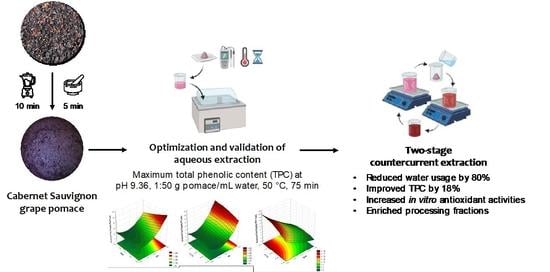
Revitalizing Unfermented Cabernet Sauvignon Pomace Using an Eco-Friendly, Two-Stage Countercurrent Process: Role of pH on the Extractability of Bioactive Phenolics
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
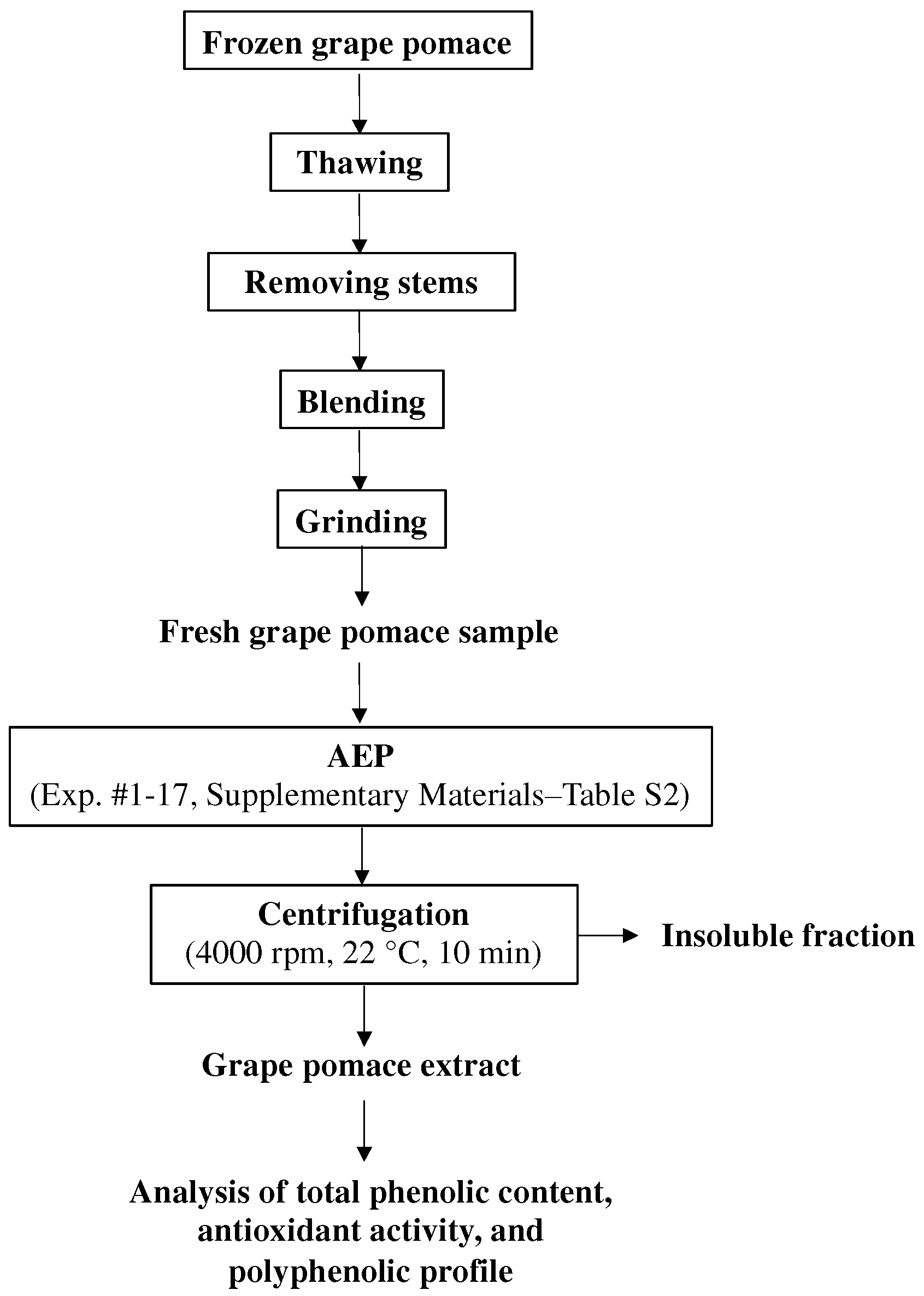
2.2. Raw Material
2.3. Initial Evaluation of the Effects of the Extraction pH on the Aqueous Extraction Process (AEP) of Grape Pomace
2.4. Effect of Conventional Solvent Extraction on TPC
2.5. Understanding the Simultaneous Effect of Extraction Parameters in the Single-Stage AEP of Grape Pomace
2.6. Two-Stage Countercurrent AEP of Grape Pomace
2.7. Chemical Analysis of Extracts
2.7.1. Total Phenolic Content (TPC)
2.7.2. ABTS and ORAC Assays for Antioxidant Capacity of the Extracts
2.7.3. Untargeted Phenolic Profiling of the Grape Pomace Extracts
2.7.4. Phenolic Quantification by RP-HPLC-DAD
2.8. Statistical Analysis
3. Results and Discussion
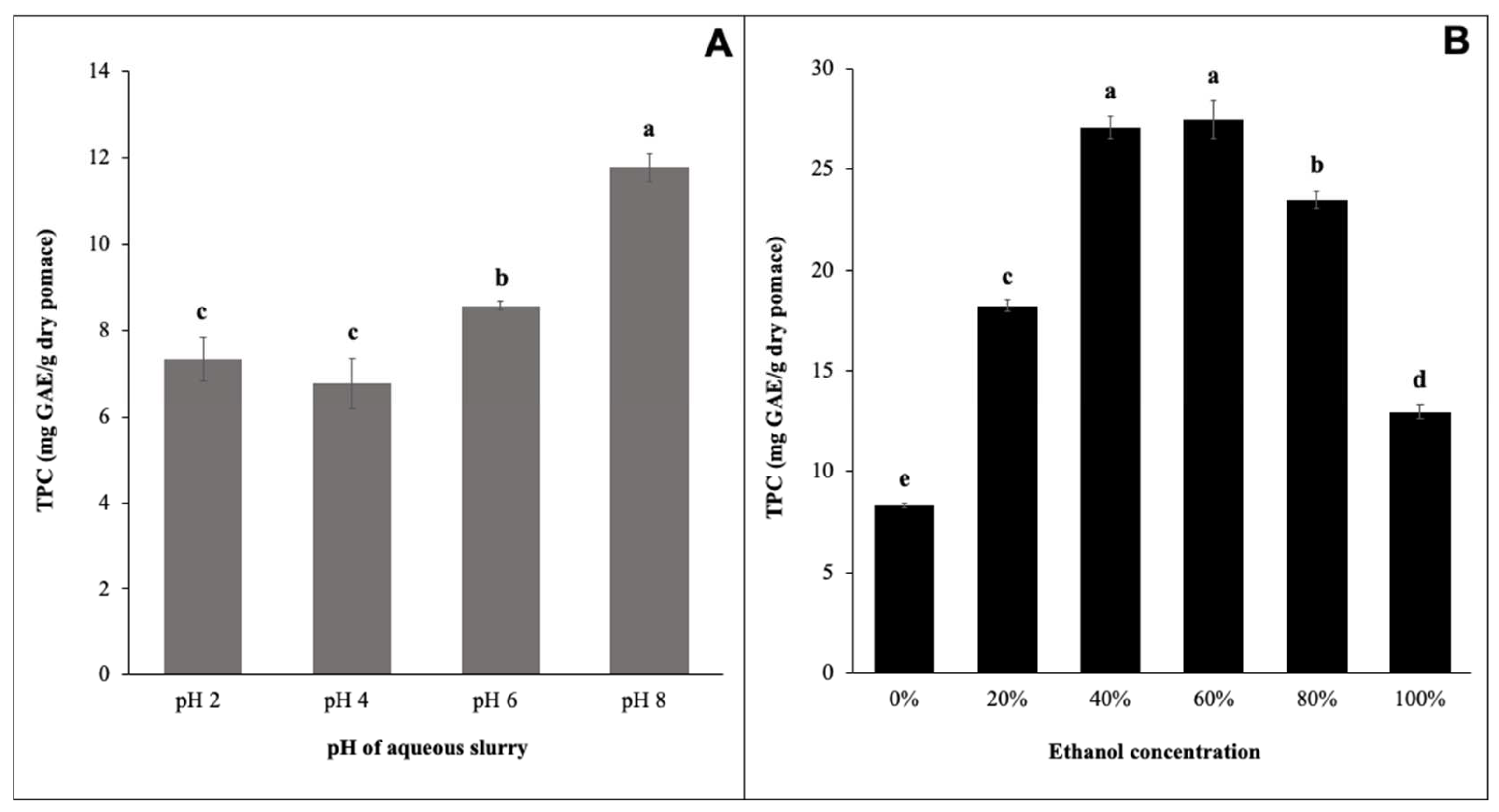
3.1. Effect of Extraction pH on TPC of AEP Grape pomace Extracts
3.2. Effect of Ethanol Concentration on TPC of Conventional Solvent Extracts
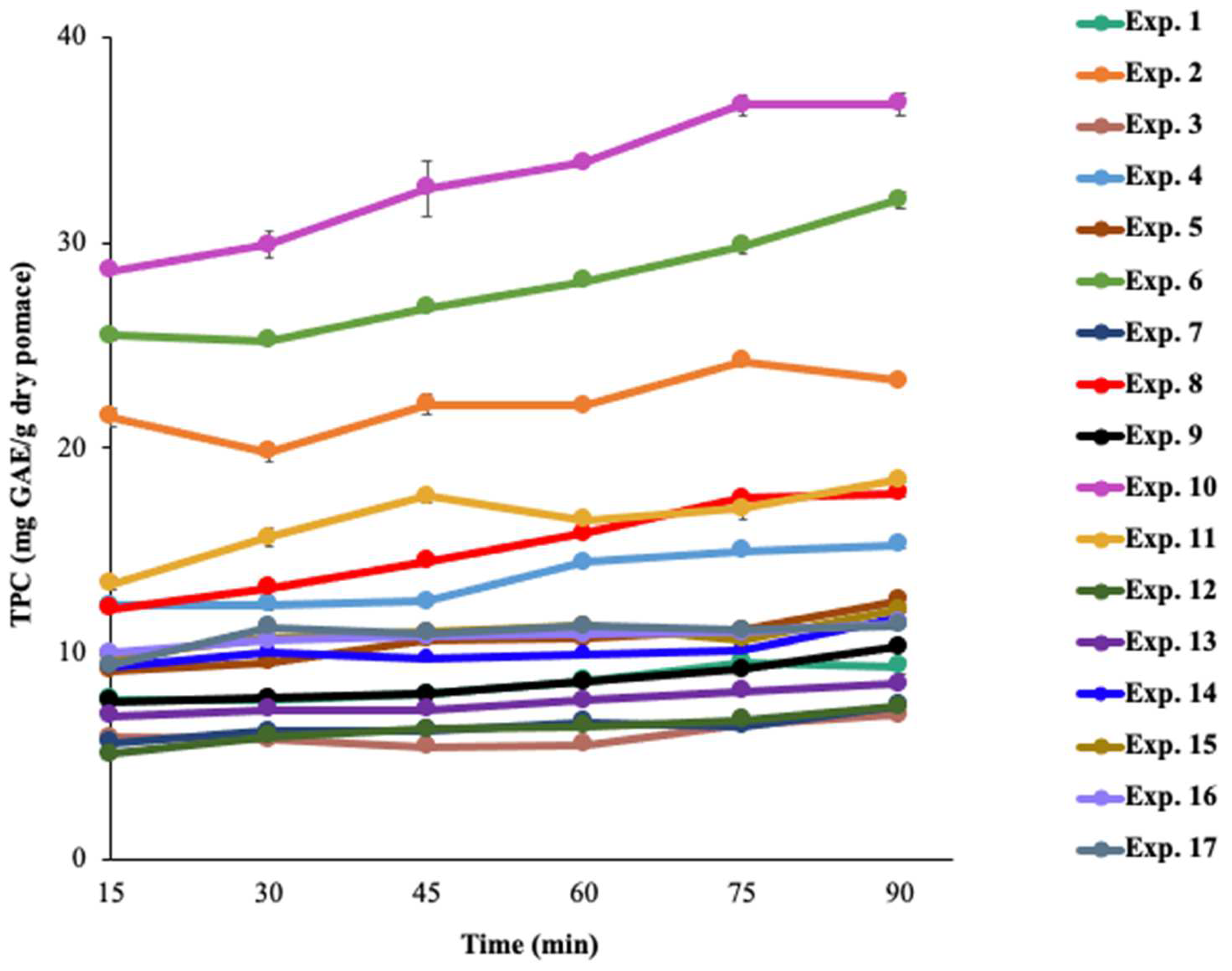
3.3. Simultaneous Effect of Extraction Parameters on TPC of Single-Stage AEP
3.4. Selecting the SLR for the Development of the Multistage Countercurrent AEP
3.5. Two-Stage Countercurrent Extraction of Phenolics from Grape Pomace
3.6. Antioxidant Activity of Single-Stage and Two-Stage Countercurrent AEP Grape Pomace Extracts
3.7. Phenolic Composition of Cabernet Sauvignon Grape Pomace Extracts
3.8. Quantification of Phenolics in Cabernet Sauvignon Grape Pomace Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- California Department of Food and Agriculture. California Grape Crush Report Final 2019; 2020. In USDA National Agricultural Statistics Service. Available online: https://www.nass.usda.gov/Statistics_by_State/California/Publications/Specialty_and_Other_Releases/Grapes/Crush/Final/2020/2020_final_grape%20crush.pdf (accessed on 4 September 2022).
- Hidalgo, P.; Pueyo, E.; Pozo-Bayón, M.A.; Martínez-Rodríguez, A.J.; Martín-Álvarez, P.; Polo, M.C. Sensory and Analytical Study of Rosé Sparkling Wines Manufactured by Second Fermentation in the Bottle. J. Agric. Food Chem. 2004, 52, 6640–6645. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, P.; Gupta, A.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Current Trends and Possibilities for Exploitation of Grape Pomace as a Potential Source for Value Addition. Environ. Pollut. 2021, 278, 116796. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Duarte, E. Integrated Approach to Winery Waste: Waste Generation and Data Consolidation. Front. Environ. Sci. Eng. 2016, 10, 168–176. [Google Scholar] [CrossRef]
- Pérez-Serradilla, J.A.; de Castro, M.D.L. Role of Lees in Wine Production: A Review. Food Chem. 2008, 111, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Saura-Calixto, F. Dietary Fiber as a Carrier of Dietary Antioxidants: An Essential Physiological Function. J. Agric. Food Chem. 2011, 59, 43–49. [Google Scholar] [CrossRef] [PubMed]
- El Achkar, J.H.; Lendormi, T.; Salameh, D.; Louka, N.; Maroun, R.G.; Lanoisellé, J.L.; Hobaika, Z. Influence of Pretreatment Conditions on Lignocellulosic Fractions and Methane Production from Grape Pomace. Bioresour. Technol. 2018, 247, 881–889. [Google Scholar] [CrossRef]
- Costa, J.R.; Amorim, M.; Vilas-Boas, A.; Tonon, R.V.; Cabral, L.M.C.; Pastrana, L.; Pintado, M. Impact of in Vitro Gastrointestinal Digestion on the Chemical Composition, Bioactive Properties, and Cytotoxicity of Vitis vinifera L. Cv. Syrah Grape Pomace Extract. Food Funct. 2019, 10, 1856–1869. [Google Scholar] [CrossRef]
- Sinrod, A.J.G.; Li, X.; Bhattacharya, M.; Paviani, B.; Wang, S.C.; Barile, D. A Second Life for Wine Grapes: Discovering Potentially Bioactive Oligosaccharides and Phenolics in Chardonnay Marc and Its Processing Fractions. LWT 2021, 144, 111192. [Google Scholar] [CrossRef]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural Bioactive Compounds from Winery By-Products as Health Promoters: A Review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef]
- Pinelo, M.; Arnous, A.; Meyer, A.S. Upgrading of Grape Skins: Significance of Plant Cell-Wall Structural Components and Extraction Techniques for Phenol Release. Trends Food Sci. Technol. 2006, 17, 579–590. [Google Scholar] [CrossRef]
- Nayak, A.; Bhushan, B.; Rosales, A.; Turienzo, L.R.; Cortina, J.L. Valorisation Potential of Cabernet Grape Pomace for the Recovery of Polyphenols: Process Intensification, Optimisation and Study of Kinetics. Food Bioprod. Process. 2018, 109, 74–85. [Google Scholar] [CrossRef]
- Srinivas, K.; King, J.W.; Monrad, J.K.; Howard, L.R.; Zhang, D.; Martin, R.E. Pressurized Solvent Extraction of Flavonoids from Grape Pomace Utilizing Organic Acid Additives. Ital. J. Food Sci. 2011, 23, 90–105. [Google Scholar]
- Naczk, M.; Shahidi, F. Extraction and Analysis of Phenolics in Food. J. Chromatogr. A 2004, 1054, 95–111. [Google Scholar] [CrossRef]
- Kallithraka, S.; Garcia-Viguera, C.; Bridle, P.; Bakker, J. Survey of Solvents for the Extraction of Grape Seed Phenolics. Phytochem. Anal. 1995, 6, 265–267. [Google Scholar] [CrossRef]
- Ding, Y.; Morozova, K.; Scampicchio, M.; Ferrentino, G. Non-Extractable Polyphenols from Food by-Products: Current Knowledge on Recovery, Characterisation, and Potential Applications. Processes 2020, 8, 925. [Google Scholar] [CrossRef]
- Li, W.; Yang, R.; Ying, D.; Yu, J.; Sanguansri, L.; Augustin, M.A. Analysis of Polyphenols in Apple Pomace: A Comparative Study of Different Extraction and Hydrolysis Procedures. Ind. Crops Prod. 2020, 147, 112250. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound Phenolics in Foods, a Review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Arranz, S.; Saura Calixto, F. Analysis of Polyphenols in Cereals May Be Improved Performing Acidic Hydrolysis: A Study in Wheat Flour and Wheat Bran and Cereals of the Diet. J. Cereal Sci. 2010, 51, 313–318. [Google Scholar] [CrossRef]
- Kim, K.H.; Tsao, R.; Yang, R.; Cui, S.W. Phenolic Acid Profiles and Antioxidant Activities of Wheat Bran Extracts and the Effect of Hydrolysis Conditions. Food Chem. 2006, 95, 466–473. [Google Scholar] [CrossRef]
- Fazary, A.E.; Ju, Y.-H. Feruloyl Esterases as Biotechnological Tools: Current and Future Perspectives. Acta Biochim. Biophys. Sin. 2007, 39, 811–828. [Google Scholar] [CrossRef]
- Panić, M.; Gunjević, V.; Cravotto, G.; Radojčić Redovniković, I. Enabling Technologies for the Extraction of Grape-Pomace Anthocyanins Using Natural Deep Eutectic Solvents in up-to-Half-Litre Batches Extraction of Grape-Pomace Anthocyanins Using NADES. Food Chem. 2019, 300, 125185. [Google Scholar] [CrossRef] [PubMed]
- Lima, Á.S.; Soares, C.M.F.; Paltram, R.; Halbwirth, H.; Bica, K. Extraction and Consecutive Purification of Anthocyanins from Grape Pomace Using Ionic Liquid Solutions. Fluid Phase Equilibria 2017, 451, 68–78. [Google Scholar] [CrossRef]
- Moro, K.I.B.; Bender, A.B.B.; da Silva, L.P.; Penna, N.G. Green Extraction Methods and Microencapsulation Technologies of Phenolic Compounds From Grape Pomace: A Review. Food Bioprocess Technol. 2021, 14, 1407–1431. [Google Scholar] [CrossRef]
- Rosenthal, A.; Pyle, D.L.; Niranjan, K. Aqueous and Enzymatic Processes for Edible Oil Extraction. Enzym. Microb. Technol. 1996, 19, 402–420. [Google Scholar] [CrossRef]
- de Moura, J.M.L.N.; Campbell, K.; Mahfuz, A.; Jung, S.; Glatz, C.E.; Johnson, L. Enzyme-Assisted Aqueous Extraction of Oil and Protein from Soybeans and Cream de-Emulsification. JAOCS J. Am. Oil Chem. Soc. 2008, 85, 985–995. [Google Scholar] [CrossRef]
- de Moura, J.M.L.N.; Campbell, K.; de Almeida, N.M.; Glatz, C.E.; Johnson, L.A. Protein Extraction and Membrane Recovery in Enzyme-Assisted Aqueous Extraction Processing of Soybeans. J. Am. Oil Chem. Soc. 2011, 88, 877–889. [Google Scholar] [CrossRef]
- Zhao, S.; Baik, O.-D.; Choi, Y.J.; Kim, S.-M. Pretreatments for the Efficient Extraction of Bioactive Compounds from Plant-Based Biomaterials. Crit. Rev. Food Sci. Nutr. 2014, 54, 1283–1297. [Google Scholar] [CrossRef]
- de Moura, J.M.L.N.; Johnson, L.A. Two-Stage Countercurrent Enzyme-Assisted Aqueous Extraction Processing of Oil and Protein from Soybeans. J. Am. Oil Chem. Soc. 2009, 86, 283–289. [Google Scholar] [CrossRef]
- Souza Almeida, F.; Furlan Goncalves Dias, F.; Kawazoe Sato, A.C.; Leite Nobrega de Moura Bell, J.M. From Solvent Extraction to the Concurrent Extraction of Lipids and Proteins from Green Coffee: An Eco-Friendly Approach to Improve Process Feasibility. Food Bioprod. Process. 2021, 129, 144–156. [Google Scholar] [CrossRef]
- Moro, K.I.B.; Bender, A.B.B.; Ferreira, D.d.F.; Speroni, C.S.; Barin, J.S.; da Silva, L.P.; Penna, N.G. Recovery of Phenolic Compounds from Grape Pomace (Vitis vinifera L.) by Microwave Hydrodiffusion and Gravity. LWT 2021, 150, 112066. [Google Scholar] [CrossRef]
- Rajha, H.N.; Ziegler, W.; Louka, N.; Hobaika, Z.; Vorobiev, E.; Boechzelt, H.G.; Maroun, R.G. Effect of the Drying Process on the Intensification of Phenolic Compounds Recovery from Grape Pomace Using Accelerated Solvent Extraction. Int. J. Mol. Sci. 2014, 15, 18640–18658. [Google Scholar] [CrossRef]
- Darra, N.e.; Grimi, N.; Vorobiev, E.; Louka, N.; Maroun, R.; Darra, N.E.; Grimi, N.; Vorobiev, E.; Louka, N.; Maroun, R.; et al. Extraction of Polyphenols from Red Grape Pomace Assisted by Pulsed Ohmic Heating. Food Bioprocess Technol. 2013, 6, 1281–1289. [Google Scholar] [CrossRef]
- Tomaz, I.; Šeparović, M.; Štambuk, P.; Preiner, D.; Maletić, E.; Karoglan Kontić, J. Effect of Freezing and Different Thawing Methods on the Content of Polyphenolic Compounds of Red Grape Skins. J. Food Process. Preserv. 2018, 42, e13550. [Google Scholar] [CrossRef]
- AOAC Official Method 925.09-1925; Solids (Total) and Loss on Drying (Moisture); Official Methods of Analysis of AOAC International: Arlington, VA, USA, 2015.
- AOCS Official Method Ba 5-49; Ash in Oilseed Cake and Meals; Official Methods and Recommended Practices of the AOCS: Urbana, IL, USA, 2017.
- AOAC Official Method 922.06 Fat in Flour; Acid Hydrolysis Method; Official Methods of Analysis of AOAC International: Arlington, VA, USA, 2012.
- Amendola, D.; de Faveri, D.M.; Spigno, G. Grape Marc Phenolics: Extraction Kinetics, Quality and Stability of Extracts. J. Food Eng. 2010, 97, 384–392. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzym. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Al-Duais, M.; Müller, L.; Böhm, V.; Jetschke, G. Antioxidant Capacity and Total Phenolics of Cyphostemma Digitatum before and after Processing: Use of Different Assays. Eur. Food Res. Technol. 2009, 228, 813–821. [Google Scholar] [CrossRef]
- Zulueta, A.; Esteve, M.J.; Frígola, A. ORAC and TEAC Assays Comparison to Measure the Antioxidant Capacity of Food Products. Food Chem. 2009, 114, 310–316. [Google Scholar] [CrossRef]
- Fiehn, O.; Wohlgemuth, G.; Scholz, M.; Kind, T.; Lee, D.Y.; Lu, Y.; Moon, S.; Nikolau, B. Quality Control for Plant Metabolomics: Reporting MSI-Compliant Studies. Plant J. 2008, 53, 691–704. [Google Scholar] [CrossRef]
- Konar, N.; Poyrazoĝlu, E.S.; Demir, K.; Artik, N. Determination of Conjugated and Free Isoflavones in Some Legumes by LC–MS/MS. J. Food Compos. Anal. 2012, 25, 173–178. [Google Scholar] [CrossRef]
- Librán, C.M.; Mayor, L.; Garcia-Castello, E.M.; Vidal-Brotons, D. Polyphenol Extraction from Grape Wastes: Solvent and PH Effect. Agric. Sci. 2013, 04, 56–62. [Google Scholar] [CrossRef]
- Oreopoulou, A.; Tsimogiannis, D.; Oreopoulou, V. Extraction of Polyphenols from Aromatic and Medicinal Plants: An Overview of the Methods and the Effect of Extraction Parameters. In Polyphenols in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 243–259. ISBN 9780128137680. [Google Scholar]
- Arranz, S.; Saura-Calixto, F.; Shaha, S.; Kroon, P.A. High Contents of Nonextractable Polyphenols in Fruits Suggest That Polyphenol Contents of Plant Foods Have Been Underestimated. J. Agric. Food Chem. 2009, 57, 7298–7303. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Y.; Toledo, R.T. Oxygen Radical Absorbance Capacities of Grape/Wine Industry Byproducts and Effect of Solvent Type on Extraction of Grape Seed Polyphenols. J. Food Compos. Anal. 2006, 19, 41–48. [Google Scholar] [CrossRef]
- Sant’Anna, V.; Brandelli, A.; Marczak, L.D.F.; Tessaro, I.C. Kinetic Modeling of Total Polyphenol Extraction from Grape Marc and Characterization of the Extracts. Sep. Purif. Technol. 2012, 100, 82–87. [Google Scholar] [CrossRef]
- White, B.L.; Howard, L.R.; Prior, R.L. Release of Bound Procyanidins from Cranberry Pomace by Alkaline Hydrolysis. J. Agric. Food Chem. 2010, 58, 7572–7579. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Yeo, J. Insoluble-Bound Phenolics in Food. Molecules 2016, 21, 1216. [Google Scholar] [CrossRef] [PubMed]
- Campos, F.; Peixoto, A.F.; Fernandes, P.A.R.; Coimbra, M.A.; Mateus, N.; de Freitas, V.; Fernandes, I.; Fernandes, A. The Antidiabetic Effect of Grape Pomace Polysaccharide-Polyphenol Complexes. Nutrients 2021, 13, 4495. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Jürgens, H.S. Effect of PH on the Stability of Plant Phenolic Compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef]
- Liyanapathirana, C.; Shahidi, F. Optimization of Extraction of Phenolic Compounds from Wheat Using Response Surface Methodology. Food Chem. 2005, 93, 47–56. [Google Scholar] [CrossRef]
- Larrauri, J.A.; Rupérez, P.; Saura-Calixto, F. Effect of Drying Temperature on the Stability of Polyphenols and Antioxidant Activity of Red Grape Pomace Peels. J. Agric. Food Chem. 1997, 45, 1390–1393. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; de Faveri, D.M. Effects of Extraction Time, Temperature and Solvent on Concentration and Antioxidant Activity of Grape Marc Phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Speisky, H.; Fuentes, J.; Dorta, E.; Lopez-Alarcón, C. Polyphenols: Sources and Main Characteristics in Advances in Technologies for Producing Food-Relevant Polyphenols; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Ky, I.; Teissedre, P.L. Characterisation of Mediterranean Grape Pomace Seed and Skin Extracts: Polyphenolic Content and Antioxidant Activity. Molecules 2015, 20, 2190. [Google Scholar] [CrossRef]
- Monagas, M.; Hernandez-Ledesma, B.; Garrido, I.; Martin-Alvarez, P.J.; Gomez-Cordoves, C.; Bartolome, B.; Monagas, M.; Hernández-Ledesma, B.; Martín-Álvarez, P.J.; Gómez-Cordovés, C.; et al. Quality Assessment of Commercial Dietary Antioxidant Products from Vitis vinifera L. Grape Seeds. Nutr. Cancer 2005, 53, 244–254. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Couti, P.; Woillez, M.; de Bélis, R.; Sen, L.; des Substances Végétales, E.; Biologique, A. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2019, 57, 1768–1774. [Google Scholar] [CrossRef]
- Lingua, M.S.; Fabani, M.P.; Wunderlin, D.A.; Baroni, M.v. In Vivo Antioxidant Activity of Grape, Pomace and Wine from Three Red Varieties Grown in Argentina: Its Relationship to Phenolic Profile. J. Funct. Foods 2015, 20, 332–345. [Google Scholar] [CrossRef]
- Valls, J.; Agnolet, S.; Haas, F.; Struffi, I.; Ciesa, F.; Robatscher, P.; Oberhuber, M. Valorization of Lagrein Grape Pomace as a Source of Phenolic Compounds: Analysis of the Contents of Anthocyanins, Flavanols and Antioxidant Activity. Eur. Food Res. Technol. 2017, 243, 2211–2224. [Google Scholar] [CrossRef]
- Monrad, J.K.; Suárez, M.; Motilva, M.J.; King, J.W.; Srinivas, K.; Howard, L.R. Extraction of Anthocyanins and Flavan-3-Ols from Red Grape Pomace Continuously by Coupling Hot Water Extraction with a Modified Expeller. Food Res. Int. 2014, 65, 77–87. [Google Scholar] [CrossRef]
- Liu, B.; Raeth, T.; Beuerle, T.; Beerhues, L. A Novel 4-Hydroxycoumarin Biosynthetic Pathway. Plant Mol. Biol. 2010, 72, 17–25. [Google Scholar] [CrossRef]
- Hroboňová, K.; Sádecká, J. Coumarins Content in Wine: Application of HPLC, Fluorescence Spectrometry, and Chemometric Approach. J. Food Sci. Technol. 2020, 57, 200–209. [Google Scholar] [CrossRef]
- Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Simple Coumarins and Analogues in Medicinal Chemistry: Occurrence, Synthesis and Biological Activity. Curr. Med. Chem. 2005, 12, 887–916. [Google Scholar] [CrossRef]
- Stój, A.; Szwajgier, D.; Baranowska-Wójcik, E.; Domagała, D. Gentisic Acid, Salicylic Acid, Total Phenolic Content and Cholinesterase Inhibitory Activities of Red Wines Made from Various Grape Varieties. S. Afr. J. Enol. Vitic. 2019, 40. [Google Scholar] [CrossRef]
- Castillo-Muñoz, N.; Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. Flavonol Profiles of Vitis vinifera Red Grapes and Their Single-Cultivar Wines. J. Agric. Food Chem. 2007, 55, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Marchante, L.; Gómez Alonso, S.; Alañón, M.E.; Pérez-Coello, M.S.; Díaz-Maroto, M.C. Natural Extracts from Fresh and Oven-Dried Winemaking by-Products as Valuable Source of Antioxidant Compounds. Food Sci. Nutr. 2018, 6, 1564–1574. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Hao, F.; Mo, H.; Jiang, J.; Wang, H.; Liu, C.; Fan, X.; Zhang, Y. Phenol Profiles and Antioxidant Properties of White Skinned Grapes and Their Coloured Genotypes during Growth. Biotechnol. Biotechnol. Equip. 2016, 31, 58–67. [Google Scholar] [CrossRef]
- Landrault, N.; Larronde, F.; Delaunay, J.C.; Castagnino, C.; Vercauteren, J.; Merillon, J.M.; Gasc, F.; Cros, G.; Teissedre, P.L. Levels of Stilbene Oligomers and Astilbin in French Varietal Wines and in Grapes during Noble Rot Development. J. Agric. Food Chem. 2002, 50, 2046–2052. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of Citrus Flavonoids, Naringin and Naringenin, on Metabolic Syndrome and Their Mechanisms of Action. Adv. Nutr. 2014, 5, 404. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, G.; Gatti, M.; Bavaresco, L.; Lucini, L. Untargeted Metabolomics to Investigate the Phenolic Composition of Chardonnay Wines from Different Origins. J. Food Compos. Anal. 2018, 71, 87–93. [Google Scholar] [CrossRef]
- Ramirez-Lopez, L.M.; DeWitt, C.A.M. Analysis of Phenolic Compounds in Commercial Dried Grape Pomace by High-Performance Liquid Chromatography Electrospray Ionization Mass Spectrometry. Food Sci. Nutr. 2014, 2, 470. [Google Scholar] [CrossRef]
- Perestrelo, R.; Lu, Y.; Santos, S.A.O.; Silvestre, A.J.D.; Neto, C.P.; Câmara, J.S.; Rocha, S.M. Phenolic Profile of Sercial and Tinta Negra Vitis vinifera L. Grape Skins by HPLC–DAD–ESI-MSn: Novel Phenolic Compounds in Vitis vinifera L. Grape. Food Chem. 2012, 135, 94–104. [Google Scholar] [CrossRef]
- Cuevas-Valenzuela, J.; González-Rojas, Á.; Wisniak, J.; Apelblat, A.; Pérez-Correa, J.R. Solubility of (+)-Catechin in Water and Water-Ethanol Mixtures within the Temperature Range 277.6–331.2 K: Fundamental Data to Design Polyphenol Extraction Processes. Fluid Ph. Equilibria 2014, 382, 279–285. [Google Scholar] [CrossRef]
- Shi, J.; Yu, J.; Pohorly, J.; Young, J.C.; Bryan, M.; Wu, Y.; Canada, A. Optimization of the Extraction of Polyphenols from Grape Seed Meal by Aqueous Ethanol Solution. J. Food Agric. Environ. 2003, 1, 42–47. [Google Scholar]
- Sato, M.; Suzuki, Y.; Okuda, T.; Yokotsuka, K. Contents of Resveratrol, Piceid, and Their Isomers in Commercially Available Wines Made from Grapes Cultivated in Japan. Biosci. Biotechnol. Biochem. 1997, 61, 1800–1805. [Google Scholar] [CrossRef]
- Vian, M.A.; Tomao, V.; Gallet, S.; Coulomb, P.O.; Lacombe, J.M. Simple and Rapid Method for Cis- and Trans-Resveratrol and Piceid Isomers Determination in Wine by High-Performance Liquid Chromatography Using Chromolith Columns. J. Chromatogr. A 2005, 1085, 224–229. [Google Scholar] [CrossRef]
- Gaensly, F.; Agustini, B.C.; da Silva, G.A.; Picheth, G.; Bonfim, T.M.B. Autochthonous Yeasts with β-Glucosidase Activity Increase Resveratrol Concentration during the Alcoholic Fermentation of Vitis Labrusca Grape Must. J. Funct. Foods 2015, 19, 288–295. [Google Scholar] [CrossRef]
- Dadáková, K.; Jurasová, L.; Tomáš Kašparovský, T.; Průšová, B.; Mojmír Baroň, M.; Sochor, J. Origin of Wine Lignans. Mater. Veg. 2021, 1, 3. [Google Scholar] [CrossRef]
- Fuentealba, C.; Figuerola, F.; Estévez, A.M.; González-Munõz, A.; Munõz, O. Optimization of Secoisolariciresinol Diglucoside Extraction from Flaxseed (Linum usitatissimum L.) and Isolation by a Simple HPLC-UV Method. Food 2015, 13, 273–281. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, W.; Meng, Y.; Zhang, Y.; Jin, G.; Fang, Z. Wine Phenolic Profile Altered by Yeast: Mechanisms and Influences. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3579–3619. [Google Scholar] [CrossRef]
- Triyastuti, M.S.; Anwar, N. Bioactive Compounds from Purple Roselle Calyx (Hibiscus sabdariffa L.) extract using multistage countercurrent method. Media Gizi Indones. 2022, 17, 1–10. [Google Scholar] [CrossRef]
- Jiang, G.; Wu, Z.; Ramachandra, K.; Zhao, C.; Ameer, K. Changes in Structural and Chemical Composition of Insoluble Dietary Fibers Bound Phenolic Complexes from Grape Pomace by Alkaline Hydrolysis Treatment. Food Sci. Technol. 2022, 42. [Google Scholar] [CrossRef]


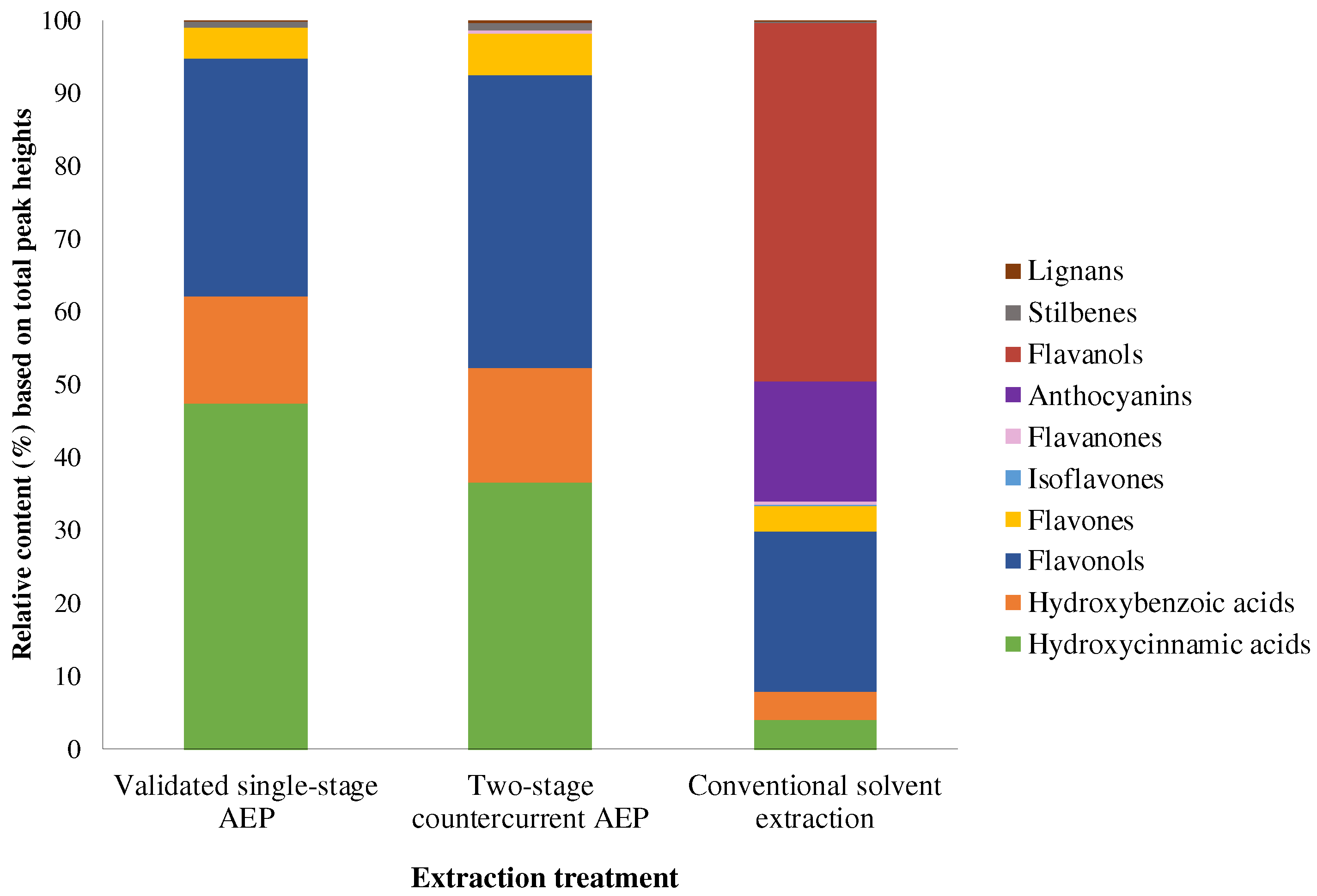
| Validated Single-Stage AEP Conditions | Theoretical Single-Stage AEP at Varying SLR Conditions | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SLR (1:x) | 1:50 | 1:45 | 1:40 | 1:35 | 1:30 | 1:25 | 1:20 | 1:15 | 1:10 | 1:5 |
| Uncoded SLR | 0.020 | 0.022 | 0.025 | 0.029 | 0.033 | 0.040 | 0.050 | 0.070 | 0.100 | 0.200 |
| Coded SLR | −1.68 | −1.64 | −1.59 | −1.52 | −1.43 | −1.31 | −1.12 | −0.802 | −0.187 | 1.68 |
| 10.72 | 10.7 | 10.7 | 10.7 | 10.7 | 10.7 | 10.7 | 10.7 | 10.7 | 10.7 | 10.7 |
| 7.26x1 | 12.2 | 12.2 | 12.2 | 12.2 | 12.2 | 12.2 | 12.2 | 12.2 | 12.2 | 12.2 |
| 4.33x12 | 12.3 | 12.3 | 12.3 | 12.3 | 12.3 | 12.3 | 12.3 | 12.3 | 12.3 | 12.3 |
| −3.41x2 | 5.7 | 5.6 | 5.4 | 5.2 | 4.9 | 4.5 | 3.8 | 2.7 | 0.6 | −5.7 |
| 0.95x3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| −1.73x1x2 | 4.9 | 4.8 | 4.6 | 4.4 | 4.2 | 3.8 | 3.3 | 2.3 | 0.5 | −4.9 |
| Predicted TPC | 45.8 | 45.6 | 45.2 | 44.8 | 44.2 | 43.5 | 42.3 | 40.3 | 36.4 | 24.6 |
| TPC (mg GAE/g Dry Pomace) | ABTS (µmol TE/g Dry Pomace) | ORAC (µmol TE/g Dry Pomace) | ||
|---|---|---|---|---|
| Predicted * | Experimental ** | |||
| Validated single-stage AEP (pH 9.36, 1:50 SLR, 50 °C, 75 min) | 45.8a | 42.9 ± 2.22 bC | 547 ± 44.5 B | 547 ± 44.5 B |
| Two-stage countercurrent extraction (pH 9.36, 1:10 SLR, 50 °C, 45/75 min) | - | 50.5 ± 1.16 B | 930 ± 168.0 B | 930 ± 168.0 B |
| (Conventional solvent extraction) 60% ethanol, 1:10 SLR, 50 °C, 75 min | - | 62.5 ± 1.27 A | 3005 ± 480.1 A | 3005 ± 480.1 A |
| Concentration (µg/g Dry Pomace) | |||
|---|---|---|---|
| Compound | Validated Single-Stage AEP | Two-Stage Countercurrent AEP | Conventional Solvent Extraction |
| Gallic acid | 3.47 ± 0.82 b | 5.78 ± 1.72 b | 34.57 ± 2.66 a |
| Protocatechuic acid | 21.94 ± 1.09 a | 23.73 ± 0.15 a | 11.57 ± 1.75 b |
| (+)-Catechin | 204.21 ± 9.41 b | 320.13 ± 15.5 a | 308.1 ± 28.52 a |
| Syringic acid | 414.2 ± 14.77 a | 332.08 ± 21.36 b | 14.34 ± 3.63 c |
| (-)-Epicatechin | not detected | not detected | 233.29 ± 58.18 |
| p-Coumaric acid | 15.38 ± 0.67 b | 18.13 ± 0.82 a | not detected |
| Ferulic acid | 10.26 ± 0.67 b | 20.64 ± 2.37 a | not detected |
| Malvidin-3-O-glucoside | not detected | not detected | 2287.45 ± 135.63 |
| (-)-Epicatechin gallate | not detected | not detected | 79.06 ± 4.09 |
| Quercetin-3-O-glucuronide | 23.24 ± 1.68 c | 37.87 ± 2.67 b | 142.94 ± 16.72 a |
| Naringenin-7-O-glucoside | not detected | 71.64 ± 1.6 b | 117.15 ± 28.73 a |
| Isorhamnetin-3-O-glucoside | 78.41 ± 6.91 a | 47.73 ± 7.87 b | 62.54 ± 10.48 a |
| Polymeric phenols | 2698.75 ± 241.59 b | 3277.18 ± 63.83 a | 2134.69 ± 45.20 c |
| Polymeric pigments | 456.85 ± 48.61 a | 404.24 ± 45.34 a | 117.22 ± 171.78 b |
| Total | 3926.71 ± 321.22 c | 4559.14 ± 156.23 b | 5542.94 ± 462.17 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinton, S.; Furlan Goncalves Dias, F.; Lerno, L.A.; Barile, D.; Leite Nobrega de Moura Bell, J.M. Revitalizing Unfermented Cabernet Sauvignon Pomace Using an Eco-Friendly, Two-Stage Countercurrent Process: Role of pH on the Extractability of Bioactive Phenolics. Processes 2022, 10, 2093. https://doi.org/10.3390/pr10102093
Pinton S, Furlan Goncalves Dias F, Lerno LA, Barile D, Leite Nobrega de Moura Bell JM. Revitalizing Unfermented Cabernet Sauvignon Pomace Using an Eco-Friendly, Two-Stage Countercurrent Process: Role of pH on the Extractability of Bioactive Phenolics. Processes. 2022; 10(10):2093. https://doi.org/10.3390/pr10102093
Chicago/Turabian StylePinton, Sophia, Fernanda Furlan Goncalves Dias, Larry A. Lerno, Daniela Barile, and Juliana Maria Leite Nobrega de Moura Bell. 2022. "Revitalizing Unfermented Cabernet Sauvignon Pomace Using an Eco-Friendly, Two-Stage Countercurrent Process: Role of pH on the Extractability of Bioactive Phenolics" Processes 10, no. 10: 2093. https://doi.org/10.3390/pr10102093
APA StylePinton, S., Furlan Goncalves Dias, F., Lerno, L. A., Barile, D., & Leite Nobrega de Moura Bell, J. M. (2022). Revitalizing Unfermented Cabernet Sauvignon Pomace Using an Eco-Friendly, Two-Stage Countercurrent Process: Role of pH on the Extractability of Bioactive Phenolics. Processes, 10(10), 2093. https://doi.org/10.3390/pr10102093