An Overview of the Potential of Medicinal Plants Used in the Development of Nutraceuticals for the Management of Diabetes Mellitus: Proposed Biological Mechanisms
Abstract
1. Introduction
2. Flavonoids
2.1. Isoflavones
2.2. Flavones
3. Saponins
4. Examples of Plants with Antidiabetic Properties
4.1. Fenugrec (Trigonella Foenum-Graecum)
4.2. Date Palm (Phoenix dactylifera L.)
4.3. Garlic
4.4. Cumin (Cuminum cyminum)
4.5. Olive (Olea europea L.)
4.6. Polysaccharides
- ▪
- Vanadium is found in all cells; it acts as an “insulin mimetic”; it is found in mushrooms, shellfish, black pepper, parsley, dill seed, beer, wine, and grains. According to animal and in vitro studies, vanadium has insulin-like effects in the liver, skeletal muscle, and adipose tissue [163]; moreover, it stimulates glucose uptake—either directly, or by inhibiting the phosphotyrosine phosphatase enzyme system—thus enhancing insulin receptor phosphorylation and insulin receptor (IR)–tyrosine kinase interaction [164];
- ▪
- ω-3 Fatty acids, which are abundant in some plants—such as sunflower and safflower—have been reported to improve insulin resistance in animal models [165].
- ▪
- Legumes are rich in fiber, protein, and nutrients, and are slowly digested; they produce relatively small blood glucose increases. Identifying the factors determining starch digestibility may be useful in the management of diabetes and disorders of carbohydrate metabolism [168]. Centrone et al. (2020) [169] reported that mice fed with a chickpea-supplemented diet displayed lower levels of glycemia.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global est mates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- World Health Organ. WHO Expert committee on diabetes mellitus: Second report. Tech. Rep. Ser 1980, 646, 1–80. [Google Scholar]
- WHO Study group. Diabetes mellitus, World Health Organ. Tech. Rep. Ser. 1985, 727, 1–113. [Google Scholar]
- Nasri, H.; Rafieian-Kopaei, M. Preventive and curative effect ofgarlic on nephrotoxic effect of gentamicin in rat. J. Babol Univ. Med. Sci. 2014, 16, 42–48. [Google Scholar]
- Rafieian-Kopaei, M.; Baradaran, A.; Nasri, H. Significance of extracapillary proliferation in IgA-nephropathy patients with regard to clinical and histopathological variable. Hippokratia 2013, 17, 258–261. [Google Scholar] [PubMed]
- Rafieian-Kopaei, M.; Ghaed-Amini, F.; Nasri, H. Erythropoietin has kidney protective efficacy beyond stimulating erythropoiesis. J. Isfahan Med. Sch. 2013, 31, 619–622. [Google Scholar]
- Srinivasan, K.; Ramarao, P. Animal models in type 2 diabetes research: An overview. Indian J. Med. Res. 2007, 125, 451–472. [Google Scholar]
- Matthaei, S.; Stumvoll, M.; Kellerer, M.; Häring, H.U. Pathophysiology and pharmacological treatment of insulin resistance. Endocr. Rev. 2000, 2, 585–618. [Google Scholar]
- Nasri, H.; Baradaran, A.; Ardalan, M.R.; Mardani, S.; Momeni, A.; Rafieian-Kopaei, M. Bright renoprotective properties of metformin: Beyond blood glucose regulatory effects. Iran J. Kidney Dis. 2013, 7, 423–428. [Google Scholar]
- Madihi, Y.; Merrikhi, A.; Baradaran, A.; Rafieian-Kopaei, M.; Fard, S.; Ansari Samani, R.; Mesripour, A. Impact of sumac on postprandial high-fat oxidative stress. Pak. J. Med. Sci. 2013, 29, 340–345. [Google Scholar] [CrossRef]
- Amini, G.F.; Rafieian-Kopaei, M.; Nematbakhsh, M.; Baradaran, A.; Nasri, H. Ameliorative effects of metformin on renal histologic and biochemical alterations of gentamicin-induced renal toxicity in Wistar rats. J. Res. Med. Sci. 2012, 17, 621–625. [Google Scholar] [PubMed]
- Mayfield, J. Diagnosis and classification of diabetes mellitus: New criteria. Am. Fam. Physician 1998, 58, 1355–1362. [Google Scholar] [PubMed]
- Nasri, H.; Rafieian-Kopaei, M. Tubular kidney protection by antioxidants. Iran J. Public Health 2013, 42, 1194–1196. [Google Scholar]
- Li, W.L.; Zheng, H.C.; Bukuru, J.; De Kimpe, N. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J. Ethnopharmacol. 2004, 92, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Nasri, H.; Shirzad, H. Toxicity and safety of medicinal plants. J. HerbMed. Plarmacol. 2013, 2, 21–22. [Google Scholar]
- Abbas, G.; Al Harrasi, A.; Hussain, H.; Hamaed, A.; Supuran, C.T. The management of diabetes mellitus-imperative role of natural products against dipeptidyl peptidase-4, α-glucosidase and sodium-dependent glucose co-transporter 2 (SGLT2). Bioorg. Chem. 2019, 86, 305–315. [Google Scholar] [CrossRef]
- Jacob, B.; Narendhirakannan, R.T. Role of medicinal plants in the management of diabetes mellitus: A review. 3 Biotech 2019, 9, 4. [Google Scholar] [CrossRef]
- Gupta, R. Active phytoconstituents for diabetes management: A review. J. Complement. Integr. Med. 2018, 19, 15. [Google Scholar] [CrossRef]
- Huang, T.H.; Teoh, A.W.; Lin, B.L.; Lin, D.S.; Roufogalis, B. The role of herbal PPAR modulators in the treatment of cardiometabolic syndrome. Pharm. Res. 2009, 60, 195–206. [Google Scholar] [CrossRef]
- Cushnie., T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Galeotti, F.; Barile, E.; Curir, P.; Dolci, M.; Lanzotti, V. Flavonoids from carnation (Dianthus caryophyllus) and their antifungal activity. Phytochem. Lett. 2008, 1, 44–48. [Google Scholar] [CrossRef]
- Testa, R.; Bonfigli, A.R.; Genovese, S.; De Nigris, V.; Ceriello, A. The Possible Role of Flavonoids in the Prevention of Diabetic Complications. Nutrients 2016, 8, 310. [Google Scholar] [CrossRef] [PubMed]
- Stahl, L.; Miller, K.B.; Apgar, J.; Sweigart, D.S.; Stuart, D.A.; McHale, N.; Ou, B.; Kondo, M.; Hurst, W.J. Preservation of cocoa antioxidant activity, total polyphenols, flavan-3-ols, and procyanidin content in foods prepared with cocoa powder. J. Food Sci. 2009, 74, C456–C461. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Mirmiran, P.; Azizi, F. Dietary polyphenols as potential nutraceuticals in management of diabetes: A review. J. Diabetes Metab. Disord. 2013, 12, 43. [Google Scholar] [CrossRef]
- Graf, B.A.; Milbury, P.E.; Blumberg, J.B. Flavonols, flavones, flavanones, and human health: Epidemiological evidence. J. Med. Food 2005, 8, 281–290. [Google Scholar] [CrossRef]
- De Bock, M.; Derraik, J.G.; Cutfield, W.S. Polyphenols and glucose homeostasis in humans. J. Acad. Nutr. Diet. 2012, 112, 808–815. [Google Scholar] [CrossRef]
- Kim, J.H.; Kang, M.J.; Choi, H.N.; Jeong, S.M.; Lee, Y.M.; Kim, J.I. Quercetin attenuates fasting and postprandial hyperglycemia in animal models of diabetes mellitus. Nutr. Res. Pract. 2011, 5, 107–111. [Google Scholar] [CrossRef]
- Arabbi, P.R.; Genovese, M.I.; Lajolo, F.M. Flavonoids in vegetablefoods commonly consumed in Brazil and estimated ingestion by the Brazilian population. J. Agric. Food Chem. 2004, 52, 1124–1131. [Google Scholar] [CrossRef]
- Ishikawa, A.; Yamashita, H.; Hiemori, M.; Inagaki, E.; Kimoto, M.; Okamoto, M.; Tsuji, H.; Memon, A.N.; Mohammadi, A.; Natori, Y. Characterization of inhibitors of postprandial hyperglycemia from the leaves of Nerium indicum. J. Nutr. Sci. Vitaminol. 2007, 53, 166–173. [Google Scholar] [CrossRef]
- Jo, S.H.; Ka, E.H.; Lee, H.S.; Apostolidis, E.; Jang, H.D.; Kwon, Y.I. Comparison of Antioxidant Potential and Rat intestinal a-Glucosidases inhibitory Activities of Quercetin, Rutin, and Isoquercetin. Int. J. Appl. Res. Nat. Prod. 2009, 2, 52–60. [Google Scholar]
- Jung, U.J.; Lee, M.K.; Jeong, K.S.; Choi, M.S. The hypoglycemic effects of hesperidin and naringin are partly mediated by hepatic glucose-regulating enzymes in C57BL/KsJ-db/db mice. J. Nutr. 2004, 134, 2499–2503. [Google Scholar] [CrossRef] [PubMed]
- Cok, A.; Plaisier, C.; Salie, M.J.; Oram, D.S.; Chenge, J.; Louters, L.L. Berberine acutely activates the glucose transport activity of GLUT1. Biochimie 2011, 93, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Shin, E.J.; Kim, E.D.; Bayaraa, T.; Frost, S.C.; Hyun, C.K. Berberine activates GLUT1-mediated glucose uptake in 3T3-L1 adipocytes. Biol. Pharm. Bull. 2007, 30, 2120–2125. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Ye, J.; Jia, W. Effects and mechanisms of berberine in diabetes treatment. Acta Pharm. Sin. B 2012, 2, 327–334. [Google Scholar] [CrossRef]
- Yin, J.; Xing, H.; Ye, J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metab. Clin. Exp. 2008, 57, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Cazarolli, L.H.; Folador, P.; Moresco, H.H.; Brighente, I.M.; Pizzolatti, M.G.; Silva, F.R. (Stimulatory effect of apigenin-6-C-beta-L-fucopyranoside on insulin secretion and glycogen synthesis. Eur. J. Med. Chem. 2009, 44, 4668–4673. [Google Scholar] [CrossRef]
- Cazarolli, L.H.; Folador, P.; Moresco, H.H.; Brighente, I.M.C.; Pizzolatti, M.G.; Silva, F.R.M.B. Mechanism of action of the stimulatory effect of apigenin6-C-(2″-O-alpha-l-rhamnopyranosyl)-beta-l-fucopyranoside on (14) C-glucose uptake. Chem. Biol. Interact. 2009, 179, 407–412. [Google Scholar] [CrossRef]
- Cazarolli, L.H.; Kappel, V.D.; Pereira, D.F.; Moresco, H.H.; Brighente, I.M.; Pizzolatti, M.G.; Silva, F.R. Anti-hyperglycemic action of apigenin-6-C-beta-fucopyranoside from Averrhoa carambola. Fitoterapia 2012, 83, 1176–1183. [Google Scholar] [CrossRef]
- Cazarolli, L.H.; Pereira, D.F.; Kappel, V.D.; Folador, P.; Figueiredo, M.D.; Pizzolatti, M.G.; Silva, F.R. Insulin signaling: A potential signaling pathway for the stimulatory effect of kaempferitrin on glucose uptake in skeletal muscle. Eur. J. Pharmacol. 2013, 712, 1–7. [Google Scholar] [CrossRef]
- Ong, K.C.; Khoo, H.E. Effects of myricetin on glycemia and glycogen metabolism in diabetic rats. Life Sci. 2000, 67, 1695–1705. [Google Scholar] [CrossRef]
- Jorge, A.P.; Horst, H.; de Sousa, E.; Pizzolatti, M.G.; Silva, F.R. Insulinomimetic effects of kaempferitrin on glycaemia and on 14C-glucose uptake in rat soleus muscle. Chem. Biol. Interact. 2004, 149, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Ashour, M.B.; Abdel-Moneim, A.; Ahmed, O.M. Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats. J. Diabetes Its Complicat. 2012, 26, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.M.; Mahmoud, A.M.; Abdel-Moneim, A.; Ashour, M.B. Antihyperglycemic and Antihyperlipidemic Effects of Hesperidin and Naringin in High Fat Diet/Streptozotocin Type 2 Diabetic Rats. Life Sci. J. 2011, 8, 91–101. [Google Scholar]
- Zhu, W.; Jia, Q.; Wang, Y.; Zhang, Y.; Xia, M. The antho-cyanin cyanidin-3-O-β-glucoside, a flavonoid, increases hepatic glutathione synthesis and protects hepatocytes against reactive oxygen species during hyperglycemia: Involvement of a cAMP–PKA-dependent signaling pathway. Free Rad. Biol. Med. 2012, 52, 314–327. [Google Scholar] [CrossRef]
- Khan, N.; Syed, D.N.; Ahmad, N.; Mukhtar, H. Fisetin: A dietary antioxidant for health promotion. Antioxid. Redox Signal. 2013, 19, 151–162. [Google Scholar] [CrossRef]
- Prasath, G.S.; Sundaram, C.S.; Subramanian, S.P. Fisetin averts oxidative stress in pancreatic tissues of streptozotocin-induced diabetic rats. Endocrine 2013, 44, 359–368. [Google Scholar] [CrossRef]
- Guo, H.; Xia, M.; Zou, T.; Ling, W.; Zhong, R.; Zhang, W. Cyanidin 3-glucoside attenuates obesity-associated insulin resistance and hepatic steatosis in high-fat diet-fed and db/db mice via the transcription factor FoxO1. J. Nutr. Biochem. 2012, 23, 349–360. [Google Scholar] [CrossRef]
- Mirshekar, M.; Roghani, M.; Khalili, M.; Baluchnejadmojarad, T.; Arab, M.S. Chronic oral pelargonidin alleviates streptozotocin-induced diabetic neuropathic hyperalgesia in rat: Involvement of oxidative stress. Iran Biomed. J. 2010, 14, 33–39. [Google Scholar]
- Pari, L.; Srinivasan, S. Antihyp erglycemic effect of diosmin on hepatic key enzymes of carbohydrate metabolism in Streptozotocin nicotinamide induced diabetic rats. Biomed. Pharm. 2010, 64, 477–481. [Google Scholar] [CrossRef]
- Hao, H.H.; Shao, Z.M.; Tang, D.Q.; Lu, Q.; Chen, X.; Yin, X.; Wu, J.; Chen, H. Preventive effects of Rutin on the development of experimental diabetic nephropathy in rats. Life Sci. 2012, 91, 959–967. [Google Scholar] [CrossRef]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced Glycation End Products and Diabetic Complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Negre-Salvayre, A.; Salvayre, R.; Augé, N.; Pamplona, R.; Portero-Otín, M. Hyperglycemia and Glycation in Diabetic Complications. Antioxid. Redox Signal. 2009, 11, 3071–3109. [Google Scholar] [CrossRef] [PubMed]
- Stanely Mainzen Prince, P.; Kannan, N.K. Protective effect of rutin on lipids, lipoproteins, lipid metabolizing enzymes and glycoproteins in streptozotocin-induced diabetic rats. J. Pharm Pharmacol. 2006, 58, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Visnagri, A.; Kandhare, A.D.; Chakravarty, S.; Ghosh, P.; Bodhankar, S.L. Hesperidin, a flavanoglycone attenuates experimental diabetic neuropathy via modulation of cellular and biochemical marker to improve nerve functions. Pharm. Biol. 2014, 52, 814–828. [Google Scholar] [CrossRef] [PubMed]
- Priscilla, D.H.; Jayakumar, M.; Thirumurugan, K. Flavanone naringenin: An effective anti-hyperglycemic and antihyperlipidemic nutraceutical agent on high fat diet fed streptozotocin induced type 2 diabetic rats. J. Funct. Foods 2015, 14, 363–373. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Forouhi, N.G.; Sharp, S.J.; A González, C.; Buijsse, B.; Guevara, M.; van der Schouw, Y.; Amiano, P.; Boeing, H.; Bredsdorff, L.; et al. Dietary intakes of individual flavanols and flavonols are inversely associated with incident type 2 diabetes in European populations. J. Nutr. 2014, 144, 335–343. [Google Scholar] [CrossRef]
- Curtis, P.J.; Sampson, M.; Potter, J.; Dhatariya, K.; Kroon, P.A.; Cassidy, A. Chronic ingestion of flavan-3-ols and isoflavones improves insulin sensitivity and lipoprotein status and attenuates estimated 10-year CVD risk in medicated postmenopausal women with type 2 diabetes: A 1-year, double-blind, randomized, controlled trial. Diabetes Care 2012, 35, 226–232. [Google Scholar] [CrossRef]
- Li, Y.; Ding, Y. Minireview: Therapeutic potential of myricetin in diabetes mellitus. Food Sci. Hum. Wellness 2012, 1, 19–25. [Google Scholar] [CrossRef]
- Al-Numair, K.S.; Chandramohan, G.; Veeramani, C.; Alsaif, M.A. Ameliorative effect of kaempferol, a flavonoid, on oxidative stress in streptozotocin-induced diabetic rats. Redox Rep. 2015, 20, 198–209. [Google Scholar] [CrossRef]
- Yang, X.; Yang, J.; Xu, C.; Huang, M.; Zhou, Q.; Lv, J.; Ma, X.; Ke, C.; Ye, Y.; Shu, G.; et al. Antidiabetic effects of flavonoids from Sophora flavescens EtOAc extract in type 2 diabetic KK-ay mice. J. Ethnopharmacol. 2015, 171, 161–170. [Google Scholar] [CrossRef]
- Fu, Z.; Gilbert, E.R.; Pfeiffer, L.; Zhang, Y.; Fu, Y.; Liu, D. Genistein ameliorates hyperglycemia in a mouse model of nongenetic type 2 diabetes. Appl. Physiol. Nutr. Metab 2012, 37, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Kar, A. Apigenin (4′,5,7-trihydroxyflavone) regulates hyperglycaemia, thyroid dysfunction and lipid peroxidation in alloxan- induced diabetic mice. J. Pharm. Pharmacol. 2007, 59, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Fraschini, F.; Demartini, G.; Esposti, D. Pharmacology of silymarin. Clin. Drug Investig. 2002, 22, 51–65. [Google Scholar] [CrossRef]
- Sirovina, D.; Oršolić, N.; Končić, M.Z.; Kovačević, G.; Benković, V.; Gregorović, G. Quercetin vs chrysin: Effect on liver histopathology in diabetic mice. Hum. Exp. Toxicol. 2013, 32, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, R.; Shanthi, P.; Sachdanandam, P. Effect of tangeretin, a polymethoxylated flavone on glucose metabolism in streptozotocininduced diabetic rats. Phytomedicine 2014, 21, 793–799. [Google Scholar] [CrossRef]
- Clark, A.; Wells, C.A.; Buley, I.D.; Cruicks-hank, J.K.; Vanhegan, R.I.; Matthews, D.R.; Cooper, G.J.S.; Holman, R.R.; Turner, R.C. Islet amyloid increased alpha-cells, reduced beta-cells and exocrine fibrosis:quantitative changes in the pancreas in type 2 diabetes. Diabetes Res. 1988, 9, 151–159. [Google Scholar]
- Ivorra, M.D.; Paya, M.; Villar, A. A review of natural products andplants as potential antidiabetic drugs. Ethnopharmacology 1989, 27, 243–275. [Google Scholar] [CrossRef]
- Francis, G.; Kerem, Z.; Makkar, H.P.S.; Becker, K. The biological action of saponins in animal systems: A review. Br. J. Nutr. 2002, 88, 587–605. [Google Scholar] [CrossRef]
- Sheweita, S.A.; Mashaly, S.; Newairy, A.A.; Abdou, H.M.; Eweda, S.M. Changes in Oxidative Stress and Antioxidant Enzyme Activities in Streptozotocin—Induced Diabetes Mellitus in Rats: Role of Alhagimaurorum Extracts. Oxid. Med. Cell. Longev. 2016, 2016, 5264064. [Google Scholar] [CrossRef]
- Coman, C.; Rugină, O.D.; Socaciu, C. Plants and Natural Compounds with Antidiabetic Action. Not. Bot. Horti. Agrob. 2012, 40, 314–325. [Google Scholar] [CrossRef]
- Patel, S.B.; Santani, D.; Shah, M.B.; Patel, V.S. Anti–hyperglycemic and Anti–hyperlipidemic Effects of Bryonia Laciniosa Seed Extract and its Saponin Fraction in Streptozotocin–induced Diabetes in Rats. J. Young Pharm. 2012, 4, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.G.; Bundey, S.E.; Macleod, A.F. Neurodegeneration and diabetes: UK nationwide study of Wolfram (DIDMOAD) syndrome. Lancet 1995, 346, 1458–1463. [Google Scholar] [CrossRef]
- Choi, M.R.; Kwak, S.M.; Bang, S.H.; Jeong, J.E.; Kim, D.J. Chronic saponin treatment attenuates damage to the pancreas in chronic alcohol–treated diabetic rats. J. Ginseng. Res. 2016, 41, 503–512. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sharma, V.; Paliwal, R. Isolation and characterization of saponin from Moringa oleifera (Moringaeceae) pods. Int. J. Pharm. Pharmacol. Sci. 2013, 5, 179–183. [Google Scholar]
- Kim, J.J.; Xiao, H.; Tan, Y.; Wang, Z.Z.; Paul Seale, J.; Qu, X. The effects and mechanism of saponins of Panax notoginsengon glucose metabolism in 3T3–L1 Cells. Am. J. Chin. Med. 2009, 37, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Shu, G.; Yang, Z.; Mo, S.; Zhao, Y.; Mei, Z. Antidiabeticeffect of total saponins from Entada phaseoloides (L.) Merr. in type2 diabetic rats. J. Ethnopharmacol. 2012, 139, 814–821. [Google Scholar] [CrossRef]
- Elekofehinti, O.O.; Kamdem, J.P.; Kade, I.J.; Rocha, J.B.T.; Adanlawo, I.G. Hypoglycemic, antiperoxidative and antihyperlipi-demic effects of saponins from Solanum anguivi Lam. Fruits in alloxan-induced diabetic rats. S. Afr. J. Bot. 2013, 88, 56–61. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Kim, Y.S.; Ryu, Y.S.; Choi, Y.H.; Cha, M.R.; Yang, H.J.; Park, S. Platyconic acid, a saponin from Platycodi radix, improves glucose homeostasis by enhancing insulin sensitivity in vitro and in vivo. Eur. J. Nutr. 2012, 51, 529–540. [Google Scholar] [CrossRef]
- Metwally, N.S.; Mohamed, A.M.; ELSharabasy, F.S. Chemical con-stituents of the Egyptian Plant Anabasis articulata (Forssk) Moq andits antidiabetic effects on rats with streptozotocin-induced diabetichepatopathy. J. Appl. Pharm. Sci. 2012, 2, 54–65. [Google Scholar]
- Marie, A.; Anuff-Harding, M.C.; Omoruyi, F. Intestinal disaccha-rides and some renal enzymes in streptozotocin-induced diabeticrats fed sapogenin extract from bitter yam. Life Sci. 2006, 78, 2595–2600. [Google Scholar]
- Mirunalini, S.; Shahira, R. Novel effect of diosgenin—A plant derivedsteroid: A review. Pharmacologyonline 2011, 1, 726–736. [Google Scholar]
- Habicht, S.D.; Kind, V.; Rudloff, S.; Borsch, C.; Mueller, A.S.; Pal-lauf, J.; Yang, R.; Krawinke, M.B. Quantification of antidiabetic extracts and compounds in bitter gourd varieties. Food Chem. 2011, 126, 172–176. [Google Scholar] [CrossRef]
- Lee, K.T.; Jung, T.W.; Lee, H.J.; Kim, S.G.; Shin, Y.S.; Whang, W.K. The antidiabetic effect of genosenoside Rb2 via activation of AMPK. Arch. Pharm. Res. 2011, 34, 1201–1208. [Google Scholar]
- Denga, Y.; Kai, H.; Xiaoli, Y.; Xin, C.; Jing, H.; Xuegang, L.; Lujiang, Y.; Yalan, J.; Qing, J.; Panpan, L. Saponin rich fractions from Polygonatumodoratum (Mill.) druce with more potential hypoglycemic effects. J. Ethnopharmacol. 2012, 141, 228–233. [Google Scholar] [CrossRef]
- McAnuff, M.A.; Harding, W.W.; Omoruyi, F.O.; Jacobs, H.; MorrisonAsemota, H.E.Y.N. Hypoglycemic effects of steroidal sapogeninsisolated from Jamaican bitter yam, Dioscorea polygonoides. Food Chem. Toxicol. 2005, 43, 1667–1672. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, S.K.; Föller, M.; Gu, S.; Vir, S.; Shah, M.B.; Bhutani, K.K.; Santani, D.D.; Lang, F. Involvement of the PI3K/AKT pathway in thehypoglycemic effects of saponins from Helicteres isora. J. Ethnopharmacol. 2009, 126, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Elekofehinti, O.O.; Omotuyi, I.O.; Kamdem, J.P.; Ejelonu, O.C.; Alves, G.V.; Adanlawo, I.G.; Rocha, J.B. Saponin as regulator of biofuel: Implication for ethnobotanical management of diabetes. J. Physiol. Biochem. 2014, 70, 555–567. [Google Scholar] [CrossRef]
- Evans, W.C. Trease and Evans’s Pharmacognosy, 14th ed.; Saunders: London, UK, 1996; pp. 124–143. [Google Scholar]
- Brownlee, M. Biochemistry and molecular cell biology of diabeticcomplications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Baynes, J.W. Role of oxidative stress in development of complicationsin diabetes. Diabetes 1991, 40, 405–412. [Google Scholar] [CrossRef]
- Forbes, J.; Coughlan, M.; Cooper, M. Oxidative stress as a major culpritin kidney disease in diabetes. Diabetes 2008, 57, 1446–1454. [Google Scholar] [CrossRef]
- Elekofehinti, O.O.; Kamdem, J.P.; Kade, I.J.; Adanlawo, I.G.; Rocha, J.B.T. Saponins from Solanum anguivi lam. fruit exhibit in vitro and in vivo antioxidant activities in alloxan-induced oxidative stress. Asian J. Pharm. Clin. Res. 2012, 6, 249–254. [Google Scholar]
- Gülcin, I.; Küfrevioglu, I.; Oktay, M.; Büyükokuroglu, M.E. Antioxi-dant, antimicrobial, antiulcer and analgesic activities of nettle (Urticadioica L.). J. Ethnopharmacol. 2004, 90, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Elekofehinti, O.O.; Kade, I.J. Aqueous extract of Solanum anguiviLam. fruits (African egg plant) inhibit Fe2+ and SNP induced lipidperoxidation in Rat’s brain—In Vitro. Der Pharm. Lett. 2012, 4, 1352–1359. [Google Scholar]
- Xi, M.; Hai, C.; Tang, H.; Chen, M.; Fang, K.; Liang, X. Antioxidant andantiglycation properties of total saponins extracted from traditional Chinese medicine used to treat diabetes mellitus. Phytother. Res. 2008, 22, 228–237. [Google Scholar] [CrossRef]
- Siddiqui, B.S.; Karzhaubekova, Z.Z.; Burasheva, G.S.; Sultanova, N.A. Chemical constituents of the aerial parts of Kalidium foliatum. Chem. Pharm. Bull. 2007, 55, 1356–1360. [Google Scholar] [CrossRef] [PubMed]
- Sakatani, T.; Shirayama, T.S.; Uzaki, Y.; Yamamoto, T.M.; Ani, H.; Kawasaki, T.; Sug, H.; Matsubara, H. The association between cholesteroland mortality in heart failure. Comparison between patients with andwithout coronary artery disease. Int. Heart J. 2005, 46, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.B.; An, Y.R.; Kim, S.J.; Park, H.W.; Jung, W.; Kyung, J.S.; Hwang, S.Y.; Kim, Y.S. Lipid metabolic effect of Korean red ginseng extract in mice fed on a high-fat diet. J. Sci. Food Agric. 2011, 92, 388–396. [Google Scholar] [CrossRef]
- Gregoire, F.M. Adipocyte differentiation: From fibroblast to endocrinecell. Exp. Biol. Med. 2001, 226, 997–1002. [Google Scholar] [CrossRef]
- Shang, W.; Ying, Y.; Boren, J.; Hua, J.; Libin, Z.; Shangquan, L.; Mingdao, C. Ginsenoside Rb1 promotes adipogenesis in 3T3-L1 cells by enhancing PPARγ2 and C/EBPα gene expression. Life Sci. 2007, 80, 618–625. [Google Scholar] [CrossRef]
- Tammi, A.; Ronnemaa, T.; Gylling, H. Plant stanol ester margarine low-ers serum total and low-density lipoprotein cholesterol concentrationsof healthy children: The STRIP project. Special Turku Coronary Risk Factors Intervention Project. J. Pediatr. 2000, 136, 503–510. [Google Scholar] [CrossRef]
- Kelley, D.E.; Goodpaster, B.H. Skeletal muscle triglyceride. An aspect of regional adiposity and insulin resistance. Diabetes Care 2001, 24, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Hamao, M.; Matsuda, H.; Nakamura, S.; Nakashima, S.; Semura, S.; Maekubo, S.; Wakasugi, S.; Yoshikawa, M. Anti-obesity effects of the methanolic extract and chaka saponins from the flower buds of Camellia sinensis in mice. Bioorg. Med. Chem. 2011, 19, 6033–6041. [Google Scholar] [CrossRef] [PubMed]
- Han, L.K.; Zheng, Y.N.; Xu, B.J.; Okuda, H.; Kimura, Y. Saponins from Platycodi radix ameliorate high fat diet-induced obesity in mice. J. Nutr. 2002, 132, 2241–2245. [Google Scholar] [CrossRef] [PubMed]
- Eu, C.H.; Lim, W.Y.; Ton, S.H.; Kadir, K.A. Glycyrrhizic acid improvedlipoprotein lipase expression, insulin sensitivity, serum lipid and lipid deposition in high—Fat diet induced obese rats. Lipids Health Dis. 2010, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Fuller, S.; Stephens, J.M. Diosgenin, 4-hydroxyisoleucine, and fiber from fenugreek: Mechanisms of actions and potential effects on metabolic syndrome. Adv. Nutr. 2015, 6, 189–197. [Google Scholar] [CrossRef]
- Kalailingam, P.; Kannaian, B.; Tamilmani, E.; Kaliaperumal, R. Efficacy of natural Diosgenin on cardiovascular risk, insulin secretion, and beta cells in Streptozotocin (STZ)-induced diabetic rats. Phytomedicine 2014, 21, 1154–1161. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Singh, S.; Chhipa, R.; Bhat, M. The hypoglycemic activity of fenugreek seed extract is mediated through the stimulation of an insulin signaling pathway. Br. J. Pharm. 2005, 146, 41–48. [Google Scholar] [CrossRef]
- Broca, C.; Gross, R.; Petit, P.; Sauvaire, Y.; Manteghetti, M.; Tournier, M.; Masiello, P.; Gomis, R.; Ribes, G. 4-Hydroxyisoleucine: Experimental evidence of its insulinotropic and antidiabetic properties. Am. J. Physiol. 1999, 277, 617–623. [Google Scholar] [CrossRef]
- Broca, C.; Manteghetti, M.; Gross, R.; Baissac, Y.; Jacob, M.; Petit, P.; Sauvaire, Y.; Ribes, G. 4-Hydroxyisoleucine: Effects of synthetic and natural analogues on insulin secretion. Eur. J. Pharm. 2000, 390, 339–345. [Google Scholar] [CrossRef]
- Gupta, A.; Gupta, R.; Lal, B. Effect of Trigonella foenum-graecum (fenugreek) seeds on glycaemic control and insulin resistance in type 2 diabetes mellitus: A double blind placebo controlled study. J. Assoc. Physicians India 2001, 49, 1057–1061. [Google Scholar]
- Kassain, N.; Azadbakht, L.; Forghani, B.; Amimi, M. Effect of fenugreek seeds on blood glucose and lipid profiles in type 2 diabetic patients. Int. J. Vitam. Nutr. Res. 2009, 79, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Masmoudi-Allouche, F.; Touati, S.; Mnafgui, K.; Gharsallah, N.; El Feki, A.; Allouche, N. Phytochemical profile, antioxidant, antibacterial, antidiabetic and anti-obesity activities of fruits and pits from date palm (Phoenix dactylifera L.) grown in south of Tunisia. J. Pharmacogn. Phytochem. 2016, 5, 15–22. [Google Scholar]
- Shobana, S.; Sreerama, Y.N.; Malleshi, N. Composition and enzyme inhibitory properties of finger millet (Eleusine coracana L.) seed coat phenolics: Mode of inhibition of α- glucosidase and pancreatic amylase. Food Chem. 2009, 115, 1268–1273. [Google Scholar] [CrossRef]
- Cazarolli, L.H.; Zanatta, L.; Alberton, E.H.; Figueiredo, M.S.; Folador, P.; Damazio, R.G. Flavonoids: Prospective drug candidates. Mini Rev. Med. Chem. 2008, 8, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Farva, D.; Goji, I.A.; Joseph, P.K.; August, K.T. Effects of garlic oil on streptozotocin-diabetic rats maintained on normal and high fat diets. Indian J. Biochem. Biophys. 1986, 23, 24–27. [Google Scholar] [PubMed]
- Srivastana, V.K.; Afao, Z. Garlic extract inhibits accumulation of polyols and hydration in diabetic rat lens. Curr. Sci. 1989, 58, 376–377. [Google Scholar]
- Reuter, H.D.; Sendl, A. Allium sativum and Allium ursinum: Chemistry, pharmacology and medicinal applications. In Economic and Medicinal Plants Research; Wagner, H., Farnsworth, N.R., Eds.; Academic Press: London, UK, 1994; Volume 6, pp. 55–113. [Google Scholar]
- Venmadhi, S.; Devaki, T. Studies on some liver enzymes in rats ingesting ethanol and treated with garlic oil. Med. Sci. Res. 1992, 20, 729–731. [Google Scholar]
- Mathew, P.T.; Augusti, K.T. Studies on the effect of allicin (diallyl disulphide-oxide) on alloxan diabetes: Part Ihypoglycaemic action and enhancement of serum insulin effect and glycogen synthesis. Indian J. Biochem. Biophys. 1973, 10, 209–212. [Google Scholar]
- Banerjee, S.K.; Maulik, S.K. Effect of garlic on cardiovascular disorder: A review. Nutr. J. 2002, 1, 4–15. [Google Scholar] [CrossRef]
- Augusti, K.T.; Sheela, C.G. Antidiabetic effect of SACS isolated from garlic Allium sativum L. Indian J. Exp. Biol. 1992, 30, 523–526. [Google Scholar]
- Andallu, B.; Suryakantham, V.; Lakshmi, S.B. Effect of mulberry (Morms indica L) therapy on plasma and erythrocyte membrane lipids in patients with Type 2 diabetes. Clin. Chem. Acta 2001, 314, 47–53. [Google Scholar] [CrossRef]
- Kumari, K.; Mathew, B.C.; Augusti, K.T. Anti-diabetic and hypolipidaemic effects of S-methyl cysteine sulfoxide isolated from Allium cepa Linn. Indian J. Biochem. Biophys. 1995, 32, 49–54. [Google Scholar] [PubMed]
- Chang, M.L.W.; Johnson, M.A. Effect of garlic on carbohydrate metabolism and lipid synthesis in rats. J. Nutr. 1980, 110, 931–936. [Google Scholar] [CrossRef]
- Carson, J.F. Chemistry and biological properties of onion and garlic. Food Rev. Intern. 1987, 3, 71–103. [Google Scholar] [CrossRef]
- Augusti, K.T. Therapeutic values of onion (Allium cepa) and Garlic (Allium sativum). Indian J. Exp. Biol. 1996, 34, 634–640. [Google Scholar]
- Dhandapani, S.; Subramanian, V.R.; Rajagopal, S.; Namasivayam, N. Hypolipidemic effect of Cuminum cyminum L. on alloxan-induced diabetic rats. Pharm. Res. 2002, 46, 251–255. [Google Scholar] [CrossRef]
- Jagtap, A.G.; Patil, P.B. Antihyperglycemic activity and inhibition of advanced glycation end product formation byCuminum cyminumin streptozotocin induced diabetic rats. Food Chem. Toxicol. 2010, 48, 2030–2036. [Google Scholar] [CrossRef] [PubMed]
- Deepak. Importance of Cuminum cyminum L. and Carum carvi L. in traditional medicaments-A review. Indian J. Tradit. Knowl. 2013, 12, 300–307. [Google Scholar]
- Saibandith, B.; Spencer, J.P.E.; Rowland, I.R.; Commane, D.M. Olive polyphenols and the metabolic syndrome. Molecules 2017, 22, 1082. [Google Scholar] [CrossRef] [PubMed]
- Javadi, H.; Yaghoobzadeh, H.; Esfahani, Z.; Reza Memarzadeh, M.; Mehdi Mirhashemi, S. Effects of olive leaf extract on metabolic response, liver and kidney functions and inflammatory biomarkers in hypertensive patients. Pak. J. Biol. Sci. PJBS 2019, 22, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, M.; Di Mise, A.; Centrone, M.; D’Agostino, M.; Tingskov, S.J.; Venneri, M.; Pellegrino, T.; Difonzo, G.; Caponio, F.; Norregaard, R.; et al. Olive Leaf Extract (OLE) impaired vasopressin-induced aquaporin-2 trafficking through the activation of the calcium-sensing receptor. Sci. Rep. 2021, 11, 4537. [Google Scholar] [CrossRef]
- Cheurfa, M.; Abdallah, H.H.; Allem, R.; Noui, A.; Picot-Allain, C.M.N.; Mahomoodally, F. Hypocholesterolaemic and antioxidant properties of Olea europaea L. leaves from Chlef province, Algeria using in vitro, in vivo and in silico approaches. Food Chem. Toxicol. 2018, 123, 98–105. [Google Scholar] [CrossRef]
- Annunziata, G.; Maisto, M.; Schisano, C.; Barrea, L.; Ciampaglia, R.; Narcis, V.; Tenore, G.C.; Novellino, E. Oleuropein as a novel anti-diabetic nutraceutical. An overview. Arch. Diabetes Obes. 2018, 1, 54–58. [Google Scholar] [CrossRef]
- Carnevale, R.; Silvestri, R.; Loffredo, L.; Novo, M.; Cammisotto, V.; Castellani, V.; Bartimoccia, S.; Nocella, C.; Violi, F. Oleuropein, a component of extra virgin olive oil, lowers postprandial glycaemia in healthy subjects. Br. J. Clin. Pharm. 2018, 84, 1566–1574. [Google Scholar] [CrossRef]
- Ranieri, M.; Di Mise, A.; Difonzo, G.; Centrone, M.; Venneri, M.; Pellegrino, T.; Russo, A.; Mastrodonato, M.; Caponio, F.; Valenti, G.; et al. Green olive leaf extract (OLE) provides cytoprotection in renal cells exposed to low doses of cadmium. PLoS ONE 2019, 14, e0214159. [Google Scholar] [CrossRef]
- Benlarbi, M.; Jemai, H.; Hajri, K.; Amri, E.; Jebbari, M.; Hammoun, I.; Baccouche, B.; Boudhrioua Mihoubi, N.; Ben Chaouacha-Chekir, R.; Dhifi, W. Neuroprotective effect of oleuropein on retina photoreceptors cells primary culture and olive leaf extract and oleuropein inhibitory effects on aldose reductase in a diabetic model: Meriones of Shaw (Meriones shawi). Arch. Physiol. Biochem. 2020, 1, 1–8. [Google Scholar] [CrossRef]
- Centrone, M.; D’Agostino, M.; Difonzo, G.; De Bruno, A.; Di Mise, A.; Ranieri, M.; Montemurro, C.; Valenti, G.; Poiana, M.; Caponio, F.; et al. Antioxidant Efficacy of Olive By-Product Extracts in Human Colon HCT8 Cells. Foods 2020, 10, 11. [Google Scholar] [CrossRef]
- Ahmadi, S.; Mainali, R.; Nagpal, R.; Sheikh-Zeinoddin, M.; Soleimanian-Zad, S.; Wang, S.; Deep, G.; Kumar Mishra, S.; Yadav, H. Dietary Polysaccharides in the Amelioration of Gut Microbiome Dysbiosis and Metabolic Diseases. Obes. Control 2017, 4. [Google Scholar] [CrossRef]
- Capuano, E. Behavior of dietary fiber in the gastrointestinal tract determines its physiological effect. Crit. Rev. Food Sci. Nutr. 2017, 57, 3543–3564. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.K.; Prasad, S.K.; Kumar, R.; Hemalatha, S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac. J. Trop. Biomed. 2012, 2, 320–330. [Google Scholar] [CrossRef]
- Rivellese, A.A.; Maffettone, A. Dietary Fiber in the Treatment of Metabolic Diseases. Eur. J. Clin. Nutr. 1995, 49, 110–112. [Google Scholar]
- Moreno Franco, B.; Leon Latre, M.; Andres Esteban, E.M.; Ordovas, J.M.; Casasnovas, J.A.; Penalvo, J.L. Soluble and insoluble dietary fibre intake and risk factors for metabolic syndrome and cardiovascular disease in middle-aged adults: The AWHS cohort. Nutr. Hosp. 2014, 30, 1279–1288. [Google Scholar] [PubMed]
- Mann, J.I.; De Leeuw, I.; Hermansen, K.D.; Karamanos, B.; Karlström, B.; Katsilambros, N.; Riccardi, G.; Rivellese, A.A.; Rizkalla, S.; Slama, G.; et al. Evidence-based nutritional approaches to the treatment and prevention of diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2004, 14, 373–394. [Google Scholar] [CrossRef]
- Gaikwad, S.B.; Krishna Mohan, G.; Sandhya Rani, M. Phytochemicals for Diabetes Management. Pharm. Crops 2014, 25, 11–28. [Google Scholar] [CrossRef]
- Chen, H.; Qu, Z.; Fu, L.; Dong, P.; Zhang, X. Physicochemical properties and antioxidant capacity of 3 polysaccharides from green tea, oolong tea, and black tea. J. Food Sci. 2009, 74, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Semwal, A.; Kumar, H.; Verma, H.C.; Kumar, A. In-vivo study for anti-hyperglycemic potential of aqueous extract of Basil seeds (Ocimum basilicum Linn) and its influence on biochemical parameters, serum electrolytes and haematological indices. Biomed. Pharm. 2016, 84, 2008–2013. [Google Scholar] [CrossRef]
- Ribes, G.; Sauvaire, Y.; Da Costa, C.; Baccou, J.C.; Loubatieres-Mariani, M.M. Antidiabetic effects of subtractions from fenugreek seeds in diabetic dogs. Proc. Soc. Exp. Biol. Med. 1986, 182, 159–166. [Google Scholar] [CrossRef]
- Quanhong, L.; Caili, F.; Yukui, R.; Guanghui, H.; Tongyi, C. Effects of proteinbound polysaccharide isolated from pumpkin on insulin in diabetic rats. Plant Foods Hum. Nutr. 2005, 60, 13–16. [Google Scholar] [CrossRef]
- Lu, Z.X.; Walker, K.Z.; Muir, J.G.; Mascara, T.; Dea, O.K. Arabinoxylan fiber, a byproduct of wheat flour processing, reduces the postprandial glucose response in normoglycemic subjects. Am. J. Clin. Nutr. 2000, 71, 1123–1128. [Google Scholar] [CrossRef]
- Behall, K.M.; Scholfield, D.J.; Hallfrisch, J.G.; Liljeberg-Elmstahl, H.G. Consumption of both resistant starch and β-glucan improves postprandial plasma glucose and insulin in women. Diabetes Care 2006, 29, 976–981. [Google Scholar] [CrossRef]
- Baldi, A.; Swapnil, G. Hypoglycemic effect of polyherbal formulation in alloxan induced diabetic rats. Pharmacologyonline 2011, 3, 764–773. [Google Scholar]
- Tapsell, L.C.; Hemphill, I.; Cobiac, L.; Patch, C.S.; Sullivan, D.R.; Fenech, M.; Roodenrys, S.; Keogh, J.B.; Clifton, P.M.; Williams, P.G.; et al. Health benefits of herbs and spices: The past, the present, the future. Med. J. Aust. 2006, 185, S1–S24. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.M.; Baviskar, D.T.; Chaudhari, P.M. Anti-Diabetic Activity of Polyherbal Formulation on Alloxan Induced Diabetes. IOSR J. Pharm. Biol. Sci. 2018, 13, 2319–7676. [Google Scholar]
- Vuksan, V.; Sievenpiper, J.L. Herbal remedies in the management of diabetes: Lessons learned from the study of ginseng. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 149–160. [Google Scholar] [CrossRef]
- Combs, G.F. Vitamin C. In The Vitamins, Fundamental Aspects in Nutrition and Health, 4th ed.; Elsevier: London, UK, 2012; pp. 233–261. [Google Scholar]
- Chen, H.; Karne, R.J.; Hall, G. High-dose oral vitamin C partially replenishes vitamin C levels in patients with type 2 diabetes and low vitamin C levels but does not improve endothelial dysfunction or insulin resistance. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, 137–145. [Google Scholar] [CrossRef]
- Khodaeian, M.; Tabatabaei-Malazy, O.; Qorbani, M.; Farzadfar, F.; Amini, P.; Larijani, B. Effect of vitamins C and E on insulin resistance in diabetes: A meta-analysis study. Eur. J. Clin. Investig. 2015, 45, 1161–1174. [Google Scholar] [CrossRef]
- Tabatabaei-Malazy, O.; Nikfar, S.; Larijani, B.; Abdollahi, M. Influence of ascorbic acid supplementation on type 2 diabetes mellitus in observational and randomized controlled trials; a systematic review with meta-analysis. J. Pharm. Pharmacol. Sci. 2014, 17, 554–582. [Google Scholar] [CrossRef]
- Anonymous. Annual Reports: Howletts and Port Lympne Zoo Parks, UK. Int. Zoo News 1992, 39, 30–41. [Google Scholar]
- Tabatabaei-Malazy, O.; Larijani, B.; Abdollahi, M. A systematic review of in vitro studies conducted on effect of herbal products on secretion of insulin from Langerhans islets. J. Pharm. Pharmacol. Sci. 2012, 15, 447–466. [Google Scholar]
- Jacques-Camarena, O.; González-Ortiz, M.; Martínez-Abundis, E.; López-Madrueño, J.P.; Medina-Santillán, R. Effect of vanadium on insulin sensitivity in patients with impaired glucose tolerance. Ann. Nutr. Metab. 2008, 53, 195–198. [Google Scholar] [CrossRef]
- Pandey, S.K.; Anand-Srivastava, M.B.; Srivastava, A.K. Vanadyl sulfate-stimulated glycogen synthesis is associated with activation of phosphatidylinositol 3-kinase and is independent of insulin receptor tyrosine phosphorylation. Biochemistry 1998, 37, 7006–7014. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Rizkalla, S.W.; Boillot, J. Dietary (n−3) polyunsaturated fatty acids improve adipocyte insulin action and glucose metabolism in insulin resistant rats: Relation to membrane fatty acids. J. Nutr. 1996, 126, 1951–1958. [Google Scholar] [PubMed]
- Wyn Snow, Managing Editor. With Diabetes Surging Some Look for Alternative Treatment, 20 October 2006.
- Ghadge, A.A.; Kuvalekar, A.A. Controversy of oral hypoglycemic agents in type 2 diabetes mellitus: Novel move towards combination therapies. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, S5–S13. [Google Scholar] [CrossRef] [PubMed]
- Thorne, M.J.; Thompson, L.U.; Jenkins, D.J. Factors affecting starch digestibility and the glycemic response with special reference to legumes. Am. J. Clin. Nutr. 1983, 38, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Centrone, M.; Gena, P.; Ranieri, M.; Di Mise, A.; D’Agostino, M.; Mastrodonato, M.; Venneri, M.; De Angelis, D.; Pavan, S.; Pasqualone, A.; et al. In Vitro and In Vivo Nutraceutical Characterization of Two Chickpea Accessions: Differential Effects on Hepatic Lipid Over-Accumulation. Antioxidants 2020, 9, 268. [Google Scholar] [CrossRef]


| Organs | Biological Mechanisms | ||||
|---|---|---|---|---|---|
| Medicinal Plants | Liver | ↑PPAR-γ | ↑Glycogen synthesis ↓Gluconeogenesis | ↓Hepatic glucose output | Management of Diabetes and its Complications |
| ↑PPAR-α and PPAR-δ | ↓Fatty acid synthesis ↑Fatty acid oxidation | ↓Liver fats ↑Hepatic insulin sensitivity | |||
| ↑Energy expenditure | |||||
| ↑AMPK | ↓Blood glucose ↑Insulin sensitivity ↓Serum lipids | ||||
| ↓ACC | ↓Blood glucose ↑Insulin sensitivity ↓Serum lipids | ||||
| Adipocytes | ↑PPAR-α and PPAR-δ | ↓Fat intake, fatty acid synthesis ↑Energy expenditure | |||
| ↑PPAR-γ, adiponectin ↑TNF-α | ↑Energy expenditure ↑Insulin sensitivity, glucose uptake | ||||
| Inhibition of AMPK Activation of Akt | ↑Differentiation of adipocytes | ↑Accumulation of fat | |||
| ↑GLUT4 | ↓Blood glucose ↑Insulin sensitivity ↓Serum lipids | ||||
| ↑Glc uptake | ↓Blood glucose ↑Insulin sensitivity ↓Serum lipids | ||||
| Skeletal Muscle | ↑PI3K and P38-MAPK | ↑Insulin sensitivity, glucose uptake | |||
| ↑Expression and allocation of GLUT4 | ↑Glucose uptake | ||||
| ↑Glc uptake | ↓Blood glucose ↑Insulin sensitivity ↓Serum lipids | ||||
| Pancreas | ↑Insulin synthesis and secretion cellular signaling | ↑Glucose clearance | |||
| ↑β-cell regeneration | ↑Glucose clearance | ||||
| Gastrointestinal Tract | ↓α-amylase and α-glucosidase | ↓Absorption of glucose | |||
| Flavonoids | Subclasses | Chemical Structure | Examples |
|---|---|---|---|
 Flavan (Basic Structure of Flavonoids) | Flavanones |  |
|
| Flavonols |  |
| |
| Isoflavones |  |
| |
| Flavan-3-ols |  |
| |
| Flavones |  |
| |
| Flavonolignans |  |
| |
| Anthocyanidins |  |
| |
| Chalcones | 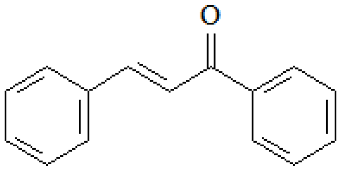 |
| Plant | Type of Polysaccharides | Antidiabetic Effect | Reference |
|---|---|---|---|
| Tea | Polysaccharides | High α-glucosidase-inhibitory activity in vitro and also in vivo (mice) Beneficial for hyperglycemia treatment in diabetes. | [147] |
| Basil seed (Ocimum basilicum) | Gum | Improvements in body weight, serum electrolytes, and hematological indices, along with increased pancreatic islets. | [148] |
| Fenugreek seeds (Trigonella foenum-graecum L.) | Fibers | The addition of the fiber-rich subfraction of fenugreek seeds to insulin treatment decreased hyperglycemia, glycosuria, plasma glucagon, and somatostatin levels in diabetic dogs. | [149] |
| Pumpkin | Protein-bound polysaccharide (PBPP) | PBPP increased serum insulin, reduced the blood glucose, and improved glucose tolerance in diabetic rats in a dose-dependent manner. | [150] |
| Wheat | Arabinoxylan | Postprandial glucose and insulin responses improved upon ingestion of arabinoxylan-rich fiber in human subjects. | [151] |
| Oatrim | β-glucan | oatrim fibers improve postprandial insulin release and glucose levels in normal and overweight persons. | [152] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhujaily, M.; Dhifi, W.; Mnif, W. An Overview of the Potential of Medicinal Plants Used in the Development of Nutraceuticals for the Management of Diabetes Mellitus: Proposed Biological Mechanisms. Processes 2022, 10, 2044. https://doi.org/10.3390/pr10102044
Alhujaily M, Dhifi W, Mnif W. An Overview of the Potential of Medicinal Plants Used in the Development of Nutraceuticals for the Management of Diabetes Mellitus: Proposed Biological Mechanisms. Processes. 2022; 10(10):2044. https://doi.org/10.3390/pr10102044
Chicago/Turabian StyleAlhujaily, Muhanad, Wissal Dhifi, and Wissem Mnif. 2022. "An Overview of the Potential of Medicinal Plants Used in the Development of Nutraceuticals for the Management of Diabetes Mellitus: Proposed Biological Mechanisms" Processes 10, no. 10: 2044. https://doi.org/10.3390/pr10102044
APA StyleAlhujaily, M., Dhifi, W., & Mnif, W. (2022). An Overview of the Potential of Medicinal Plants Used in the Development of Nutraceuticals for the Management of Diabetes Mellitus: Proposed Biological Mechanisms. Processes, 10(10), 2044. https://doi.org/10.3390/pr10102044









