Removal of Hydrogen Sulfide and Ammonia Using a Biotrickling Filter Packed with Modified Composite Filler
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Modified Activated Carbon
2.2. Gas Static Adsorption Experiment
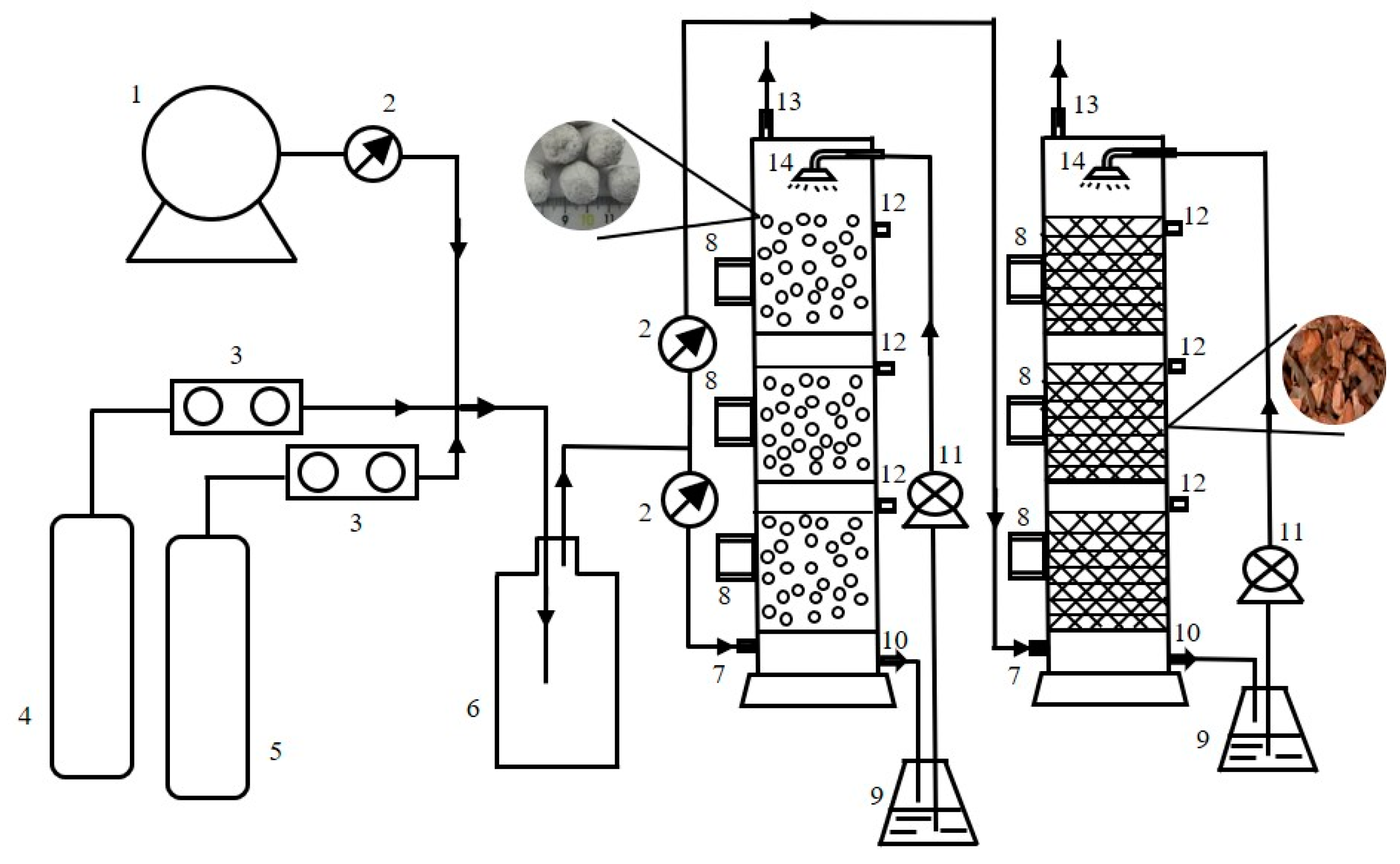
2.3. Experimental Set up
2.4. Analytical Methods
2.5. Statistical Analysis
3. Results
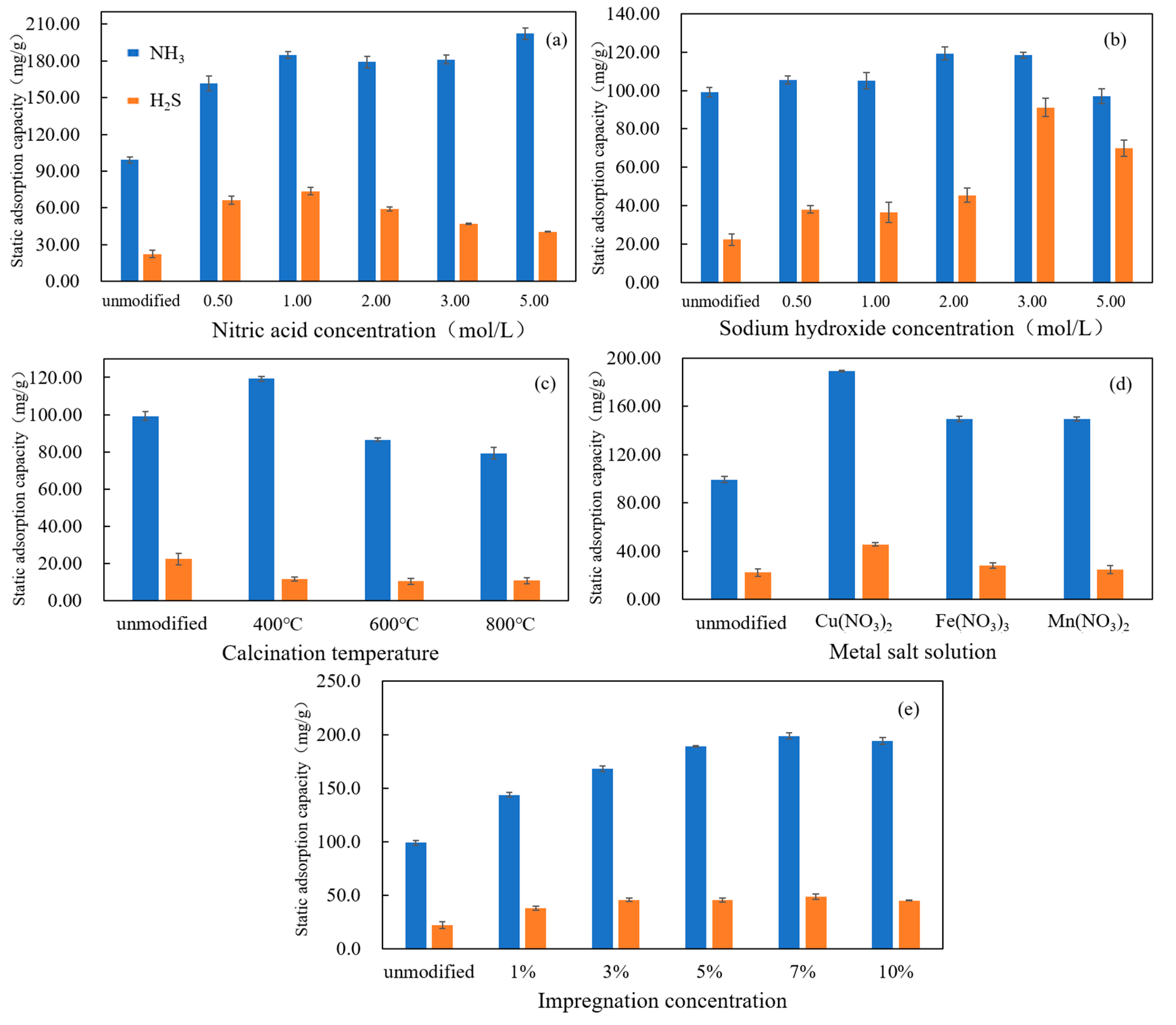
3.1. Performance of Static Adsorption in the Removal of H2S and NH3
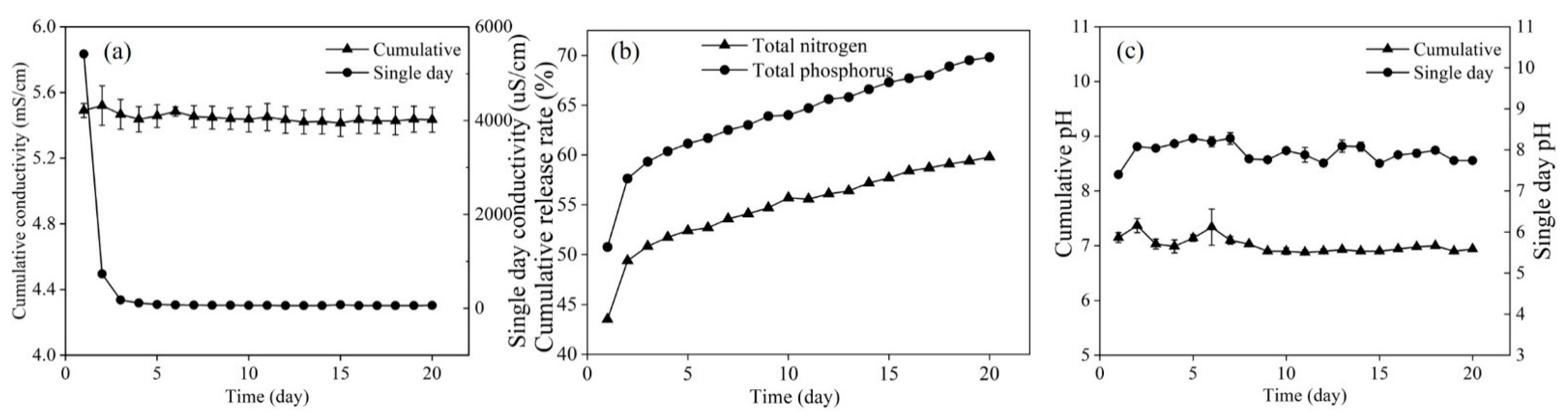
3.2. Performance of Composite Fillers
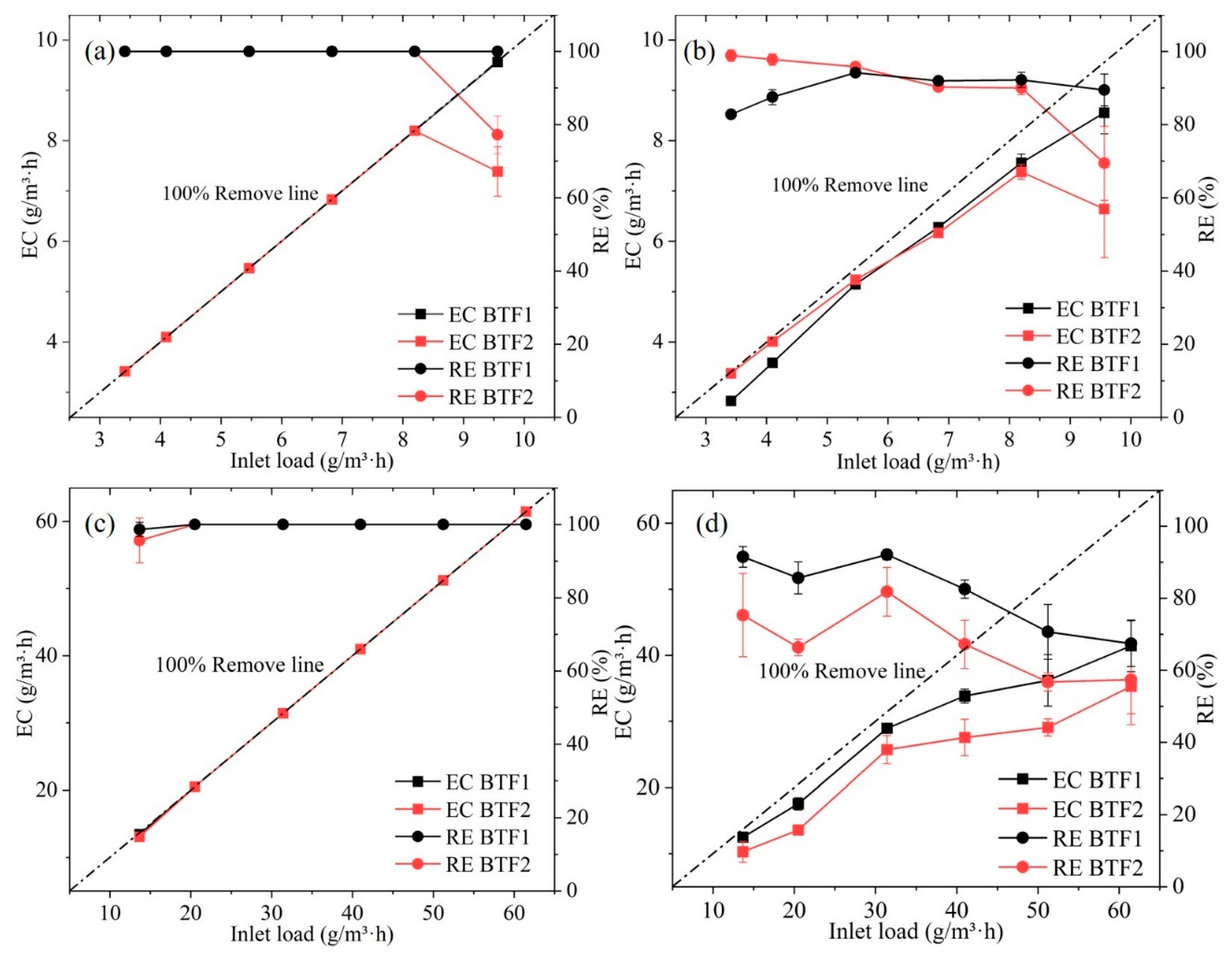
3.3. H2S and NH3 Removal Performance of the Two BTFs
3.4. Analysis of Microbial Community Structure
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chai, F.; Li, P.; Li, L.; Qiu, Z.; Han, Y.; Yang, K. Dispersion, olfactory effect, and health risks of VOCs and odors in a rural domestic waste transfer station. Environ. Res. 2022, 209, 112879. [Google Scholar] [CrossRef]
- Yuan, J.; Li, Y.; Zhang, H.; Zhang, D.; Chadwick, D.; Li, G.; Wang, G.; Chi, M.; Yang, F. Effects of adding bulking agents on the biodrying of kitchen waste and the odor emissions produced. J. Environ. Sci. 2018, 67, 344–355. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, Z.; Tang, C.; Liao, W.; Yu, Y.; Li, G.; Yang, Y.; An, T. Malodorous gases production from food wastes decomposition by indigenous microorganisms. Sci. Total Environ. 2020, 717, 137175. [Google Scholar] [CrossRef]
- Rybarczyk, P.; Szulczyński, B.; Gębicki, J.; Hupka, J. Treatment of malodorous air in biotrickling filters: A review. Biochem. Eng. J. 2019, 141, 146–162. [Google Scholar] [CrossRef]
- Gao, X.; Yang, F.; Cheng, J.; Xu, Z.; Zang, B.; Li, G.; Xie, X.; Luo, W. Emission of volatile sulphur compounds during swine manure composting: Source identification, odour mitigation and assessment. Waste Manag. 2022, 153, 129–137. [Google Scholar] [CrossRef]
- Zheng, T.; Li, L.; Chai, F.; Wang, Y. Factors impacting the performance and microbial populations of three biofilters for co-treatment of H2S and NH3 in a domestic waste landfill site. Process Saf. Environ. Prot. 2021, 149, 410–421. [Google Scholar] [CrossRef]
- Huan, C.; Fang, J.; Tong, X.; Zeng, Y.; Liu, Y.; Jiang, X.; Ji, G.; Xu, L.; Lyu, Q.; Yan, Z. Simultaneous elimination of H2S and NH3 in a biotrickling filter packed with polyhedral spheres and best efficiency in compost deodorization. J. Clean. Prod. 2021, 284, 124708. [Google Scholar] [CrossRef]
- Ying, S.; Kong, X.; Cai, Z.; Man, Z.; Xin, Y.; Liu, D. Interactions and microbial variations in a biotrickling filter treating low concentrations of hydrogen sulfide and ammonia. Chemosphere 2020, 255, 126931. [Google Scholar] [CrossRef]
- Huan, C.; Lyu, Q.; Tong, X.; Li, H.; Zeng, Y.; Liu, Y.; Jiang, X.; Ji, G.; Xu, L.; Yan, Z. Analyses of deodorization performance of mixotrophic biotrickling filter reactor using different industrial and agricultural wastes as packing material. J. Hazard. Mater. 2021, 420, 126608. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.; Han, Y.; Qu, Q.; Li, J.; Zhong, C.; Peng, D. Characteristics of low H2S concentration biogas desulfurization using a biotrickling filter: Performance and modeling analysis. Bioresour. Technol. 2019, 280, 143–150. [Google Scholar] [CrossRef]
- Lebrero, R.; Gondim, A.; Pérez, R.; García-Encina, P.; Muñoz, R. Comparative assessment of a biofilter, a biotrickling filter and a hollow fiber membrane bioreactor for odor treatment in wastewater treatment plants. Water Res. 2014, 49, 339–350. [Google Scholar] [CrossRef]
- Dorado, A.; Lafuente, J.; Gabriel, D.; Gamisans, X. The role of water in the performance of biofilters: Parameterization of pressure drop and sorption capacities for common packing materials. J. Hazard. Mater. 2010, 180, 693–702. [Google Scholar] [CrossRef]
- Pérez, M.; Álvarez-hornos, F.; Portune, K.; Gabaldn, C. Abatement of styrene waste gas emission by biofilter and biotrickling filter: Comparison of packing materials and inoculation procedures. Appl. Microbiol. Biotechnol. 2015, 99, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Parseh, I.; Hajizadeh, Y.; Jaafarzadeh, N.; Goudarzi, Z.; Shakerinejad, G.; Badeenezhad, A.; Mengelizadeh, N.; Fallahizadeh, S. Removal behavior of gaseous furfural using a biofilter packed with perlite, ripe compost, and oak woodchips. Process Saf. Environ. Prot. 2021, 149, 135–143. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Qin, Y.; Yang, Z.; Cao, J.; Xing, Y.; Li, J. Performance and microbial community evolution of toluene degradation using a fungi-based bio-trickling filter. J. Hazard. Mater. 2019, 365, 642–649. [Google Scholar] [CrossRef]
- Daneshyar, A.; Ghaedi, M.; Sabzehmeidani, M.; Daneshyar, A. H2S adsorption onto Cu-Zn-Ni nanoparticles loaded activated carbon and Ni-Co nanoparticles loaded gamma-Al2O3: Optimization and adsorption isotherms. J. Colloid Interface Sci. 2017, 490, 553–561. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, M.; Li, J.; Zheng, Z. Influence of moderate pre-oxidation treatment on the physical, chemical and phosphate adsorption properties of iron-containing activated carbon. J. Environ. Sci. 2014, 26, 519–528. [Google Scholar] [CrossRef]
- Hassani, A.; Khataee, A. 10-Activated carbon fiber for environmental protection. In Activated Carbon Fiber and Textiles; Woodhead Publishing Series in Textiles; Woodhead Publishing: Sawston, UK, 2017; pp. 245–280. [Google Scholar]
- Cheng, Z.; Feng, K.; Xu, D.; Kennes, C.; Chen, J.; Chen, D.; Zhang, S.; Ye, J.; Dionysiou, D. An innovative nutritional slow-release packing material with functional microorganisms for biofiltration: Characterization and performance evaluation. J. Hazard. Mater. 2019, 366, 16–26. [Google Scholar] [CrossRef]
- Bakhta, S.; Sadaoui, Z.; Lassi, U.; Romar, H.; Kupila, R.; Vieillard, J. Performances of metals modified activated carbons for fluoride removal from aqueous solutions. Chem. Phys. Lett. 2020, 754, 137705. [Google Scholar] [CrossRef]
- Pongkua, W.; Thiravetyan, P.; Dumont, E. New tool for the determination of the nitrogen accumulation rate in the washing liquid of a biotrickling filter treating ammonia emissions. Chem. Eng. J. 2020, 397, 125399. [Google Scholar] [CrossRef]
- Hamisi, R.; Renman, A.; Renman, G.; Wörman, A.; Thunvik, R. Long-term phosphorus sorption and leaching in sand filters for onsite treatment systems. Sci. Total Environ. 2022, 833, 155254. [Google Scholar] [CrossRef]
- Shin, J.; Lee, Y.; Lee, S.; Kim, S.; Ochir, D.; Park, Y.; Kim, J.; Chon, K. Single and competitive adsorptions of micropollutants using pristine and alkali-modified biochars from spent coffee grounds. J. Hazard. Mater. 2020, 400, 123102. [Google Scholar] [CrossRef]
- Qu, Y.; Xu, L.; Chen, Y.; Sun, S.; Wang, Y.; Guo, L. Efficient toluene adsorption/desorption on biochar derived from in situ acid-treated sugarcane bagasse. Environ. Sci. Pollut. Res. 2021, 28, 62616–62627. [Google Scholar] [CrossRef] [PubMed]
- Zhan, M.; Liu, Y.; Ye, W.; Chen, T.; Jiao, W. Modification of activated carbon using urea to enhance the adsorption of dioxins. Environ. Res. 2022, 204, 112035. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Gao, N.; Zhao, D.; An, N.; Li, L.; Xiao, J. Bromate reduction and reaction-enhanced perchlorate adsorption by FeCl3-impregnated granular activated carbon. Water Res. 2019, 149, 149–158. [Google Scholar] [CrossRef]
- Zhang, W.; He, Y.; Wang, Y.; Li, G.; Chen, J.; Zhu, Y. Comprehensive investigation on the gasification reactivity of pyrolysis residue derived from Ca-rich petrochemical sludge: Roles of microstructure characteristics and calcium evolution. Energy Convers. Manag. 2022, 253, 115150. [Google Scholar] [CrossRef]
- Yu, G.; Wang, G.; Wang, S.; Yang, C.; Chen, H.; Zhu, Y.; Yu, L.; Li, J.; Kazemian, H. Performance promotion and its mechanism for n-hexane removal in a lab-scale biotrickling filter with reticular polyurethane sponge under intermittent spraying mode. Process Saf. Environ. Prot. 2021, 152, 654–662. [Google Scholar] [CrossRef]
- Jia, T.; Zhang, L.; Zhao, Q.; Peng, Y. The effect of biofilm growth on the sulfur oxidation pathway and the synergy of microorganisms in desulfurization reactors under different pH conditions. J. Hazard. Mater. 2022, 432, 128638. [Google Scholar] [CrossRef]
- Hendrych, J.; Buzková, A.; Špaček, P. Innovative packing material for waste gas biofiltration. Environ. Technol. Innov. 2021, 24, 101837. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, X.; Jin, Z.; Yang, S.; Zhang, J. The performance and microbial community in a slightly alkaline biotrickling filter for the removal of high concentration H2S from biogas. Chemosphere 2020, 249, 126127. [Google Scholar] [CrossRef]
- Khanongnuch, R.; Di, C.; Lakaniemi, A.; Rene, E.; Lens, P. H2S removal and microbial community composition in an anoxic biotrickling filter under autotrophic and mixotrophic conditions. J. Hazard. Mater. 2019, 367, 397–406. [Google Scholar] [CrossRef]
- Ben, J.; Couvert, A.; Amrane, A.; Rouxel, F.; Cloirec, P.; Dumont, E. Biofiltration of H2S in air—Experimental comparisons of original packing materials and modeling. Biochem. Eng. J. 2016, 112, 153–160. [Google Scholar]
- Huynh, N.; Le, T.; Tran, L. Removal of H2S in biogas using biotrickling filter: Recent development. Process Saf. Environ. 2020, 144, 297–309. [Google Scholar] [CrossRef]
- Xia, G.; Zhou, X.; Hu, J.; Sun, Z.; Yao, J.; Chen, D.; Wang, J. Simultaneous removal of carbon disulfide and hydrogen sulfide from viscose fibre waste gas with a biotrickling filter in pilot scale. J. Clean. Prod. 2019, 230, 21–28. [Google Scholar] [CrossRef]
- Yao, X.; Shi, Y.; Wang, K.; Wang, C.; He, L.; Li, C.; Yao, Z. Highly efficient degradation of hydrogen sulfide, styrene, and m-xylene in a bio-trickling filter. Sci. Total Environ. 2022, 808, 152130. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Yang, J.; Zhu, R.; Nie, Y.; Jin, B.; Li, S. Performance evaluation and microbial community analysis of the composite filler micro-embedded with Pseudomonas putida for the biodegradation of toluene. Process Biochem. 2020, 92, 10–16. [Google Scholar] [CrossRef]
- Bu, H.; Carvalho, G.; Huang, C.; Sharma, K.; Yuan, Z.; Song, Y.; Bond, P.; Keller, J.; Yu, M.; Jiang, G. Evaluation of continuous and intermittent trickling strategies for the removal of hydrogen sulfide in a biotrickling filter. Chemosphere 2022, 291, 132723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Chen, C.; Xu, X.; Lee, D.; Ren, N. The interaction between Pseudomonas C27 and Thiobacillus denitrificans in the integrated autotrophic and heterotrophic denitrification process. Sci. Total Environ. 2022, 811, 152360. [Google Scholar] [CrossRef] [PubMed]
- Treinyte, J.; Bridziuviene, D.; Fataraite-urboniene, E.; Rainosalo, E.; Rajan, R.; Cesoniene, L.; Grazuleviciene, V. Forestry wastes filled polymer composites for agricultural use. J. Clean. Prod. 2018, 205, 388–406. [Google Scholar] [CrossRef]
- Wu, H.; Yin, Z.; Quan, Y.; Fang, Y.; Yin, C. Removal of methyl acrylate by ceramic-packed biotrickling filter and their response to bacterial community. Bioresour. Technol. 2016, 209, 237–245. [Google Scholar] [CrossRef]
- Zagorskis, A.; Januševičius, T.; Danila, V. Removal of acetone vapor from air using a biotrickling filter packed with polymeric bioballs. Processes 2022, 10, 57. [Google Scholar] [CrossRef]
- Wen, X.; Xu, H.; Huang, S.; Sun, C.; Tong, N.; Zhang, Y. Simultaneous removal of sulphur dioxide and nitric oxide at different oxygen concentrations in a thermophilic biotrickling filter (BTF): Evaluation of removal efficiency, intermediates interaction and characterisation of microbial communities. Bioresour. Technol. 2019, 294, 122150. [Google Scholar] [CrossRef]


| HNO3 (mol/L) | NaOH (mol/L) | Calcination Temperature (°C) | Metal Salt Solution | Impregnation Concentration (wt%) |
|---|---|---|---|---|
| 0.5 | 0.5 | 400 | Cu(NO3)2 | 1 |
| 1.0 | 1.0 | 3 | ||
| 2.0 | 2.0 | 600 | Fe(NO3)3 | 5 |
| 3.0 | 3.0 | 7 | ||
| 5.0 | 5.0 | 800 | Mn(NO3)2 | 10 |
| Phase | Period of Operation (Days) | EBRT (s) | Inlet Concentration (mg/m3) | |
|---|---|---|---|---|
| H2S | NH3 | |||
| I | 1~10 | 100 | 83.6 | 83.6 |
| II | 11~28 | 100~34 | 83.6 | 83.6 |
| III | 29~46 | 40 | 139.4~627.2 | 139.3~626.9 |
| Particle Size (mm) | Bulk Density (kg/m³) | True Density (kg/m³) | Porosity (%) | Moisture Content (%) | pH | Mechanical Strength (N) | |
|---|---|---|---|---|---|---|---|
| Composite filler | 15 | 164.8 | 414 | 60.2 | 86 | 6.9~7.4 | 203 |
| bark | 30~50 | 244 | — | 59.9 | 56.3 | 5.7 | — |
| Sampling Time (Day)/Inlet Load (g/m3·h) | Chao | ACE | Shannon | Simpson | Coverage |
|---|---|---|---|---|---|
| 10/3.4 | 119.5/645.6 | 189.2/691.1 | 0.4/3.6 | 0.88/0.08 | 0.998/0.988 |
| 13/3.4 | 388.8/720.5 | 521.9/755.6 | 1.9/4.1 | 0.32/0.04 | 0.992/0.987 |
| 16/4.1 | 237.3/587.0 | 317.0/793.8 | 1.8/3.9 | 0.34/0.05 | 0.995/0.987 |
| 19/5.5 | 328.6/782.3 | 430.5/774.5 | 2.5/4.7 | 0.20/0.02 | 0.993/0.987 |
| 22/6.8 | 197.3/806.9 | 279.1/923.2 | 1.7/4.6 | 0.32/0.02 | 0.995/0.984 |
| 25/8.2 | 546.5/801.0 | 564.9/989.9 | 3.0/4.2 | 0.12/0.04 | 0.990/0.984 |
| 28/9.6 | 620.2/856.0 | 810.4/876.0 | 3.8/5.1 | 0.05/0.01 | 0.986/0.984 |
| 31/13.7 | 632.5/980.2 | 516.0/1097.9 | 3.3/4.8 | 0.07/0.02 | 0.991/0.981 |
| 34/20.5 | 551.77/712.28 | 678.81/731.36 | 3.5/4.7 | 0.08/0.02 | 0.988/0.986 |
| 37/31.4 | 565.0/1002.0 | 633.7/1099.2 | 3.8/4.5 | 0.05/0.03 | 0.988/0.981 |
| 40/41.0 | 526.9/717.6 | 651.5/641.8 | 3.7/4.3 | 0.06/0.04 | 0.989/0.987 |
| 43/51.2 | 523.7/488.1 | 642.2/512.7 | 2.5/3.9 | 0.29/0.05 | 0.989/0.990 |
| 46/61.5 | 631.1/552.1 | 588.8/543.5 | 2.8/3.9 | 0.21/0.06 | 0.989/0.989 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Cui, R.; Jiang, H.; Bai, M.; Lin, K.; Zhang, M.; Ren, L. Removal of Hydrogen Sulfide and Ammonia Using a Biotrickling Filter Packed with Modified Composite Filler. Processes 2022, 10, 2016. https://doi.org/10.3390/pr10102016
Wang Y, Cui R, Jiang H, Bai M, Lin K, Zhang M, Ren L. Removal of Hydrogen Sulfide and Ammonia Using a Biotrickling Filter Packed with Modified Composite Filler. Processes. 2022; 10(10):2016. https://doi.org/10.3390/pr10102016
Chicago/Turabian StyleWang, Yue, Ruoqi Cui, Hairong Jiang, Miao Bai, Kaizong Lin, Minglu Zhang, and Lianhai Ren. 2022. "Removal of Hydrogen Sulfide and Ammonia Using a Biotrickling Filter Packed with Modified Composite Filler" Processes 10, no. 10: 2016. https://doi.org/10.3390/pr10102016
APA StyleWang, Y., Cui, R., Jiang, H., Bai, M., Lin, K., Zhang, M., & Ren, L. (2022). Removal of Hydrogen Sulfide and Ammonia Using a Biotrickling Filter Packed with Modified Composite Filler. Processes, 10(10), 2016. https://doi.org/10.3390/pr10102016






