Optimization and Potentials of Kraft Lignin Hydrolysates Obtained by Subcritical Water at Moderate Temperatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Chemicals and Reagents
2.3. Hydrothermal Treatment of Lignin
2.4. Determination of Total Phenols Content
2.5. Determination of Other Aromatics
2.6. Determination of Total Antioxidant Capacity
3. Results
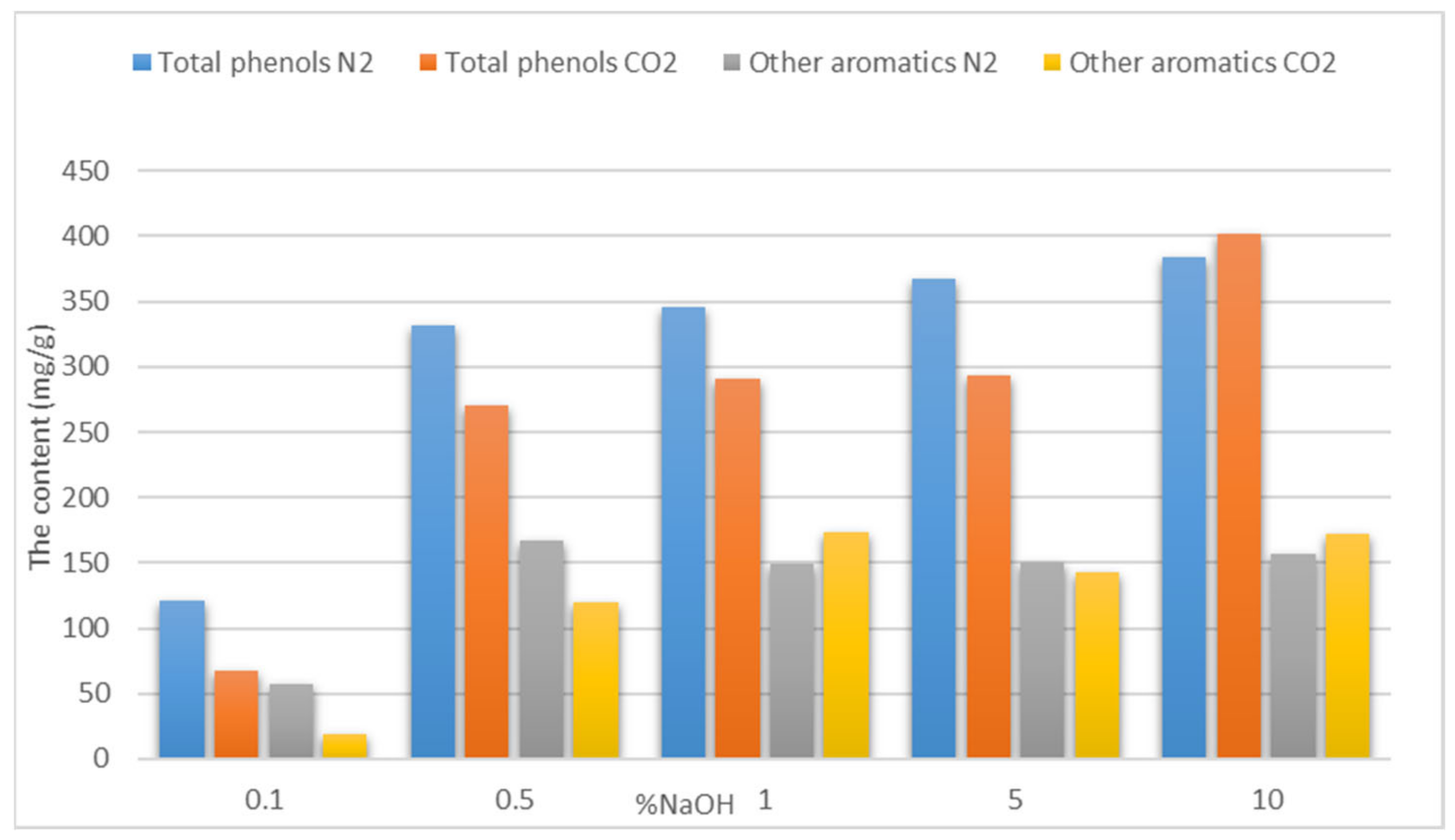
3.1. The Influence of the Catalyst
The Influence of the Catalyst Concentration
3.2. Optimization of Lignin Depolymerization by Subcritical Water
3.2.1. Optimization of the Temperature
3.2.2. Optimization of the Treatment Time
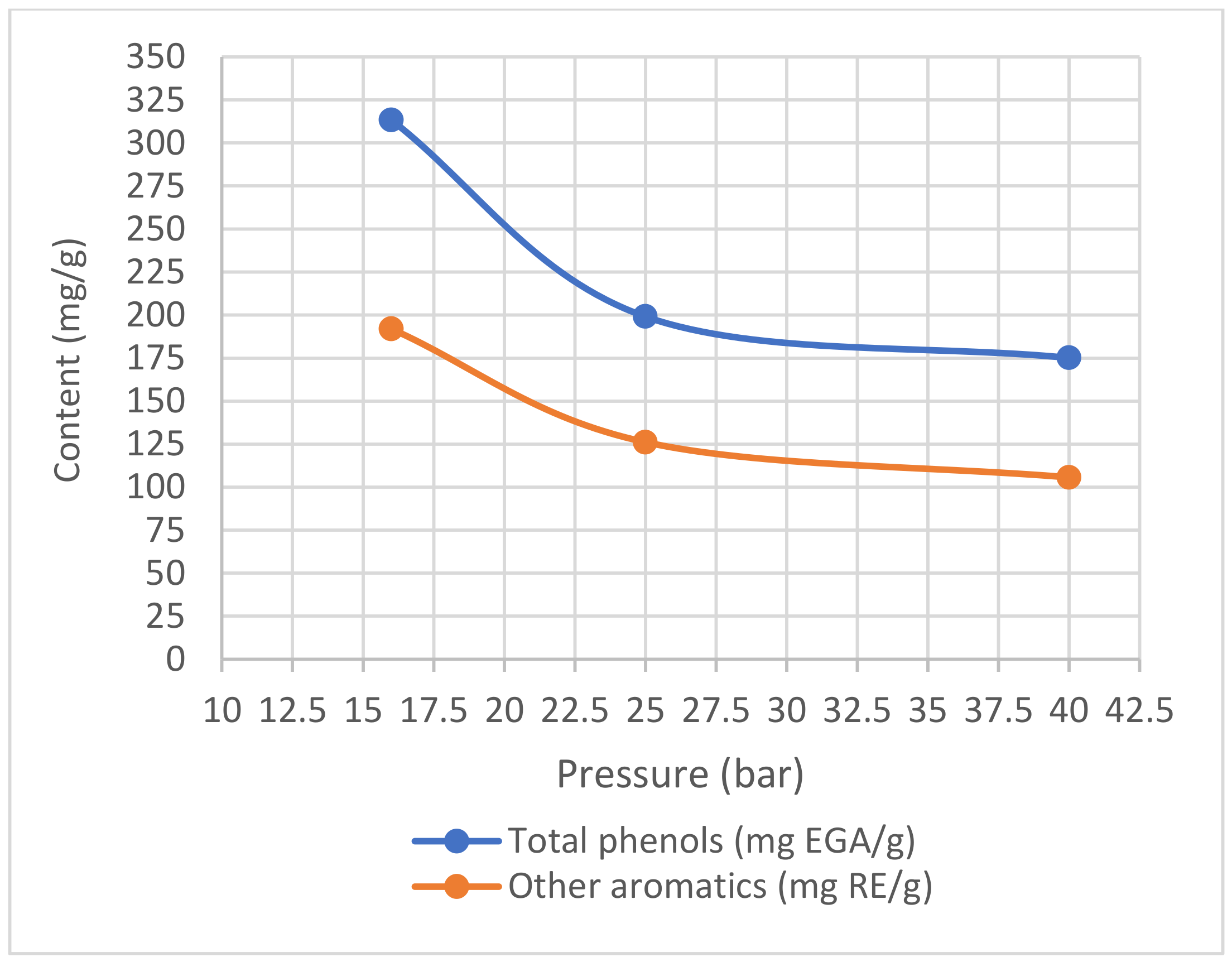
3.2.3. Pressure Optimization
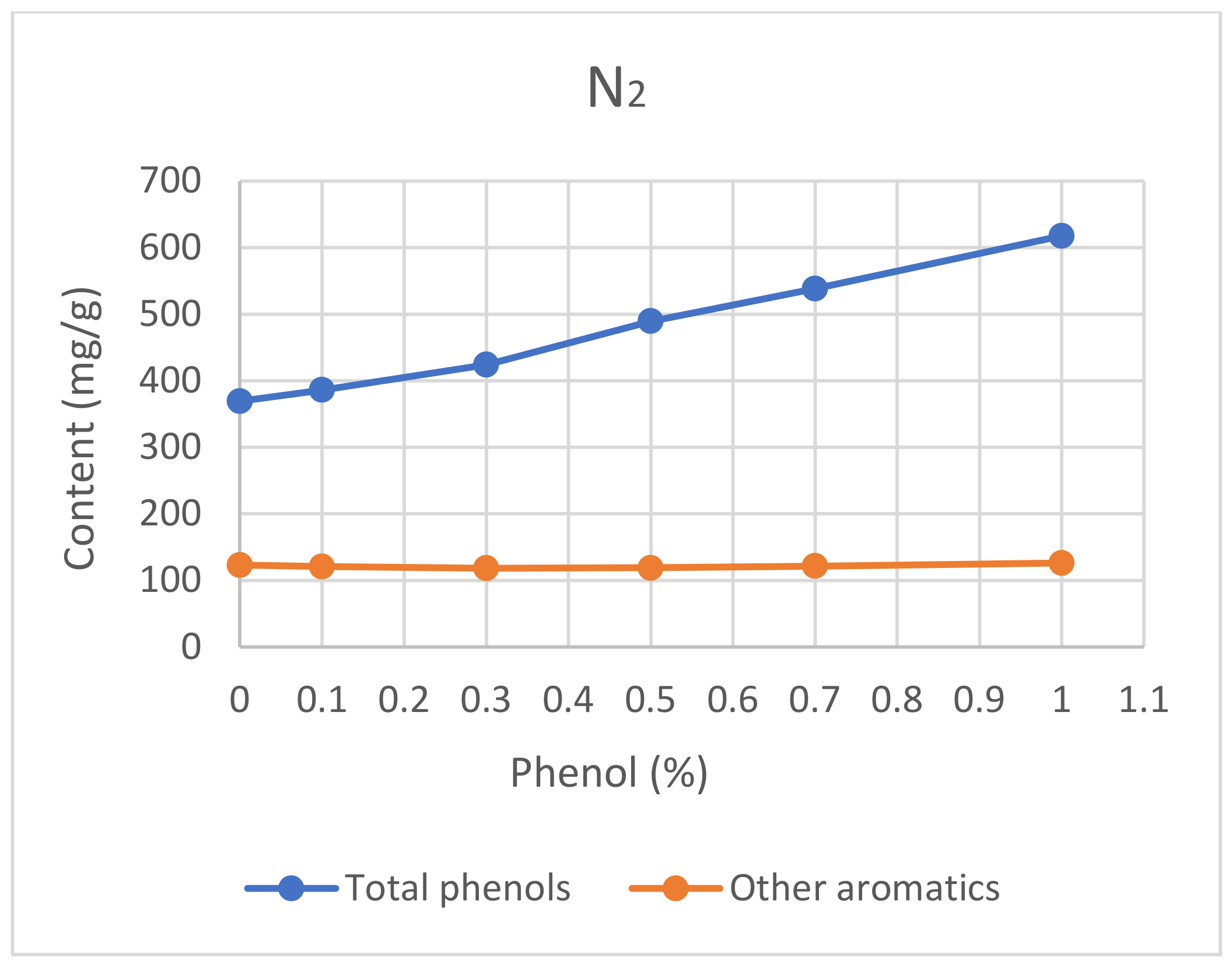
3.2.4. Capping Agent
4. Total Antioxidant Activity
5. Discussion
5.1. The Influence of the Catalyst
The Influence of the Catalyst Concentration
5.2. Optimization of Lignin Depolymerization by Subcritical Water
5.2.1. Optimization of the Temperature
5.2.2. Optimization of the Treatment Time
5.2.3. Pressure Optimization
5.2.4. Capping Agent
6. Antioxidant Activity
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Negi, H.; Singh, R.K. A review on lignin utilization in petroleum exploration, petroleum products formulation, bio-fuel production, and oil spill clean-up. Biomass Conv. 2020. [Google Scholar] [CrossRef]
- Yao, H.; Wang, Y.; Liu, J.; Xu, M.; Ma, P.; Ji, J.; You, Z. Review on Applications of Lignin in Pavement Engineering: A Recent Survey. Front. Matter. 2022, 8, 803524. [Google Scholar] [CrossRef]
- Kus, N.S. Organic reactions in subcritical and supercritical water. Tetrahedron 2012, 68, 949–958. [Google Scholar]
- Švarc-Gajić, J. Sampling and Sample Preparation in Analytical Chemistry; Nova Science Publishers: New York, NY, USA, 2012. [Google Scholar]
- Iwamura, H.; Sato, T.; Okada, M.; Sue, K.; Hiaki, T. Organic Reactions in Sub- and Supercritical Water in the Absence of Any Added Catalyst. J. Res. Inst. Sci. Tech. 2014, 132, 1–9. [Google Scholar] [CrossRef]
- Belkheiri, T.; Andersson, S.I.; Mattsson, C.; Olausson, L.; Theliander, H.; Vamling, L. Hydrothermal liquefaction of kraft lignin in sub-critical water: The influence of the sodium and potassium fraction. Biomass Conv. Bioref. 2018, 8, 585–595. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.N.; Taki, G.; Rana, M.; Park, J.H. Yield of Phenolic Monomers from Lignin Hydrothermolysis in Subcritical Water System. Ind. Eng. Chem. Res. 2018, 57, 4779–4784. [Google Scholar] [CrossRef]
- Balawanthrao, J. Screening of Catalysts for the Subcritical Water Depolymerization of Lignin. In Electronic Theses and Dissertations; South Dakota State University: Brookings, SD, USA, 2020. [Google Scholar]
- Dell’Orco, S.; Miliotti, E.; Lotti, G.; Rizzo, A.M.; Rosi, L.; Chiaramonti, D. Hydrothermal Depolymerization of Biorefinery Lignin-Rich Streams: Influence of Reaction Conditions and Catalytic Additives on the Organic Monomers Yields in Biocrude and Aqueous Phase. Energies 2020, 13, 1241. [Google Scholar] [CrossRef] [Green Version]
- Ivakhnov, A.D.; Ul’yanovskii, S.A.; Pokryshkin, I.S.; Shavrina, I.S.; Pikovskoi, I.I.; Kosyakov, D.S. Promissing solvents for lignin depolymerisation: Stability under supercritical conditiona. Russ. J. Phys. Chem. 2018, 13, 1147–1149. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.; Islam, M.N.; Agarwal, A.; Taki, G.; Park, S.J.; Dong, S.; Jo, Y.T.; Park, J.H. Production of Phenol-Rich Monomers from Kraft Lignin Hydrothermolysates in Basic-Subcritical Water over MoO3/SBA-15 Catalyst. Energy Fuels 2018, 32, 11564–11575. [Google Scholar] [CrossRef]
- Jiang, W.; Lyu, G.; Wu, S.; Lucia, L.A.; Yang, G.; Liu, Y. Supercritical water-induced lignin decomposition reactions: A structural and quantitative study. Bioresources 2016, 11, 5660–5675. [Google Scholar] [CrossRef] [Green Version]
- Pandey, M.P.; Kim, C.S. Lignin depolymerization and conversion: A review of thermochemical methods. Chem. Eng. Technol. 2011, 34, 29–41. [Google Scholar] [CrossRef]
- Pérez, E.; Tuck, C.O. Quantitative analysis of products from lignin depolymerisation in high temperature water. Eur. Polym. J. 2018, 99, 38–48. [Google Scholar] [CrossRef]
- Pérez, E.; Tuck, C.O.; Poliakoff, M. Valorisation of lignin by depolymerisation and fractionation using supercritical fluids and conventional solvents. J. Supercrit. Fluids. 2018, 133, 690–695. [Google Scholar] [CrossRef]
- Mauriello, F.; Paone, E.; Pietropaolo, R.; Balu, A.; Luque, R. Catalytic transfer hydrogenolysis of lignin-derived aromatic ethers promoted by bimetallic Pd/Ni systems. ACS Sustain. Chem. Eng. 2018, 6, 9269–9276. [Google Scholar] [CrossRef]
- Hu, Y.; Jiang, G.; Xu, G.; Mu, X. Hydrogenolysis of lignin model compounds into aromatics with bimetallic Ru-Ni supported onto nitrogen-doped activated carbon catalyst. Mol. Catal. 2018, 445, 316–326. [Google Scholar] [CrossRef]
- Kong, X.; Chen, L. Hydrogenation of aromatic aldehydes to aromatic hydrocarbons over Cu-HZSM-5 catalyst. Catal. Commun. 2014, 57, 45–49. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Mimura, N.; Segawa, A.; Mazaki, H.; Sato, O. Lignin depolymerisation into aromatic monomers using supported metal cacalysts in supercritical water. J. Jpn. Pet. Inst. 2020, 63, 221–227. [Google Scholar] [CrossRef]
- Güvenatam, B.; Heeres, E.; Pidko, E.; Hensen, E. Lewis-acid catalyzed depolymerization of Protobind lignin in supercritical water and ethanol. Catal. Today 2016, 259, 460–466. [Google Scholar] [CrossRef]
- Kong, X.; Li, W.; Li, X.; Liu, L.; Geng, W.; Liu, L. Hydrothermal depolymerization of alkali lignin to high-yield monomers over nickel nitrate modified commercial hydrotalcites catalyst. J. Energy Inst. 2020, 93, 658–665. [Google Scholar] [CrossRef]
- Švarc-Gajić, J.; Cvetanović, A.; Segura-Carretero, A.; Linares, I.B.; Mašković, P. Characterisation of Ginger Extracts Obtained by Subcritical Water. J. Supercrit. Fluids 2017, 123, 92–100. [Google Scholar] [CrossRef]
- Cvetanović, A.; Švarc-Gajić, J.; Zeković, Z.; Gašić, U.; Tešić, Ž.; Zengin, G.; Mašković, P.; Mahomoodally, M.F.; Đurović, S. Subcritical water extraction as a cutting edge technology for the extraction of bioactive compounds from chamomile: Influence of pressure on chemical composition and bioactivity of extracts. Food Chem. 2018, 266, 389–396. [Google Scholar] [CrossRef]
- Okuda, K.; Umetsu, M.; Takami, S.; Adschiri, T. Disassembly of lignin and chemical recovery—Rapid depolymerisation of lignin without char formation in water-phenol mixtures. Fuel Process. Technol. 2021, 85, 803–813. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Pan, X.; Kadla, J.; Ehara, K.; Gilkes, N.; Saddler, J. Organosolv ethanol lignin from hybrid poplar as a radical scavenger: Relationship between lignin structure, extraction conditions, and antioxidant activity. J. Agric. Food Chem. 2006, 54, 5806–5813. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Liu, W.; Huang, J.; Lou, H.; Qiu, H. Study on the Antioxidant Activity of Lignin and Its Application Performance in SBS Elastomer. Ind. Eng. Chem. Res. 2021, 60, 790–797. [Google Scholar] [CrossRef]
- Švarc-Gajić, J.; Cerdà, V.; Clavijo, S.; Suárez, R.; Mašković, P.; Cvetanović, A.; Delerue-Matos, C.; Carvalho, A.; Novakov, V. Bioactive compounds of sweet and sour cherry stems obtained by subcritical water extraction. J. Chem. Techn. Biotechn. 2017, 93, 1627–1635. [Google Scholar] [CrossRef] [Green Version]
- Švarc-Gajić, J.; Cerdà, V.; Delerue-Matos, C.; Mašković, P.; Clavijo, S.; Suarez, R.; Cvetanović, A.; João Ramalhosa, M.; Barroso, F.; Moreira, M.; et al. Chemical characterization and in vitro bioactivity of apple bark extracts obtained by subcritical water. Waste Biomass Valori. 2021, 12, 6781–6794. [Google Scholar] [CrossRef]
- Aspé, E.; Fernández, K. The effect of different extraction techniques on extraction yield, total phenolic, and anti-radical capacity of extracts from Pinus radiata bark. Ind. Crop. Prod. 2011, 34, 838–844. [Google Scholar] [CrossRef]
- Ku, C.S.; Jang, J.P.; Mun, S.P. Exploitation of polyphenol-rich pine barks for potent antioxidant activity. J. Wood Sci. 2007, 53, 524–528. [Google Scholar] [CrossRef]
- Moreira, M.M.; Barroso, M.F.; Boeykens, A.; Withouck, H.; Morais, S.; Delerue-Matos, C. Valorization of apple tree wood residues by polyphenols extraction: Comparison between conventional and microwave-assisted extraction. Ind. Crop. Prod. 2017, 104, 210–220. [Google Scholar] [CrossRef] [Green Version]
- Lamounier, K.C.; Cunha, L.C.S.; de Morais, S.A.L.; de Aquino, F.J.T.; Chang, R.; do Nascimento, E.A.; de Souza, M.G.M.; Martins, C.H.G.; Cunha, W.R. Chemical analysis and study of phenolics, antioxidant activity, and antibacterial effect of the wood and bark of Maclura tinctoria (L.) D. Don ex Steud. Altern. Med. 2012, 2012, 451039. [Google Scholar]
- Hartonen, K.; Parshintsev, J.; Sandberg, K.; Bergelin, E.; Nisula, L.; Riekkola, M.L. Isolation of flavonoids from aspen knotwood by pressurized hot water extraction and comparison with other extraction techniques. Talanta 2007, 74, 32–38. [Google Scholar] [CrossRef]
- Abdelaziz, O.Y.; Li, K.; Tunå, P. Continuous catalytic depolymerisation and conversion of industrial kraft lignin into low-molecular-weight aromatics. Biomass Conv. Bioref. 2018, 8, 455–470. [Google Scholar] [CrossRef] [Green Version]
- Abad-Fernández, N.; Pérez, E.; Cocero, M.J. Aromatics from lignin through ultrafast reactions in water. Green Chem. 2019, 21, 1351–1360. [Google Scholar] [CrossRef] [Green Version]
- Yong, T.L.K.; Matsumura, Y. Reaction Kinetics of the Lignin Conversion in Supercritical Water. Ind. Eng. Chem. Res. 2012, 51, 11975–11988. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Li, X.H.; Wu, S.B.; Li, Y.M. Temperature impact on the hydrothermal depolymerisation of Cunninghamia lanceolata enzymatic/mild acidolysis lignin in subcritical water. Bioresources 2016, 11, 21–32. [Google Scholar]
- Erdocia, X.; Prado, R.; Corcuera, M.Á.; Labidi, J. Influence of reaction conditions on lignin hydrothermal treatment. Front. Energy Res. 2014, 2, 13. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Korányi, T.I.; Bootb, M.D.; Hensen, E.L.M. Ethanol as capping agent and formaldehyde scavenger for efficient depolymerization of lignin to aromatics. Green Chem. 2015, 17, 4941–4950. [Google Scholar] [CrossRef] [Green Version]
- Ahlbom, A.; Maschietti, M.; Nielsen, R.; Lyckeskog, H.; Hasani, M.; Theliander, H. Using Isopropanol as a Capping Agent in the Hydrothermal Liquefaction of Kraft Lignin in Near-Critical Water. Energies 2021, 14, 932. [Google Scholar] [CrossRef]
- Qazi, S.S.; Li, D.; Briens, C.; Berruti, F.; Abou-Zaid, M.M. Antioxidant Activity of the Lignins Derived from Fluidized-Bed Fast Pyrolysis. Molecules 1999, 22, 372. [Google Scholar] [CrossRef] [Green Version]
- Juárez, M.; Ángel, L.; Quijada, M.; Dulce, M.A.; Sánchez, T.; Carmen, L.; Aguilar, G.; Gámez, G.; Nohemí, M. Antioxidant activity of peppers (Capsicum annuum L.) extracts and characterization of their phenolic constituents. Interciencia 2012, 37, 588–593. [Google Scholar]
- Nurlinda, K.; Handayani, V.; Rasyid, F.A. Spectrophotometric Determination of Total Flavonoid Content in Biancaea Sappan (Caesalpinia sappan L.) Leaves. J. Fitofarmaka Indones. 2021, 8, 3. [Google Scholar] [CrossRef]



| Total Phenols (mg GAE/g ± 2SD) | ||
|---|---|---|
| Catalyst | N2 | CO2 |
| Without catalyst | 35.30 ± 0.31 | 42.25 ± 0.63 |
| 0.5% Phenol | 153.09 ± 1.29 | 168.48 ±0.34 |
| 1% HCl | 112.85 ± 0.27 | 122.82 ± 0.28 |
| 1% NaOH | 336.07 ± 1.05 | 240.81 ± 2.95 |
| 0.2% Zeolite | 34.52 ± 0.03 | 35.30 ± 0.41 |
| 0.2% TiO2 | 35.55 ± 0.51 | 37.52 ± 0.69 |
| 0.2% ZrO2 | 34.72 ± 0.70 | 45.01 ± 0.73 |
| 0.2% CeO2 | 31.20 ± 0.25 | 35.44 ± 0.13 |
| 0.2% (CeO2-ZrO2 = 80:20) | 33.59 ± 0.88 | 34.30 ± 0.30 |
| T (°C) | Total Phenols ± 2SD * (mg GAE/g) | Other Aromatics ± 2SD (mg RE/g) | ||
|---|---|---|---|---|
| N2 | CO2 | N2 | CO2 | |
| 120 | 341.88 ± 0.71 | 164.88 ± 1.40 | 114.29 ± 0.54 | 58.48 ± 1.15 |
| 150 | 426.01 ± 0.35 | 249.00 ± 0.61 | 209.43 ± 4.41 | 125.21 ± 0.32 |
| 180 | 336.07 ± 1.05 | 240.81 ± 2.95 | 173.43 ± 0.80 | 103.06 ± 0.91 |
| 200 | 387.70 ± 0.36 | 317.54 ± 1.31 | 160.41 ± 0.00 | 205.67 ± 3.03 |
| 220 | 399.95 ± 2.49 | 289.31 ± 3.06 | 160.06 ± 0.37 | 193.65 ± 2.18 |
| t (min) | Total Phenols (mg EGA/g) | Other Aromatics (mg RE/g) | ||
|---|---|---|---|---|
| N2 (150 °C) | CO2 (200 °C) | N2 (150 °C) | CO2 (200 °C) | |
| 30 | 349.75 ± 0.83 | 287.52 ± 1.24 | 156.97 ± 1.10 | 165.85 ± 1.45 |
| 40 | 350.13 ± 0.83 | 279.19 ± 3.31 | 156.29 ± 2.24 | 172.64 ± 2.04 |
| 60 | 426.01 ± 0.35 | 317.54 ± 1.31 | 209.43 ± 4.41 | 205.67 ± 3.03 |
| 90 | 327.25 ± 0.84 | 294.62 ± 1.71 | 140.30 ± 1.34 | 207.63 ± 3.16 |
| 120 | 332.10 ± 0.42 | 265.38 ± 3.14 | 136.87 ± 0.54 | 187.37 ± 3.89 |
| t (°C) | mg AAE/g ± 2SD | |
|---|---|---|
| N2 | CO2 | |
| 120 | 633.69 ± 3.58 | 371.21 ± 5.39 |
| 150 | 700.42 ± 3.48 | 574.11 ± 1.72 |
| 180 | 544.44 ± 21.28 | 476.77 ± 6.36 |
| 200 | 608.18 ± 7.21 | 548.34 ± 2.28 |
| 220 | 604.82 ± 0.00 | 483.00 ± 10.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Švarc-Gajić, J.; Brezo-Borjan, T.; Gosselink, R.J.A.; Slaghek, T.M.; Šojić-Merkulov, D.; Ivetić, T.; Bognár, S.; Stojanović, Z. Optimization and Potentials of Kraft Lignin Hydrolysates Obtained by Subcritical Water at Moderate Temperatures. Processes 2022, 10, 2049. https://doi.org/10.3390/pr10102049
Švarc-Gajić J, Brezo-Borjan T, Gosselink RJA, Slaghek TM, Šojić-Merkulov D, Ivetić T, Bognár S, Stojanović Z. Optimization and Potentials of Kraft Lignin Hydrolysates Obtained by Subcritical Water at Moderate Temperatures. Processes. 2022; 10(10):2049. https://doi.org/10.3390/pr10102049
Chicago/Turabian StyleŠvarc-Gajić, Jaroslava, Tanja Brezo-Borjan, Richard J. A. Gosselink, Ted M. Slaghek, Daniela Šojić-Merkulov, Tamara Ivetić, Szabolcs Bognár, and Zorica Stojanović. 2022. "Optimization and Potentials of Kraft Lignin Hydrolysates Obtained by Subcritical Water at Moderate Temperatures" Processes 10, no. 10: 2049. https://doi.org/10.3390/pr10102049
APA StyleŠvarc-Gajić, J., Brezo-Borjan, T., Gosselink, R. J. A., Slaghek, T. M., Šojić-Merkulov, D., Ivetić, T., Bognár, S., & Stojanović, Z. (2022). Optimization and Potentials of Kraft Lignin Hydrolysates Obtained by Subcritical Water at Moderate Temperatures. Processes, 10(10), 2049. https://doi.org/10.3390/pr10102049











