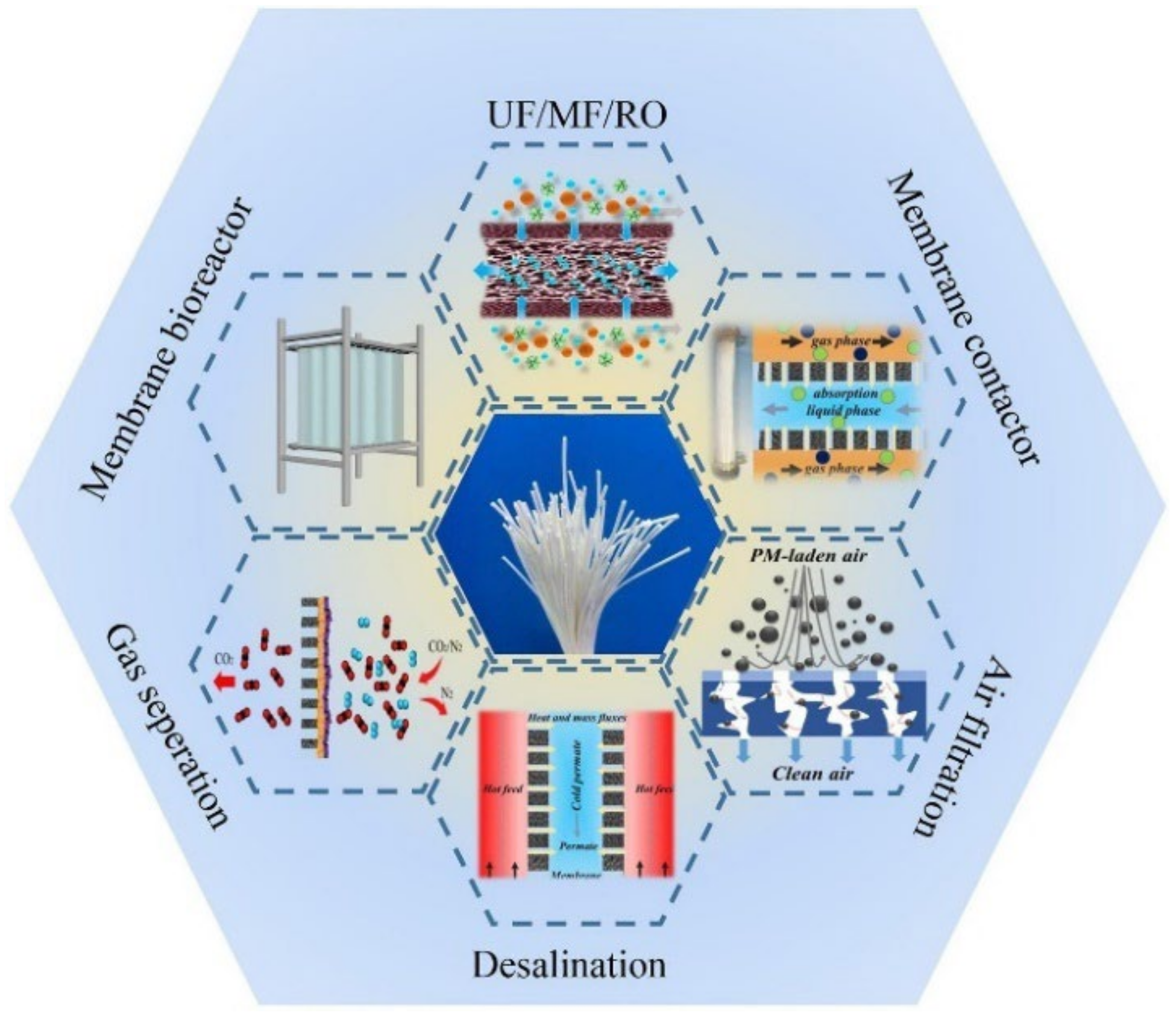
A Review on Hollow Fiber Membrane Contactors for Carbon Capture: Recent Advances and Future Challenges
Abstract
:1. Introduction
2. Membrane Materials
- The success of membrane gas absorption over other processes will largely depend on the type of membrane materials and fabrication method used.
- At high temperatures, ceramic hollow fiber membranes have better mechanical and thermal stability than polymeric membranes.
- PTFE offers better resistance than PP when wetting is a matter of importance.
- The plasma-treated membranes exhibited a lower degradation rate and a higher mass transfer rate compared to untreated membranes.
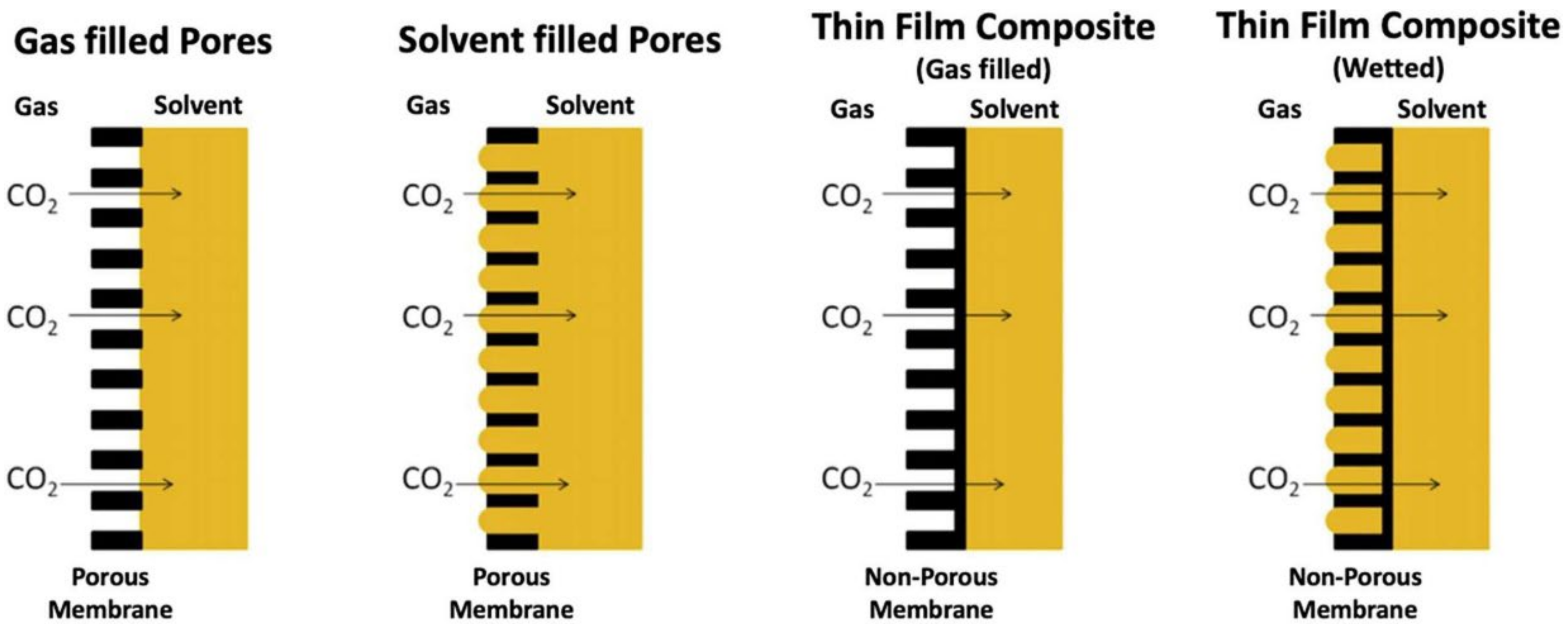
- Pore wetting significantly reduces the CO2 mass transfer rate, as the mass transfer coefficient through liquid-filled pores is orders of magnitude lower than that if the pores are gas-filled.
- Membrane materials with a high contact angle with the solvent could be resistant to pore wetting.
- A membrane with small pores will resist wetting, but there is a trade-off as smaller pores will also reduce the rate of CO2 mass transfer.
- From a wetting point of view, hydrophobic membrane materials are preferred due to their relatively high contact angle.
- PP and PE membranes can be chemically degraded during long-term operation in contact with an amine-based solvent.
3. Membrane Modification
3.1. Role of Additives
3.2. Surface Modifying Macromolecules
3.3. Composite Membranes
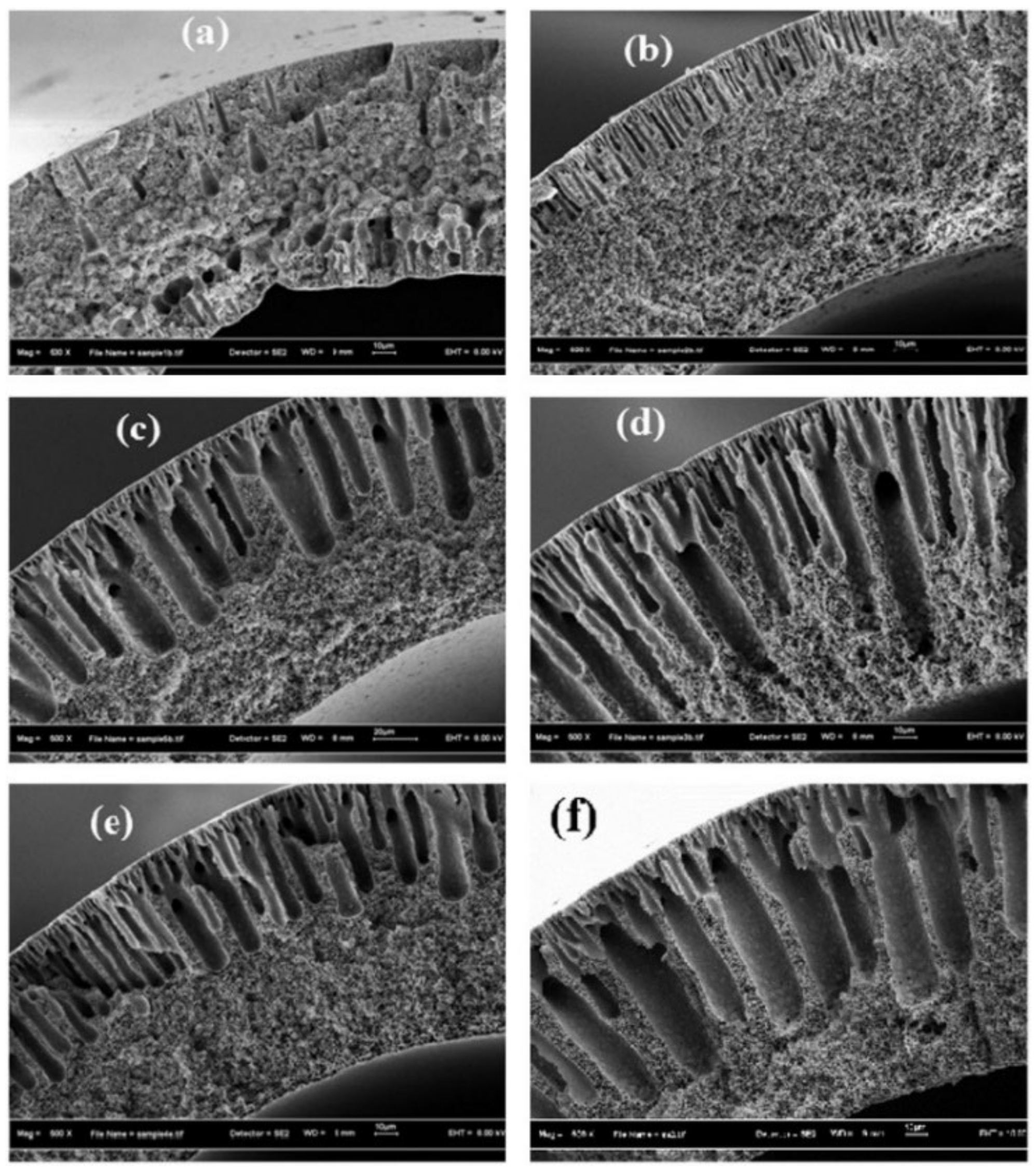
- Permeability, wetting resistance, and pore size are important factors, which can seriously affect the performance of the membranes.
- By controlling the phase inversion rate of the spinning dope using the appropriate amount of non-solvent additives, an improved membrane structure can be achieved.
- Membranes that are composed of microporous support and an ultrathin dense layer could prevent membrane wetting, due to the dense thin film on the liquid side of the membrane, which prevents the liquid breakthrough.
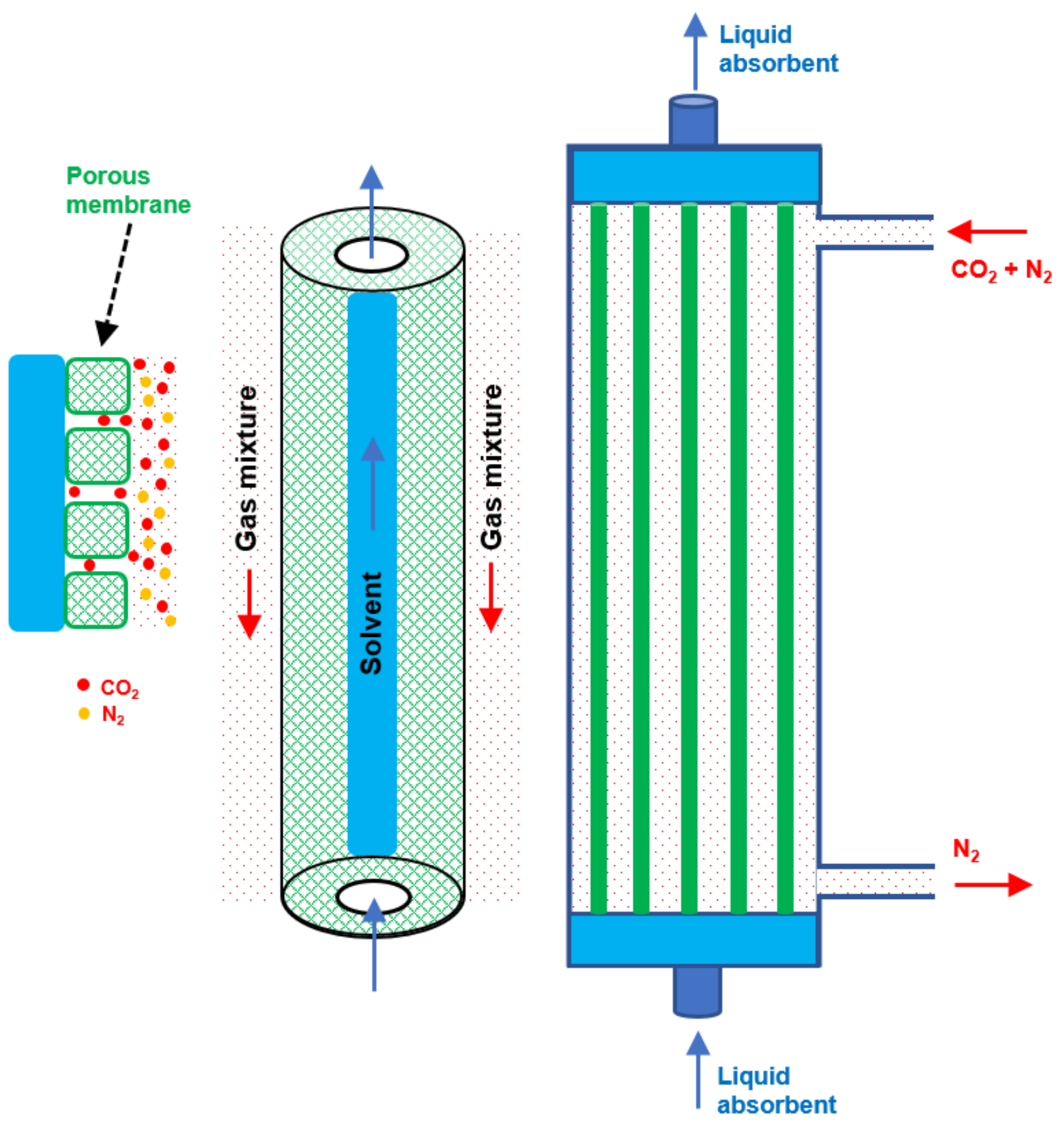
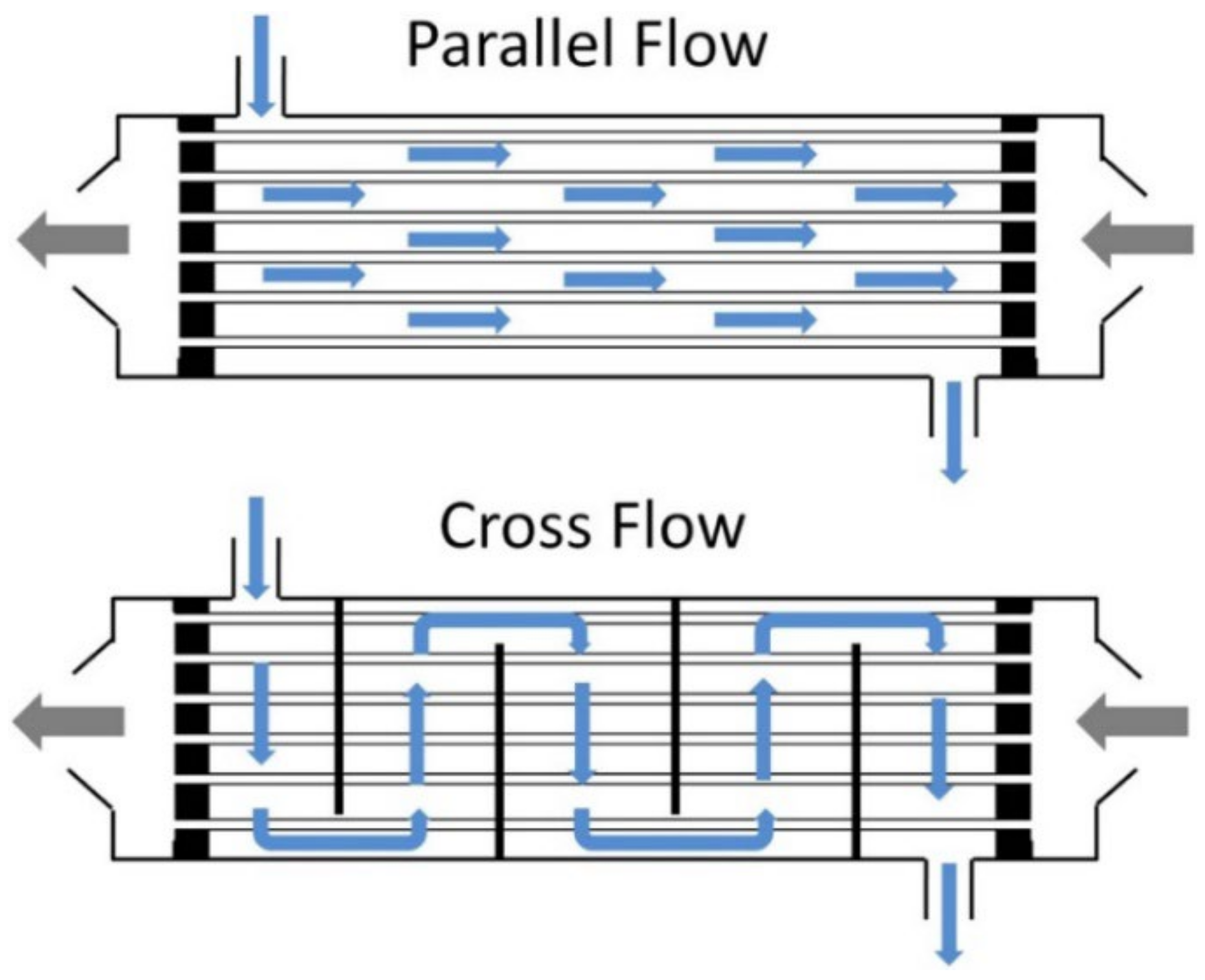
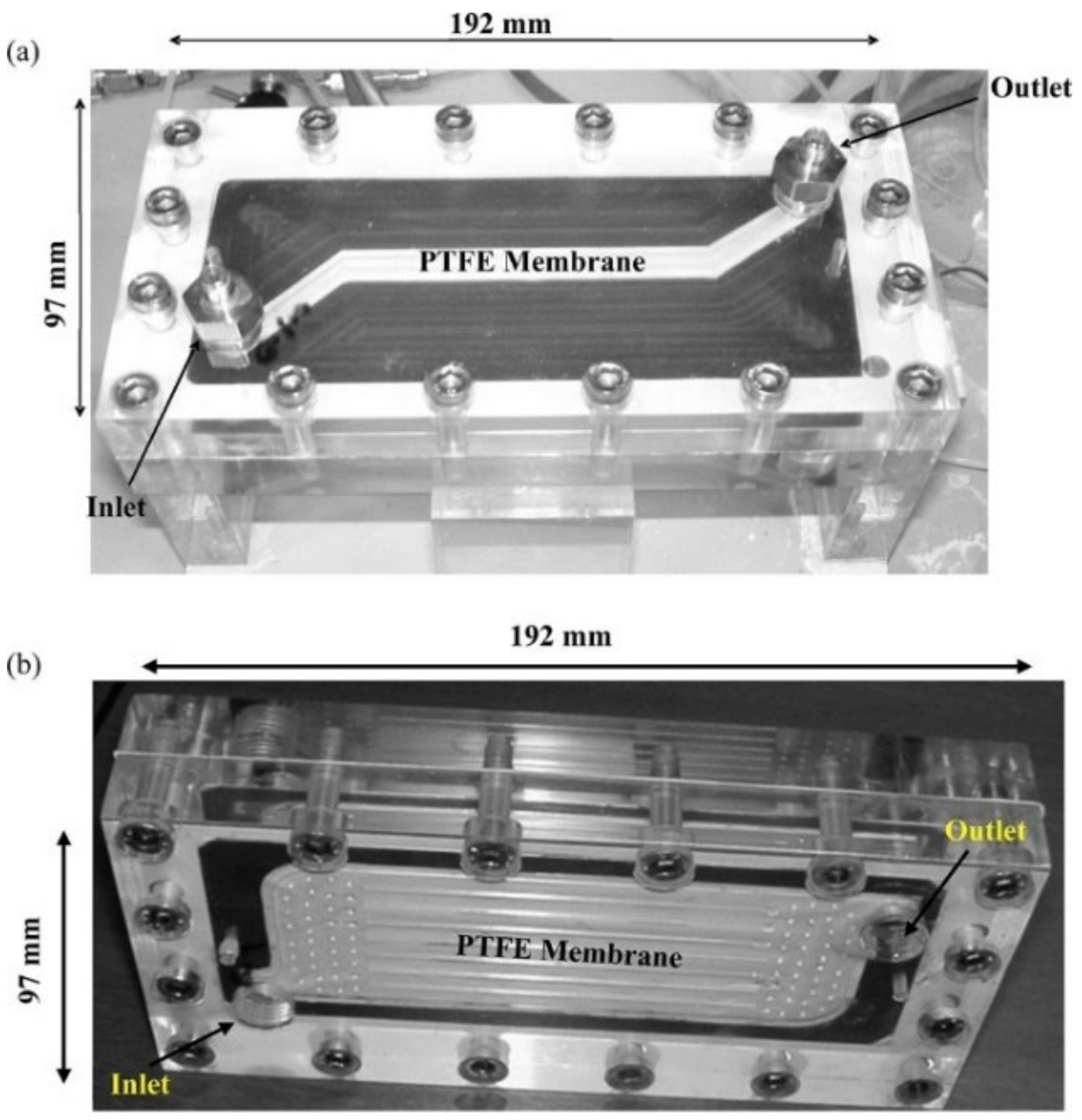
4. Module Shape
5. Absorbents for Membrane Contactors
5.1. Alkanolamines
5.2. Amino Acids
5.3. Ionic Liquids
5.4. Deep Eutectic Solvents (DES)
5.5. Inorganic Solvents
- The choice of proper absorbent plays a critical role in membrane gas absorption as it can strongly influence mass transfer, efficiency, and operating cost.
- High surface tension and good compatibility with the membrane are the most important criteria for selecting an absorbent.
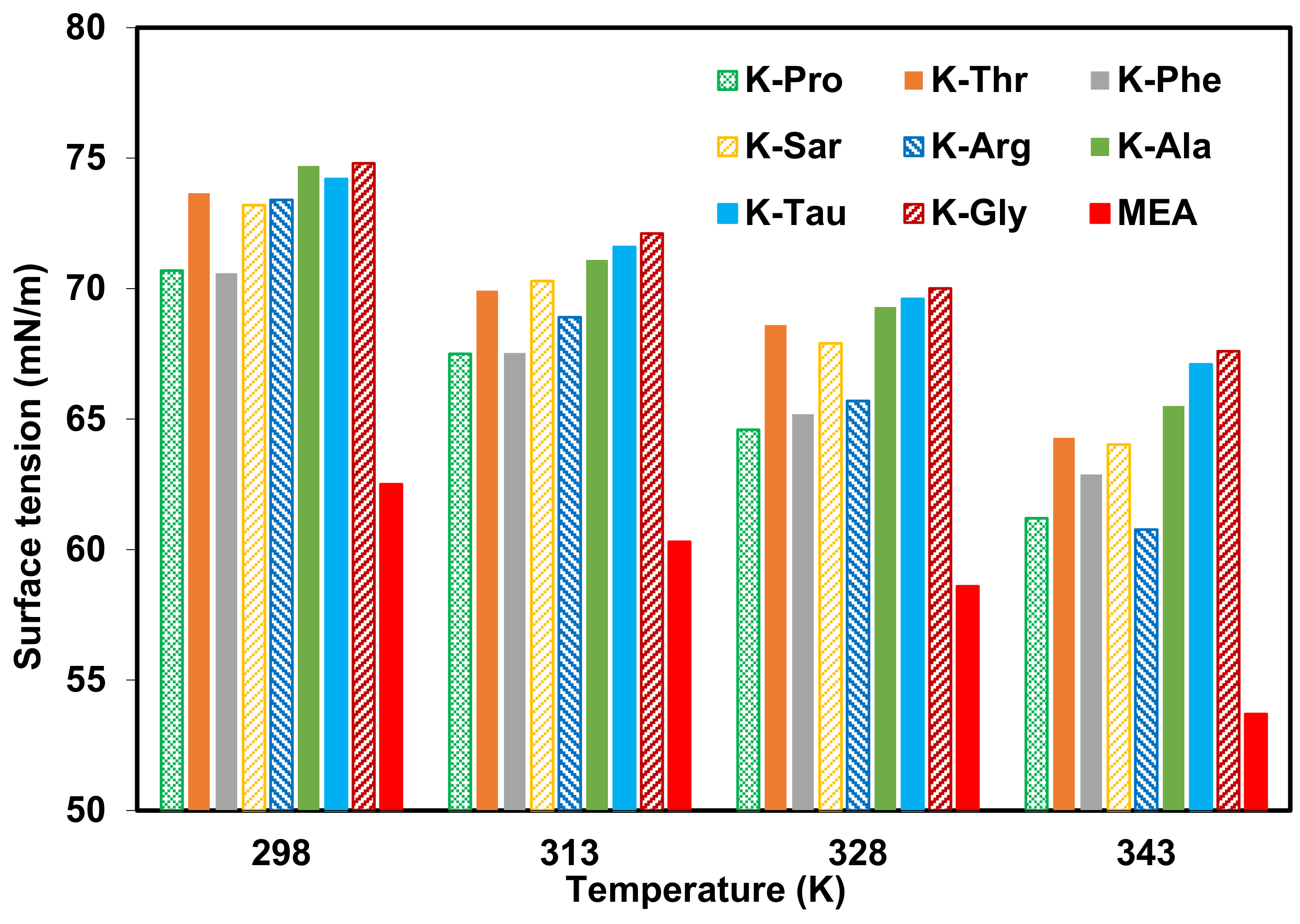
- Proline is the most promising amino acid for bulk absorption of CO2 with faster reaction kinetics than other amino acids while glycine exhibits the highest surface tension.
- Better absorption and desorption performance can be expected when a mixture of two solvents is used.
- Potassium salt of amino acids has greater absorption properties than sodium salt of amino acids.
- Amino acid salts are a better choice than amines for use in membrane contactors from the surface tension point of view.
- Absorbent properties and their interaction with membrane material are essential factors in determining the extent of wetting.
- The interaction between chemical solvent and membrane pores leads to a change in the internal pore structure, which enhances membrane wetting.
6. Operating Parameters
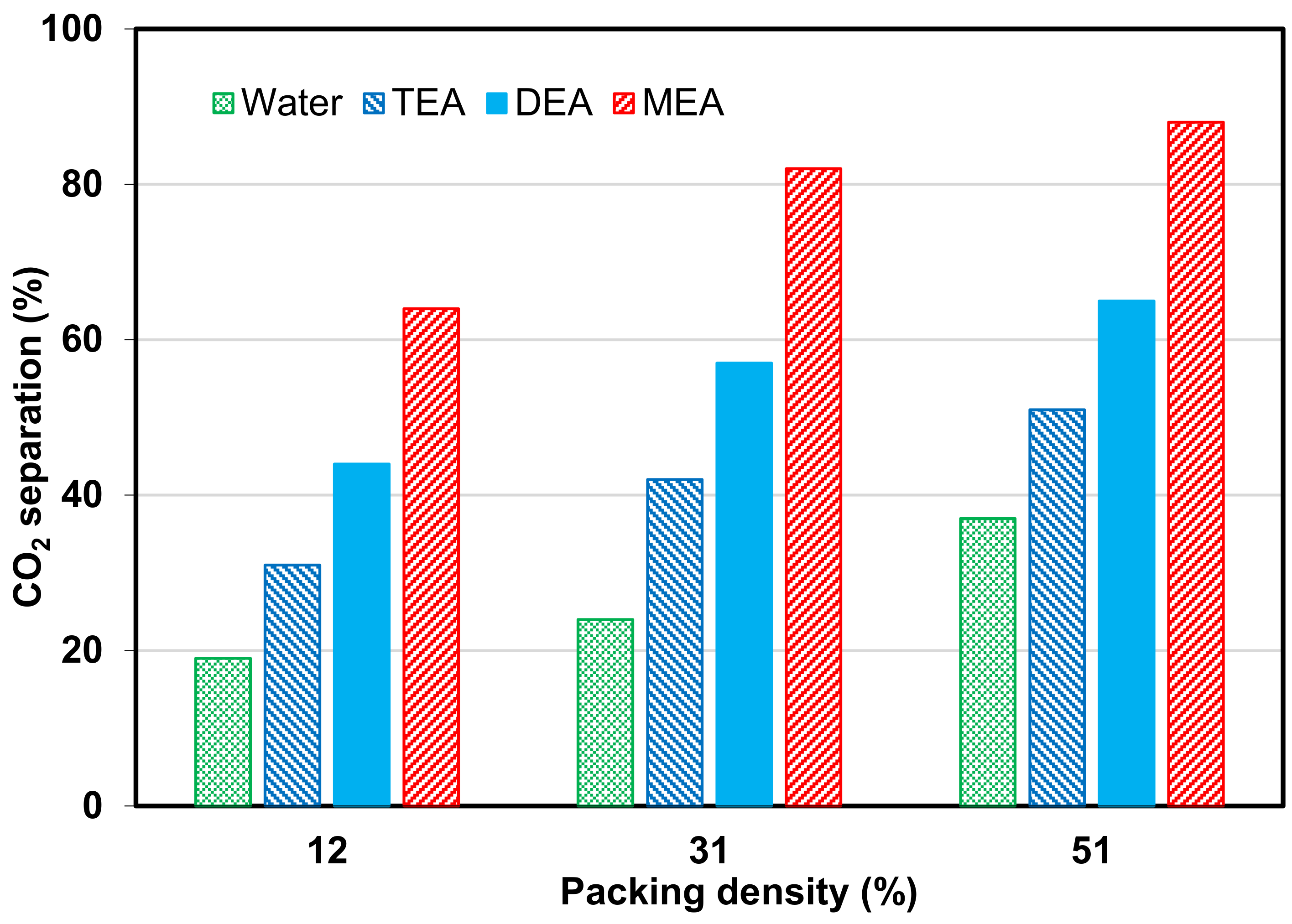
- An increase in operating pressure, liquid temperature, and packing density favor CO2 flux.
- Membrane wetting becomes more serious with the increase in solvent flow rate.
- Higher gas flow rates can improve CO2 absorption flux, but at the cost of reducing CO2 removal efficiency.
- Improving CO2 absorption performance by increasing the liquid flow rate to a very high level may not be ideal because of the risk of membrane wetting.
- The module with a smaller diameter hollow fiber membrane can accommodate a much greater interfacial area per unit volume, which makes it a more efficient absorber unit.
- Increasing module length, number of fibers, and porosity provide better CO2 separation performance.
7. Membrane-Based Hybrid Processes
- The hybrid process offers a high degree of flexibility, with respect to the capture ratio and/or final CO2 purity.
- The improvement to either membrane or cryogenic technologies will improve the hybrid system as well.
- Combining membranes and absorption technologies could result in significant energy savings by reducing the steam required for amine regeneration.
- The hybrid process showed a higher CO2 removal efficiency than the conventional absorption tower.
- The membrane contactor hybrid process was proved to be economic by evaluation through CO2 recovery cost and operating power consumption.
- The membrane-absorption unit can improve the absorption process by generating 400–1500% greater mass transfer area per unit volume leading to smaller equipment sizes.
- Compared to standalone methods, hybrid processes showed superiority not only in CO2 recovery and energy penalty but also in installation investment.
8. Long-Term Stability of Membranes
- The membrane wetting phenomena increase the mass transfer resistance and limit the long-term process stability.
- Membrane material and absorbent compatibility are important to secure long-term absorption performance.
- The long-term absorption performance of the membrane contactors is predominantly related to the hydrophobicity of the hollow fibers and subsequently the membrane wetting.
9. Challenges in Membrane Contactors
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| ArgK | Potassium argininate |
| AEEA | (2-Aminoethyl)ethanolamine |
| AMP | 2-amino-2-methyl-1-propanol |
| CH4 | Methane |
| CO2 | Carbon dioxide |
| DGA | Diglycolamine |
| DEAB | Diethylamino-2-butanol |
| DMEA | Dimethylethanolamine |
| DEEA | Diethylethanolamine |
| DIPA | Diisopropanolamine |
| DEA | Diethanolamine |
| EDA | Ethylenediamine |
| GlyK | Potassium glycinate |
| K-Pro | Potassium prolinate |
| K-Thr | Potassium threoninate |
| K-Phe | Potassium phenylalanine |
| K-Arg | Potassium argininate |
| K-Ala | Potassium alaninate |
| K-Tau | Potassium taurinate |
| K2CO3 | Potassium carbonate |
| LysK | Potassium lysinate |
| LiCl | Lithium chloride |
| MDEA | Methyldiethanolamine |
| MEA | Monoethanolamine |
| N2 | Nitrogen |
| NaOH | Sodium hydroxide |
| NH3 | Ammonia |
| PI | Polyimide |
| PES | Polyethersulfone |
| PS | Polysulfone |
| PTFE | Polytetrafluoroethylene |
| PVDF | Polyvinylidene fluoride |
| PP | Polypropylene |
| PEI | Polyetherimide |
| PSF | Polysulfone |
| PE | Polyethylene |
| PPO | Poly(phenylene oxide) |
| PMP | Poly(4-methyl-1-pentene) |
| PDMS | Polydimethylsiloxane |
| PVP | Polyvinyl pirrolidone |
| PEG | Polyethylene glycol |
| PA | Phosphoric acid |
| PEG-400 | Polyethylene glycol |
| PZ | Piperazine |
| PZEA | 2-(1-piperazinyl)-ethylamine |
| SO2 | Sulfur dioxide |
| SarK | Potassium sarcosinate |
| TEA | Triethanolamine |
| 1DMA2P | 1-Dimethylamino-2-propanol |
| 2MPZ | 2-methylpiperazine |
| [emim][EtSO4] | 1-ethyl-3-methylimidazolium ethylsulfate |
| [Bmim][BF4] | 1-butyl-3-methylimidazolium tetrafluoroborate |
| [Emim][BF4] | 1-Ethyl-3-methylimidazolium tetrafluoroborate |
| [apmim][BF4] | 1-(3-aminopropyl)-3-methyl-imidazolium tetrafluoroborate |
| [Emim][Ac] | 1-ethyl-3-methylimidazolium acetate |
References
- Wu, X.; Fan, H.; Sharif, M.; Yu, Y.; Wei, K.; Zhang, Z.; Liu, G. Electrochemically-Mediated Amine Regeneration of CO2 Capture: From Electrochemical Mechanism to Bench-Scale Visualization Study. Appl. Energy 2021, 302, 117554. [Google Scholar] [CrossRef]
- Gao, W.; Liang, S.; Wang, R.; Jiang, Q.; Zhang, Y.; Zheng, Q.; Xie, B.; Toe, C.Y.; Zhu, X.; Wang, J.; et al. Industrial Carbon Dioxide Capture and Utilization: State of the Art and Future Challenges. Chem. Soc. Rev. 2020, 49, 8584–8686. [Google Scholar] [CrossRef]
- Luis Míguez, J.; Porteiro, J.; Pérez-Orozco, R.; Patiño, D.; Rodríguez, S. Evolution of CO2 Capture Technology between 2007 and 2017 through the Study of Patent Activity. Appl. Energy 2018, 211, 1282–1296. [Google Scholar] [CrossRef]
- Kang, J.S.; Kim, S.; Hatton, T.A. Redox-Responsive Sorbents and Mediators for Electrochemically Based CO2 Capture. Curr. Opin. Green Sustain. Chem. 2021, 31, 100504. [Google Scholar] [CrossRef]
- Sreenivasulu, B.; Gayatri, D.V.; Sreedhar, I.; Raghavan, K.V. A Journey into the Process and Engineering Aspects of Carbon Capture Technologies. Renew. Sustain. Energy Rev. 2015, 41, 1324–1350. [Google Scholar] [CrossRef]
- Li, G.; Liu, Q.; Liao, B.; Chen, L.; Zhou, H.; Zhou, Z.; Xia, B.; Huang, J.; Liu, B. Synthesis of Novel Ferrocene-Based Conjugated Microporous Polymers with Intrinsic Magnetism. Eur. Polym. J. 2017, 93, 556–560. [Google Scholar] [CrossRef]
- Abu-Zahra, M.R.M.; Schneiders, L.H.J.; Niederer, J.P.M.; Feron, P.H.M.; Versteeg, G.F. CO2 Capture from Power Plants: Part I. A Parametric Study of the Technical Performance Based on Monoethanolamine. Int. J. Greenh. Gas Control 2007, 1, 37–46. [Google Scholar] [CrossRef]
- Vadillo, J.M.; Gómez-Coma, L.; Garea, A.; Irabien, A. Hollow Fiber Membrane Contactors in CO2 Desorption: A Review. Energy Fuels 2021, 35, 111–136. [Google Scholar] [CrossRef]
- Liu, M.; Nothling, M.D.; Zhang, S.; Fu, Q.; Qiao, G.G. Thin Film Composite Membranes for Postcombustion Carbon Capture: Polymers and Beyond. Prog. Polym. Sci. 2022, 126, 101504. [Google Scholar] [CrossRef]
- Kumar, P.S. Development and Design of Membrane Gas Absorption Processes; Twente University Press: Enschede, The Netherlands, 2002. [Google Scholar]
- Ibrahim, M.H.; El-Naas, M.H.; Zhang, Z.; van der Bruggen, B. CO2 Capture Using Hollow Fiber Membranes: A Review of Membrane Wetting. Energy Fuels 2018, 32, 963–978. [Google Scholar] [CrossRef]
- Li, G.; Liu, Q.; Xia, B.; Huang, J.; Li, S.; Guan, Y.; Zhou, H.; Liao, B.; Zhou, Z.; Liu, B. Synthesis of Stable Metal-Containing Porous Organic Polymers for Gas Storage. Eur. Polym. J. 2017, 91, 242–247. [Google Scholar] [CrossRef]
- Li, J.L.; Chen, B.H. Review of CO2 Absorption Using Chemical Solvents in Hollow Fiber Membrane Contactors. Sep. Purif. Technol. 2005, 41, 109–122. [Google Scholar] [CrossRef]
- Zhang, Y.; Sunarso, J.; Liu, S.; Wang, R. Current Status and Development of Membranes for CO2/CH4 Separation: A Review. Int. J. Greenh. Gas Control 2013, 12, 84–107. [Google Scholar] [CrossRef]
- Cui, Z.; Demontigny, D. Part 7: A Review of CO2 Capture Using Hollow Fiber Membrane Contactors. Carbon Manag. 2014, 4, 69–89. [Google Scholar] [CrossRef]
- Qi, Z.; Cussler, E.L. Microporous Hollow Fibers for Gas Absorption: I. Mass Transfer in the Liquid. J. Membr. Sci. 1985, 23, 321–332. [Google Scholar] [CrossRef]
- Qi, Z.; Cussler, E.L. Microporous Hollow Fibers for Gas Absorption: II. Mass Transfer across the Membrane. J. Membr. Sci. 1985, 23, 333–345. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, R. Gas–Liquid Membrane Contactors for Acid Gas Removal: Recent Advances and Future Challenges. Curr. Opin. Chem. Eng. 2013, 2, 255–262. [Google Scholar] [CrossRef]
- Simons, K.; Nijmeijer, K.; Wessling, M. Gas–Liquid Membrane Contactors for CO2 Removal. J. Membr. Sci. 2009, 340, 214–220. [Google Scholar] [CrossRef]
- Li, L.; Ma, G.; Pan, Z.; Zhang, N.; Zhang, Z. Research Progress in Gas Separation Using Hollow Fiber Membrane Contactors. Membranes 2020, 10, 380. [Google Scholar] [CrossRef] [PubMed]
- Falk-Pedersen, O.; Dannström, H. Separation of Carbon Dioxide from Offshore Gas Turbine Exhaust. Energy Convers. Manag. 1997, 38, S81–S86. [Google Scholar] [CrossRef]
- Ansaloni, L.; Hartono, A.; Awais, M.; Knuutila, H.K.; Deng, L. CO2 Capture Using Highly Viscous Amine Blends in Non-Porous Membrane Contactors. Chem. Eng. J. 2019, 359, 1581–1591. [Google Scholar] [CrossRef]
- Huang, J.; Tan, Z.-Q.; Su, H.-M.; Guo, Y.-W.; Huan, L.; Liao, B.; Liu, Q.-Q. Ferrocenyl Building Block Constructing Porous Organic Polymer for Gas Capture and Methyl Violet Adsorption. J. Cent. S. Univ. 2020, 27, 1247–1261. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, C.; Huang, Q.; Liu, H.; Zhao, J. Progress on Polymeric Hollow Fiber Membrane Preparation Technique from the Perspective of Green and Sustainable Development. Chem. Eng. J. 2021, 403, 126295. [Google Scholar] [CrossRef]
- Ghadiri, M.; Marjani, A.; Shirazian, S. Development of a Mechanistic Model for Prediction of CO2 Capture from Gas Mixtures by Amine Solutions in Porous Membranes. Environ. Sci. Pollut. Res. 2017, 24, 14508–14515. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhu, Z.; Zhou, M.; Jie, X.; Wang, L.; Kang, G.; Cao, Y. Removal of CO2 from Biogas by Membrane Contactor Using PTFE Hollow Fibers with Smaller Diameter. J. Membr. Sci. 2021, 627, 119232. [Google Scholar] [CrossRef]
- Kaneko, K.; Yadav, N.; Takeuchi, K.; Maira, B.; Terano, M.; Taniike, T. Versatile Strategy for Fabrication of Polypropylene Nanocomposites with Inorganic Network Structures Based on Catalyzed In-Situ Sol–Gel Reaction during Melt Mixing. Compos. Sci. Technol. 2014, 102, 120–125. [Google Scholar] [CrossRef]
- De Montigny, D.; Tontiwachwuthikul, P.; Chakma, A. Using Polypropylene and Polytetrafluoroethylene Membranes in a Membrane Contactor for CO2 Absorption. J. Membr. Sci. 2006, 277, 99–107. [Google Scholar] [CrossRef]
- Li, S.; Pyrzynski, T.J.; Klinghoffer, N.B.; Tamale, T.; Zhong, Y.; Aderhold, J.L.; James Zhou, S.; Meyer, H.S.; Ding, Y.; Bikson, B. Scale-up of PEEK Hollow Fiber Membrane Contactor for Post-Combustion CO2 Capture. J. Membr. Sci. 2017, 527, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.C.; Lin, S.H.; der Chien, R.; Hsu, P.S. Effects of Shape, Porosity, and Operating Parameters on Carbon Dioxide Recovery in Polytetrafluoroethylene Membranes. J. Hazard. Mater. 2010, 179, 692–700. [Google Scholar] [CrossRef]
- Franco, J.A.; Demontigny, D.D.; Kentish, S.E.; Perera, J.M.; Stevens, G.W. Polytetrafluoroethylene (PTFE)-Sputtered Polypropylene Membranes for Carbon Dioxide Separation in Membrane Gas Absorption: Hollow Fiber Configuration. Ind. Eng. Chem. Res. 2012, 51, 1376–1382. [Google Scholar] [CrossRef]
- Hedayat, M.; Soltanieh, M.; Mousavi, S.A. Simultaneous Separation of H2S and CO2 from Natural Gas by Hollow Fiber Membrane Contactor Using Mixture of Alkanolamines. J. Membr. Sci. 2011, 377, 191–197. [Google Scholar] [CrossRef]
- Bakeri, G.; Ismail, A.F.; Shariaty-Niassar, M.; Matsuura, T. Effect of Polymer Concentration on the Structure and Performance of Polyetherimide Hollow Fiber Membranes. J. Membr. Sci. 2010, 363, 103–111. [Google Scholar] [CrossRef]
- Bakeri, G.; Matsuura, T.; Ismail, A.F. The Effect of Phase Inversion Promoters on the Structure and Performance of Polyetherimide Hollow Fiber Membrane Using in Gas–Liquid Contacting Process. J. Membr. Sci. 2011, 383, 159–169. [Google Scholar] [CrossRef]
- Ghasem, N.; Al-Marzouqi, M.; Duidar, A. Effect of PVDF Concentration on the Morphology and Performance of Hollow Fiber Membrane Employed as Gas–Liquid Membrane Contactor for CO2 Absorption. Sep. Purif. Technol. 2012, 98, 174–185. [Google Scholar] [CrossRef]
- Lin, S.H.; Hsieh, C.F.; Li, M.H.; Tung, K.L. Determination of Mass Transfer Resistance during Absorption of Carbon Dioxide by Mixed Absorbents in PVDF and PP Membrane Contactor. Desalination 2009, 249, 647–653. [Google Scholar] [CrossRef]
- Fashandi, H.; Ghodsi, A.; Saghafi, R.; Zarrebini, M. CO2 Absorption Using Gas-Liquid Membrane Contactors Made of Highly Porous Poly(Vinyl Chloride) Hollow Fiber Membranes. Int. J. Greenh. Gas Control 2016, 52, 13–23. [Google Scholar] [CrossRef]
- Chen, Z.; Shen, Q.; Gong, H.; Du, M. Preparation of a Novel Dual-Layer Polyvinylidene Fluoride Hollow Fiber Composite Membrane with Hydrophobic Inner Layer for Carbon Dioxide Absorption in a Membrane Contactor. Sep. Purif. Technol. 2020, 248, 117045. [Google Scholar] [CrossRef]
- Xia, Q.C.; Liu, M.L.; Cao, X.L.; Wang, Y.; Xing, W.; Sun, S.P. Structure Design and Applications of Dual-Layer Polymeric Membranes. J. Membr. Sci. 2018, 562, 85–111. [Google Scholar] [CrossRef]
- Faiz, R.; Fallanza, M.; Ortiz, I.; Li, K. Separation of Olefin/Paraffin Gas Mixtures Using Ceramic Hollow Fiber Membrane Contactors. Ind. Eng. Chem. Res. 2013, 52, 7918–7929. [Google Scholar] [CrossRef]
- Novitskii, E.G.; Bazhenov, S.D.; Volkov, A.v. Optimization of Methods for Purification of Gas Mixtures to Remove Carbon Dioxide (A Review). Pet. Chem. 2021, 61, 407–423. [Google Scholar] [CrossRef]
- Rezaei-Dashtarzhandi, M.; Ismail, A.F.; Goh, P.S.; Wan Azelee, I.; Abbasgholipourghadim, M.; Ur Rehman, G.; Matsuura, T. Zeolite ZSM5-Filled PVDF Hollow Fiber Mixed Matrix Membranes for Efficient Carbon Dioxide Removal via Membrane Contactor. Ind. Eng. Chem. Res. 2016, 55, 12632–12643. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, Y.G.; Kim, M.K.; Lee, S.H.; Park, J.H. Study on CO2 Absorption Performance of Lab-Scale Ceramic Hollow Fiber Membrane Contactor by Gas/Liquid Flow Direction and Module Design. Sep. Purif. Technol. 2019, 220, 189–196. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, M.K.; Park, J.H.; Magnone, E. Temperature and Pressure Dependence of the CO2 Absorption through a Ceramic Hollow Fiber Membrane Contactor Module. Chem. Eng. Process. Process Intensif. 2020, 150, 107871. [Google Scholar] [CrossRef]
- Koonaphapdeelert, S.; Wu, Z.; Li, K. Carbon Dioxide Stripping in Ceramic Hollow Fibre Membrane Contactors. Chem. Eng. Sci. 2009, 64, 1–8. [Google Scholar] [CrossRef]
- Magnone, E.; Lee, H.J.; Shin, M.C.; Park, J.H. A Performance Comparison Study of Five Single and Sixteen Blended Amine Absorbents for CO2 Capture Using Ceramic Hollow Fiber Membrane Contactors. J. Ind. Eng. Chem. 2021, 100, 174–185. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, M.K.; Lee, S.H.; Park, J.H. CH4/CO2 Separation from Biogas Stream Using Porous Hydrophobic Ceramic Hollow Fiber Membrane Contactors. Korean J. Chem. Eng. 2020, 37, 1036–1041. [Google Scholar] [CrossRef]
- Kong, X.; Gong, D.; Ke, W.; Qiu, M.; Fu, K.; Xu, P.; Chen, X.; Fan, Y. Investigation of Mass Transfer Characteristics of SO2Absorption into NaOH in a Multichannel Ceramic Membrane Contactor. Ind. Eng. Chem. Res. 2020, 59, 11054–11062. [Google Scholar] [CrossRef]
- Bottino, A.; Capannelli, G.; Comite, A.; di Felice, R.; Firpo, R. CO2 Removal from a Gas Stream by Membrane Contactor. Sep. Purif. Technol. 2008, 59, 85–90. [Google Scholar] [CrossRef]
- Fougerit, V.; Pozzobon, V.; Pareau, D.; Théoleyre, M.A.; Stambouli, M. Experimental and Numerical Investigation Binary Mixture Mass Transfer in a Gas–Liquid Membrane Contactor. J. Membr. Sci. 2019, 572, 1–11. [Google Scholar] [CrossRef]
- Nakhjiri, A.T.; Heydarinasab, A.; Bakhtiari, O.; Mohammadi, T. Experimental Investigation and Mathematical Modeling of CO2 Sequestration from CO2/CH4 Gaseous Mixture Using MEA and TEA Aqueous Absorbents through Polypropylene Hollow Fiber Membrane Contactor. J. Membr. Sci. 2018, 565, 1–13. [Google Scholar] [CrossRef]
- Ghobadi, J.; Ramirez, D.; Khoramfar, S.; Jerman, R.; Crane, M.; Hobbs, K. Simultaneous Absorption of Carbon Dioxide and Nitrogen Dioxide from Simulated Flue Gas Stream Using Gas-Liquid Membrane Contacting System. Int. J. Greenh. Gas Control 2018, 77, 37–45. [Google Scholar] [CrossRef]
- Gomez-Coma, L.; Garea, A.; Irabien, A. Carbon Dioxide Capture by [Emim][Ac] Ionic Liquid in a Polysulfone Hollow Fiber Membrane Contactor. Int. J. Greenh. Gas Control 2016, 52, 401–409. [Google Scholar] [CrossRef] [Green Version]
- Talavari, A.; Ghanavati, B.; Azimi, A.; Sayyahi, S. Preparation and Characterization of PVDF-Filled MWCNT Hollow Fiber Mixed Matrix Membranes for Gas Absorption by Al2O3 Nanofluid Absorbent via Gas–Liquid Membrane Contactor. Chem. Eng. Res. Des. 2020, 156, 478–494. [Google Scholar] [CrossRef]
- Elhambakhsh, A.; Keshavarz, P. Sono-Hollow Fiber Membrane Contactors: A New Approach for CO2 Separation by Physical/Chemical Absorbents. J. Nat. Gas. Sci. Eng. 2022, 101, 104538. [Google Scholar] [CrossRef]
- Hwang, H.Y.; Nam, S.Y.; Koh, H.C.; Ha, S.Y.; Barbieri, G.; Drioli, E. The Effect of Operating Conditions on the Performance of Hollow Fiber Membrane Modules for CO2/N2 Separation. J. Ind. Eng. Chem. 2012, 18, 205–211. [Google Scholar] [CrossRef]
- Kosaraju, P.; Kovvali, A.S.; Korikov, A.; Sirkar, K.K. Hollow Fiber Membrane Contactor Based CO2 Absorption-Stripping Using Novel Solvents and Membranes. Ind. Eng. Chem. Res. 2005, 44, 1250–1258. [Google Scholar] [CrossRef]
- Abdulhameed, M.A.; Othman, M.H.D.; Ismail, A.F.; Matsuura, T.; Harun, Z.; Rahman, M.A.; Puteh, M.H.; Jaafar, J.; Rezaei, M.; Hubadillah, S.K. Carbon Dioxide Capture Using a Superhydrophobic Ceramic Hollow Fibre Membrane for Gas-Liquid Contacting Process. J. Clean. Prod. 2017, 140, 1731–1738. [Google Scholar] [CrossRef]
- Naim, R.; Ismail, A.F.; Cheer, N.B.; Abdullah, M.S. Polyvinylidene Fluoride and Polyetherimide Hollow Fiber Membranes for CO2 Stripping in Membrane Contactor. Chem. Eng. Res. Des. 2014, 92, 1391–1398. [Google Scholar] [CrossRef] [Green Version]
- Naim, R.; Ismail, A.F.; Mansourizadeh, A. Effect of Non-Solvent Additives on the Structure and Performance of PVDF Hollow Fiber Membrane Contactor for CO2 Stripping. J. Membr. Sci. 2012, 423–424, 503–513. [Google Scholar] [CrossRef] [Green Version]
- Naim, R.; Ismail, A.F.; Mansourizadeh, A. Preparation of Microporous PVDF Hollow Fiber Membrane Contactors for CO2 Stripping from Diethanolamine Solution. J. Membr. Sci. 2012, 392–393, 29–37. [Google Scholar] [CrossRef]
- Rahbari-Sisakht, M.; Ismail, A.F.; Rana, D.; Matsuura, T. Effect of Different Additives on the Physical and Chemical CO2 Absorption in Polyetherimide Hollow Fiber Membrane Contactor System. Sep. Purif. Technol. 2012, 98, 472–480. [Google Scholar] [CrossRef]
- Rahbari-Sisakht, M.; Ismail, A.F.; Matsuura, T. Development of Asymmetric Polysulfone Hollow Fiber Membrane Contactor for CO2 Absorption. Sep. Purif. Technol. 2012, 86, 215–220. [Google Scholar] [CrossRef]
- Rahbari-Sisakht, M.; Ismail, A.F.; Matsuura, T. Effect of Bore Fluid Composition on Structure and Performance of Asymmetric Polysulfone Hollow Fiber Membrane Contactor for CO2 Absorption. Sep. Purif. Technol. 2012, 88, 99–106. [Google Scholar] [CrossRef]
- Mansourizadeh, A.; Ismail, A.F. Effect of Additives on the Structure and Performance of Polysulfone Hollow Fiber Membranes for CO2 Absorption. J. Membr. Sci. 2010, 348, 260–267. [Google Scholar] [CrossRef]
- Mansourizadeh, A.; Ismail, A.F. A Developed Asymmetric PVDF Hollow Fiber Membrane Structure for CO2 Absorption. Int. J. Greenh. Gas Control 2011, 5, 374–380. [Google Scholar] [CrossRef]
- Mansourizadeh, A.; Ismail, A.F. Preparation and Characterization of Porous PVDF Hollow Fiber Membranes for CO2 Absorption: Effect of Different Non-Solvent Additives in the Polymer Dope. Int. J. Greenh. Gas Control 2011, 5, 640–648. [Google Scholar] [CrossRef]
- Mansourizadeh, A.; Ismail, A.F.; Abdullah, M.S.; Ng, B.C. Preparation of Polyvinylidene Fluoride Hollow Fiber Membranes for CO2 Absorption Using Phase-Inversion Promoter Additives. J. Membr. Sci. 2010, 355, 200–207. [Google Scholar] [CrossRef]
- Mansourizadeh, A.; Mousavian, S. Structurally Developed Microporous Polyvinylidene Fluoride Hollow-Fiber Membranes for CO2 Absorption with Diethanolamine Solution. J. Polym. Res. 2013, 20, 99. [Google Scholar] [CrossRef]
- Mansourizadeh, A.; Jazebizadeh, M.H.; Vaseghi, M.R.; Aghili, A. A Comparative Study on the Structure of Developed Porous PVDF and PEI Hollow Fiber Membrane Contactors for CO2 Absorption. J. Polym. Res. 2016, 23, 4. [Google Scholar] [CrossRef]
- DashtArzhandi, M.R.; Ismail, A.F.; Matsuura, T.; Ng, B.C.; Abdullah, M.S. Fabrication and Characterization of Porous Polyetherimide/Montmorillonite Hollow Fiber Mixed Matrix Membranes for CO2 Absorption via Membrane Contactor. Chem. Eng. J. 2015, 269, 51–59. [Google Scholar] [CrossRef]
- Atchariyawut, S.; Feng, C.; Wang, R.; Jiraratananon, R.; Liang, D.T. Effect of Membrane Structure on Mass-Transfer in the Membrane Gas–Liquid Contacting Process Using Microporous PVDF Hollow Fibers. J. Membr. Sci. 2006, 285, 272–281. [Google Scholar] [CrossRef]
- Shi, L.; Wang, R.; Cao, Y.; Liang, D.T.; Tay, J.H. Effect of Additives on the Fabrication of Poly(Vinylidene Fluoride-Co-Hexafluropropylene) (PVDF-HFP) Asymmetric Microporous Hollow Fiber Membranes. J. Membr. Sci. 2008, 315, 195–204. [Google Scholar] [CrossRef]
- Bakeri, G.; Rezaei-DashtArzhandi, M.; Ismail, A.F.; Matsuura, T.; Abdullah, M.S.; Cheer, N.B. Porous Polyethersulfone Hollow Fiber Membrane in CO2 Separation Process via Membrane Contactor—The Effect of Nonsolvent Additives. Korean J. Chem. Eng. 2016, 34, 160–169. [Google Scholar] [CrossRef]
- Wang, K.Y.; Foo, S.W.; Chung, T.S. Mixed Matrix PVDF Hollow Fiber Membranes with Nanoscale Pores for Desalination through Direct Contact Membrane Distillation. Ind. Eng. Chem. Res. 2009, 48, 4474–4483. [Google Scholar] [CrossRef]
- Fontananova, E.; Jansen, J.C.; Cristiano, A.; Curcio, E.; Drioli, E. Effect of Additives in the Casting Solution on the Formation of PVDF Membranes. Desalination 2006, 192, 190–197. [Google Scholar] [CrossRef]
- Loh, C.H.; Wang, R. Effects of Additives and Coagulant Temperature on Fabrication of High Performance PVDF/Pluronic F127 Blend Hollow Fiber Membranes via Nonsolvent Induced Phase Separation. Chin. J. Chem. Eng. 2012, 20, 71–79. [Google Scholar] [CrossRef]
- Ghasem, N.; Al-Marzouqi, M.; Zhu, L. Preparation and Properties of Polyethersulfone Hollow Fiber Membranes with O-Xylene as an Additive Used in Membrane Contactors for CO2 Absorption. Sep. Purif. Technol. 2012, 92, 1–10. [Google Scholar] [CrossRef]
- Pang, H.; Gong, H.; Du, M.; Shen, Q.; Chen, Z. Effect of Non-Solvent Additive Concentration on CO2 Absorption Performance of Polyvinylidenefluoride Hollow Fiber Membrane Contactor. Sep. Purif. Technol. 2018, 191, 38–47. [Google Scholar] [CrossRef]
- Nabian, N.; Ghoreyshi, A.A.; Rahimpour, A.; Shakeri, M. Performance Evaluation and Mass Transfer Study of CO2 Absorption in Flat Sheet Membrane Contactor Using Novel Porous Polysulfone Membrane. Korean J. Chem. Eng. 2015, 32, 2204–2211. [Google Scholar] [CrossRef]
- Kianfar, E.; Pirouzfar, V.; Sakhaeinia, H. An Experimental Study on Absorption/Stripping CO2 Using Mono-Ethanol Amine Hollow Fiber Membrane Contactor. J. Taiwan. Inst. Chem. Eng. 2017, 80, 954–962. [Google Scholar] [CrossRef]
- Edwie, F.; Teoh, M.M.; Chung, T.S. Effects of Additives on Dual-Layer Hydrophobic–Hydrophilic PVDF Hollow Fiber Membranes for Membrane Distillation and Continuous Performance. Chem. Eng. Sci. 2012, 68, 567–578. [Google Scholar] [CrossRef]
- Gomez-Coma, L.; Garea, A.; Rouch, J.C.; Savart, T.; Lahitte, J.F.; Remigy, J.C.; Irabien, A. Membrane Modules for CO2 Capture Based on PVDF Hollow Fibers with Ionic Liquids Immobilized. J. Membr. Sci. 2016, 498, 218–226. [Google Scholar] [CrossRef] [Green Version]
- Ismail, A.F.; Mansourizadeh, A. A Comparative Study on the Structure and Performance of Porous Polyvinylidene Fluoride and Polysulfone Hollow Fiber Membranes for CO2 Absorption. J. Membr. Sci. 2010, 365, 319–328. [Google Scholar] [CrossRef]
- Bakeri, G.; Rana, D.; Ismail, A.F.; Matsuura, T.; Ghaee, A. Performance of Surface-Modified Poly(Etherimide) Hollow-Fiber Membranes in a Membrane Gas–Liquid Contacting Process with Response Surface Methodology. J. Appl. Polym. Sci. 2013, 128, 1313–1325. [Google Scholar] [CrossRef]
- Rahbari-Sisakht, M.; Ismail, A.F.; Rana, D.; Matsuura, T.; Emadzadeh, D. Effect of SMM Concentration on Morphology and Performance of Surface Modified PVDF Hollow Fiber Membrane Contactor for CO2 Absorption. Sep. Purif. Technol. 2013, 116, 67–72. [Google Scholar] [CrossRef]
- Rahbari-Sisakht, M.; Rana, D.; Matsuura, T.; Emadzadeh, D.; Padaki, M.; Ismail, A.F. Study on CO2 Stripping from Water through Novel Surface Modified PVDF Hollow Fiber Membrane Contactor. Chem. Eng. J. 2014, 246, 306–310. [Google Scholar] [CrossRef]
- Rahbari-Sisakht, M.; Ismail, A.F.; Rana, D.; Matsuura, T. Effect of Novel Surface Modifying Macromolecules on Morphology and Performance of Polysulfone Hollow Fiber Membrane Contactor for CO2 Absorption. Sep. Purif. Technol. 2012, 99, 61–68. [Google Scholar] [CrossRef]
- Zheng, Z.; Gu, Z.; Huo, R.; Luo, Z. Superhydrophobic Poly(Vinylidene Fluoride) Film Fabricated by Alkali Treatment Enhancing Chemical Bath Deposition. Appl. Surf. Sci. 2010, 256, 2061–2065. [Google Scholar] [CrossRef]
- Bakeri, G.; Ismail, A.F.; Rana, D.; Matsuura, T. Development of High Performance Surface Modified Polyetherimide Hollow Fiber Membrane for Gas–Liquid Contacting Processes. Chem. Eng. J. 2012, 198–199, 327–337. [Google Scholar] [CrossRef]
- Bakeri, G.; Matsuura, T.; Ismail, A.F.; Rana, D. A Novel Surface Modified Polyetherimide Hollow Fiber Membrane for Gas–Liquid Contacting Processes. Sep. Purif. Technol. 2012, 89, 160–170. [Google Scholar] [CrossRef]
- Mansourizadeh, A.; Aslmahdavi, Z.; Ismail, A.F.; Matsuura, T. Blend Polyvinylidene Fluoride/Surface Modifying Macromolecule Hollow Fiber Membrane Contactors for CO2 Absorption. Int. J. Greenh. Gas Control 2014, 26, 83–92. [Google Scholar] [CrossRef]
- Nordin, N.A.H.M.; Ismail, A.F.; Mustafa, A.; Surya Murali, R.; Matsuura, T. Utilizing Low ZIF-8 Loading for an Asymmetric PSf/ZIF-8 Mixed Matrix Membrane for CO2/CH4 Separation. RSC Adv. 2015, 5, 30206–30215. [Google Scholar] [CrossRef]
- Scholes, C.A.; Kentish, S.E.; Stevens, G.W.; deMontigny, D. Comparison of Thin Film Composite and Microporous Membrane Contactors for CO2 Absorption into Monoethanolamine. Int. J. Greenh. Gas Control 2015, 42, 66–74. [Google Scholar] [CrossRef]
- Rezaei, M.; Ismail, A.F.; Bakeri, G.; Hashemifard, S.A.; Matsuura, T. Effect of General Montmorillonite and Cloisite 15A on Structural Parameters and Performance of Mixed Matrix Membranes Contactor for CO2 Absorption. Chem. Eng. J. 2015, 260, 875–885. [Google Scholar] [CrossRef]
- Amirabedi, P.; Akbari, A.; Yegani, R. Fabrication of Hydrophobic PP/CH3SiO2 Composite Hollow Fiber Membrane for Membrane Contactor Application. Sep. Purif. Technol. 2019, 228, 115689. [Google Scholar] [CrossRef]
- Zhao, S.; Feron, P.H.M.; Deng, L.; Favre, E.; Chabanon, E.; Yan, S.; Hou, J.; Chen, V.; Qi, H. Status and Progress of Membrane Contactors in Post-Combustion Carbon Capture: A State-of-the-Art Review of New Developments. J. Membr. Sci. 2016, 511, 180–206. [Google Scholar] [CrossRef]
- Dibrov, G.A.; Volkov, V.V.; Vasilevsky, V.P.; Shutova, A.A.; Bazhenov, S.D.; Khotimsky, V.S.; van de Runstraat, A.; Goetheer, E.L.V.; Volkov, A.V. Robust High-Permeance PTMSP Composite Membranes for CO2 Membrane Gas Desorption at Elevated Temperatures and Pressures. J. Membr. Sci. 2014, 470, 439–450. [Google Scholar] [CrossRef]
- Dai, Z.; Ansaloni, L.; Deng, L. Precombustion CO2 Capture in Polymeric Hollow Fiber Membrane Contactors Using Ionic Liquids: Porous Membrane versus Nonporous Composite Membrane. Ind. Eng. Chem. Res. 2016, 55, 5983–5992. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, Y.; Zhang, Z.; Yao, J. Preparation and Characterization of OH/SiO2-TiO2/PES Composite Hollow Fiber Membrane Using Gas-Liquid Membrane Contactor for CO2/CH4 Separation. Sep. Purif. Technol. 2022, 288, 120551. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, B.; Wang, L.; Zhang, Z.; Li, J.; He, X.; Zhang, H.; Zhao, X.; Wang, H. Superhydrophobic PVDF Membrane Induced by Hydrophobic SiO2 Nanoparticles and Its Use for CO2 Absorption. Sep. Purif. Technol. 2018, 190, 108–116. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Lin, Y.; Malde, C.; Wang, R. Synthesis of ZIF-8 Based Composite Hollow Fiber Membrane with a Dense Skin Layer for Facilitated Biogas Upgrading in Gas-Liquid Membrane Contactor. J. Membr. Sci. 2019, 585, 238–252. [Google Scholar] [CrossRef]
- Fosi-Kofal, M.; Mustafa, A.; Ismail, A.F.; Rezaei-DashtArzhandi, M.; Matsuura, T. PVDF/CaCO3 Composite Hollow Fiber Membrane for CO2 Absorption in Gas–Liquid Membrane Contactor. J. Nat. Gas. Sci. Eng. 2016, 31, 428–436. [Google Scholar] [CrossRef]
- Scholes, C.A.; Shen, S. Mass Transfer Correlations for Membrane Gas-Solvent Contactors Undergoing Carbon Dioxide Desorption. Chin. J. Chem. Eng. 2018, 26, 2337–2343. [Google Scholar] [CrossRef]
- Yang, M.-C.; Cussler, E.L. Designing Hollow-Fiber Contactors. AIChE J. 1986, 32, 1910–1916. [Google Scholar] [CrossRef]
- Constantinou, A.; Barrass, S.; Gavriilidis, A. CO2 Absorption in Flat Membrane Microstructured Contactors of Different Wettability Using Aqueous Solution of NaOH. Green Process. Synth. 2018, 7, 471–476. [Google Scholar] [CrossRef]
- Wan, C.F.; Yang, T.; Lipscomb, G.G.; Stookey, D.J.; Chung, T.-S. Design and Fabrication of Hollow Fiber Membrane Modules. In Hollow Fiber Membranes: Fabrication and Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 225–252. [Google Scholar] [CrossRef]
- Paul, S.; Ghoshal, A.K.; Mandal, B. Theoretical Studies on Separation of CO2 by Single and Blended Aqueous Alkanolamine Solvents in Flat Sheet Membrane Contactor (FSMC). Chem. Eng. J. 2008, 144, 352–360. [Google Scholar] [CrossRef]
- Malakhov, A.O.; Bazhenov, S.D. Carbon Dioxide Desorption from Amine Solution in a Nonporous Membrane Contactor. Pet. Chem. 2018, 58, 330–337. [Google Scholar] [CrossRef]
- Bougie, F.; Iliuta, I.; Iliuta, M.C. Flat Sheet Membrane Contactor (FSMC) for CO2 Separation Using Aqueous Amine Solutions. Chem. Eng. Sci. 2015, 123, 255–264. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Sunarti, A.R.; Lee, K.T.; Fernando, W.J.N. CO2 Removal Using Membrane Gas Absorption. Int. J. Greenh. Gas Control 2010, 4, 495–498. [Google Scholar] [CrossRef]
- Lin, S.H.; Tung, K.L.; Chang, H.W.; Lee, K.R. Influence of Fluorocarbon Flat-Membrane Hydrophobicity on Carbon Dioxide Recovery. Chemosphere 2009, 75, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Ghasem, N.; Al-Marzouqi, M. Modeling and Experimental Study of Carbon Dioxide Absorption in a Flat Sheet Membrane Contactor. J. Membr. Sci. Res. 2017, 3, 57–63. [Google Scholar] [CrossRef]
- Constantinou, A.; Barrass, S.; Gavriilidis, A. CO2 Absorption in Polytetrafluoroethylene Membrane Microstructured Contactor Using Aqueous Solutions of Amines. Ind. Eng. Chem. Res. 2014, 53, 9236–9242. [Google Scholar] [CrossRef]
- Hafeez, S.; Safdar, T.; Pallari, E.; Manos, G.; Aristodemou, E.; Zhang, Z.; Al-Salem, S.M.; Constantinou, A. CO2 Capture Using Membrane Contactors: A Systematic Literature Review. Front. Chem. Sci. Eng. 2020, 15, 720–754. [Google Scholar] [CrossRef]
- Mansourizadeh, A.; Ismail, A.F. Hollow Fiber Gas–Liquid Membrane Contactors for Acid Gas Capture: A Review. J. Hazard. Mater. 2009, 171, 38–53. [Google Scholar] [CrossRef]
- Xu, Y.; Goh, K.; Wang, R.; Bae, T.H. A Review on Polymer-Based Membranes for Gas-Liquid Membrane Contacting Processes: Current Challenges and Future Direction. Sep. Purif. Technol. 2019, 229, 115791. [Google Scholar] [CrossRef]
- He, F.; Wang, T.; Fang, M.; Wang, Z.; Yu, H.; Ma, Q. Screening Test of Amino Acid Salts for CO2 Absorption at Flue Gas Temperature in a Membrane Contactor. Energy Fuels 2017, 31, 770–777. [Google Scholar] [CrossRef]
- Gusnawan, P.; Ganegamage, S.; Heagy, M.; Yu, J. Reactive CO2 Absorption Mechanism of a Soybean-Based (SBB) Solvent Containing 18 Amino Acid Salts in Polyvinylidene Fluoride (PVDF) Hollow Fiber Membrane-Based Gas-Liquid Membrane Contactor. Chem. Eng. J. 2020, 399, 125819. [Google Scholar] [CrossRef]
- Lu, J.G.; Chen, M.D.; Ji, Y.; Zhang, H. Membrane-Based CO2 Absorption into Blended Amine Solutions. J. Fuel Chem. Technol. 2009, 37, 740–746. [Google Scholar] [CrossRef]
- Nakhjiri, A.T.; Heydarinasab, A.; Bakhtiari, O.; Mohammadi, T. Modeling and Simulation of CO2 Separation from CO2/CH4 Gaseous Mixture Using Potassium Glycinate, Potassium Argininate and Sodium Hydroxide Liquid Absorbents in the Hollow Fiber Membrane Contactor. J. Env. Chem. Eng. 2018, 6, 1500–1511. [Google Scholar] [CrossRef]
- Nakhjiri, A.T.; Heydarinasab, A. Efficiency Evaluation of Novel Liquid Potassium Lysinate Chemical Solution for CO2 Molecular Removal inside the Hollow Fiber Membrane Contactor: Comprehensive Modeling and CFD Simulation. J. Mol. Liq 2020, 297, 111561. [Google Scholar] [CrossRef]
- Masoumi, S.; Rahimpour, M.R.; Mehdipour, M. Removal of Carbon Dioxide by Aqueous Amino Acid Salts Using Hollow Fiber Membrane Contactors. J. CO2 Util. 2016, 16, 42–49. [Google Scholar] [CrossRef]
- Yan, S.P.; Fang, M.X.; Zhang, W.F.; Wang, S.Y.; Xu, Z.K.; Luo, Z.Y.; Cen, K.F. Experimental Study on the Separation of CO2 from Flue Gas Using Hollow Fiber Membrane Contactors without Wetting. Fuel Process. Technol. 2007, 88, 501–511. [Google Scholar] [CrossRef]
- Lu, J.G.; Zheng, Y.F.; Cheng, M.D. Membrane Contactor for CO2 Absorption Applying Amino-Acid Salt Solutions. Desalination 2009, 249, 498–502. [Google Scholar] [CrossRef]
- Simons, K.; Nijmeijer, K.; Mengers, H.; Brilman, W.; Wessling, M. Highly Selective Amino Acid Salt Solutions as Absorption Liquid for CO2 Capture in Gas–Liquid Membrane Contactors. ChemSusChem 2010, 3, 939–947. [Google Scholar] [CrossRef]
- Rahim, N.A.; Ghasem, N.; Al-Marzouqi, M. Absorption of CO2 from Natural Gas Using Different Amino Acid Salt Solutions and Regeneration Using Hollow Fiber Membrane Contactors. J. Nat. Gas. Sci. Eng. 2015, 26, 108–117. [Google Scholar] [CrossRef]
- Nieminen, H.; Järvinen, L.; Ruuskanen, V.; Laari, A.; Koiranen, T.; Ahola, J. Mass Transfer Characteristics of a Continuously Operated Hollow-Fiber Membrane Contactor and Stripper Unit for CO2 Capture. Int. J. Greenh. Gas Control 2020, 98, 103063. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, M.; Yan, S.; Yu, H.; Wei, C.C.; Luo, Z. Optimization of Blended Amines for CO2 Absorption in a Hollow-Fiber Membrane Contactor. Ind. Eng. Chem. Res. 2013, 52, 12170–12182. [Google Scholar] [CrossRef]
- Paul, S.; Ghoshal, A.K.; Mandal, B. Removal of CO2 by Single and Blended Aqueous Alkanolamine Solvents in Hollow-Fiber Membrane Contactor: Modeling and Simulation. Ind. Eng. Chem. Res. 2007, 46, 2576–2588. [Google Scholar] [CrossRef]
- Albarracin Zaidiza, D.; Belaissaoui, B.; Rode, S.; Favre, E. Intensification Potential of Hollow Fiber Membrane Contactors for CO2 Chemical Absorption and Stripping Using Monoethanolamine Solutions. Sep. Purif. Technol. 2017, 188, 38–51. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, Y.; Chen, Y.; Zhang, L. Investigation of CO2 Absorption in Methyldiethanolamine and 2-(1-Piperazinyl)-Ethylamine Using Hollow Fiber Membrane Contactors: Part C. Effect of Operating Variables. J. Nat. Gas. Sci. Eng. 2014, 20, 58–66. [Google Scholar] [CrossRef]
- Nakhjiri, A.T.; Heydarinasab, A. Computational Simulation and Theoretical Modeling of CO2 Separation Using EDA, PZEA and PS Absorbents inside the Hollow Fiber Membrane Contactor. J. Ind. Eng. Chem. 2019, 78, 106–115. [Google Scholar] [CrossRef]
- Cao, F.; Gao, H.; Ling, H.; Huang, Y.; Liang, Z. Theoretical Modeling of the Mass Transfer Performance of CO2 Absorption into DEAB Solution in Hollow Fiber Membrane Contactor. J. Membr. Sci. 2020, 593, 117439. [Google Scholar] [CrossRef]
- Akan, A.P.; Chau, J.; Sirkar, K.K. Post-Combustion CO2 Capture and Recovery by Pure Activated Methyldiethanolamine in Crossflow Membrane Contactors Having Coated Hollow Fibers. Sep. Purif. Technol. 2020, 244, 116427. [Google Scholar] [CrossRef]
- Lu, J.; Wang, L.; Sun, X.; Li, J.; Liu, X. Absorption of CO2 into Aqueous Solutions of Methyldiethanolamine and Activated Methyldiethanolamine from a Gas Mixture in a Hollow Fiber Contactor. Ind. Eng. Chem. Res. 2005, 44, 9230–9238. [Google Scholar] [CrossRef]
- Cao, F.; Gao, H.; Xiong, Q.; Liang, Z. Experimental Studies on Mass Transfer Performance for CO2 Absorption into Aqueous N,N-Dimethylethanolamine (DMEA) Based Solutions in a PTFE Hollow Fiber Membrane Contactor. Int. J. Greenh. Gas Control 2019, 82, 210–217. [Google Scholar] [CrossRef]
- Lin, S.H.; Chiang, P.C.; Hsieh, C.F.; Li, M.H.; Tung, K.L. Absorption of Carbon Dioxide by the Absorbent Composed of Piperazine and 2-Amino-2-Methyl-1-Propanol in PVDF Membrane Contactor. J. Chin. Inst. Chem. Eng. 2008, 39, 13–21. [Google Scholar] [CrossRef]
- Rongwong, W.; Jiraratananon, R.; Atchariyawut, S. Experimental Study on Membrane Wetting in Gas–Liquid Membrane Contacting Process for CO2 Absorption by Single and Mixed Absorbents. Sep. Purif. Technol. 2009, 69, 118–125. [Google Scholar] [CrossRef]
- Yang, J.; Yu, X.; Yan, J.; Tu, S.T.; Dahlquist, E. Effects of SO2 on CO2 Capture Using a Hollow Fiber Membrane Contactor. Appl. Energy 2013, 112, 755–764. [Google Scholar] [CrossRef]
- Khaisri, S.; deMontigny, D.; Tontiwachwuthikul, P.; Jiraratananon, R. CO2 Stripping from Monoethanolamine Using a Membrane Contactor. J. Membr. Sci. 2011, 376, 110–118. [Google Scholar] [CrossRef]
- Scholes, C.A.; Simioni, M.; Qader, A.; Stevens, G.W.; Kentish, S.E. Membrane Gas–Solvent Contactor Trials of CO2 Absorption from Syngas. Chem. Eng. J. 2012, 195–196, 188–197. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, R.; Li, H.; Gao, H.; Liang, Z. Mass Transfer Performance for CO2 Absorption into Aqueous Blended DMEA/MEA Solution with Optimized Molar Ratio in a Hollow Fiber Membrane Contactor. Sep. Purif. Technol. 2019, 211, 628–636. [Google Scholar] [CrossRef]
- Gao, H.; Liu, S.; Gao, G.; Luo, X.; Liang, Z. Hybrid Behavior and Mass Transfer Performance for Absorption of CO2 into Aqueous DEEA/PZ Solutions in a Hollow Fiber Membrane Contactor. Sep. Purif. Technol. 2018, 201, 291–300. [Google Scholar] [CrossRef]
- Cao, F.; Gao, H.; Gao, G.; Liang, Z. Mass Transfer Performance and Correlation for CO2 Absorption into Aqueous 1-Dimethylamino-2-Propanol (1DMA2P) Solution in a PTFE Hollow Fiber Membrane Contactor. Chem. Eng. Process. Process Intensif. 2019, 136, 226–233. [Google Scholar] [CrossRef]
- Makhloufi, C.; Lasseuguette, E.; Remigy, J.C.; Belaissaoui, B.; Roizard, D.; Favre, E. Ammonia Based CO2 Capture Process Using Hollow Fiber Membrane Contactors. J. Membr. Sci. 2014, 455, 236–246. [Google Scholar] [CrossRef] [Green Version]
- Niknam, M.; Zare, P.; Keshavarz, P. Experimental and Modeling Study of CO2 Absorption by L-Proline Promoted Potassium Carbonate Using Hollow Fiber Membrane Contactor. Int. J. Greenh. Gas Control 2020, 93, 102877. [Google Scholar] [CrossRef]
- Mesbah, M.; Momeni, M.; Soroush, E.; Shahsavari, S.; Galledari, S.A. Theoretical Study of CO2 Separation from CO2/CH4 Gaseous Mixture Using 2-Methylpiperazine-Promoted Potassium Carbonate through Hollow Fiber Membrane Contactor. J. Env. Chem. Eng. 2019, 7, 102781. [Google Scholar] [CrossRef]
- Jin, P.; Huang, C.; Shen, Y.; Zhan, X.; Hu, X.; Wang, L.; Wang, L. Simultaneous Separation of H2S and CO2 from Biogas by Gas-Liquid Membrane Contactor Using Single and Mixed Absorbents. Energy Fuels 2017, 31, 11117–11126. [Google Scholar] [CrossRef]
- Izaddoust, A.; Keshavarz, P. Experimental and Theoretical Study of CO2 Absorption with Piperazine-Promoted Potassium Carbonate Solution in Hollow Fiber Membrane Contactors. Energy Fuels 2017, 31, 9790–9799. [Google Scholar] [CrossRef]
- Masoumi, S.; Keshavarz, P.; Ayatollahi, S.; Mehdipour, M.; Rastgoo, Z. Enhanced Carbon Dioxide Separation by Amine-Promoted Potassium Carbonate Solution in a Hollow Fiber Membrane Contactor. Energy Fuels 2013, 27, 5423–5432. [Google Scholar] [CrossRef]
- Mehdipour, M.; Karami, M.R.; Keshavarz, P.; Ayatollahi, S. Analysis of CO2 Separation with Aqueous Potassium Carbonate Solution in a Hollow Fiber Membrane Contactor. Energy Fuels 2013, 27, 2185–2193. [Google Scholar] [CrossRef]
- Saidi, M. Kinetic Study and Process Model Development of CO2 Absorption Using Hollow Fiber Membrane Contactor with Promoted Hot Potassium Carbonate. J. Env. Chem. Eng. 2017, 5, 4415–4430. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Zhang, Z.; Hu, X.; Cheng, Y.; Zhong, C. Carbon Dioxide Absorption from Biogas by Amino Acid Salt Promoted Potassium Carbonate Solutions in a Hollow Fiber Membrane Contactor: A Numerical Study. Energy Fuels 2018, 32, 3637–3646. [Google Scholar] [CrossRef]
- Qazi, S.; Gómez-Coma, L.; Albo, J.; Druon-Bocquet, S.; Irabien, A.; Sanchez-Marcano, J. CO2 Capture in a Hollow Fiber Membrane Contactor Coupled with Ionic Liquid: Influence of Membrane Wetting and Process Parameters. Sep. Purif. Technol. 2020, 233, 115986. [Google Scholar] [CrossRef]
- Pahlavanzadeh, H.; Darabi, M.; Ghaleh, V.R.; Bakhtiari, O. CFD Modeling of CO2 Absorption in Membrane Contactors Using Aqueous Solutions of Monoethanolamine-Ionic Liquids. Ind. Eng. Chem. Res. 2020, 59, 18629–18639. [Google Scholar] [CrossRef]
- Yang, J.; Yu, X.; Yan, J.; Tu, S.T. CO2 Capture Using Amine Solution Mixed with Ionic Liquid. Ind. Eng. Chem. Res. 2014, 53, 2790–2799. [Google Scholar] [CrossRef]
- Rostami, S.; Keshavarz, P.; Raeissi, S. Experimental Study on the Effects of an Ionic Liquid for CO2 Capture Using Hollow Fiber Membrane Contactors. Int. J. Greenh. Gas Control 2018, 69, 1–7. [Google Scholar] [CrossRef]
- Bazhenov, S.; Malakhov, A.; Bakhtin, D.; Khotimskiy, V.; Bondarenko, G.; Volkov, V.; Ramdin, M.; Vlugt, T.J.H.; Volkov, A. CO2 Stripping from Ionic Liquid at Elevated Pressures in Gas-Liquid Membrane Contactor. Int. J. Greenh. Gas Control 2018, 71, 293–302. [Google Scholar] [CrossRef]
- Lu, J.G.; Lu, C.T.; Chen, Y.; Gao, L.; Zhao, X.; Zhang, H.; Xu, Z.W. CO2 Capture by Membrane Absorption Coupling Process: Application of Ionic Liquids. Appl. Energy 2014, 115, 573–581. [Google Scholar] [CrossRef]
- Gómez-Coma, L.; Garea, A.; Irabien, Á. Hybrid Solvent ([Emim][Ac]+water) to Improve the CO2 Capture Efficiency in a PVDF Hollow Fiber Contactor. ACS Sustain. Chem. Eng. 2017, 5, 734–743. [Google Scholar] [CrossRef]
- Ling, H.; Liu, S.; Gao, H.; Zhang, H.; Liang, Z. Solubility of N2O, Equilibrium Solubility, Mass Transfer Study and Modeling of CO2 Absorption into Aqueous Monoethanolamine (MEA)/1-Dimethylamino-2-Propanol (1DMA2P) Solution for Post-Combustion CO2 Capture. Sep. Purif. Technol. 2020, 232, 115957. [Google Scholar] [CrossRef]
- Chen, S.; Han, X.; Sun, X.; Luo, X.; Liang, Z. The Comparative Kinetics Study of CO2 Absorption into Non-Aqueous DEEA/MEA and DMEA/MEA Blended Systems Solution by Using Stopped-Flow Technique. Chem. Eng. J. 2020, 386, 121295. [Google Scholar] [CrossRef]
- Alivand, M.S.; Mazaheri, O.; Wu, Y.; Stevens, G.W.; Scholes, C.A.; Mumford, K.A. Development of Aqueous-Based Phase Change Amino Acid Solvents for Energy-Efficient CO2 Capture: The Role of Antisolvent. Appl. Energy 2019, 256, 113911. [Google Scholar] [CrossRef]
- Hu, G.; Smith, K.H.; Wu, Y.; Mumford, K.A.; Kentish, S.E.; Stevens, G.W. Carbon Dioxide Capture by Solvent Absorption Using Amino Acids: A Review. Chin. J. Chem. Eng. 2018, 26, 2229–2237. [Google Scholar] [CrossRef]
- Gupta, M.; Svendsen, H.F. Understanding Carbamate Formation Reaction Thermochemistry of Amino Acids as Solvents for Postcombustion CO2 Capture. J. Phys. Chem. B 2019, 123, 8433–8447. [Google Scholar] [CrossRef] [PubMed]
- Sang Sefidi, V.; Luis, P. Advanced Amino Acid-Based Technologies for CO2 Capture: A Review. Ind. Eng. Chem. Res. 2019, 58, 20181–20194. [Google Scholar] [CrossRef]
- Lu, J.G.; Ji, Y.; Zhang, H.; Chen, M.D. CO2 Capture Using Activated Amino Acid Salt Solutions in a Membrane Contactor. Sep. Sci. Technol. 2010, 45, 1240–1251. [Google Scholar] [CrossRef]
- Xiao, M.; Liu, H.; Gao, H.; Olson, W.; Liang, Z. CO2 Capture with Hybrid Absorbents of Low Viscosity Imidazolium-Based Ionic Liquids and Amine. Appl. Energy 2019, 235, 311–319. [Google Scholar] [CrossRef]
- Fu, D.; Zhang, P. Investigation of the Absorption Performance and Viscosity for CO2 Capture Process Using [Bmim][Gly] Promoted MDEA (N-Methyldiethanolamine) Aqueous Solution. Energy 2015, 87, 165–172. [Google Scholar] [CrossRef]
- Wang, C.; Luo, H.; Jiang, D.-E.; Li, H.; Dai, S.; Wang, C.; Li, H.; Wang, C.; Jiang, D.; Dai, S.; et al. Carbon Dioxide Capture by Superbase-Derived Protic Ionic Liquids. Angew. Chem. Int. Ed. 2010, 49, 5978–5981. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; de Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep Eutectic Solvents: Syntheses, Properties and Applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef]
- Carriazo, D.; Serrano, M.C.; Gutiérrez, M.C.; Ferrer, M.L.; del Monte, F. Deep-Eutectic Solvents Playing Multiple Roles in the Synthesis of Polymers and Related Materials. Chem. Soc. Rev. 2012, 41, 4996–5014. [Google Scholar] [CrossRef]
- Trivedi, T.J.; Lee, J.H.; Lee, H.J.; Choi, J.W. Deep Eutectic Solvents as Attractive Media for CO2 Capture †. Green Chem. 2016, 18, 2834. [Google Scholar] [CrossRef]
- Gu, Y.; Hou, Y.; Ren, S.; Sun, Y.; Wu, W. Hydrophobic Functional Deep Eutectic Solvents Used for Efficient and Reversible Capture of CO2. ACS Omega 2020, 5, 6809–6816. [Google Scholar] [CrossRef] [Green Version]
- Ishaq, M.; Gilani, M.A.; Afzal, Z.M.; Bilad, M.R.; Nizami, A.S.; Rehan, M.; Tahir, E.; Khan, A.L. Novel Poly Deep Eutectic Solvents Based Supported Liquid Membranes for CO2 Capture. Front. Energy Res. 2020, 8, 272. [Google Scholar] [CrossRef]
- Vatanpour, V.; Dehqan, A.; Harifi-Mood, A.R. Ethaline Deep Eutectic Solvent as a Hydrophilic Additive in Modification of Polyethersulfone Membrane for Antifouling and Separation Improvement. J. Membr. Sci. 2020, 614, 118528. [Google Scholar] [CrossRef]
- Craveiro, R.; Neves, L.A.; Duarte, A.R.C.; Paiva, A. Supported Liquid Membranes Based on Deep Eutectic Solvents for Gas Separation Processes. Sep. Purif. Technol. 2021, 254, 117593. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, N.; Zhang, L.; Sun, Y.; Huang, Z.; Wang, B.; Dou, H.; Guan, H. Enhanced Separation Performance of PES Ultrafiltration Membranes by Imidazole-Based Deep Eutectic Solvents as Novel Functional Additives. J. Membr. Sci. 2018, 564, 247–258. [Google Scholar] [CrossRef]
- Kaur, H.; Chen, C.C. Thermodynamic Modeling of CO2 Absorption in Aqueous Potassium Carbonate Solution with Electrolyte NRTL Model. Fluid Phase Equilib. 2020, 505, 112339. [Google Scholar] [CrossRef]
- Ayittey, F.K.; Obek, C.A.; Saptoro, A.; Perumal, K.; Wong, M.K. Process Modifications for a Hot Potassium Carbonate-Based CO2 Capture System: A Comparative Study. Greenh. Gases Sci. Technol. 2020, 10, 130–146. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Z. Thermodynamic Modeling for the Carbonate System K-HCO3-CO3 in Alkanolamine Solutions: An Extended Application for CO2 Scrubbing. Chem. Eng. J. 2020, 384, 123250. [Google Scholar] [CrossRef]
- Mohammadi Saadat, M.; Norouzbahari, S.; Esmaeili, M. CO2/N2 Separation by Glycerol Aqueous Solution in a Hollow Fiber Membrane Contactor Module: CFD Simulation and Experimental Validation. Fuel 2022, 323, 124370. [Google Scholar] [CrossRef]
- Chen, G.; Chen, G.; Cao, F.; Zhang, R.; Gao, H.; Liang, Z. Mass Transfer Performance and Correlation for CO2 Absorption into Aqueous 3-Diethylaminopropylamine Solution in a Hollow Fiber Membrane Contactor. Chem. Eng. Process. Process Intensif. 2020, 152, 107932. [Google Scholar] [CrossRef]
- Cao, F.; Gao, H.; Li, H.; Liang, Z. Experimental and Theoretical Studies on Mass Transfer Performance for CO2 Absorption into Aqueous N, N-Dimethylethanolamine Solution in the Polytetrafluoroethylene Hollow-Fiber Membrane Contactor. Ind. Eng. Chem. Res. 2018, 57, 16862–16874. [Google Scholar] [CrossRef]
- Ghobadi, J.; Ramirez, D.; Jerman, R.; Crane, M.; Khoramfar, S. CO2 Separation Performance of Different Diameter Polytetrafluoroethylene Hollow Fiber Membranes Using Gas-Liquid Membrane Contacting System. J. Membr. Sci. 2018, 549, 75–83. [Google Scholar] [CrossRef]
- Clausse, M.; Merel, J.; Meunier, F. Numerical Parametric Study on CO2 Capture by Indirect Thermal Swing Adsorption. Int. J. Greenh. Gas Control 2011, 5, 1206–1213. [Google Scholar] [CrossRef] [Green Version]
- Song, C.; Liu, Q.; Ji, N.; Deng, S.; Zhao, J.; Li, Y.; Song, Y.; Li, H. Alternative Pathways for Efficient CO2 Capture by Hybrid Processes—A Review. Renew. Sustain. Energy Rev. 2018, 82, 215–231. [Google Scholar] [CrossRef]
- Yeon, S.H.; Lee, K.S.; Sea, B.; Park, Y.I.; Lee, K.H. Application of Pilot-Scale Membrane Contactor Hybrid System for Removal of Carbon Dioxide from Flue Gas. J. Membr. Sci. 2005, 257, 156–160. [Google Scholar] [CrossRef]
- Anantharaman, R.; Berstad, D.; Roussanaly, S. Techno-Economic Performance of a Hybrid Membrane—Liquefaction Process for Post-Combustion CO2 Capture. Energy Procedia 2014, 61, 1244–1247. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Kim, M.K.; Lee, S.H.; Park, T.S.; do Park, S.; Park, J.H. Integrated Membrane Contactor Absorber/Regeneration Column Process for CO2 Capture with Large Scale Module at Various Operating Conditions. Catal. Today 2020, 358, 316–323. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, M.; Yu, H.; Ma, Q.; Luo, Z. Modeling of CO2 Stripping in a Hollow Fiber Membrane Contactor for CO2 Capture. Energy Fuels 2013, 27, 6887–6898. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Z.; Fan, J.; Yang, L.; Liu, J. Experimental Study on Postcombustion Systems Including a Hollow Fiber Membrane and a Packed Column. ACS Omega 2020, 5, 17692–17702. [Google Scholar] [CrossRef]
- Burdyny, T.; Struchtrup, H. Hybrid Membrane/Cryogenic Separation of Oxygen from Air for Use in the Oxy-Fuel Process. Energy 2010, 35, 1884–1897. [Google Scholar] [CrossRef]
- Yan, S.; Fang, M.; Zhang, W.; Zhong, W.; Luo, Z.; Cen, K. Comparative Analysis of CO2 Separation from Flue Gas by Membrane Gas Absorption Technology and Chemical Absorption Technology in China. Energy Convers. Manag. 2008, 49, 3188–3197. [Google Scholar] [CrossRef]
- Belaissaoui, B.; le Moullec, Y.; Willson, D.; Favre, E. Hybrid Membrane Cryogenic Process for Post-Combustion CO2 Capture. J. Membr. Sci. 2012, 415–416, 424–434. [Google Scholar] [CrossRef]
- Kundu, P.K.; Chakma, A.; Feng, X. Effectiveness of Membranes and Hybrid Membrane Processes in Comparison with Absorption Using Amines for Post-Combustion CO2 Capture. Int. J. Greenh. Gas Control 2014, 28, 248–256. [Google Scholar] [CrossRef]
- Rajabzadeh, S.; Yoshimoto, S.; Teramoto, M.; Al-Marzouqi, M.; Ohmukai, Y.; Maruyama, T.; Matsuyama, H. Effect of Membrane Structure on Gas Absorption Performance and Long-Term Stability of Membrane Contactors. Sep. Purif. Technol. 2013, 108, 65–73. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, R. Novel Method for Incorporating Hydrophobic Silica Nanoparticles on Polyetherimide Hollow Fiber Membranes for CO2 Absorption in a Gas–Liquid Membrane Contactor. J. Membr. Sci. 2014, 452, 379–389. [Google Scholar] [CrossRef]
- Chabanon, E.; Roizard, D.; Favre, E. Membrane Contactors for Postcombustion Carbon Dioxide Capture: A Comparative Study of Wetting Resistance on Long Time Scales. Ind. Eng. Chem. Res. 2011, 50, 8237–8244. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Kim, K.; Lee, J.; Koh, D.Y.; Bae, T.H. CO2 Absorption Using Membrane Contactors: Recent Progress and Future Perspective. Ind. Eng. Chem. Res. 2020, 59, 6773–6794. [Google Scholar] [CrossRef]
- Zhang, L.; Qu, R.; Sha, Y.; Wang, X.; Yang, L. Membrane Gas Absorption for CO2 Capture from Flue Gas Containing Fine Particles and Gaseous Contaminants. Int. J. Greenh. Gas Control 2015, 33, 10–17. [Google Scholar] [CrossRef]
- Naim, R.; Sean, G.P.; Nasir, Z.; Mokhtar, N.M.; Muhammad, N.A.S. Recent Progress and Challenges in Hollow Fiber Membranes for Wastewater Treatment and Resource Recovery. Membranes 2021, 11, 839. [Google Scholar] [CrossRef]





| Polymer | Chemical Structure | Polymer | Chemical Structure |
|---|---|---|---|
| Polysulfone (PSF) | 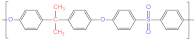 | Polypropylene (PP) |  |
| Polyethersulfone (PES) | 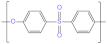 | Polyvinylidene (PVDF) | 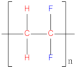 |
| Polyether ether ketone (PEEK) |  | Polytetrafluoroethylene (PTFE) |  |
| Polyetherimide (PEI) |  | Polymethyl pentene (PMP) | 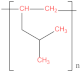 |
| Polyethylene (PE) | 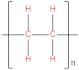 | Polydimethylsiloxane (PDMS) | 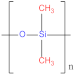 |
| Material | Pore Size | Porosity | Preparation Method | CO2 Flux | Ref. |
|---|---|---|---|---|---|
| PTFE | N.A. | 50% | Commercial, Sumitomo Polymer | 0.1 | [28] |
| PTFE | 0.15–0.27 µm | 44–58% | Paste extrusion-stretching-sintering | 0.7–1.3 | [26] |
| PP | 0.2 µm | 60% | Commercial, Accurel Membrana | N.A. | [49] |
| PP | 0.03 µm | 40% | Phase inversion technique | 0.5–2.6 | [50] |
| PTFE | 0.11–1.3 µm | 33–52% | Stretching and heating methods | 0.8–1.8 | [30] |
| PP | 0.1 µm | 20% | Commercial, Parsian Co. | N.A. | [51] |
| PVDF, PSF | N.A. | 50% | Commercial, Ecofine Co. | N.A. | [32] |
| PTFE | 0.48 µm | 52% | Commercial, Markel Corporation | 3–12 | [52] |
| PP | 0.2 µm | 50% | Commercial, Memtec | 0.4–1.2 | [31] |
| PTFE | 2.5 µm | 34% | Stretching | N.A. | [15] |
| PEI | 3.9–9.3 nm | 72% | Wet phase inversion method | 2–2.7 | [33] |
| PS | N.A. | 43% | Commercial, VWR Eurolab | N.A. | [53] |
| PP, PPO | 0.27 µm | 50% | Commercial, Parker Filtration | N.A. | [19] |
| PVDF | 78–222 nm | 67–85% | Arkema | 0.5–4 | [54] |
| PVDF | 50–470 nm | 32–45% | Thermally induced phase separation | 1–3 | [35] |
| PP | 200 nm | 50% | N.A. | 1–5 | [55] |
| PSF | N.A. | 50% | Commercial, Airrane Co. | N.A. | [56] |
| PVC | 0.89 µm | 75% | Phase inversion technique | 0.2–1 | [37] |
| PP, PVDF | 0.2 µm | 40–50% | Commercial, Pall Co. | 0.5–3 | [36] |
| PMP | N.A. | 30–40% | Commercial, Celgard | N.A. | [57] |
| PEEK | N.A. | 59–71% | Phase inversion technique | 0.1–0.3 | [29] |
| Ceramic | 68 nm | 46% | Phase inversion technique | 0.2–8 | [43] |
| Ceramic | N.A. | 40% | Phase inversion technique | N.A. | [45] |
| Ceramic | 0.9 µm | 49% | Phase inversion technique | 5–6.5 | [46] |
| Ceramic | 300 nm | 54% | Phase inversion spinning | 7.5 | [47] |
| Ceramic | N.A. | N.A. | Phase inversion and sintering | 0.18 | [58] |
| Ceramic | 100 nm | N.A. | Phase inversion spinning | 0.1 | [48] |
| Material | Additive | Pore Size | Preparation Method | CO2 Flux | Ref. |
|---|---|---|---|---|---|
| PVDF, PEI | PEG | 0.14–0.35 µm | Spinning process | 3.5–4 | [59] |
| PVDF | LiCl, Glycerol, (PEG-400), Methanol, Phosphoric acid | 0.036–4.42 µm | Wet spinning process | 4.03 | [60] |
| PVDF | LiCl | 0.014–0.43 µm | Wet-spinning Process. | 1.61 | [61] |
| PEI | Ethanol, Glycerol, Acetone | 9.02–26.84 nm | Phase inversion method | 1–8 | [62] |
| PSF | Glycerol | 47–373 nm | Wet spinning process | 2.9 | [63,64] |
| PSF | Glycerol, PEG200, Ethanol, Acetic acid | 3–10.52 nm | Phase inversion method | 0.98 | [65] |
| PVDF | LiCl | 3.96 nm | Wet spinning process | 4.1 | [66] |
| PVDF | Glycerol, phosphoric acid, ethanol, PEG-400 | 5.22–9.62 nm | Commercial: Arkema | 7.8 | [67] |
| PVDF | phosphoric acid, LiCl | 5.66–9.46 nm | Wet spinning method | 5.4 | [68] |
| PVDF | Glycerol | 0.07–0.1 µm | Dry–wet phase inversion method | 3–8 | [69] |
| PEI, PVDF | Glycerol | 0.09–0.05 µm | Wet spinning method | 1.5 | [70] |
| PEI | MMT | 44–331 nm | Wet phase inversion method. | 2.35 | [71] |
| PVDF | Water, Glycerol, Phosphoric acid | 9.25–20 nm | Commercial: Elf Autochem | 3–7 | [72] |
| PVDF | LiCl, glycerol | 5–25 nm | Commercial: ARKEM | N.A. | [73] |
| PEI | Water, Glycerol, Acetic acid, Ethanol, Methanol | 101–140 nm | Wet-spinning method | 0.9–1.7 | [34] |
| PES | Water, methanol, ethanol, glycerol, acetic acid, acetone | 160–630 nm | Commercial: Arkema | 0.5–4.5 | [74] |
| PVDF | Ethylene glycol | 1 µm | Dry-jet wet phase inversion | N.A. | [75] |
| PVDF | PVP, LiCl | N.A. | Phase inversion technique | N.A. | [76] |
| PVDF | LiCl and water | N.A. | Commercial | 1.2 | [77] |
| PES | o-xylene | 0.2 µm | Commercial, BASF | 0.1–4 | [78] |
| PVDF | LiCl + phosphoric acid | 17–53 nm | Dry-jet wet spinning phase inversion | 1.31 | [79] |
| PSF | PVP | 117–129 nm | Non-solvent phase inversion method | 2.5–5 | [80] |
| PSF | Ethanol | 19–24 nm | Commercial | 1–4 | [81] |
| PVDF | Methanol | 0.35–0.48 µm | Commercial Arkema | N.A. | [82] |
| PVDF | LiCl | N.A. | Phase inversion | 1.6 | [83] |
| PVDF, PSF | Glycerol | 6.1–9.6 nm | Wet spinning method | N.A. | [84] |
| PEI | SMM | 20–640 nm | Commercial | 3.5 | [85] |
| PVDF | SMM | 158–650 nm | Dry–wet phase inversion process | 5.4 | [86,87] |
| PSF | SMM | 250–268 nm | Dry–wet phase inversion process | 2.5 | [88] |
| PVDF | DDS, MTS | N.A. | Alkali treatment | N.A. | [89] |
| PEI | SMM | 77–280 nm | Dry–wet phase inversion process | 3.2 | [90,91] |
| PVDF | SMM | 90–300 | Dry–wet phase inversion process | 6.8 | [92] |
| Absorbent | Molecular Structure | Absorbent | Molecular Structure |
|---|---|---|---|
| Arginine (Arg) |  | Alanine (Ala) | 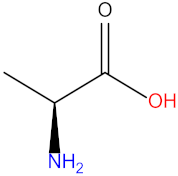 |
| Phenylalanine (Phe) | 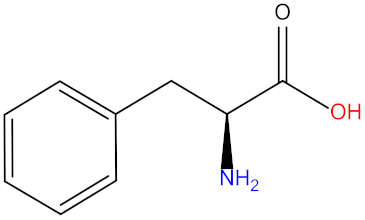 | Threonine (Thr) |  |
| Methionine (Met) | 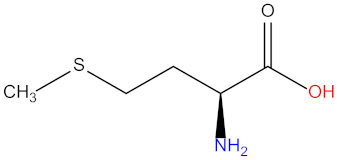 | Glycine (Gly) |  |
| Glutamine (Glu) | 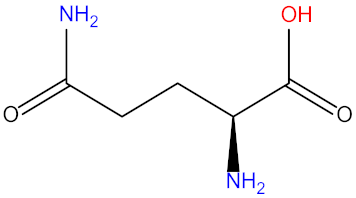 | Proline (Pro) |  |
| Leucine (Leu) | 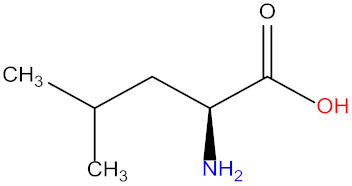 | Tryptophan (Try) | 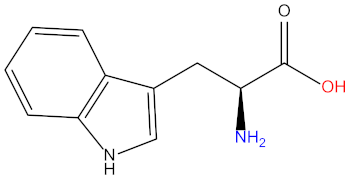 |
| Lysine (Lys) |  | Valine (Val) |  |
| Diisopropanolamine (DIPA) |  | (2-Aminoethyl)ethanolamine (AEEA) | 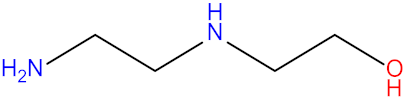 |
| Methyldiethanolamine (MDEA) | 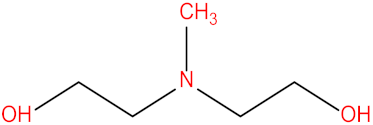 | Monoethanolamine (MEA) |  |
| Diethanolamine (DEA) | 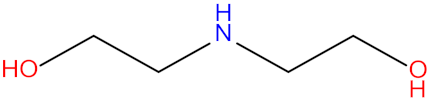 | Piperazine (PZ) | 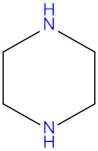 |
| 2-amino-2-methyl-1-propanol (AMP) | 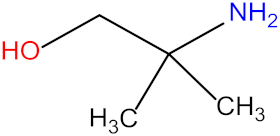 | Diethylethanolamine (DEEA) | 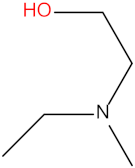 |
| Methylamino)propylamine (MAPA) | 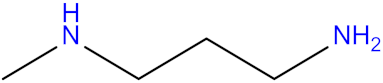 | 2-(1-piperazinyl)-ethylamine (PZEA) |  |
| Solvent Category | Liquid Absorbent | Concentration | Gas Mixture | CO2 Flux | Membrane Material | Ref. |
|---|---|---|---|---|---|---|
| Amino acid salt | Proline, alanine, sarcosine, glycine | 1 kmol/m3 | CO2/N2 | N.A. | PP | [118] |
| Amino acid salt | Arginine, serine, threonine, alanine | 0.5–1 kmol/m3 | CO2/N2 | 0.8–2 | PVDF | [119] |
| Amino acid salt | Glycine + MEA | 1 kmol/m3 | CO2 | N.A. | PP | [120] |
| Amino acid salt | Arginine, glycine | 1 kmol/m3 | CO2/CH4 | 5–8 | PVDF | [121] |
| Amino acid salt | Lysine | 1 kmol/m3 | CO2/CH4 | N.A. | PP | [122] |
| Amino acid salt | Sarcosine | 1 kmol/m3 | CO2/N2 | 2–2.5 | PP | [123] |
| Amino acid salt | Glycine | 1–3 kmol/m3 | CO2/N2/O2 | 5.5–6.1 | PP | [124] |
| Amino acid salt | Glycine + PZ | 1 kmol/m3 | CO2/N2 | N.A. | PP | [125] |
| Amino acid salt | Sarcosine | 1.5 kmol/m3 | CO2/CH4 | N.A. | PP | [126] |
| Amino acid salt | Sarcosine, glycine | 0.5 kmol/m3 | CO2/CH4 | 17–19 | PVDF | [127] |
| Amino acid salt | Glycine | 1 kmol/m3 | CO2 | 1–3.5 | PP | [128] |
| Alkanolamines | MDEA + MEA, DEA + AMP | 30 wt% | CO2/N2 | N.A. | PP | [129] |
| Alkanolamines | MEA, DEA, MDEA, AMP | 10 wt% | CO2 | N.A. | PP | [130] |
| Alkanolamgines | MEA | 30 wt% | CO2 | 5 | PP | [131] |
| Alkanolamines | MDEA + PZEA | 1 kmol/m3 | 5–7.2 | PP | [132] | |
| Alkanolamines | EDA, PZEA | 1 kmol/m3 | CO2/CH4 | N.A. | PP | [133] |
| Alkanolamines | DEAB | 2 kmol/m3 | CO2 | 0.3–0.4 | PTFE | [134] |
| Alkanolamines | MDEA + PZ | N.A. | CO2/N2 | N.A. | PP | [135] |
| Alkanolamines | MDEA + PZ | 2.5 kmol/m3 | CO2/N2 | N.A. | PP | [136] |
| Alkanolamines | DMEA | 2 kmol/m3 | CO2/N2 | 0.1–0.15 | PTFE | [137] |
| Alkanolamines | AMP + PZ | 1.1 kmol/m3 | CO2/N2 | 2.5–3.08 | PVDF | [138] |
| Alkanolamines | MEA, AMP, DEA | 1 kmol/m3 | CO2/N2 | 1–4 | PVDF | [139] |
| Alkanolamines | MEA | 1 kmol/m3 | SO2/CO2 | 0.45 | PP | [140] |
| Alkanolamines | MEA | 5 kmol/m3 | CO2 | N.A. | PTFE | [141] |
| Alkanolamines | MEA | 30 wt% | CO2/N2 | N.A. | PTFE | [142] |
| Alkanolamines | MEA + DMEA | 2 kmol/m3 | CO2/N2 | 0.01–0.03 | PTFE | [143] |
| Alkanolamines | DEEA + PZ | 2 kmol/m3 | CO2/N2 | 0.016–0.03 | PTFE | [144] |
| Alkanolamines | 1DMA2P | 2 kmol/m3 | CO2/air | 0.011 | PTFE | [145] |
| Ammonia | NH3 | 5 wt% | CO2/N2 | N.A. | PP | [146] |
| Inorganic solvent | K2CO3 + proline | 5 wt% | CO2/N2 | 3.5–5.2 | PP | [147] |
| Inorganic solvent | K2CO3 + 2MPZ | 0.5 kmol/m3 | CO2/CH4 | N.A. | PP | [148] |
| Inorganic solvent | K2CO3 + SarK | 0.12 kmol/m3 | CO2/H2S | 2.4 | PVDF | [149] |
| Inorganic solvent | K2CO3 + PZ | 5 wt% | CO2/N2 | N.A. | PP | [150] |
| Inorganic solvent | K2CO3 + MDEA | 3 kmol/m3 | CO2/N2 | 0.005–0.01 | pp | [151] |
| Inorganic solvent | K2CO3 | 1 kmol/m3 | CO2/N2 | 8–10 | PP | [152] |
| Inorganic solvent | K2CO3 + DEA | N.A. | CO2/CH4 | N.A. | PP | [153] |
| Inorganic solvent | K2CO3 + Gly-K, Lys-K, Arg-K | 1.3 kmol/m3 | CO2/CH4 | 9–19 | PP | [154] |
| Ionic liquid | [emim][EtSO4] | N.A. | CO2/N2 | 0.3 | PP | [155] |
| Ionic liquid | [Bmim][BF4] | 10–50 wt% | 35% CO2 | 1.5–10 | pp | [156] |
| Ionic liquid | [Bmim][BF4] + MEA | 40 wt% | CO2/SO2/N2/O2 | 0.4 | PP | [157] |
| Ionic liquid | [Bmim][BF4] + MDEA | 25 wt% | CO2/N2 | 4.5–7 | PP | [158] |
| Ionic liquid | [Emim][BF4] | 0.5 kmol/m3 | Pure CO2 | N.A. | PTMSP | [159] |
| Ionic liquid | [Emim][BF4], [apmim][BF4] | 1–5 kmol/m3 | CO2/N2 | 0.1–8 | PP | [160] |
| Ionic liquid | [Emim][Ac] | N.A. | CO2/N2 | N.A. | PVDF | [161] |
| Membrane Parameters Affecting Membrane Performance | |
|---|---|
| Fiber inner radius (μm) | Membrane area (m2) |
| Membrane thickness (μm) | Number of fibers |
| Module inner diameter (m) | Membrane porosity |
| Membrane length (m) | Membrane tortuosity |
| Packing density | |
| Operating Parameters Affecting Membrane Performance | |
| Gas temperature (K) | Gas flow rate (L/h) |
| Liquid temperature (K) | Liquid flow rate (L/h) |
| Operating pressure (MPa) | Liquid concentration |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramezani, R.; Di Felice, L.; Gallucci, F. A Review on Hollow Fiber Membrane Contactors for Carbon Capture: Recent Advances and Future Challenges. Processes 2022, 10, 2103. https://doi.org/10.3390/pr10102103
Ramezani R, Di Felice L, Gallucci F. A Review on Hollow Fiber Membrane Contactors for Carbon Capture: Recent Advances and Future Challenges. Processes. 2022; 10(10):2103. https://doi.org/10.3390/pr10102103
Chicago/Turabian StyleRamezani, Rouzbeh, Luca Di Felice, and Fausto Gallucci. 2022. "A Review on Hollow Fiber Membrane Contactors for Carbon Capture: Recent Advances and Future Challenges" Processes 10, no. 10: 2103. https://doi.org/10.3390/pr10102103
APA StyleRamezani, R., Di Felice, L., & Gallucci, F. (2022). A Review on Hollow Fiber Membrane Contactors for Carbon Capture: Recent Advances and Future Challenges. Processes, 10(10), 2103. https://doi.org/10.3390/pr10102103








