Antibacterial, Antifungal, and Antibiotic Adsorption Properties of Graphene-Modified Nonwoven Materials for Application in Wastewater Treatment Plants
Abstract
1. Introduction
2. Experimental
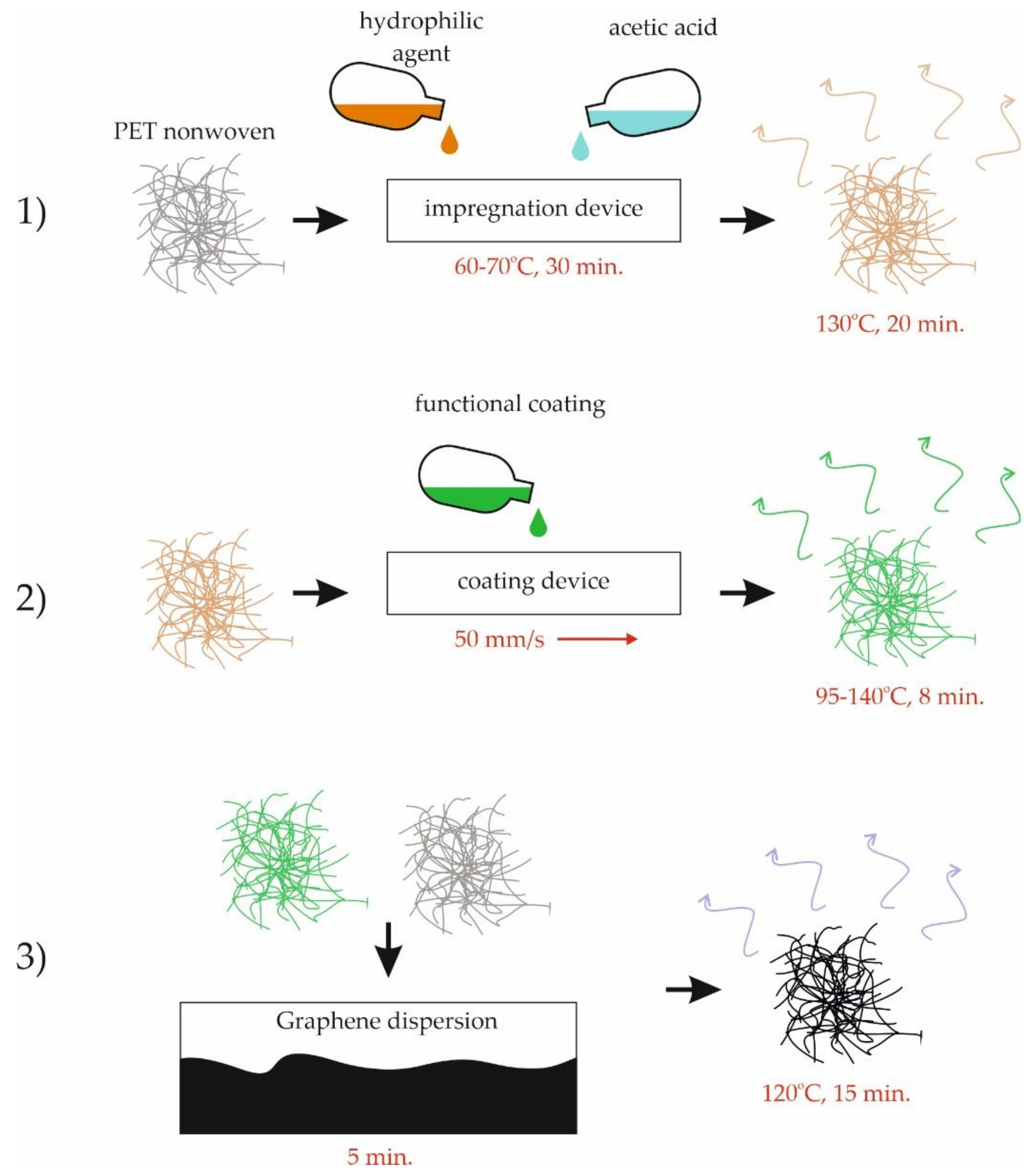
2.1. Fabrication of the Graphene-Modified Nonwoven Materials and Materials Used
2.2. Characterization of Nonwoven Morphology
2.3. Determination of Antibacterial and Antifungal Properties
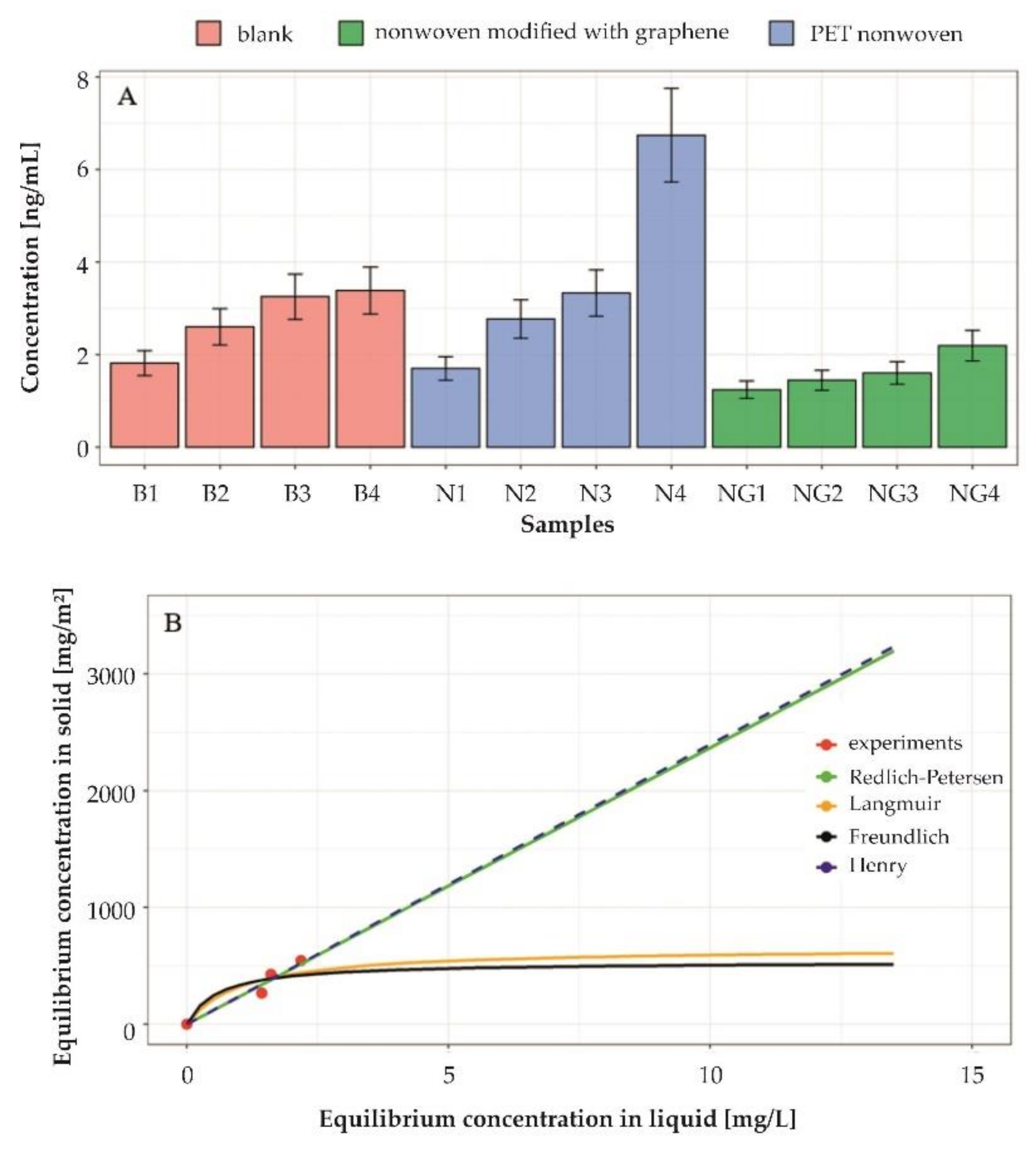
2.4. Evaluation of Adsorption Properties
3. Results and Discussion
3.1. Quantification of Fiber Morphology via SEM and Digital Optical Microscopy
3.2. Pore Distributions of Nonwoven Materials
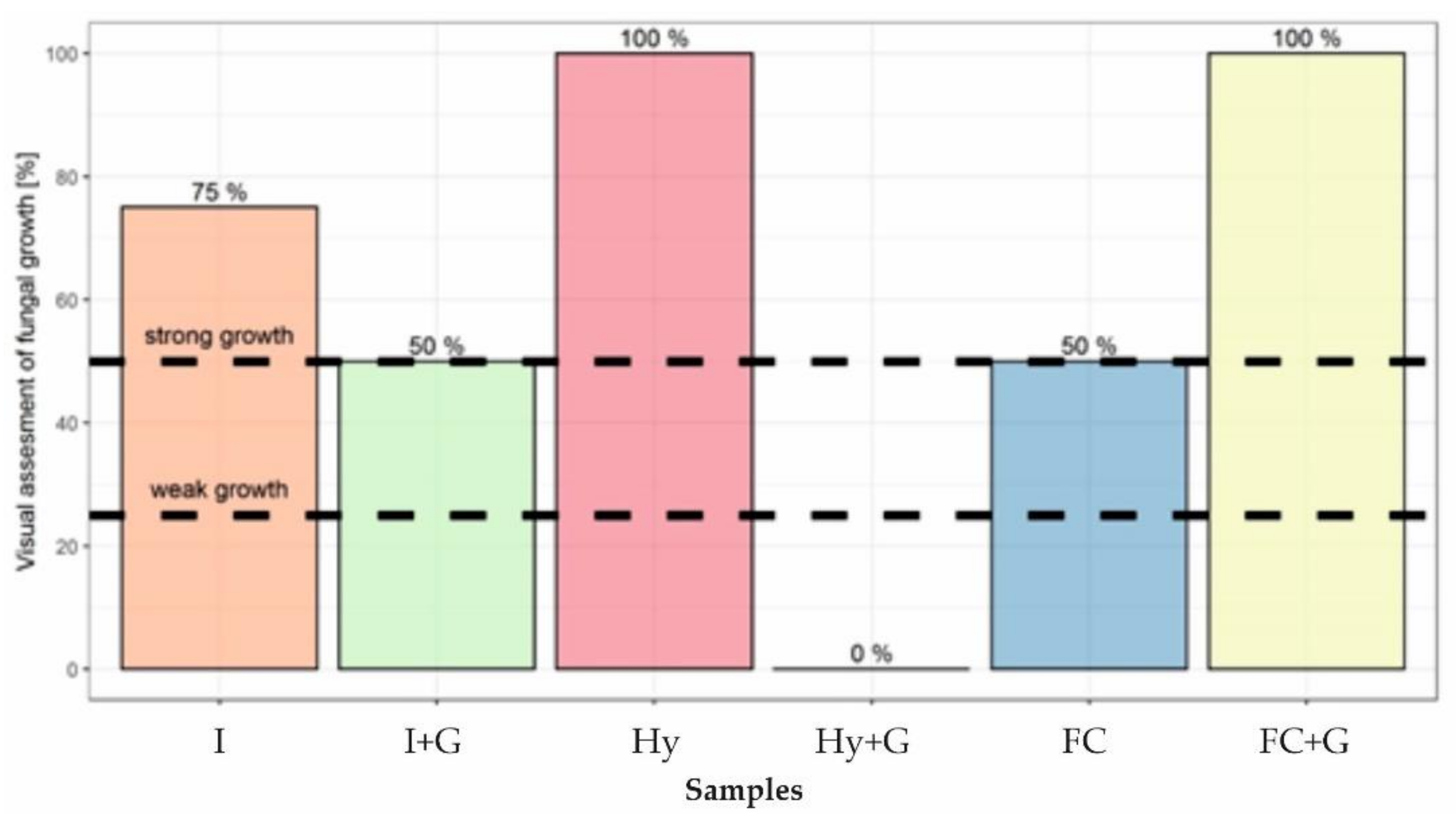
3.3. Antibacterial and Antifungal Properties
3.4. Adsorption Properties of Graphene-Modified Nonwovens
4. Conclusions
- The experimental GMN systems, which we present in this study, behave in agreement with statistical patterns—namely, logistic and lognormal fittings to describe the histograms of fiber diameter distributions;
- For the industrial design of graphene-modified nonwovens, several strategies and textile auxiliaries may be considered, which allows us to state that the statistical classification strategy is a fast and feasible strategy for an initial screening of the prioritized structure–properties relationships;
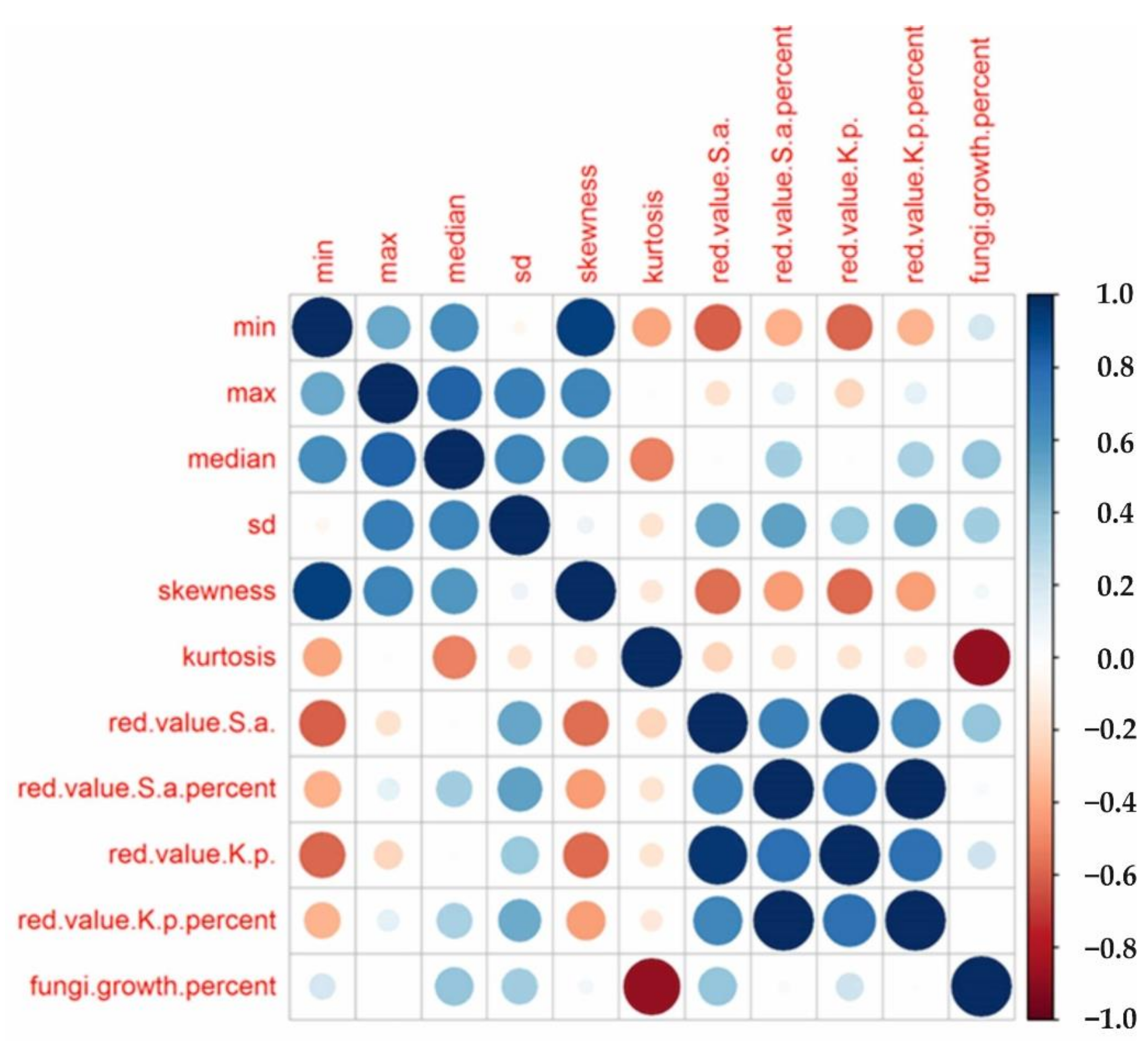
- The basic statistical classification of nonwoven morphology in two separate groups (i.e., different skewness and kurtosis parameters) allows us to reveal some correlations between kurtosis and fungi growth, indicating that physical mechanistic pathways exhibit a dominant role in fungi growth;
- The morphological analysis did not allow us to correlate bacterial growth with the structural features of the two nonwoven classes. As a result, we conclude that chemical mechanisms may be more pronounced in inactivating bacteria;
- Both different bacteria and fungi are important for fouling processes in MBR. Further research on optimizing the statistical analysis (e.g., machine learning algorithms) of morphology-related data and the correlation of those data to a broad range of microorganisms should be done for a fundamental and all-embracing understanding of biological fouling processes;
- It was concluded that Henry and Redlich–Petersen isotherms describe the tetracycline adsorption process by graphene-modified nonwovens in a suitable way. The obtained results are of significant practical importance for wastewater treatment engineering combined with the adsorption process;
- Data provided in this study will help in the effective initial tailoring of adsorptive nonwovens for applications in sewage plants.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
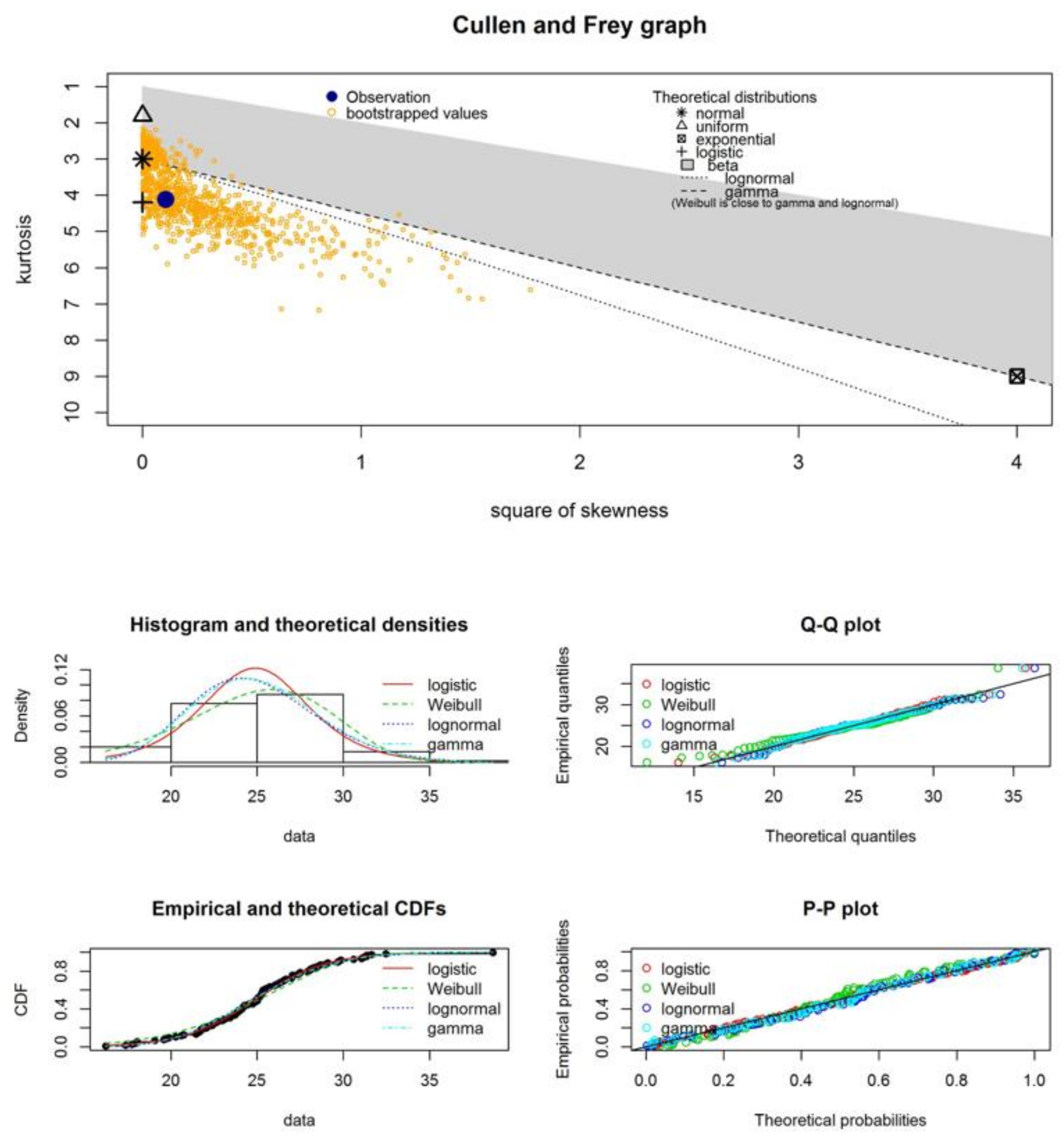
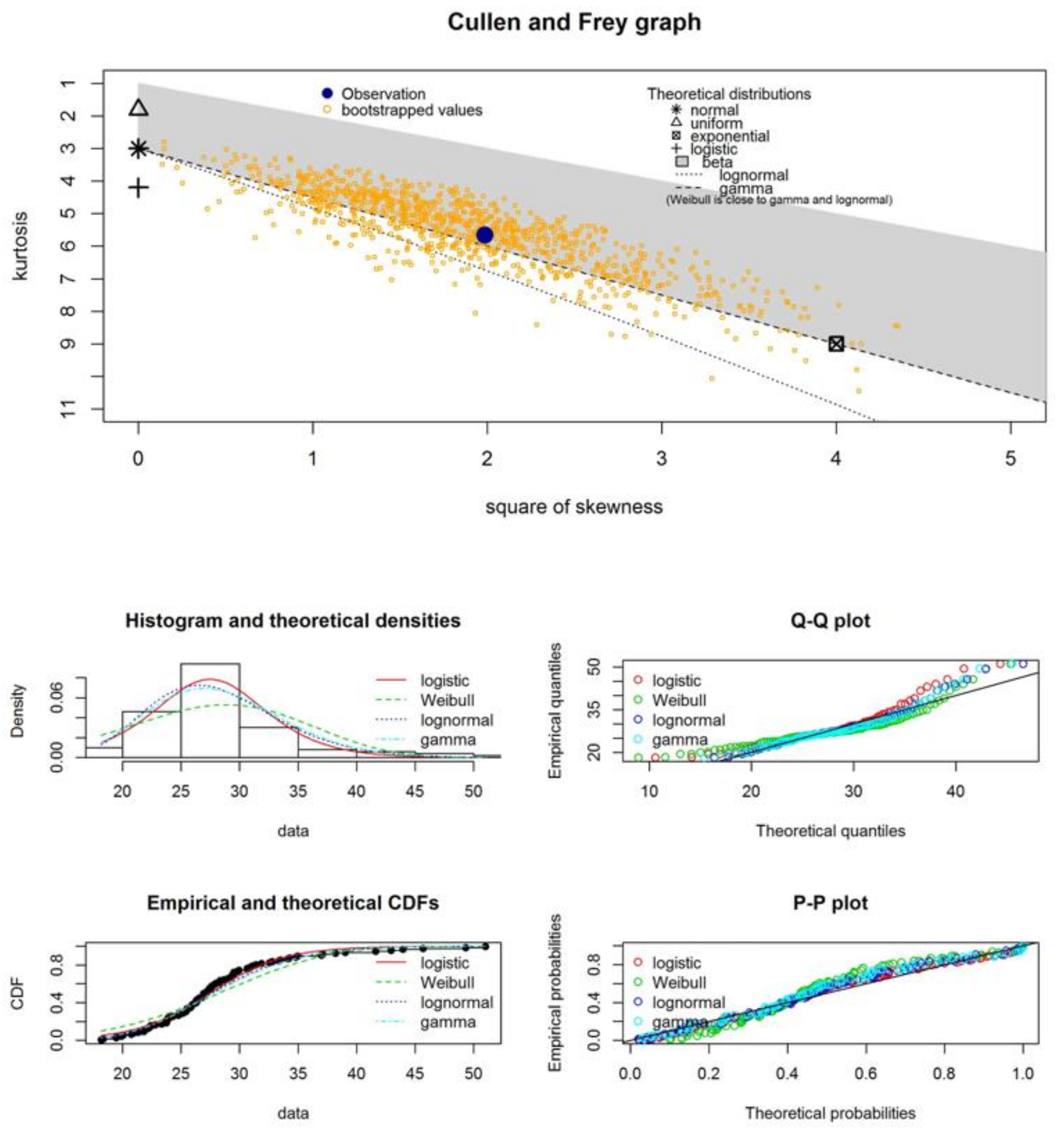
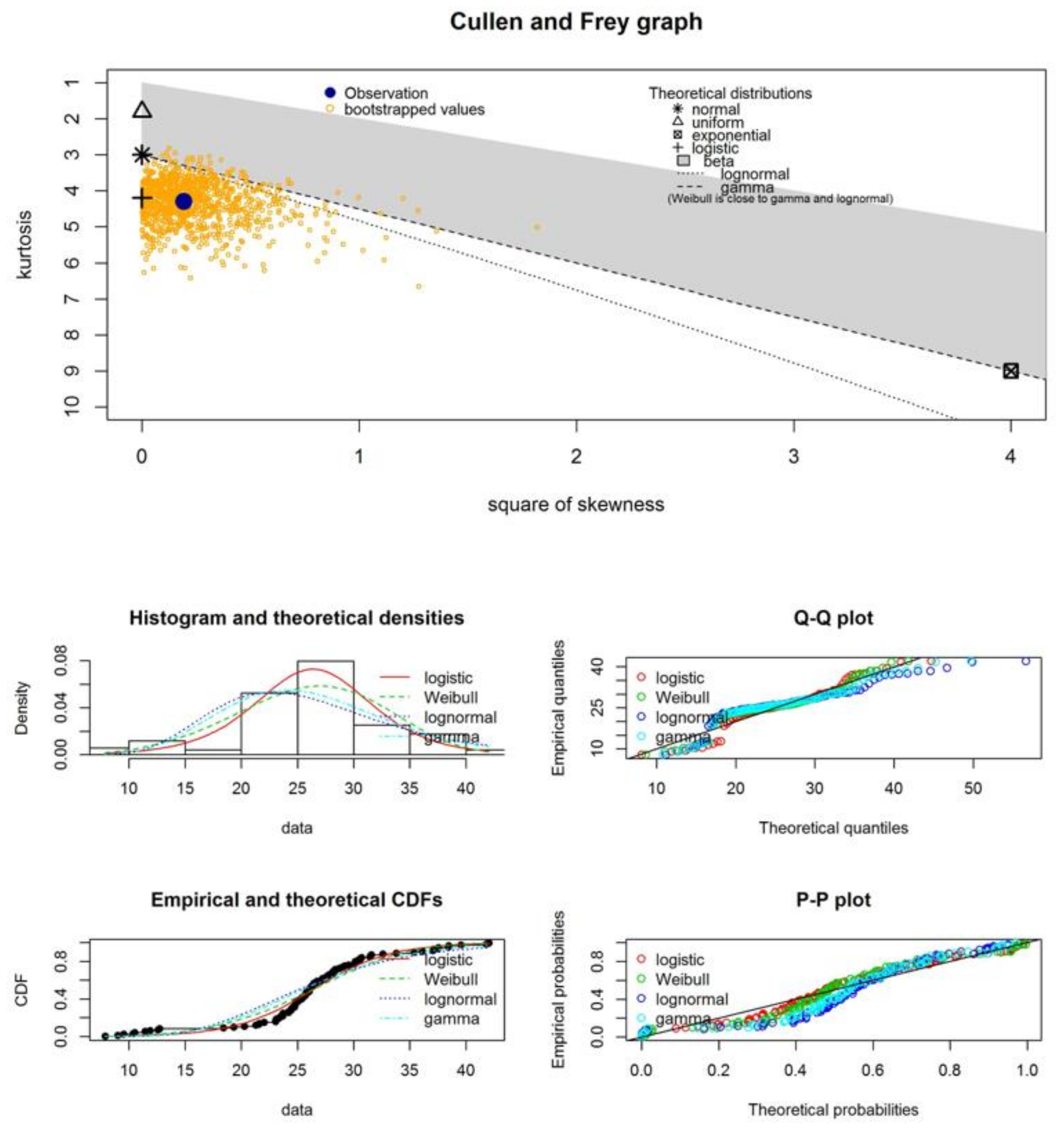
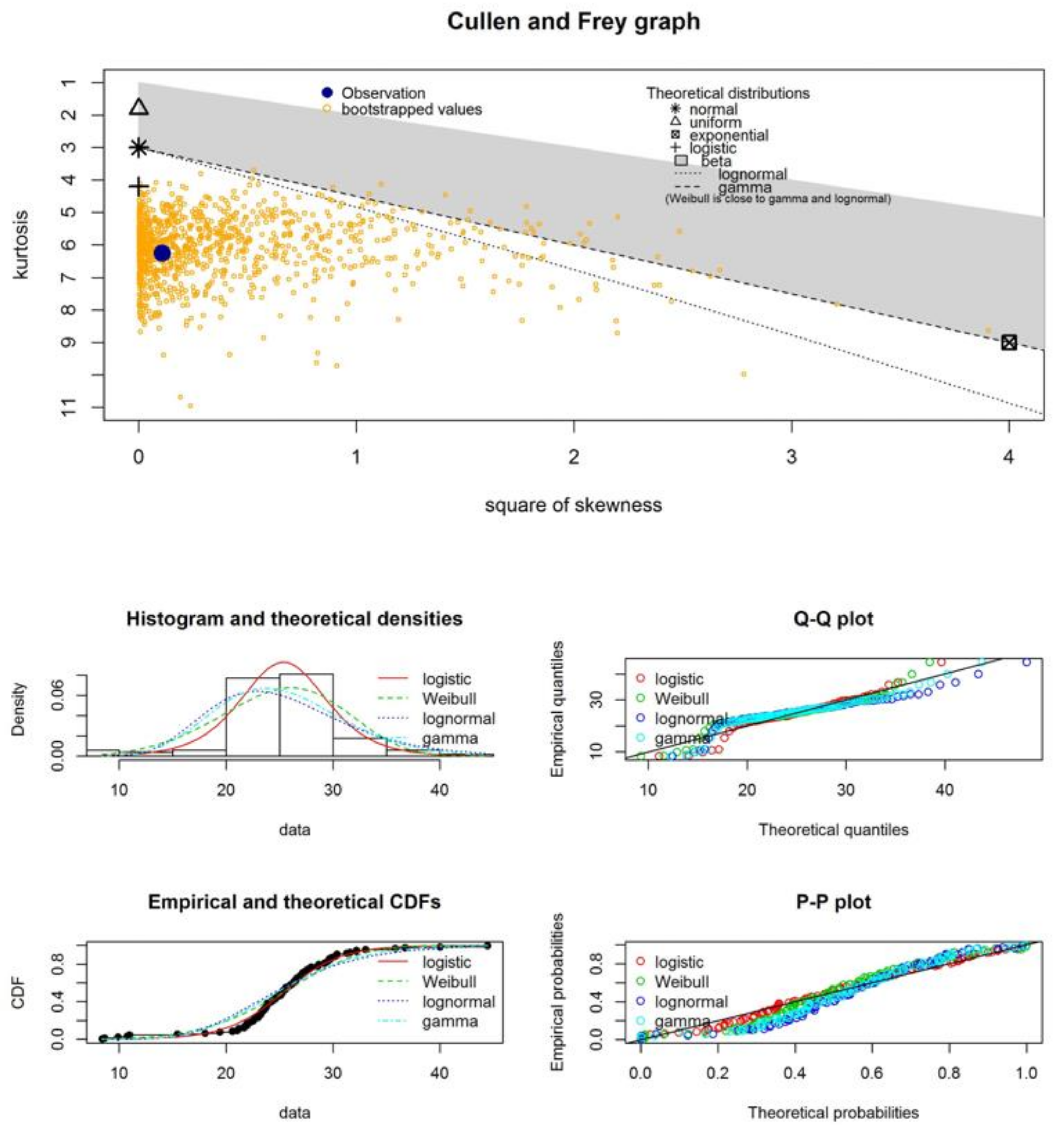

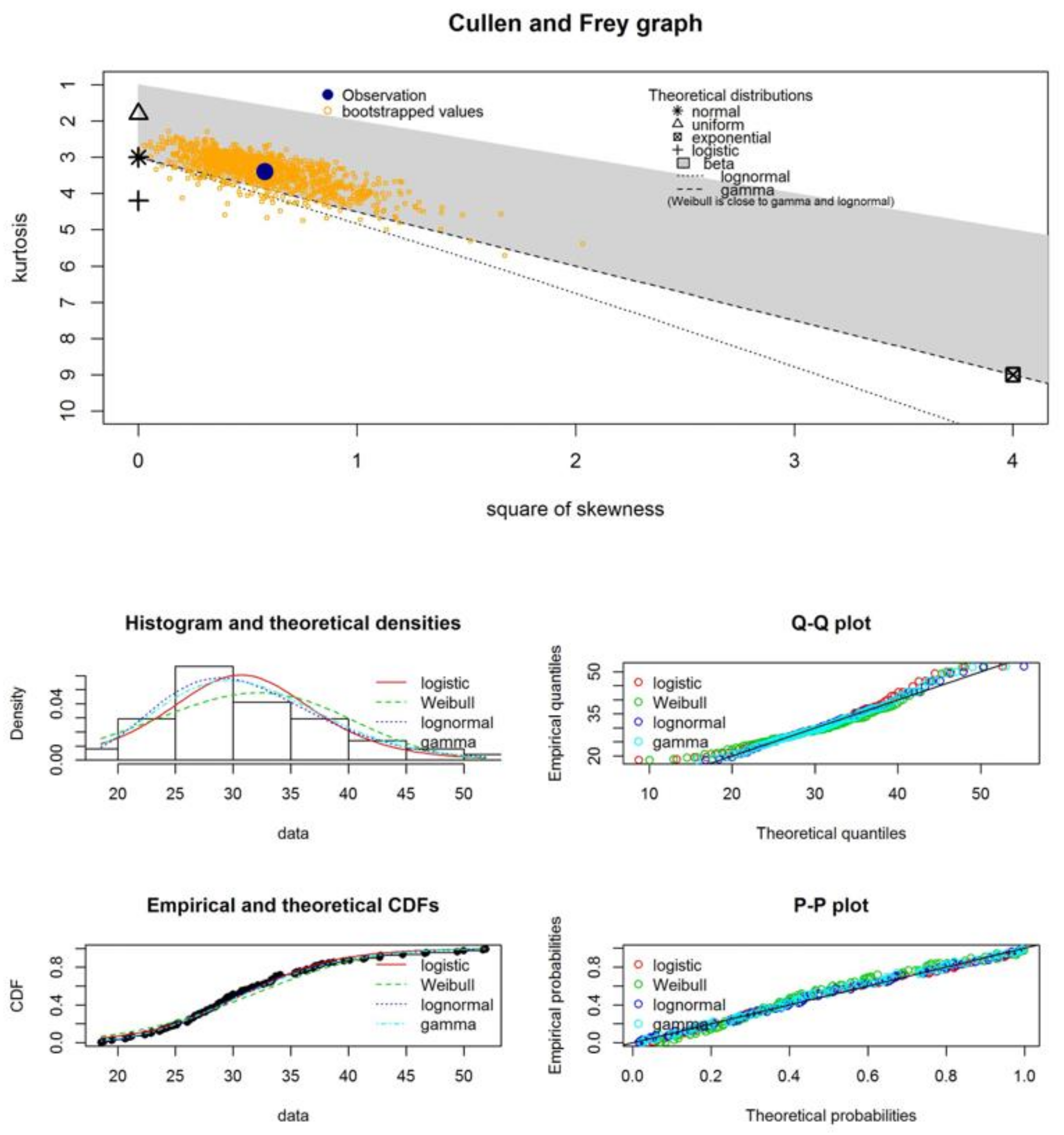
| Samples | Statistical Test | Normal Distribution | Logistic Distribution | Weibull Distribution | Lognormal Distribution | Gamma Distribution |
|---|---|---|---|---|---|---|
| I | KS | 0.0591 | 0.0464 | 0.0851 | — | 0.0580 |
| CVM | 0.0529 | 0.0215 | 0.2078 | — | 0.0598 | |
| AD | 0.3288 | 0.1634 | 1.3147 | — | 0.3957 | |
| AIC | 549.9551 | 547.6779 | 562.8373 | — | 548.9707 | |
| BIC | 555.1654 | 552.8882 | 568.0477 | — | 554.1810 | |
| I + G | KS | — | 0.0736 | 0.1604 | 0.0989 | 0.1142 |
| CVM | — | 0.1363 | 0.7570 | 0.1798 | 0.2592 | |
| AD | — | 1.3444 | 4.4728 | 1.0542 | 1.5239 | |
| AIC | — | 641.4852 | 668.6332 | 632.6182 | 637.9469 | |
| BIC | — | 646.6956 | 673.8435 | 637.8285 | 643.1572 | |
| Hy | KS | — | 0.1230 | 0.1654 | 0.1664 | 0.2153 |
| CVM | — | 0.2837 | 0.5657 | 0.5341 | 0.8937 | |
| AD | — | 1.9730 | 3.2062 | 3.0975 | 5.0934 | |
| AIC | — | 660.6151 | 669.6108 | 669.4142 | 687.5085 | |
| BIC | — | 665.8254 | 674.6246 | 674.6246 | 692.7189 | |
| Hy + G | KS | — | 0.0861 | 0.1607 | 0.2057 | 0.1824 |
| CVM | — | 0.1270 | 0.5942 | 1.0462 | 0.7776 | |
| AD | — | 1.1656 | 3.5652 | 6.3650 | 4.8938 | |
| AIC | — | 610.3368 | 632.5411 | 661.0108 | 645.9053 | |
| BIC | — | 615.5471 | 637.7514 | 666.2211 | 651.1157 | |
| FC | KS | — | 0.0849 | 0.1500 | 0.0967 | 0.1095 |
| CVM | — | 0.1544 | 0.5549 | 0.1456 | 0.2030 | |
| AD | — | 1.3250 | 3.3217 | 0.8219 | 1.1429 | |
| AIC | — | 622.3762 | 642.8090 | 612.2101 | 615.9995 | |
| BIC | — | 627.5865 | 648.0194 | 617.4204 | 621.2099 | |
| FC + G | KS | — | 0.0570 | 0.1086 | 0.0459 | 0.0589 |
| CVM | — | 0.0626 | 0.2737 | 0.0231 | 0.0513 | |
| AD | — | 0.6694 | 1.8214 | 0.1809 | 0.3605 | |
| AIC | — | 686.1610 | 695.2037 | 676.5396 | 678.5678 | |
| BIC | — | 691.3713 | 700.4141 | 681.7499 | 683.7781 |
References
- Chee-Sanford, J.C.; Mackie, R.I.; Koike, S.; Krapac, I.G.; Lin, Y.-F.; Yannarell, A.C.; Maxwell, S.; Aminov, R.I. Fate and transport of antibiotic residues and antibiotic resistance genes following land application of manure waste. J. Environ. Qual. 2009, 38, 1086–1108. [Google Scholar] [CrossRef] [PubMed]
- Ocampo-Pérez, R.; Rivera-Utrilla, J.; Gómez-Pacheco, C.; Sánchez-Polo, M.; López-Peñalver, J.J. Kinetic study of tetracycline adsorption on sludge-derived adsorbents in aqueous phase. Chem. Eng. J. 2012, 213, 88–96. [Google Scholar] [CrossRef]
- Karthick, S.P.; Radha, K.V. Brief review of spectrophotometric methods for the detection of tetracycline antibiotics. Int. J. Pharm. Pharm. 2014, 6, 48–51. [Google Scholar]
- Aga, D.S.; O’Connor, S.; Ensley, S.; Payero, J.O.; Snow, D.; Tarkalson, D. Determination of the persistence of tetracycline antibiotics and their degradates in manure-amended soil using enzyme-linked immunosorbent assay and liquid chromatography−mass spectrometry. J. Agric. Food Chem. 2005, 53, 7165–7171. [Google Scholar] [CrossRef] [PubMed]
- Pena, A.; Paulo, M.; Silva, L.; Seifrtová, M.; Lino, C.; Solich, P. Tetracycline antibiotics in hospital and municipal wastewaters: A pilot study in Portugal. Anal. Bioanal. Chem. 2010, 396, 2929–2936. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y.; Asselman, J.; Jeong, T.-Y.; Yu, S.; De Schamphelaere, K.A.C.; Kim, S.D. Multigenerational effects of the antibiotic tetracycline on transcriptional responses of daphnia magna and its relationship to higher levels of biological organizations. Environ. Sci. Technol. 2017, 51, 12898–12907. [Google Scholar] [CrossRef] [PubMed]
- Nunes, B.; Antunes, S.C.; Gomes, R.; Campos, J.C.; Braga, M.R.; Ramos, A.S.; Correia, A.T. Acute effects of tetracycline exposure in the freshwater fish Gambusia holbrooki: Antioxidant effects, neurotoxicity and histological alterations. Arch. Environ. Contam. Toxicol. 2014, 68, 371–381. [Google Scholar] [CrossRef]
- Lesage, N.; Spérandio, M.; Cabassud, C. Study of a hybrid process: Adsorption on activated carbon/membrane bioreactor for the treatment of an industrial wastewater. Chem. Eng. Process. Process Intensif. 2008, 47, 303–307. [Google Scholar] [CrossRef]
- Lee, J.; Chae, H.-R.; Won, Y.-J.; Lee, K.; Lee, J.; Lee, H.; Kim, I.-C.; Lee, J.-M. Graphene oxide nanoplatelets composite membrane with hydrophilic and antifouling properties for wastewater treatment. J. Membr. Sci. 2013, 448, 223–230. [Google Scholar] [CrossRef]
- Cheng, D.; Ngo, H.-H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Zhang, X.; Varjani, S.; Liu, Y. Feasibility study on a new pomelo peel derived biochar for tetracycline antibiotics removal in swine wastewater. Sci. Total Environ. 2020, 720, 137662. [Google Scholar] [CrossRef] [PubMed]
- Khadem, M.; Husni Ibrahim, A.; Mokashi, I.; Hasan Fahmi, A.; Noeman Taqui, S.; Mohanavel, V.; Hossain, N.; Baba Koki, I.B.; Elfasakhany, A.; Dhaif-Allah, M.A.H.; et al. Removal of heavy metals from wastewater using low-cost biochar prepared from jackfruit seed waste. Biomass Conv. Bioref. 2022, 1–10. [Google Scholar] [CrossRef]
- Taqui, S.N.; Mohan, C.S.; Khatoon, B.A.; Soudagar, M.E.M.; Khan, T.M.; Mujtaba, M.A.; Ahmed, W.; Elfasakhany, A.; Kumar, R.; Pruncu, C.I. Sustainable adsorption method for the remediation of malachite green dye using nutraceutical industrial fenugreek seed spent. Biomass Conv. Bioref. 2021, 1–12. [Google Scholar] [CrossRef]
- Taqui, S.N.; Cs, M.; Goodarzi, M.S.; Elkotb, M.A.; Khatoon, B.A.; Soudagar, M.E.M.; Baba Koki, I.; Elfasakhany, A.; Salah Khalifa, A.; Ashraf Ali, M.; et al. Sustainable adsorption method for the remediation of crystal violet dye using nutraceutical industrial fenugreek seed spent. Appl. Sci. 2021, 11, 7635. [Google Scholar] [CrossRef]
- Joshi, N.C. Synthesis of r-GO/PANI/ZnO based material and its application in the treatment of wastewater containing Cd2+ and Cr6+ ions. Sep. Sci. Technol. 2022, 57, 1–12. [Google Scholar] [CrossRef]
- Kumar, N.; Joshi, N.C. Potential of PTH-Fe3O4 Based Nanomaterial for the Removal of Pb (II), Cd (II), and Cr (VI) Ions. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1234–1245. [Google Scholar] [CrossRef]
- Joshi, N.C.; Gururani, P. Advances of graphene oxide based nanocomposite materials in the treatment of wastewater containing heavy metal ions and dyes. Curr. Res. Green Sustain. Chem. 2022, 5, 100306. [Google Scholar] [CrossRef]
- Kumar, N.; Joshi, N.C. Adsorption applications of synthetically prepared PANI-CuO based nanocomposite material. J. Indian Chem. Soc. 2022, 99, 100551. [Google Scholar] [CrossRef]
- Hou, J.; Chen, Z.; Gao, J.; Xie, Y.; Li, L.; Qin, S.; Wang, Q.; Mao, D.; Luo, Y. Simultaneous removal of antibiotics and antibiotic resistance genes from pharmaceutical wastewater using the combinations of up-flow anaerobic sludge bed, anoxic-oxic tank, and advanced oxidation technologies. Water Res. 2019, 159, 511–520. [Google Scholar] [CrossRef]
- Wang, J.; Zhuan, R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total Environ. 2019, 701, 135023. [Google Scholar] [CrossRef]
- Gururani, P.; Bhatnagar, P.; Bisht, B.; Kumar, V.; Joshi, N.C.; Tomar, M.S.; Pathak, B. Cold plasma technology: Advanced and sustainable approach for wastewater treatment. Environ. Sci. Pollut. Res. 2021, 28, 65062–65082. [Google Scholar] [CrossRef]
- Horng, R.-Y.; Shao, H.; Chang, W.-K.; Chang, M.-C. The feasibility study of using non-woven MBR for reduction of hydrolysed biosolids. Water Sci. Technol. 2006, 54, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-K.; Hu, A.Y.-J.; Horng, R.-Y.; Tzou, W.-Y. Membrane bioreactor with nonwoven fabrics as solid–liquid separation media for wastewater treatment. Desalination 2006, 202, 122–128. [Google Scholar] [CrossRef]
- Khajavi, R.; Bahadoran, M.; Bahador, A.; Khosravi, A. Removal of microbes and air pollutants passing through nonwoven polypropylene filters by activated carbon and nanosilver colloidal layers. J. Ind. Text. 2013, 42, 219–230. [Google Scholar] [CrossRef]
- Bouazizi, N.; Mohammad, N.M.; El Achari, A.; Behary, N.; Campagne, C.; Vieillard, J.; Thoumire, O.; Azzouz, A. Development of new multifunctional filter based nonwovens for organics pollutants reduction and detoxification: High catalytic and antibacterial activities. Chem. Eng. J. 2019, 356, 702–716. [Google Scholar]
- Luo, J.; Lv, P.; Zhang, J.; Fane, A.G.; McDougald, D.; Rice, S.A. Succession of biofilm communities responsible for biofouling of membrane bio-reactors (MBRs). PLoS ONE 2017, 12, e0179855. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Chen, L.; Que, C. Adsorption of antibiotics on graphene and biochar in aqueous solutions induced by π–π interactions. Sci. Rep. 2016, 6, 31920. [Google Scholar] [CrossRef] [PubMed]
- Al-Khateeb, L.A.; Almotiry, S.; Salam, M.A. Adsorption of pharmaceutical pollutants onto graphene nanoplatelets. Chem. Eng. J. 2014, 248, 191–199. [Google Scholar] [CrossRef]
- Zhao, J.; Ren, W.; Chenga, H.-M. Graphene sponge for efficient and repeatable adsorption and desorption of water contaminations. J. Mater. Chem. 2012, 22, 20197–20202. [Google Scholar] [CrossRef]
- Kang, X.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y. A graphene-based electrochemical sensor for sensitive detection of paracetamol. Talanta 2010, 81, 754–759. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Z.; Chen, B. Adsorption of polycyclic aromatic hydrocarbons by graphene and graphene oxide nanosheets. Environ. Sci. Technol. 2014, 48, 4817–4825. [Google Scholar] [CrossRef]
- Yu, F.; Ma, J.; Bi, D. Enhanced adsorptive removal of selected pharmaceutical antibiotics from aqueous solution by activated graphene. Environ. Sci. Pollut. Res. 2015, 22, 4715–4724. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Hu, J.; Wang, J. Removal of sulfamethazine antibiotics using CeFe-graphene nanocomposite as catalyst by Fenton-like process. J. Environ. Manag. 2016, 182, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Wang, X.; Zhang, C.; Zeng, G.; Peng, Z.; Zhou, J.; Cheng, M.; Wang, R.; Hu, Z.; Qin, X. Sorptive removal of ionizable antibiotic sulfamethazine from aqueous solution by graphene oxide-coated biochar nanocomposites: Influencing factors and mechanism. Chemosphere 2017, 186, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Bytesnikova, Z.; Richtera, L.; Smerkova, K.; Vojtech, A. Graphene oxide as a tool for antibiotic-resistant gene removal: A review. Environ. Sci. Pollut. Res. 2019, 26, 20148–20163. [Google Scholar] [CrossRef]
- Wang, X.; Yin, R.; Zeng, L.; Zhu, M. A review of graphene-based nanomaterials for removal of antibiotics from aqueous environments. Environ. Pollut. 2019, 253, 100–110. [Google Scholar] [CrossRef]
- Nakata, H.; Kannan, K.; Jones, P.D.; Giesy, J.P. Determination of fluoroquinolone antibiotics in wastewater effluents by liquid chromatography-mass spectrometry and fluorescence detection. Chemosphere 2005, 58, 759–766. [Google Scholar] [CrossRef]
- Dixon-Holland, D.E. ELISA and its application for residue analysis of antibiotics and drugs in products of animal origin. In Analysis of Antibiotic/Drug Residues in Food Products of Animal Origin; Agarwal, V.K., Ed.; Springer: Boston, MA, USA, 1992. [Google Scholar]
- Aga, D.S.; Lenczewski, M.; Snow, D.; Muurinen, J.; Sallach, J.B.; Wallace, J.S. Challenges in the measurement of antibiotics and in evaluating their impacts in agroecosystems: A critical review. J. Environ. Qual. 2016, 45, 407–419. [Google Scholar] [CrossRef]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-response analysis using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef]
- Saroyda, J.R.V.; Cruz, R.Y.S.; Antonio, R.J.C.; Flestado, C.L.P.; Magalong, J.R.S.; Zagala, K.Z.P.; Barbacena, C.L.; Bumatay, J.M.; Bautista, L.F.; Deocaris, C.C. PUPAIM: A Collection of Physical and Chemical Adsorption Isotherm Models. Version 3.6.0. 2020. Available online: https://cran.r-project.org/web/packages/PUPAIM/index.html (accessed on 12 September 2022).
- Dąbrowski, A.; Goworek, J.; Podkościelny, P.; Garbacz, J.K. Application of adsorption from solutions for characterizing inorganic sorbents. In Fundamentals of Adsorption; LeVan, M.D., Ed.; The Kluwer International Series in Engineering and Computer Science, 356; Springer: Boston, MA, USA, 1996. [Google Scholar]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and interpretation of adsorption isotherms. J. Chem. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Delignette-Muller, M.L.; Dutang, C. fitdistrplus: An R package for fitting distributions. J. Stat. Softw. 2015, 64, 1–34. [Google Scholar] [CrossRef]
- Hegab, H.M.; ElMekawy, A.; Zou, L.; Mulcahy, D.; Saint, C.P.; Ginic-Markovic, M. The controversial antibacterial activity of graphene-based materials. Carbon 2016, 105, 362–376. [Google Scholar] [CrossRef]
- Pan, Q.; Shim, E.; Pourdeyhimi, B.; Gao, W. Highly conductive polypropylene–graphene nonwoven composite via interface engineering. Langmuir 2017, 33, 7452–7458. [Google Scholar] [CrossRef] [PubMed]
- Harruddin, N.; Saufi, S.M.; Faizal, C.K.M.; Mohammad, A.W. Effect of VIPS fabrication parameters on the removal of acetic acid by supported liquid membrane using a PES–graphene membrane support. RSC Adv. 2018, 8, 25396–25408. [Google Scholar] [CrossRef] [PubMed]
- Polak, D.; Zielińska, I.; Szwast, M.; Kogut, I.; Małolepszy, A. Modification of Ceramic Membranes with Carbon Compounds for Pharmaceutical Substances Removal from Water in a Filtration—Adsorption System. Membranes 2021, 11, 481. [Google Scholar] [CrossRef]
- Kumar, P.; Huo, P.; Zhang, R.; Liu, B. Antibacterial Properties of Graphene-Based Nanomaterials. Nanomaterials 2019, 9, 737. [Google Scholar] [CrossRef]
- Qiu, J.; Liu, L.; Zhu, H.; Liu, X. Combination types between graphene oxide and substrate affect the antibacterial activity. Bioact. Mater. 2018, 3, 341–346. [Google Scholar] [CrossRef]
- de Moraes, A.C.; Lima, B.A.; de Faria, A.F.; Brocchi, M.; Alves, O.L. Graphene oxide-silver nanocomposite as a promising biocidal agent against methicillin-resistant Staphylococcus aureus. Int. J. Nanomed. 2015, 10, 6847–6861. [Google Scholar] [CrossRef]
- Pranno, N.; La Monaca, G.; Polimeni, A.; Sarto, M.S.; Uccelletti, D.; Bruni, E.; Cristalli, M.P.; Cavallini, D.; Vozza, I. Antibacterial activity against Staphylococcus aureus of titanium surfaces coated with graphene nanoplatelets to prevent peri-implant diseases. An in-vitro pilot study. Int. J. Environ. Res. Public Health 2020, 17, 1568. [Google Scholar] [CrossRef]
- Agarwalla, S.V.; Ellepola, K.; de Costa, M.; Fechine, G.J.M.; Morin, J.; Castro Neto, A.H.; Seneviratne, C.J.; Vinicius, R. Hydrophobicity of graphene as a driving force for inhibiting biofilm formation of pathogenic bacteria and fungi. Dent. Mater. J. 2019, 35, 403–413. [Google Scholar] [CrossRef]
- Zhu, L.; Perwuelz, A.; Lewandowski, M.; Campagne, C. Static and dynamic aspects of liquid capillary flow in thermally bonded polyester nonwoven fabrics. J. Adhes. Sci. Technol. 2008, 22, 745–760. [Google Scholar]
- Shahbazi, Y.; Ahmadi, F.; Karami, N. Screening, determination and confirmation of tetracycline residues in chicken tissues using four-plate test, ELISA and HPLC-UV methods: Comparison between correlation results. Food Agric. Immunol. 2015, 26, 821–834. [Google Scholar] [CrossRef]
- Liu, X.; Ji, Y.; Zhang, H.; Liu, M. Elimination of matrix effects in the determination of bisphenol A in milk by solid-phase microextraction-high-performance liquid chromatography. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk. Assess. 2008, 25, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Pailler, J.Y.; Krein, A.; Pfister, L.; Hoffmann, L.; Guignard, C. Solid phase extraction coupled to liquid chromatography-tandem mass spectrometry analysis of sulfonamides, tetracyclines, analgesics and hormones in surface water and wastewater in Luxembourg. Sci. Total Environ. 2009, 407, 4736–4743. [Google Scholar] [CrossRef] [PubMed]
- Jasper, E.; Ajibola, V.O.; Onwuka, J. Nonlinear regression analysis of the sorption of crystal violet and methylene blue from aqueous solutions onto an agro-waste derived activated carbon. Appl. Water Sci. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Bridelli, M.; Crippa, P. Theoretical analysis of the adsorption of metal ions to the surface of melanin particles. Adsorption 2008, 14, 101–109. [Google Scholar] [CrossRef]
- Abdullah, M.A.; Chiang, L.; Nadeem, M. Comparative evaluation of adsorption kinetics and isotherms of a natural product removal by Amberlite polymeric adsorbents. Chem. Eng. J. 2009, 146, 370–376. [Google Scholar]
- Zielińska, I.; Polak, D.; Szwast, M. Analysis of the adsorption of selected pharmaceuticals on a composite material PEBAX/GO. J. Water Process Eng. 2021, 44, 102272. [Google Scholar] [CrossRef]



| Statistical Parameters | I | I + G | Hy | Hy + G | FC | FC + G |
|---|---|---|---|---|---|---|
| minimum | 16 | 18 | 8 | 8 | 20 | 19 |
| maximum | 39 | 51 | 42 | 45 | 47 | 52 |
| median | 25.01 | 26.98 | 26.05 | 25.28 | 28.10 | 29.87 |
| estimated standard deviation | 3.73 | 6.25 | 6.77 | 5.50 | 5.47 | 7.41 |
| estimated skewness | 0.33 | 1.41 | −0.41 | −0.35 | 1.07 | 0.78 |
| estimated kurtosis | 4.12 | 5.66 | 4.23 | 6.34 | 4.26 | 3.49 |
| Statistical Parameters | I | I + G | Hy | Hy + G | FC | FC + G |
|---|---|---|---|---|---|---|
| minimum | 75 | 70 | 70 | 75 | 20 | 30 |
| maximum | 155 | 160 | 175 | 170 | 100 | 85 |
| median | 115.00 | 115.00 | 122.50 | 122.50 | 60.00 | 57.50 |
| mean | 99.64 | 105.71 | 108.66 | 106.97 | 32.29 | 59.71 |
| estimated standard deviation | 17.87 | 21.58 | 23.23 | 21.38 | 16.98 | 17.18 |
| estimated skewness | 0.89 | 0.42 | 0.62 | 0.70 | −0.75 | −0.90 |
| estimated kurtosis | −0.15 | −0.81 | −0.29 | −0.08 | −0.01 | −0.71 |
| Statistical Parameters | Langmuir | Freundlich | Henry | Redlich–Peterson |
|---|---|---|---|---|
| α [L/mg] | 655 | 535 | – | 0.0001 |
| β [–] | – | – | – | 0.5 |
| KHE, KL, KF, KRP [L/m2] | 0.95 | 1.7 | 239.5 | 237 |
| R2 [–] | 0.86 | 0.83 | 0.95 | 0.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kogut, I.; Armbruster, F.; Polak, D.; Kaur, S.; Hussy, S.; Thiem, T.; Gerhardts, A.; Szwast, M. Antibacterial, Antifungal, and Antibiotic Adsorption Properties of Graphene-Modified Nonwoven Materials for Application in Wastewater Treatment Plants. Processes 2022, 10, 2051. https://doi.org/10.3390/pr10102051
Kogut I, Armbruster F, Polak D, Kaur S, Hussy S, Thiem T, Gerhardts A, Szwast M. Antibacterial, Antifungal, and Antibiotic Adsorption Properties of Graphene-Modified Nonwoven Materials for Application in Wastewater Treatment Plants. Processes. 2022; 10(10):2051. https://doi.org/10.3390/pr10102051
Chicago/Turabian StyleKogut, Igor, Friederike Armbruster, Daniel Polak, Sandeep Kaur, Stephan Hussy, Tobias Thiem, Anja Gerhardts, and Maciej Szwast. 2022. "Antibacterial, Antifungal, and Antibiotic Adsorption Properties of Graphene-Modified Nonwoven Materials for Application in Wastewater Treatment Plants" Processes 10, no. 10: 2051. https://doi.org/10.3390/pr10102051
APA StyleKogut, I., Armbruster, F., Polak, D., Kaur, S., Hussy, S., Thiem, T., Gerhardts, A., & Szwast, M. (2022). Antibacterial, Antifungal, and Antibiotic Adsorption Properties of Graphene-Modified Nonwoven Materials for Application in Wastewater Treatment Plants. Processes, 10(10), 2051. https://doi.org/10.3390/pr10102051










