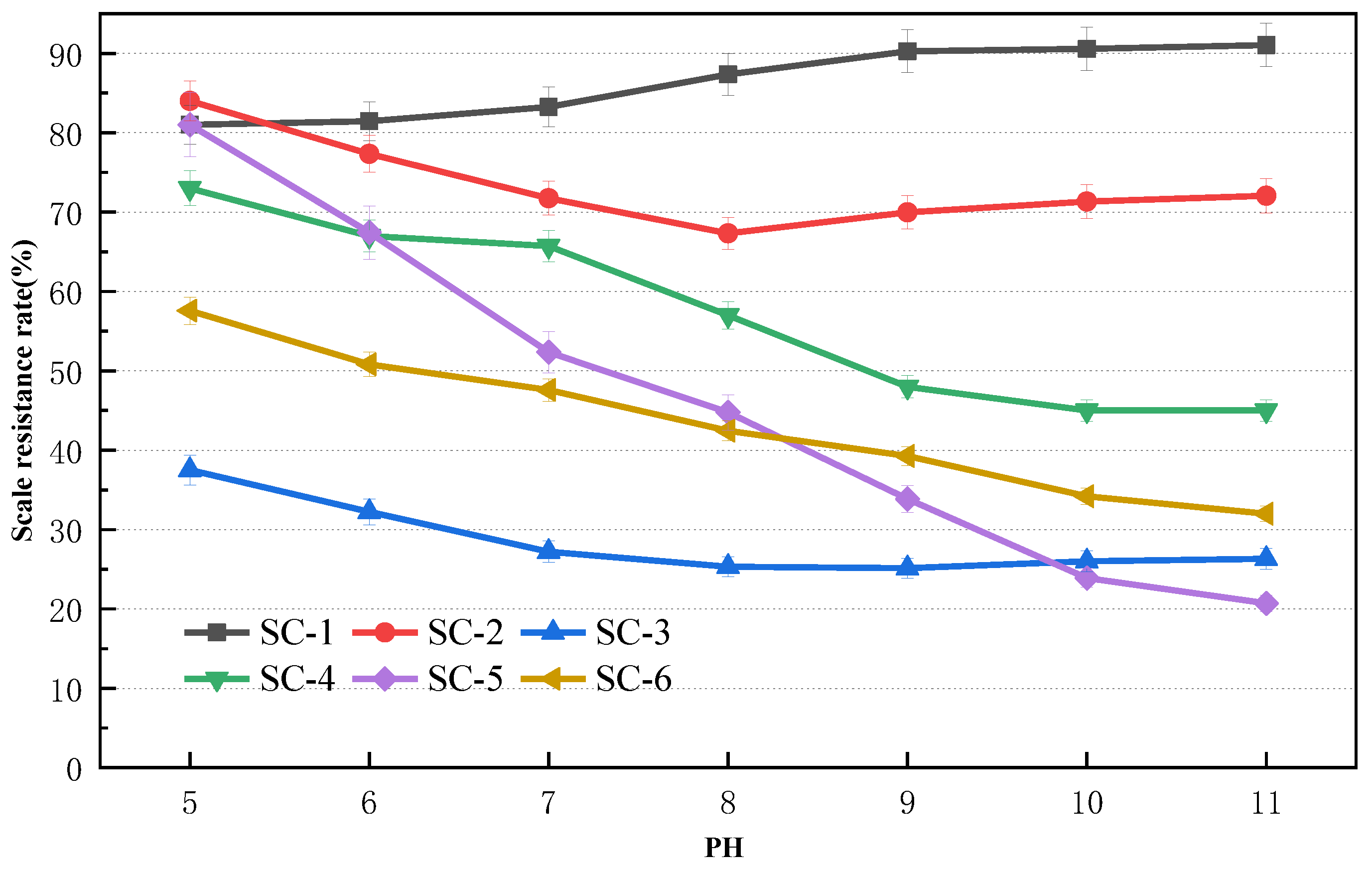
2.1.3. Effect of pH on CaSO4 Scale Inhibition
2.1.4. Effect of pH on CaCO3 Scale Inhibition
2.1.5. Mixed-Scale Preparation, Scale-Inhibition Ability Test, and Optimal Test of Scale Inhibitor Concentration
2.2. Fracturing Fluid and Its Compatibility with Scale Inhibitor
2.3. Performance Evaluation of Scale Inhibition
2.3.1. Rock Sample Preparation
2.3.2. Performance Evaluation of Scale-Inhibiting Fracturing Fluid
2.4. Drag-Reduction Test of Scale-Inhibiting Fracturing Fluid
2.4.1. Loop Friction Test Device
2.4.2. Drag-Reduction Test
3. Results and Discussion
3.1. Experimental Evaluation of Scale Inhibitor
3.1.1. Effect of Temperature on Scale Inhibition
3.1.2. Effect of pH on Scale Inhibition
3.1.3. Influence of Scale-Inhibitor Concentration and Anti-Mixing Scale Evaluation Experiment
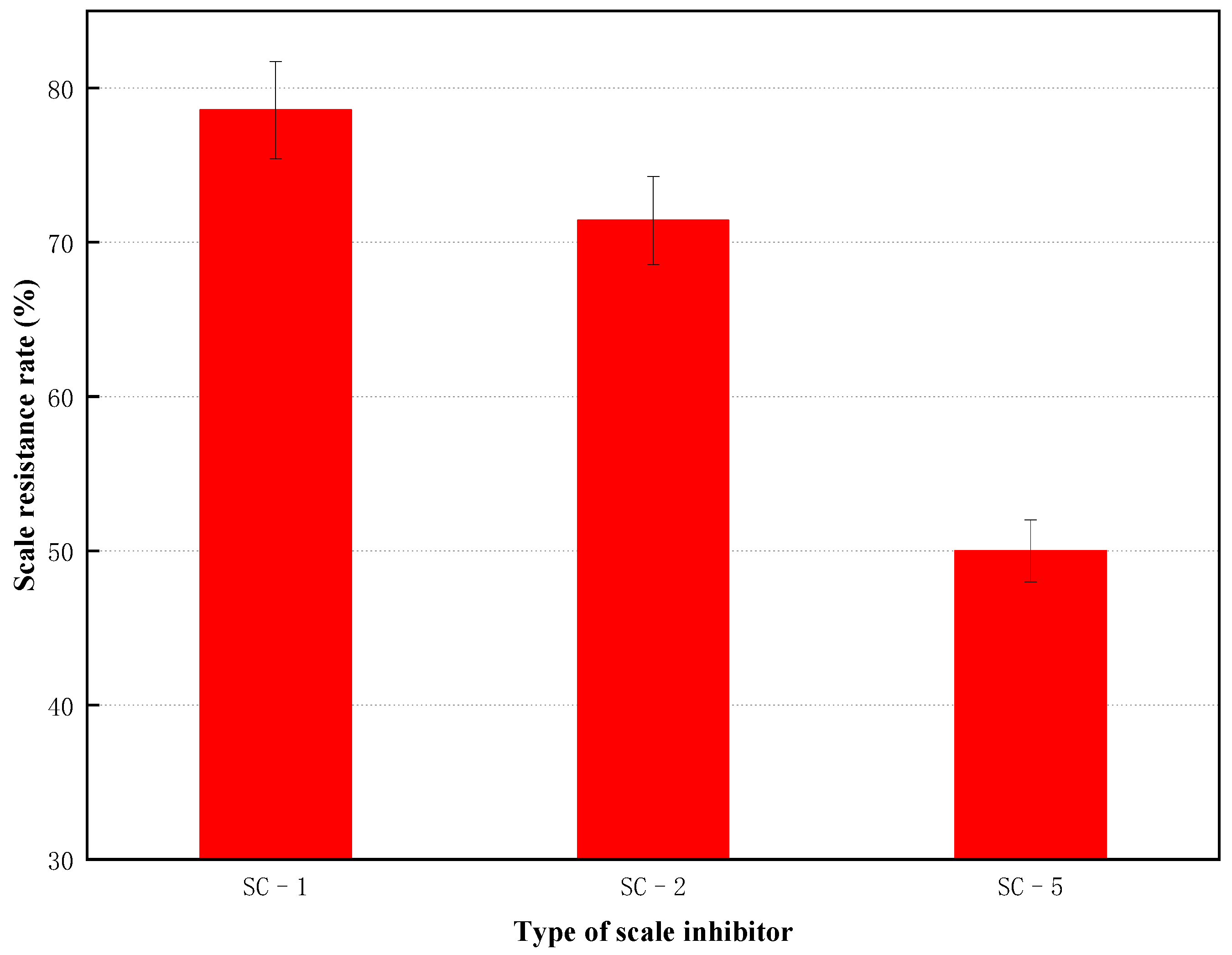
3.2. Compatibility Study
3.3. Scale-Inhibiting Effect of Fracturing Fluid
3.4. Drag-Reduction Evaluation of Scale-Inhibiting Fracturing Fluid
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, M.; Guo, Z.; Jiao, C.; Lu, S.; Li, J.; Xue, H.; Li, J.; Li, J.; Chen, G. Exploration progress and geochemical features of lacustrine shale oils in China. J. Pet. Sci. Eng. 2019, 178, 975–986. [Google Scholar] [CrossRef]
- Qijun, H.; Haiqing, H.; Jianzhong, L.; Tao, Y. Recent progress and prospect of oil and gas exploration by PetroChina Company Limited. China Pet. Explor. 2018, 23, 1–13. [Google Scholar]
- Wu, Q.; Xu, Y.; Wang, X.; Wang, T.; Zhang, S. Volume fracturing technology of unconventional reservoirs: Connotation, design optimization and implementation. Pet. Explor. Dev. 2012, 39, 377–384. [Google Scholar] [CrossRef]
- Zhang, A.; Yang, Z.; Li, X.; Xia, D.; Zhang, Y.; Luo, Y.; He, Y.; Chen, T.; Zhao, X. An evaluation method of volume fracturing effects for vertical wells in low permeability reservoirs. Pet. Explor. Dev. 2020, 47, 441–448. [Google Scholar] [CrossRef]
- Haghtalab, A.; Kamali, M.J.; Shahrabadi, A.; Golghanddashti, H. Investigation of the Precipitation of Calcium Sulfate in Porous Media: Experimental and Mathematical Modeling. Chem. Eng. Commun. 2015, 202, 1221–1230. [Google Scholar] [CrossRef]
- Olajire, A.A. A review of oilfield scale management technology for oil and gas production. J. Pet. Sci. Eng. 2015, 135, 723–737. [Google Scholar] [CrossRef]
- El-Said, M.; Ramzi, M.; Abdel-Moghny, T. Analysis of oilfield waters by ion chromatography to determine the composition of scale deposition. Desalination 2009, 249, 748–756. [Google Scholar] [CrossRef]
- Kamal, M.S.; Hussein, I.; Mahmoud, M.; Sultan, A.S.; Saad, M.A.S. Oilfield scale formation and chemical removal: A review. J. Pet. Sci. Eng. 2018, 171, 127–139. [Google Scholar] [CrossRef]
- Li, M. Synthesis and Performance Evaluation of High-Efficiency Scale Inhibitors for Oilfields; Ocean University of China: Qingdao, China, 2011. [Google Scholar]
- Jing, G.; Tang, S.; Li, X.; Wang, H. The analysis of scaling mechanism for water-injection pipe columns in the Daqing Oilfield. Arab. J. Chem. 2017, 10, S1235–S1239. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Jungang, L.; Qianya, Z.; Jinlai, F.; Yingli, L.; Jingxin, S. The analysis and prediction of scale accumulation for water-injection pipelines in the Daqing Oilfield. J. Pet. Sci. Eng. 2009, 66, 161–164. [Google Scholar] [CrossRef]
- Fan, C.; Shi, W.; Zhang, P.; Lu, H.; Zhang, N.; Work, S.; Al-Saiari, H.A.; Kan, A.T.; Tomson, M.B. Ultra-HTHP Scale Control for Deepwater Oil and Gas Production. In Proceedings of the SPE International Symposium on Oilfield Chemistry, Galveston, TX, USA, 11–13 April 2011. [Google Scholar]
- Li, J.; Tang, M.; Ye, Z.; Chen, L.; Zhou, Y. Scale formation and control in oil and gas fields: A review. J. Dispers. Sci. Technol. 2017, 38, 661–670. [Google Scholar] [CrossRef]
- Xiong, W.; Gill, M.; Moore, J.; Crandall, D.; Hakala, J.A.; Lopano, C. Influence of Reactive Flow Conditions on Barite Scaling in Marcellus Shale during Stimulation and Shut-In Periods of Hydraulic Fracturing. Energy Fuels 2020, 34, 13625–13635. [Google Scholar] [CrossRef]
- Paukert Vankeuren, A.N.; Hakala, J.A.; Jarvis, K.; Moore, J.E. Mineral Reactions in Shale Gas Reservoirs: Barite Scale Formation from Reusing Produced Water As Hydraulic Fracturing Fluid. Environ. Sci. Technol. 2017, 51, 9391–9402. [Google Scholar] [CrossRef]
- Ju, X. Research on Multi-Layer Water Mixing Injection and Anti-Scaling Technology in Dingbian Oil Production Plant; Xi’an Shiyou University: Xi’an, China, 2018. [Google Scholar]
- Peng, Y.; Yue, Z.; Ozuruigbo, C.Q.; Fan, C. Carbonate Scale Control under High Level of Dissolved Iron and Calcium in the Bakken Formation. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 14 April 2015. [Google Scholar]
- BinMerdhah, A.B.; Yassin, A.A.M.; Muherei, M.A. Laboratory and prediction of barium sulfate scaling at high-barium formation water. J. Pet. Sci. Eng. 2010, 70, 79–88. [Google Scholar] [CrossRef]
- Cui, C.; Zhang, S. Preparation, Characterization and Performance Evaluation of a Novel Scale Inhibiting and Dispersing Copolymer Containing Natural Tannin. J. Polym. Environ. 2020, 28, 1869–1879. [Google Scholar] [CrossRef]
- Amjad, Z.; Hooley, J. Influence of polyelectrolytes on the crystal growth of calcium sulfate dihydrate. J. Colloid Interface Sci. 1986, 111, 496–503. [Google Scholar] [CrossRef]
- Wylde, J.J.; Mahmoudkhani, A. Development of a Scale Inhibitor for Zr-Crosslinked Seawater Systems: A Case History of Successful Testing to Failure and Field Applications. In Proceedings of the SPE International Oilfield Scale Conference and Exhibition, Aberdeen, UK, 25–26 May 2016. [Google Scholar]
- Jing, Z.; Shao, X.; Gao, Y. Development of scale-resistant recyclable fracturing fluid systems. In Proceedings of the International Field Exploration and Development Conference 2021, Chengdu, China, 23–25 September 2021; pp. 288–294. [Google Scholar]
- Senthilmurugan, B.; Ghosh, B.; Sanker, S. High performance maleic acid based oil well scale inhibitors—Development and comparative evaluation. J. Ind. Eng. Chem. 2011, 17, 415–420. [Google Scholar] [CrossRef]
- Wang, X.-L.; Zhang, C.; Ouyang, P. The possibility of separating saccharides from a NaCl solution by using nanofiltration in diafiltration mode. J. Membr. Sci. 2002, 204, 271–281. [Google Scholar] [CrossRef]
- Vazquez, O.; Herrero, P.; Mackay, E.; Jordan, M. Non-aqueous vs aqueous overflush scale inhibitor squeeze treatment in an oilfield offshore Norway. J. Pet. Sci. Eng. 2016, 138, 1–10. [Google Scholar] [CrossRef]
- Liu, L.; Lan, X.; Li, N.; Luo, Z.; Liu, S. Study on the mechanism of acid pretreatment to reduce injury in hydraulic fracturing. J. Southwest Pet. Univ. (Sci. Technol. Ed.) 2016, 38, 150–155. [Google Scholar]
- Li, L.; Wang, Y.; Sun, Y.; Yang, W.; Yin, X.; Chen, Y.; Liu, Y. Novel and Green Hydroxyperylene Imide Based Fluorescent Polymer for Calcium Sulfate Scale Inhibition. J. Mol. Liq. 2021, 344, 117730. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, M.; Liu, J.; Chen, H.; Chen, Q.; Wang, B. Fluorescent-Tagged Hyper-Branched Polyester for Inhibition of CaSO4 Scale and the Scale Inhibition Mechanism. Mater. Today Commun. 2020, 25, 101359. [Google Scholar] [CrossRef]
- Sun, Y.; Wei, L.n.; Dai, C.; Yu, Z.; Xin, Y. The carbonic acid-rock reaction in feldspar/dolomite-rich tightsand and its impact on CO2-water relative permeability during geological carbon storage. Chem. Geol. 2021, 584, 120527. [Google Scholar] [CrossRef]
- Moayedi, H.; Aghel, B.; Vaferi, B.; Foong, L.K.; Bui, D.T. The feasibility of Levenberg–Marquardt algorithm combined with imperialist competitive computational method predicting drag reduction in crude oil pipelines. J. Pet. Sci. Eng. 2020, 185, 106634. [Google Scholar] [CrossRef]
- Kiaei, Z.; Haghtalab, A. Experimental study of using Ca-DTPMP nanoparticles in inhibition of CaCO3 scaling in a bulk water process. Desalination 2014, 338, 84–92. [Google Scholar] [CrossRef]
- Cui, K.; Li, C.; Yao, B.; Yang, F.; Sun, G. Synthesis and evaluation of an environment-friendly terpolymer CaCO3 scale inhibitor for oilfield produced water with better salt and temperature resistance. J. Appl. Polym. Sci. 2019, 137, 48460. [Google Scholar] [CrossRef]
- Mpelwa, M.; Tang, S.-F. State of the art of synthetic threshold scale inhibitors for mineral scaling in the petroleum industry: A review. Pet. Sci. 2019, 16, 830–849. [Google Scholar] [CrossRef]





| Ionic Types | CO32− | HCO3− | Cl− | SO42− | Ca2+ | Mg2+ | pH |
|---|---|---|---|---|---|---|---|
| Injected water (mg/L) | 8 | 287.3 | 11,574 | 540 | 320 | 67 | 8 |
| Core No. | Length/cm | Diameter/cm | Weight/g | Porosity /% | Pore Volume /cm3 | Quartz/% | Potash Feldspar/% | Plagioclase/% | Dolomite/% | Clay/% |
|---|---|---|---|---|---|---|---|---|---|---|
| b-1 | 5.71 | 2.38 | 58.11 | 8.22 | 2.09 | 17.23 | 1.10 | 13.20 | 67.20 | 1.27 |
| b-2 | 5.42 | 2.31 | 57.68 | 8.17 | 1.85 | 18.57 | 2.13 | 11.80 | 65.90 | 1.60 |
| Scale-Inhibitor Concentration (%) | 0.01% | 0.03% | 0.05% | 0.07% | 0.09% |
|---|---|---|---|---|---|
| Scale-Inhibiting Rate (%) | 70% | 71% | 73% | 77% | 78% |
| Scale-Inhibitor Concentration (%) | 0.1% | 0.2% | 0.3% | 0.5% | 0.7% |
|---|---|---|---|---|---|
| Scale-Inhibiting Rate (%) | 65% | 70% | 87% | 90% | 91% |
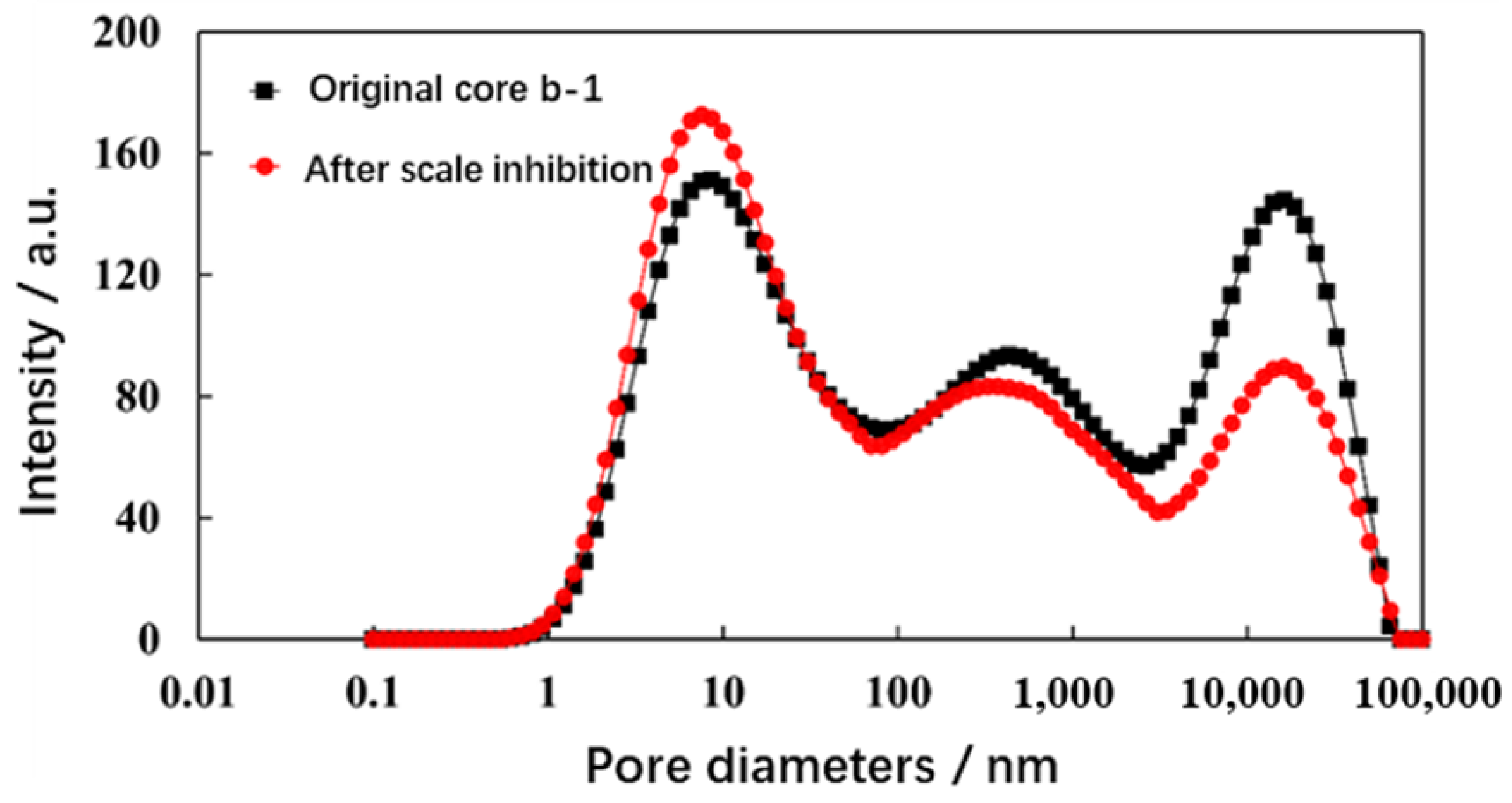
| Integral Area of Core Relaxation Signal | Damage Rate/% | Scale-Inhibition Rate/% | ||
|---|---|---|---|---|
| Original Core | Cores after Scaling | |||
| Without scale inhibitor | 10,299.63 | 7089.97 | 31.16 | 0 |
| With scale inhibitor | 5983.31 | 5864.28 | 1.99 | 96.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, M.; Sheng, L.; Ren, H.; Yiming, A.; Yao, E.; Zhang, K.; Zhao, L. Development and Performance Evaluation of Scale-Inhibiting Fracturing Fluid System. Processes 2022, 10, 2135. https://doi.org/10.3390/pr10102135
Zheng M, Sheng L, Ren H, Yiming A, Yao E, Zhang K, Zhao L. Development and Performance Evaluation of Scale-Inhibiting Fracturing Fluid System. Processes. 2022; 10(10):2135. https://doi.org/10.3390/pr10102135
Chicago/Turabian StyleZheng, Miao, Lianqi Sheng, Hongda Ren, Abulimiti Yiming, Erdong Yao, Kun Zhang, and Longhao Zhao. 2022. "Development and Performance Evaluation of Scale-Inhibiting Fracturing Fluid System" Processes 10, no. 10: 2135. https://doi.org/10.3390/pr10102135
APA StyleZheng, M., Sheng, L., Ren, H., Yiming, A., Yao, E., Zhang, K., & Zhao, L. (2022). Development and Performance Evaluation of Scale-Inhibiting Fracturing Fluid System. Processes, 10(10), 2135. https://doi.org/10.3390/pr10102135







