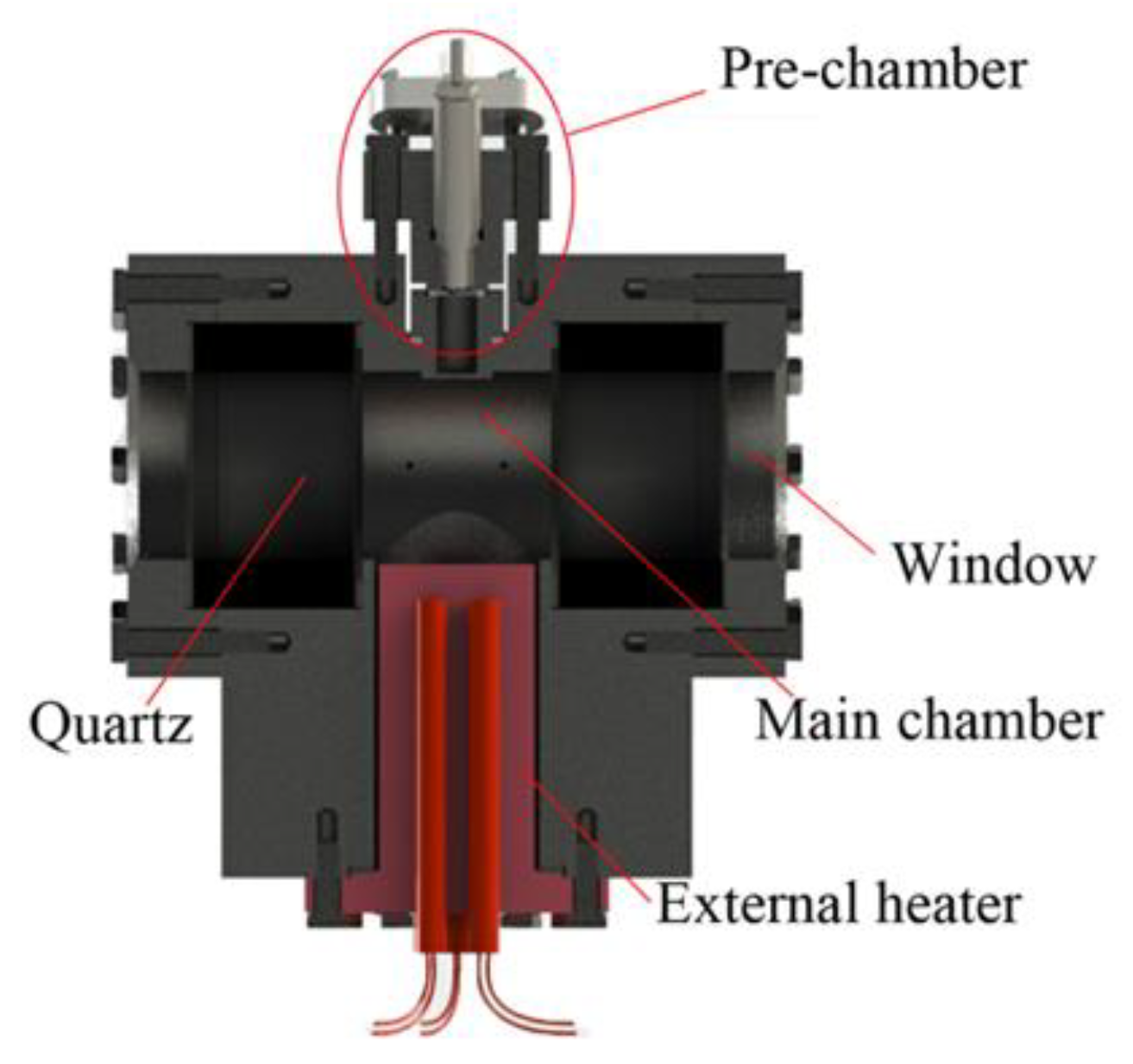
3.2. Effects of Pre-Chamber Volume
Figure 6 shows the shadow images of jet ignition processes with different pre-chamber volumes, and other experimental parameters were
T0 = 500 K,
p0 = 1.1 MPa, and
d = 2 mm. The time shown in
Figure 6 starts from the jet appearance, and the subsequent time is marked after the jet appeared (AJA).
As shown in
Figure 6, the flame propagation in the main chamber with the 8 mL pre-chamber was the fastest at all equivalence ratios. Under the condition of
Φ = 0.7, the ignition in the main chamber with the 8 mL pre-chamber was much earlier than that using the 4 mL and 6 mL pre-chambers. With an increase in the equivalence ratio, the pre-mixture became more combustible, the ignition advanced, the flame propagation was accelerated, and the combustion became more intense. When the flame nearly filled the window, a bright light was emitted. At the conditions of
Φ = 0.9 and
Φ = 1.0, the ignition in the main chamber with the 4 mL pre-chamber was still the latest, but the ignition timings using the 6 mL and 8 mL pre-chambers were close to each other when
Φ = 1.0.
Figure 7 shows the accurate jet appearance timings, the main chamber ignition timings after spark ignition (ASI), and the ignition delays in the main chamber; these data were obtained from the shadow images. The ignition delay is defined as the interval between the jet appearance and ignition in the main chamber. The jet appearance timings were determined by the time needed for flame propagation inside the pre-chamber, and the time was affected by both the flame propagation distance and the flame velocity. The ignition timings and the ignition delays were determined by the ignition energy carried by the jet flame.
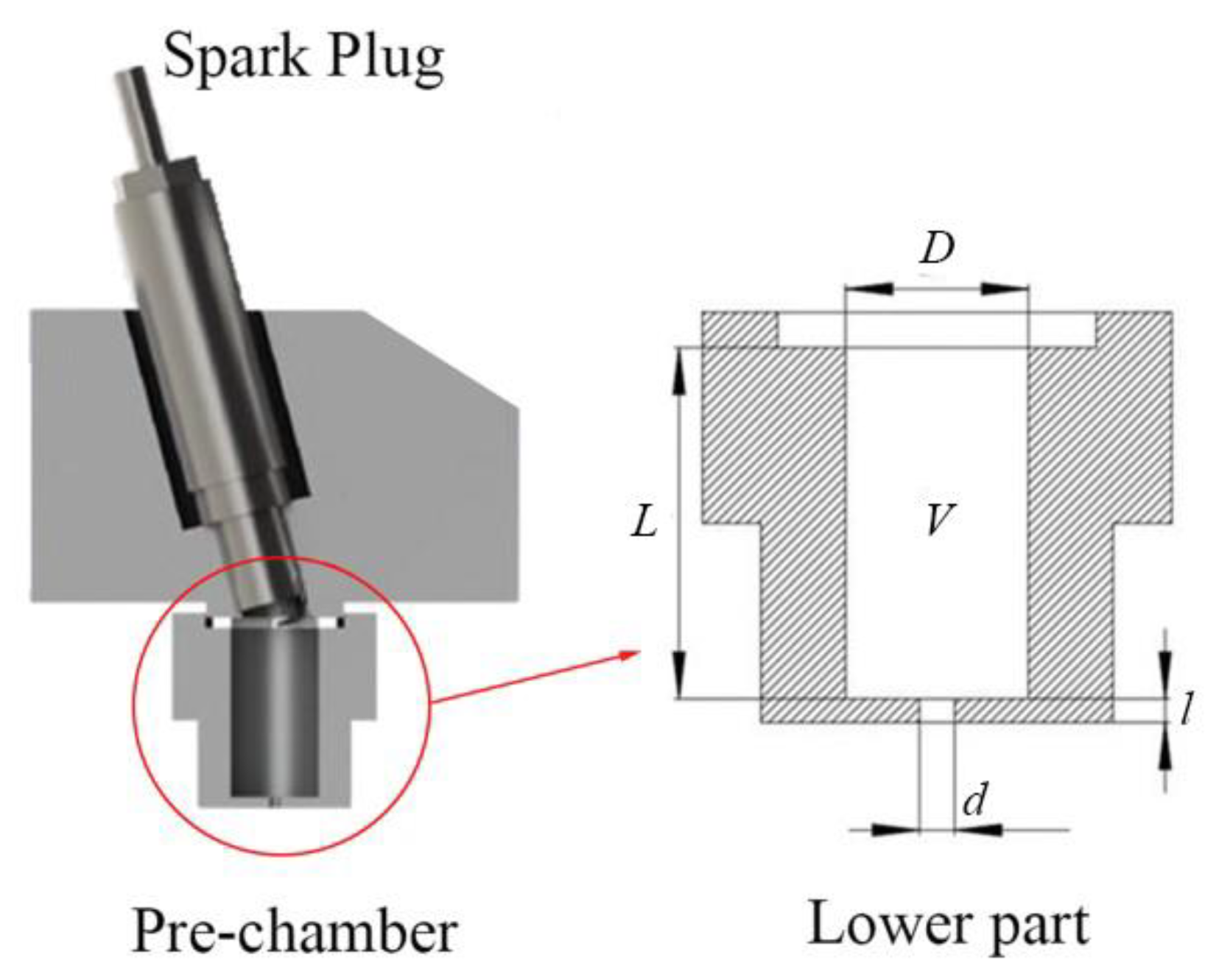
Figure 8 shows the schematic diagram of flame generation and propagation inside the pre-chamber. After the spark plug was discharged, an initial flame kernel was formed near the electrodes, and the flame propagated downwards. When the flame touched the wall of the pre-chamber, heat would transfer rapidly to the pre-chamber. The flame propagation during the period before the flame touched the wall can be regarded as an adiabatic process. For a larger volume pre-chamber, its diameter (
D) was larger, so the period of the adiabatic flame propagation process was longer and the heat loss was smaller. Moreover, the pre-chamber can be regarded as a relatively enclosed space, and the heat dissipation rate per unit volume
qL can be described by Equation (3). The convective heat transfer coefficient
h is a constant value.
F is the internal surface area of the pre-chamber,
V is the pre-chamber volume,
Tb is the temperature of the burnt gas, and
Tw is the temperature of the pre-chamber wall. The interior of the pre-chamber was an approximate cylinder, so Equation (1) can be transformed to Equation (2). The heat release rate by unit volume of the pre-mixture can be described by Equation (3). Subtract Equation (3) from Equation (2) to get the net heat release rate per unit volume of the pre-mixture (Equation (4)).
H is the reaction heat per unit volume of the pre-mixture, which is a constant value, and
k0 is the frequency factor, which is related to the number of molecular collisions. [
A] is the concentration of the pre-mixture, and the
n is the total reaction order.
E is the activation energy, and
Ru is the molar gas constant.
L and
D of the pre-chamber were proportionally changed when the pre-chamber volume changed; thus, a larger volume pre-chamber exhibited a smaller
qL. Before the reaction started, the
qG of each pre-chamber was the same because of the same initial pre-mixture parameters.
At
Φ = 0.7, it can be seen from
Figure 7 that the jet appearance timing, ignition timing, and ignition delay decreased with the increase in pre-chamber volume. Under this condition, the flame propagation velocity of ammonia was low due to the low equivalence ratio. Thus, the time needed for flame propagation was extended. In other words, the heat exchange time was long, and thus, the heat loss would play an important role. For a larger volume pre-chamber, due to it having a lower
qL, the heat loss was less. Additionally, as mentioned in
Section 3.1, when the spark plug discharged, some of the pre-mixed gas emerged from the pre-chamber through the orifice before being ignited, causing the concentration of the pre-mixture ([
A]) in the pre-chamber to be reduced. As for a larger volume pre-chamber, the ratio of the amount of emerged gas to the original pre-mixture amount in the pre-chamber would be less. Therefore, when the ignition started in the pre-chamber, the concentration ([
A]) of the ammonia/air pre-mixture of the larger volume pre-chamber would be higher, which means that the heat release rate (
qG) of the larger volume pre-chamber would also be higher. Thus, the burnt area temperature (
Tb) and the flame velocity of the larger volume pre-chamber would be higher, which made the jet appearance timing for the 8 mL pre-chamber the earliest, followed by the 6 mL and then the 4 mL pre-chambers. The turbulence kinetic energy and temperature of the jet flame from a larger volume pre-chamber would also be larger, so it could ignite the pre-mixture in the main chamber earlier.
Under the condition of Φ = 0.9, the jet appearance of the 6 mL pre-chamber was the earliest. When the flammability of the pre-mixture increased with the increase in the equivalence ratio, ignition inside the pre-chamber advanced, and the flame propagation inside the pre-chamber was accelerated, causing a reduction in the heat exchange time and the amount of emerged gas. The negative effects caused by heat loss and emerged gas were diminished to some extent, and the advantages of shorter flame propagation distance caused the jet appearance timing of the 6 mL pre-chamber to occur earlier than for the 8 mL pre-chamber. As for the 4 mL pre-chamber, even though it had the shortest flame propagation distance, under this condition, the heat loss and emerged gas still had a great influence on the flame propagation velocity inside the pre-chamber and the ignition energy of the jet flame. Thus, its jet appearance timing was still the latest among the three pre-chambers. The ignition delay of the 8 mL pre-chamber was still the shortest, followed by that of the 6 mL and then the 4 mL pre-chambers.
When the equivalence ratio rose to 1.0, the flammability was further enhanced. Therefore, the heat loss and the amount of emerged gas were reduced to small values, exerting only a tiny influence on the flame velocity inside the pre-chambers. The flame propagation distance inside the pre-chamber became the most important factor affecting the appearance of the jet, so the jet appearance timing retarded with the increase in pre-chamber volume. The orders of ignition timings and ignition delays from small to large changed to the 6 mL pre-chamber, the 8 mL pre-chamber, and the 4 mL pre-chamber, respectively. Compared to the 8 mL pre-chamber, the flame propagation of the 6 mL pre-chamber had a shorter vertical length, so that when the flame propagated from the top to the orifice, the propagation time required for the 6 mL pre-chamber was shorter than for the 8 mL pre-chamber. The cumulative heat loss in the 6 mL pre-chamber would be less, and the temperature and kinetic energy of the jet flame from the 6 mL pre-chamber would be larger than those for the 8 mL pre-chamber, so the ignition delay for the 6 mL pre-chamber was shorter than that for the 8 mL pre-chamber. For the 4 mL pre-chamber, at these ambient parameters, the cumulative heat loss of the 4 mL pre-chamber would still be the largest among the three pre-chambers, due to its having the largest qL, and the emerged gas ratio of the 4 mL pre-chamber would still be the largest. The advantages of having the shortest flame propagation distance could not counteract the negative effects of it having the largest heat loss and emerged gas ratio. Even though its jet appearance timing was the earliest, its ignition delay was still the longest.
It can also be noticed that when the equivalence ratio increased from 0.7 to 0.9, and then to 1.0, the values of jet appearance timing, ignition timing, and ignition delay of the 8 mL pre-chamber decreased more slightly than those of the 4 mL and the 6 mL pre-chambers. As discussed above, the 8 mL pre-chamber had the smallest emerged gas ratio, qL, and the longest adiabatic flame propagation process. It can be speculated that the jet characteristics of the 8 mL pre-chamber were inherently little affected by these negative factors. Thus, the change in the equivalence ratio would not have a significant influence on the 8 mL pre-chamber. However, for the 4 mL and the 6 mL pre-chambers, the increase in the flammability of the pre-mixture would significantly reduce the negative impacts of both the heat loss and emerged gas.
Figure 9 shows the main chamber pressure (MCP) traces and pressure rise rates (PRR) in the main chambers with different pre-chamber volumes. At
Φ = 0.7, the maximum MCPs of the three pre-chambers were close to each other. However, the maximum PRR of the 8 mL pre-chamber was much higher than those of the 4 mL and the 6 mL pre-chambers. Moreover, the combustion duration (PRR > 0) decreased as the pre-chamber volume increased. The combustion duration of the 4 mL pre-chamber was more than twice that of the 8 mL pre-chamber. At
Φ = 0.9 and
Φ = 1.0, the maximum MCPs increased as the pre-chamber volume increased. The PRRs of the 8 mL pre-chamber were still the largest, and its combustion durations were the shortest, especially at
Φ = 1.0, although the ignitions in the main chamber with the 4 mL and the 6 mL pre-chambers occurred earlier than with the 8 mL pre-chamber; due to it having the shortest combustion duration, the MCP of the 8 mL pre-chamber reached the maximum at the earliest time.
Figure 10 shows the shadow images of the jet ignition processes of the pre-chambers with three volumes under different initial thermodynamic parameters; the equivalence ratio was kept the same as 1.0. Combined with the data in
Figure 11, it was found that under the condition of
T0 = 500 K and
p0 = 0.8 MPa, the jet appearance and ignition orders were the 8 mL pre-chamber, the 6 mL pre-chamber, and the 4 mL pre-chamber, respectively. When the initial pressure was increased to 1.1 MPa, the experimental parameters and results were the same as under the condition of
Φ = 1.0, as shown in
Figure 7c.
Increasing the temperature to 550 K, the flammability of the pre-mixture was further enhanced. It can be noticed in
Figure 11c that the jet appearance timing and the ignition timing of the 4 mL pre-chamber were the earliest, and its ignition delay was the shortest. This may be because when the flammability of the ammonia/air pre-mixture was enough to ensure rapid ignition and fast flame propagation in the pre-chamber, the amount of emerged gas would be very tiny and the heat exchange timing would be short. Thus, the flame velocity and flame temperature in the pre-chamber would not be significantly affected by these two factors. Moreover, as the initial temperature was increased to 550 K, the temperature of the pre-chamber wall (
Tw) was also increased, to some extent. Therefore, the decrease in (
Tb −
Tw) caused the heat dissipation rate to decrease, and the differences in
qL among the three pre-chambers were narrowed. The distance required for flame propagation inside the 4 mL pre-chamber was the shortest, enabling the flame to pass through the pre-chamber in the shortest period of time; therefore, the cumulative heat loss of the 4 mL pre-chamber would be the least. Thus, the 4 mL pre-chamber could generate the jet faster than the 6 mL pre-chamber and the 8 mL pre-chamber, igniting the pre-mixture in the main chamber earlier after the ejection of the jet. The differences in the ignition delay among the three pre-chambers were not large.
The MCP and PRR traces are displayed in
Figure 12. It can be seen that under each of the three conditions, the maximum MCP and PRRs of the 8 mL pre-chamber were the largest, and its combustion durations were the shortest. Although under the condition of
T0 = 550 K and
p0 = 1.1 MPa, the 8 mL pre-chamber exhibited disadvantages with regards to the jet appearance and ignition delay, the larger volume caused the chemical energy stored in the 8 mL pre-chamber to be greater than that in the 4 mL pre-chamber and the 6 mL pre-chamber. As a result, the jet from the 8 mL pre-chamber could carry the largest ignition energy. Moreover, because the three pre-chambers have the same orifice area, it took a longer time for the flame in the 8 mL pre-chamber to be completely ejected than for the 4 mL pre-chamber and the 6 mL pre-chamber, which prolonged the duration of the ignition and the turbulence disturbance in the main chamber.
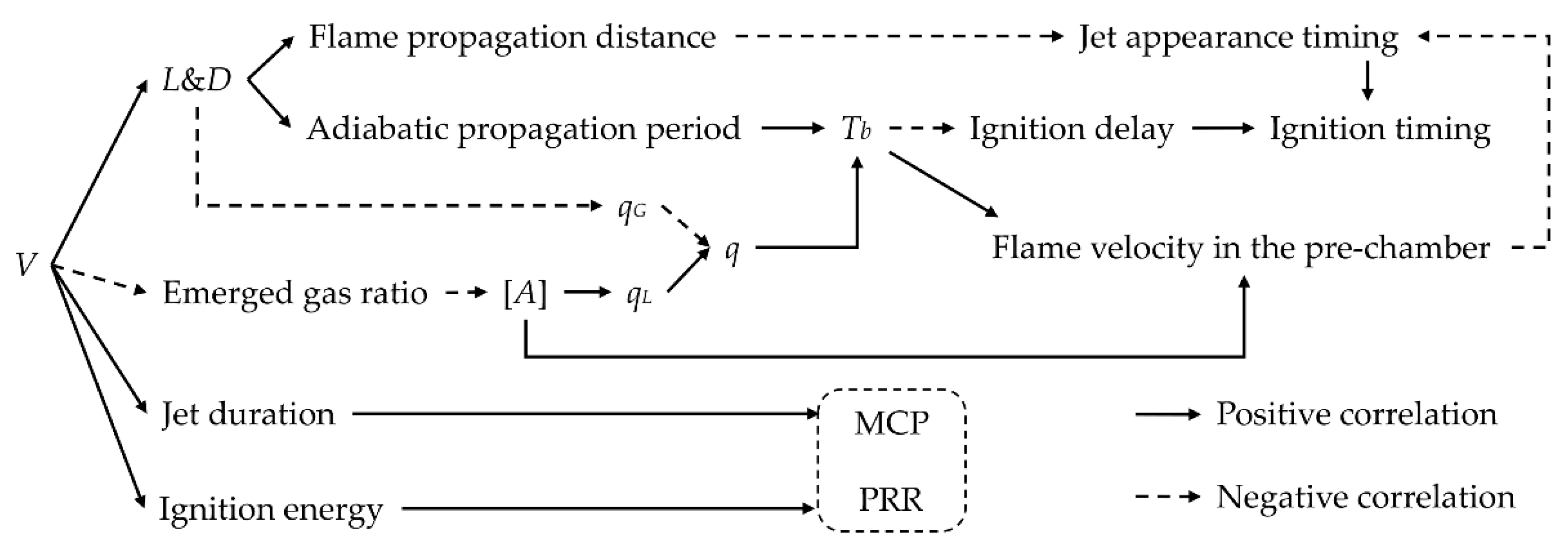
The effects of pre-chamber volume on the process variables, the jet ignition characteristics, and the combustion characteristics are summarized and shown in
Figure 13.
3.3. Effects of Pre-Chamber Orifice Diameter
Figure 14 shows the jet appearance timings, ignition timings, and ignition delays for different pre-chamber orifice diameters under different initial pressures and temperatures. The other experimental parameters were
V = 6 mL and
Φ = 1.0. The pre-chamber of 1 mm orifice diameter could not ignite the pre-mixture in the main chamber under any of these conditions.
It was found that under the condition of T0 = 500 K & p0 = 0.8 MPa, the jet appearance timing of the 3 mm pre-chamber was earlier, and at the other two conditions, the jet appearance timings of the other two pre-chambers were close. Under all the three conditions, the values of the ignition timings and ignition delays decreased as the orifice diameter changed from 2 mm to 3 mm. The 3 mm orifice pre-chamber had a larger outlet area than that of the 2 mm orifice, so the throttling effect of the 3 mm orifice pre-chamber was less than that of the 2 mm orifice pre-chamber. When the flame passed through the 3 mm orifice, the heat loss and kinetic energy dissipation would be less. As a result, the jet generated from the 3 mm orifice pre-chamber could cause the earlier ignition of the pre-mixture in the main chamber.
Figure 15 displays the MCP and PRR traces. As can be seen from these graphs, under the three conditions, the MCPs and PRRs of the 3 mm orifice pre-chamber developed first, which corresponds with the data shown in
Figure 14. Before the PRRs of the 3 mm orifice pre-chamber reached the maximums, the slopes of the PRR curves for the 3 mm orifice pre-chamber were greater than those of the 2 mm orifice pre-chamber, indicating the earlier combustion in the main chamber with the 3 mm orifice pre-chamber. However, the maximums of both the MCPs and PRRs of the 2 mm orifice pre-chamber were higher than that of the 3 mm orifice pre-chamber. As for the combustion durations, when
T = 500 K and
p = 0.8 MPa, the combustion durations of the two pre-chamber were close, and the ignition timing of the 3 mm orifice pre-chamber was earlier, so the MCP of the 3 mm orifice pre-chamber reached the maximum earlier too. Under the conditions of 500 K and 1.1 MPa and 550 K and 1.1 MPa, the combustion durations of the 2 mm orifice pre-chamber were shorter, and the MCPs of the 2 mm orifice pre-chamber reached their maximums earlier.
Taking the condition of
T0 = 500 K and
p0 = 1.1 MPa as an example, the natural luminescence images of the process from the time of the appearance the jet flame to the ignition in the main chamber are shown in
Figure 16, and the jet penetration lengths are measured. The time when the jet flame obviously propagated laterally was regarded as the time when the main chamber ignition began. The luminous intensity of the jet from the 3 mm orifice pre-chamber was higher, and before ignition in the main chamber with the 3 mm orifice pre-chamber, the jet penetration length of the 3 mm orifice pre-chamber was also longer. We noticed a phenomenon that, for the 3 mm orifice pre-chamber, after about 5 ms AJA, the portion of the jet near the orifice became wide, and the jet flame oscillated. This may be because as the combustion in the pre-chamber progressed, the pressure difference between both sides of the orifice increased, the speed of jet flame was accelerated, and the increased air resistance caused the vertical velocity of the flame edge to decrease. This phenomenon for the 2 mm orifice pre-chamber occurred later, and the oscillation degree of jet flame was relatively small. After about 9 ms AJA, the jet penetration length of the 2 mm orifice pre-chamber grew at a faster rate. The area of the 2 mm orifice was less than half that of the 3 mm orifice; therefore, the resistance of the 2 mm orifice was much larger when the flame passed through it. As a result, the pressure was easier to establish inside the 2 mm orifice pre-chamber after ignition. The ignition delay of the 2 mm orifice pre-chamber was larger; thus, the jet remained affected by the main chamber flame for a longer period time, and the pressure difference between both sides of the 2 mm orifice was further increased, which caused the jet to grow faster in the late stage. Before the ignition in the main chamber using the 2 mm orifice pre-chamber, the jet penetration length of the 2 mm orifice pre-chamber was larger than that of the 3 mm orifice pre-chamber.
The ignition in the main chamber always occurred at the jet flame front, so the ignition position in the main chamber with the 2 mm orifice pre-chamber was located close to the center of the main chamber, while that of the 3 mm orifice pre-chamber was close to the top. The centrally near ignition positions caused the flame propagation distance in the main chamber with the 2 mm orifice pre-chamber to be shorter, shortened the combustion duration, and increased the PRR of the 2 mm orifice pre-chamber. Additionally, the jet duration of the 2 mm orifice pre-chamber was longer, and the jet continued to provide turbulence fluctuation for some time after the pre-mixture in the main chamber was ignited, which also contributed to the higher PRR maximum. Under the conditions of 500 K and 1.1 MPa and 550 K and 1.1 MPa, the PRR curves of the 3 mm orifice pre-chamber showed the same development trend—the PRR first rose rapidly, fell to a plateau, and finally reached zero. It can be speculated that due to the turbulence fluctuation of the jet, the burning rate of the pre-mixture in the main chamber was high at the early stage. When the fluctuation effects dissipated, the PRR decreased, and the flame in the main chamber continued to propagate at a lower rate, which caused the plateau of the PRR curves. For the 2 mm orifice pre-chamber, due to its longer jet durations, the PRRs could continue to rise, and the curves were unimodal.
The effects of pre-chamber orifice diameter on the process variables, the jet ignition characteristics, and the combustion characteristics were summarized and are shown in
Figure 17.