Physicochemical Analysis of Cold Brew and Hot Brew Peaberry Coffee
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Coffee Bean Preparation
2.3. Coffee Bean Extract Preparation
2.4. pH and Total Titratable Acidity
2.5. Total Antioxidant Capacity Measurements
2.5.1. ABTS Assay
2.5.2. DPPH Assay
2.5.3. Folin–Ciocalteu Assay (TPC)
2.5.4. Ferric Ion Reducing Antioxidant Power (FRAP)
2.5.5. Total Flavonoid Capacity Assay
2.5.6. Cyclic Voltammetry Analysis
2.6. HPLC Analysis
2.7. Statistical Analysis
3. Results and Discussion
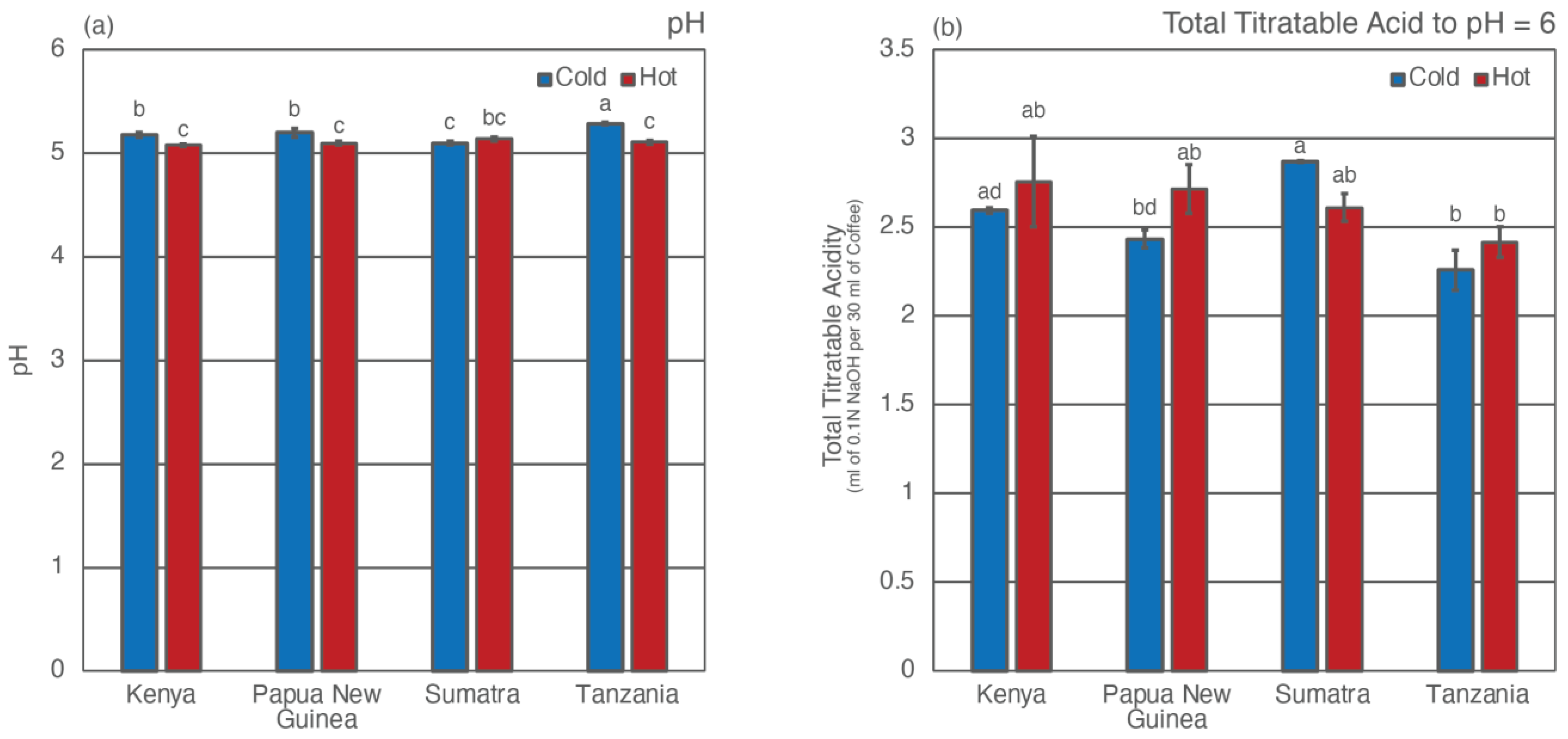
3.1. pH and Total Titratable Acidity
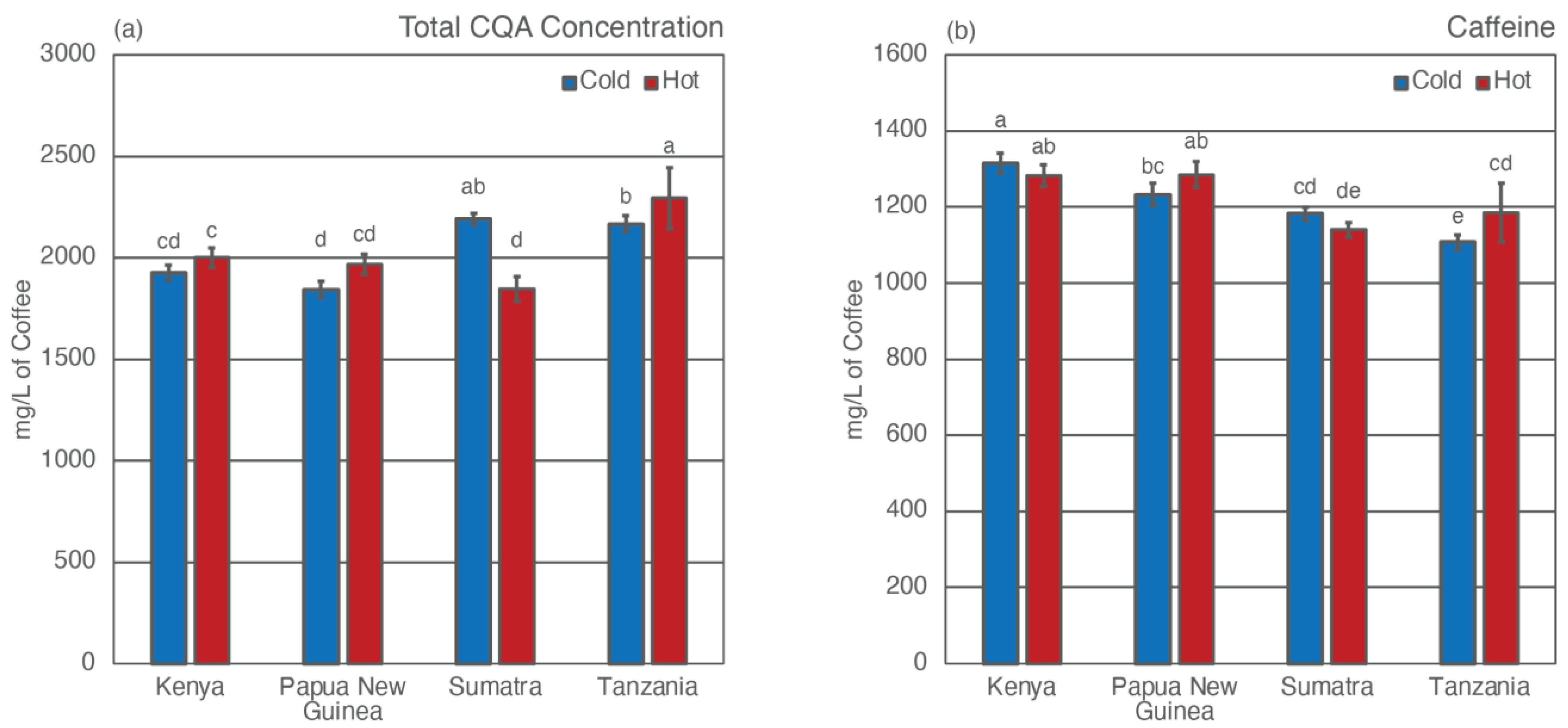
3.2. Total Caffeine and CQA Concentrations
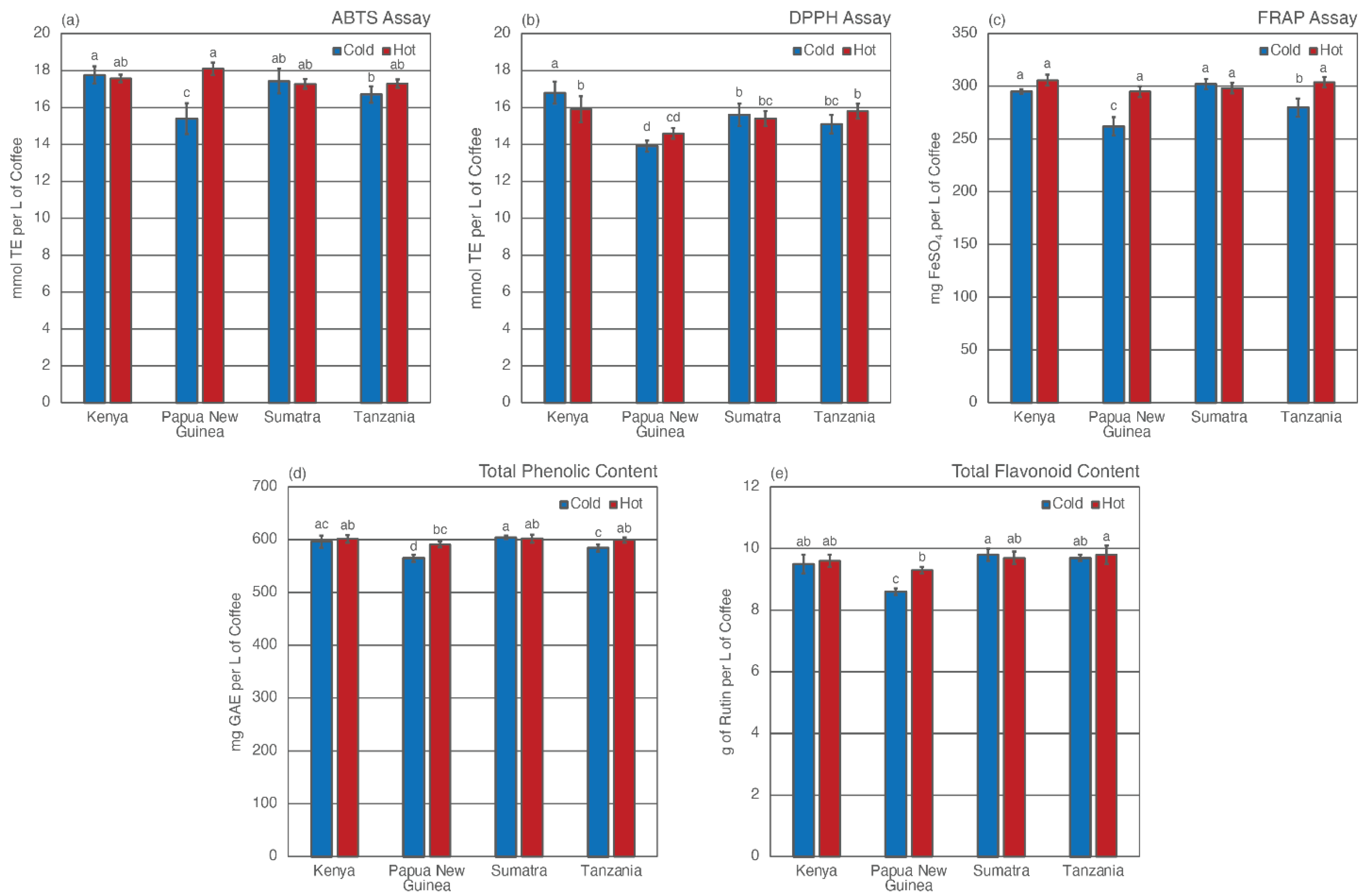
3.3. Total Antioxidant Capacity (TAC)
3.4. Cyclic Voltammetry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trade Statistics Tables, International Coffee Organization. 2021. Available online: https://www.ico.org/trade_statistics.asp?section=Statistics (accessed on 24 June 2022).
- National Coffee Association. NCA-SCA 2022 Consumption Report; National Coffee Association: New York, NY, USA, 2022. [Google Scholar]
- D’Cruze, N.; Toole, J.; Mansell, K.; Schmidt-Burbach, J. What is the true cost of the world’s most expensive coffee? Oryx 2014, 48, 170–171. [Google Scholar] [CrossRef] [Green Version]
- Price Crisis Response Initiative, SCA Publishes PCR Summary of Work and Recommendations, The Specialty Coffee Association. 2020. Available online: https://sca.coffee/sca-news/news/price-crisis-summary-of-work (accessed on 1 August 2022).
- Suhandy, D.; Yulia, M. Peaberry coffee discrimination using UV-visible spectroscopy combined with SIMCA and PLS-DA. Int. J. Food Prop. 2017, 20, S331–S339. [Google Scholar] [CrossRef]
- Suhandy, D.; Yulia, M. Kusumiyati Chemometric quantification of peaberry coffee in blends using UV–visible spectroscopy and partial least squares regression. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2018; Volume 2021, p. 060010. [Google Scholar] [CrossRef]
- Ram, A.S.; Sreenivasan, M.S.; Ramaiah, P.K. A study of peaberry development: Its implications in coffee breeding. J. Coffee Res. 1990, 20, 69–76. [Google Scholar]
- Yilma, A.; Kufa, T. Coffee Peaberry as A Potential Seed Source for Production. Int. J. Res. Stud. Agric. Sci. 2020, 7, 30–35. [Google Scholar]
- Gope, H.L.; Fukai, H. Peaberry and normal coffee bean classification using CNN, SVM, and KNN: Their implementation in and the limitations of Raspberry Pi 3. AIMS Agric. Food 2022, 7, 149–167. [Google Scholar] [CrossRef]
- Gope, H.L.; Fukai, H. Normal and peaberry coffee beans classification from green coffee bean images using convolutional neural networks and support vector machine. Int. J. Comput. Inf. Eng. 2020, 14, 189–196. Available online: https://publications.waset.org/10011255/normal-and-peaberry-coffee-beans-classification-from-green-coffee-bean-images-using-convolutional-neural-networks-and-support-vector-machine (accessed on 1 August 2022).
- Bhandarkar, N.S.; Brown, L.; Panchal, S.K. Chlorogenic acid attenuates high-carbohydrate, high-fat diet–induced cardiovascular, liver, and metabolic changes in rats. Nutr. Res. 2018, 62, 78–88. [Google Scholar] [CrossRef]
- Cano-Marquina, A.; Tarín, J.J.; Cano, A. The impact of coffee on health. Maturitas 2013, 75, 7–21. [Google Scholar] [CrossRef]
- De Melo Pereira, G.V.; de Carvalho Neto, D.P.; Magalhães Júnior, A.I.; do Prado, F.G.; Pagnoncelli, M.G.B.; Karp, S.G.; Soccol, C.R. Chemical composition and health properties of coffee and coffee by-products. Adv. Food Nutr. Res. 2020, 91, 65–96. [Google Scholar] [CrossRef]
- Depaula, J.; Farah, A. Caffeine Consumption through Coffee: Content in the Beverage, Metabolism, Health Benefits and Risks. Beverages 2019, 5, 37. [Google Scholar] [CrossRef] [Green Version]
- Farah, A.; Lima, J.D.P. Consumption of Chlorogenic Acids through Coffee and Health Implications. Beverages 2019, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamuela-Raventós, R.M. Folin-Ciocalteu method for the measurement of total phenolic content and antioxidant capacity. In Measurement of Antioxidant Activity and Capacity: Recent Trends and Applications; Apak, R., Capanoglu, E., Shahidi, F., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 107–116. [Google Scholar]
- Mikołajczak, N.; Tańska, M.; Ogrodowska, D. Phenolic compounds in plant oils: A review of composition, analytical methods, and effect on oxidative stability. Trends Food Sci. Technol. 2021, 113, 110–138. [Google Scholar] [CrossRef]
- Shraim, A.M.; Ahmed, T.A.; Rahman, M.; Hijji, Y.M. Determination of total flavonoid content by aluminum chloride assay: A critical evaluation. LWT 2021, 150, 111932. [Google Scholar] [CrossRef]
- Mekonnen, A.; Desta, W. Comparative study of the antioxidant and antibacterial activities of Rumex abyssinicus with commercially available Zingiber officinale and Curcuma longa in Bahir Dar city, Ethiopia. Chem. Biol. Technol. Agric. 2021, 8, 1–11. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Methods involving forced convection-hydrodynamic methods. In Electrochemical Methods: Fun-Damentals and Applications, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2001; pp. 331–367. [Google Scholar]
- Chevion, S.; Roberts, M.A.; Chevion, M. The use of cyclic voltammetry for the evaluation of antioxidant capacity. Free Radic. Biol. Med. 2000, 28, 860–870. [Google Scholar] [CrossRef]
- Kilmartin, P.A. Electrochemical Detection of Natural Antioxidants: Principles and Protocols. Antioxid. Redox Signal. 2001, 3, 941–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.-B.; Yuan, S.-J.; Du, J.-Q.; Hu, X.-R.; Wang, Y. Voltammetric and spectral characterization of two flavonols for assay-dependent antioxidant capacity. Bioelectrochemistry 2009, 75, 110–116. [Google Scholar] [CrossRef]
- Głód, B.K.; Kiersztyn, I.; Piszcz, P. Total antioxidant potential assay with cyclic voltammetry and/or differential pulse voltammetry measurements. J. Electroanal. Chem. 2014, 719, 24–29. [Google Scholar] [CrossRef]
- Ma, X.; Yang, H.; Xiong, H.; Li, X.; Gao, J.; Gao, Y. Electrochemical Behavior and Determination of Chlorogenic Acid Based on Multi-Walled Carbon Nanotubes Modified Screen-Printed Electrode. Sensors 2016, 16, 1797. [Google Scholar] [CrossRef] [Green Version]
- Kilmartin, P.A.; Zou, H.; Waterhouse, A.L. A Cyclic Voltammetry Method Suitable for Characterizing Antioxidant Properties of Wine and Wine Phenolics. J. Agric. Food Chem. 2001, 49, 1957–1965. [Google Scholar] [CrossRef]
- Roginsky, V.; Barsukova, T.; Hsu, C.F.; Kilmartin, P.A. Chain-Breaking Antioxidant Activity and Cyclic Voltammetry Characterization of Polyphenols in a Range of Green, Oolong, and Black Teas. J. Agric. Food Chem. 2003, 51, 5798–5802. [Google Scholar] [CrossRef] [PubMed]
- Kilmartin, P. Characterisation of polyphenols in green, oolong, and black teas, and in coffee, using cyclic voltammetry. Food Chem. 2003, 82, 501–512. [Google Scholar] [CrossRef]
- Sousa, W.R.; da Rocha, C.; Cardoso, C.L.; Silva, D.H.S.; Zanoni, M.V.B. Determination of the relative contribution of phenolic antioxidants in orange juice by voltammetric methods. J. Food Compos. Anal. 2004, 17, 619–633. [Google Scholar] [CrossRef]
- Zielinska, D.; Szawara-Nowak, D.; Ornatowska, A.; Wiczkowski, W. Use of Cyclic Voltammetry, Photochemiluminescence, and Spectrophotometric Methods for the Measurement of the Antioxidant Capacity of Buckwheat Sprouts. J. Agric. Food Chem. 2007, 55, 9891–9898. [Google Scholar] [CrossRef]
- Zielinska, D.; Szawara-Nowak, D.; Zielinski, H. Comparison of Spectrophotometric and Electrochemical Methods for the Evaluation of the Antioxidant Capacity of Buckwheat Products after Hydrothermal Treatment. J. Agric. Food Chem. 2007, 55, 6124–6131. [Google Scholar] [CrossRef]
- Yakovleva, K.E.; Kurzeev, S.A.; Stepanova, E.V.; Fedorova, T.; Kuznetsov, B.A.; Koroleva, O.V. Characterization of plant phenolic compounds by cyclic voltammetry. Appl. Biochem. Microbiol. 2007, 43, 661–668. [Google Scholar] [CrossRef]
- Piljac-Žegarac, J.; Valek, L.; Stipčević, T.; Martinez, S. Electrochemical determination of antioxidant capacity of fruit tea infusions. Food Chem. 2010, 121, 820–825. [Google Scholar] [CrossRef]
- Amidi, S.; Mojab, F.; Moghaddam, A.B.; Tabib, K.; Kobarfard, F. A simple electrochemical method for the rapid esti-mation of antioxidant potentials of some selected medicinal plants. Iran. J. Pharm. Res. 2012, 11, 117–121. Available online: https://www.ncbi.nlm.nih.gov/pubmed/25317192 (accessed on 1 August 2022). [PubMed]
- Yardım, Y. Electrochemical Behavior of Chlorogenic Acid at a Boron-Doped Diamond Electrode and Estimation of the Antioxidant Capacity in the Coffee Samples Based on Its Oxidation Peak. J. Food Sci. 2012, 77, C408–C413. [Google Scholar] [CrossRef] [PubMed]
- Ziyatdinova, G.; Aytuganova, I.; Nizamova, A.; Budnikov, H. Differential Pulse Voltammetric Assay of Coffee Antioxidant Capacity with MWNT-Modified Electrode. Food Anal. Methods 2013, 6, 1629–1638. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Hernanz, D.; Escudero-Gilete, M.L.; Heredia, F.J. Antioxidant potential of white grape pomaces: Phenolic composition and antioxidant capacity measured by spectrophotometric and cyclic voltammetry methods. Food Res. Int. 2014, 66, 150–157. [Google Scholar] [CrossRef]
- Pilipczuk, T.; Kusznierewicz, B.; Zielińska, D.; Bartoszek, A. The influence of roasting and additional processing on the content of bioactive components in special purpose coffees. J. Food Sci. Technol. 2014, 52, 5736–5744. [Google Scholar] [CrossRef] [Green Version]
- Oliveira-Neto, J.R.; Rezende, S.G.; de Fátima Reis, C.; Benjamin, S.R.; Rocha, M.L.; de Souza Gil, E. Electrochemical behavior and determination of major phenolic antioxidants in selected coffee samples. Food Chem. 2016, 190, 506–512. [Google Scholar] [CrossRef]
- Hoyos-Arbeláez, J.; Vázquez, M.; Contreras-Calderón, J. Electrochemical methods as a tool for determining the antioxidant capacity of food and beverages: A review. Food Chem. 2017, 221, 1371–1381. [Google Scholar] [CrossRef]
- Jiao, Y.; Kilmartin, P.A.; Fan, M.; Quek, S.Y. Assessment of phenolic contributors to antioxidant activity of new kiwifruit cultivars using cyclic voltammetry combined with HPLC. Food Chem. 2018, 268, 77–85. [Google Scholar] [CrossRef]
- Zielińska, D.; Turemko, M. Electroactive Phenolic Contributors and Antioxidant Capacity of Flesh and Peel of 11 Apple Cultivars Measured by Cyclic Voltammetry and HPLC–DAD–MS/MS. Antioxidants 2020, 9, 1054. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Gómez, J.; Ramírez-Silva, M.T.; Guzmán-Hernández, D.S.; Romero-Romo, M.; Palomar-Pardavé, M. Novel electrochemical method to evaluate the antioxidant capacity of infusions and beverages, based on in situ formation of free superoxide radicals. Food Chem. 2020, 332, 127409. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Wada, R.; Muguruma, H.; Osakabe, N. Analysis of Chlorogenic Acids in Coffee with a Multi-walled Carbon Nanotube Electrode. Food Anal. Methods 2020, 13, 923–932. [Google Scholar] [CrossRef]
- Yildirim, S.; Demir, E.; Gok, I.; Aboul-Enein, H.Y. Use of electrochemical methods to determine the effect of brewing techniques (Espresso, Turkish and Filter coffee) and roasting levels on the antioxidant capacity of coffee beverage. J. Food Sci. Technol. 2022, 1–11. [Google Scholar] [CrossRef]
- Babova, O.; Occhipinti, A.; Maffei, M.E. Chemical partitioning and antioxidant capacity of green coffee (Coffea arabica and Coffea canephora) of different geographical origin. Phytochemistry 2016, 123, 33–39. [Google Scholar] [CrossRef]
- Uman, E.; Colonna-Dashwood, M.; Colonna-Dashwood, L.; Perger, M.; Klatt, C.; Leighton, S.; Miller, B.; Butler, K.T.; Melot, B.C.; Speirs, R.W.; et al. The effect of bean origin and temperature on grinding roasted coffee. Sci. Rep. 2016, 6, 24483. [Google Scholar] [CrossRef] [Green Version]
- Cordoba, N.; Pataquiva, L.; Osorio, C.; Moreno, F.L.M.; Ruiz, R.Y. Effect of grinding, extraction time and type of coffee on the physicochemical and flavour characteristics of cold brew coffee. Sci. Rep. 2019, 9, 8440. [Google Scholar] [CrossRef] [Green Version]
- Moeenfard, M.; Rocha, L.; Alves, A. Quantification of Caffeoylquinic Acids in Coffee Brews by HPLC-DAD. J. Anal. Methods Chem. 2014, 2014, 965353. [Google Scholar] [CrossRef]
- Rao, N.Z.; Fuller, M.; Grim, M.D. Physiochemical Characteristics of Hot and Cold Brew Coffee Chemistry: The Effects of Roast Level and Brewing Temperature on Compound Extraction. Foods 2020, 9, 902. [Google Scholar] [CrossRef]
- Odžaković, B.; Džinić, N.; Kukrić, Z.; Grujić, S. Effect of roasting degree on the antioxidant activity of different Arabica coffee quality classes. Acta Sci. Pol. Technol. Aliment. 2015, 15, 409–417. [Google Scholar] [CrossRef] [Green Version]
- Muzykiewicz, A.; Zielonka-Brzezicka, J.; Klimowicz, A. The antioxidant potential of flesh, albedo and flavedo extracts from different varieties of grapefruits. Acta Sci. Pol. Technol. Aliment. 2015, 18, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Saeed, N.; Khan, M.R.; Shabbir, M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement. Altern. Med. 2012, 12, 221. [Google Scholar] [CrossRef]
- Takahash, R. Analysis of Chlorogenic Acid in Coffee by HPLC, GL Science Inc. (n.d.). Available online: https://www.glsciences.com/technique/app/detail.php?data_number=LT067 (accessed on 7 October 2018).
- Farah, A.; de Paulis, T.; Trugo, L.C.; Martin, P.R. Effect of Roasting on the Formation of Chlorogenic Acid Lactones in Coffee. J. Agric. Food Chem. 2005, 53, 1505–1513. [Google Scholar] [CrossRef]
- Trugo, L.C.; Macrae, R. Chlorogenic acid composition of instant coffees. Analyst 1984, 109, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Monente, C.; Ludwig, I.A.; Irigoyen, A.; De Peña, M.-P.; Cid, C. Assessment of Total (Free and Bound) Phenolic Compounds in Spent Coffee Extracts. J. Agric. Food Chem. 2015, 63, 4327–4334. [Google Scholar] [CrossRef]
- Wessa, P. Free Statistics Software, Office for Research Development and Education, Version 1.2.1. 2020. Available online: https://www.wessa.net (accessed on 3 August 2022).
- Rao, N.Z.; Fuller, M. Acidity and Antioxidant Activity of Cold Brew Coffee. Sci. Rep. 2018, 8, 16030. [Google Scholar] [CrossRef] [Green Version]
- Bilge, G. Investigating the effects of geographical origin, roasting degree, particle size and brewing method on the physicochemical and spectral properties of Arabica coffee by PCA analysis. J. Food Sci. Technol. 2020, 57, 3345–3354. [Google Scholar] [CrossRef] [PubMed]
- Muzykiewicz-Szymańska, A.; Nowak, A.; Wira, D.; Klimowicz, A. The Effect of Brewing Process Parameters on Antioxidant Activity and Caffeine Content in Infusions of Roasted and Unroasted Arabica Coffee Beans Originated from Different Countries. Molecules 2021, 26, 3681. [Google Scholar] [CrossRef]
- Córdoba, N.; Moreno, F.L.; Osorio, C.; Velásquez, S.; Ruiz, Y. Chemical and sensory evaluation of cold brew coffees using different roasting profiles and brewing methods. Food Res. Int. 2021, 141, 110141. [Google Scholar] [CrossRef] [PubMed]
- Maier, H.G. Electrophoretic clean-up of organic acids from coffee for the GC/MS analysis. Anal. Bioanal. Chem. 1996, 355, 190–193. [Google Scholar] [CrossRef]
- Maier, H.G.; Balcke, C.; Thies, F.-C. Die Säuren Des Kaffees. VI. Abhängigkeit des sauren Geschmacks von pH-Vert und Säuregrad. Lebensm. Gerichtl. Chem. 1983, 37, 81–83. [Google Scholar]
- Balzer, H.H. Acids in coffee. In Coffee: Recent Development; Clarke, R.J., Vitzthum, O.G., Eds.; Blackwell Science: Oxford, UK, 2001; pp. 18–32. [Google Scholar]
- Batali, M.E.; Cotter, A.R.; Frost, S.C.; Ristenpart, W.D.; Guinard, J.-X. Titratable Acidity, Perceived Sourness, and Liking of Acidity in Drip Brewed Coffee. ACS Food Sci. Technol. 2021, 1, 559–569. [Google Scholar] [CrossRef]
- Cordoba, N.; Fernandez-Alduenda, M.; Moreno, F.L.; Ruiz, Y. Coffee extraction: A review of parameters and their influence on the physicochemical characteristics and flavour of coffee brews. Trends Food Sci. Technol. 2020, 96, 45–60. [Google Scholar] [CrossRef]
- Yeager, S.E.; Batali, M.E.; Guinard, J.-X.; Ristenpart, W.D. Acids in coffee: A review of sensory measurements and meta-analysis of chemical composition. Crit. Rev. Food Sci. Nutr. 2021, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Gloess, A.N.; Schönbächler, B.; Klopprogge, B.; D`ambrosio, L.; Chatelain, K.; Bongartz, A.; Strittmatter, A.; Rast, M.; Yeretzian, C. Comparison of nine common coffee extraction methods: Instrumental and sensory analysis. Eur. Food Res. Technol. 2013, 236, 607–627. [Google Scholar] [CrossRef] [Green Version]
- Wonorahardjo, S.; Yuniawati, N.; Molo, A.D.P.; Rusdi, H.O.; Purnomo, H. Different Chemical Compound Profiles of Indonesian Coffee Beans as Studied Chromatography/Mass Spectrometry. IOP Conf. Ser. Earth Environ. Sci. 2019, 276, 012065. [Google Scholar] [CrossRef]
- Stanek, N.; Zarębska, M.; Biłos, Ł.; Barabosz, K.; Nowakowska-Bogdan, E.; Semeniuk, I.; Błaszkiewicz, J.; Kulesza, R.; Matejuk, R.; Szkutnik, K. Influence of coffee brewing methods on the chromatographic and spectroscopic profiles, antioxidant and sensory properties. Sci. Rep. 2021, 11, 21377. [Google Scholar] [CrossRef]
- Fuller, M.; Rao, N.Z. The Effect of Time, Roasting Temperature, and Grind Size on Caffeine and Chlorogenic Acid Concentrations in Cold Brew Coffee. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Angeloni, G.; Guerrini, L.; Masella, P.; Innocenti, M.; Bellumori, M.; Parenti, A. Characterization and comparison of cold brew and cold drip coffee extraction methods. J. Sci. Food Agric. 2018, 99, 391–399. [Google Scholar] [CrossRef]
- Lane, S.; Palmer, J.; Christie, B.; Ehlting, J.; Le, C. Can Cold Brew Coffee Be Convenient? A Pilot Study For Caffeine Content in Cold Brew Coffee Concentrate Using High Performance Liquid Chromatography. Arbutus Rev. 2017, 8, 15–23. [Google Scholar] [CrossRef]
- Fox, G.P.; Wu, A.; Yiran, L.; Force, L. Variation in Caffeine Concentration in Single Coffee Beans. J. Agric. Food Chem. 2013, 61, 10772–10778. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.S.; Kim, H.T.; Jeong, I.H.; Hong, S.R.; Oh, M.S.; Park, K.H.; Shim, J.H.; Abd El-Aty, A.M. Determination of chlorogenic acids and caffeine in homemade brewed coffee prepared under various conditions. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1064, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Ciaramelli, C.; Palmioli, A.; Airoldi, C. Coffee variety, origin and extraction procedure: Implications for coffee beneficial effects on human health. Food Chem. 2018, 278, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Olechno, E.; Puścion-Jakubik, A.; Zujko, M.; Socha, K. Influence of Various Factors on Caffeine Content in Coffee Brews. Foods 2021, 10, 1208. [Google Scholar] [CrossRef] [PubMed]
- Gebeyehu, B.T. Determination of Caffeine Content and Antioxidant Activity of Coffee. Am. J. Appl. Chem. 2015, 3, 69. [Google Scholar] [CrossRef] [Green Version]
- Angeloni, G.; Guerrini, L.; Masella, P.; Bellumori, M.; Daluiso, S.; Parenti, A.; Innocenti, M. What kind of coffee do you drink? An investigation on effects of eight different extraction methods. Food Res. Int. 2018, 116, 1327–1335. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Antioxidant Property of Coffee Components: Assessment of Methods that Define Mechanisms of Action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef] [Green Version]
- Yashin, A.; Yashin, Y.; Wang, J.Y.; Nemzer, B. Antioxidant and Antiradical Activity of Coffee. Antioxidants 2013, 2, 230–245. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Van Acker, S.A.B.E.; Van Den Berg, D.-J.; Tromp, M.N.J.L.; Griffioen, D.H.; Van Bennekom, W.P.; Van Der Vijgh, W.J.F.; Bast, A. Structural aspects of antioxidant activity of flavonoids. Free Radic. Biol. Med. 1996, 20, 331–342. [Google Scholar] [CrossRef]
- Burda, S.; Oleszek, W. Antioxidant and Antiradical Activities of Flavonoids. J. Agric. Food Chem. 2001, 49, 2774–2779. [Google Scholar] [CrossRef]
- Miłek, M.; Młodecki, Ł.; Dżugan, M. Caffeine content and antioxidant activity of various brews of specialty grade coffee. Acta Sci. Pol. Technol. Aliment. 2015, 20, 179–188. [Google Scholar] [CrossRef]
- Kang, D.-E.; Lee, H.-U.; Davaatseren, M.; Chung, M.-S. Comparison of acrylamide and furan concentrations, antioxidant activities, and volatile profiles in cold or hot brew coffees. Food Sci. Biotechnol. 2019, 29, 141–148. [Google Scholar] [CrossRef] [PubMed]
- García-Márquez, E.; Román-Guerrero, A.A.; Pérez-Alonso, C.; Cruz-Sosa, F.; Jiménez-Alvarado, R.; Vernon-Carter, E.J. Effect of solvent-temperature extraction conditions on the initial antioxidant activity and total phenolic content of muitle extracts and their decay upon storage at different pH. Rev. Mex. Anal. Conducta. 2012, 11, 1–10. [Google Scholar]
- Sripum, C.; Kukreja, R.K.; Charoenkiatkul, S.; Kriengsinyos, W.; Suttisansanee, U. The effect of extraction conditions on anti-oxidant activities and total phenolic contents of different processed Thai Jasmine rice. Int. Food. Res. J. 2017, 24, 1644–1650. [Google Scholar]
- Pinelo, M.; Rubilar, M.; Jerez, M.; Sineiro, A.J.; Núñez, M.J. Effect of Solvent, Temperature, and Solvent-to-Solid Ratio on the Total Phenolic Content and Antiradical Activity of Extracts from Different Components of Grape Pomace. J. Agric. Food Chem. 2005, 53, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, K.; Ahmed, I.A.M.; Doğu, S.; Uslu, N.; Fadimu, G.J.; Al Juhaimi, F.; Babiker, E.E.; Özcan, M.M.; Jamiu, F.G. The Effect of Heating Temperature on Total Phenolic Content, Antioxidant Activity, and Phenolic Compounds of Plum and Mahaleb Fruits. Int. J. Food Eng. 2019, 15. [Google Scholar] [CrossRef]
- Vignoli, J.A.; Viegas, M.C.; Bassoli, D.G.; Benassi, M.D.T. Roasting process affects differently the bioactive compounds and the antioxidant activity of arabica and robusta coffees. Food Res. Int. 2014, 61, 279–285. [Google Scholar] [CrossRef] [Green Version]
- Vignoli, J.A.; Bassoli, D.G.; Benassi, M.T. Antioxidant activity, polyphenols, caffeine and melanoidins in soluble coffee: The influence of processing conditions and raw material. Food Chem. 2011, 124, 863–868. [Google Scholar] [CrossRef]
- López-Galilea, I.; De Peña, M.P.; Cid, C. Correlation of Selected Constituents with the Total Antioxidant Capacity of Coffee Beverages: Influence of the Brewing Procedure. J. Agric. Food Chem. 2007, 55, 6110–6117. [Google Scholar] [CrossRef]
- Parras, P.; Martínez-Tomé, M.; Jiménez, A.; Murcia, M. Antioxidant capacity of coffees of several origins brewed following three different procedures. Food Chem. 2007, 102, 582–592. [Google Scholar] [CrossRef]
- Stelmach, E.; Pohl, P.; Szymczycha-Madeja, A. The content of Ca, Cu, Fe, Mg and Mn and antioxidant activity of green coffee brews. Food Chem. 2015, 182, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Somporn, C.; Kamtuo, A.; Theerakulpisut, P.; Siriamornpun, S. Effect of shading on yield, sugar content, phenolic acids and antioxidant property of coffee beans (Coffea Arabica L. cv. Catimor) harvested from north-eastern Thailand. J. Sci. Food Agric. 2012, 92, 1956–1963. [Google Scholar] [CrossRef] [PubMed]
- Hallmann, E.; Ożga, M.; Rembiałkowska, E. The content of bioactive compounds in selected kind of coffee from organic and conven-tional production. J. Res. Appl. Agric. Eng. 2010, 53, 99–104. [Google Scholar]
- Górecki, M.; Hallmann, E. The Antioxidant Content of Coffee and Its In Vitro Activity as an Effect of Its Production Method and Roasting and Brewing Time. Antioxidants 2020, 9, 308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte, S.M.D.S.; De Abreu, C.M.P.; De Menezes, H.C.; Dos Santos, M.H.; Gouvêa, C.M.C.P. Effect of processing and roasting on the antioxidant activity of coffee brews. Food Sci. Technol. 2005, 25, 387–393. [Google Scholar] [CrossRef]
- Haile, M.; Bae, H.M.; Kang, W.H. Comparison of the Antioxidant Activities and Volatile Compounds of Coffee Beans Obtained Using Digestive Bio-Processing (Elephant Dung Coffee) and Commonly Known Processing Methods. Antioxidants 2020, 9, 408. [Google Scholar] [CrossRef]
- Król, K.; Gantner, M.; Tatarak, A.; Hallmann, E. The content of polyphenols in coffee beans as roasting, origin and storage effect. Eur. Food Res. Technol. 2019, 246, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Kitzberger, C.S.G.; Scholz, M.B.D.S.; Benassi, M.D.T. Bioactive compounds content in roasted coffee from traditional and modern Coffea arabica cultivars grown under the same edapho-climatic conditions. Food Res. Int. 2014, 61, 61–66. [Google Scholar] [CrossRef]
- Yildirim, S.; Gok, I.; Demir, E.; Tokusoglu, O. Use of electrochemical techniques for determining the effect of brewing techniques (espresso, Turkish and filter coffee) and roasting levels on total antioxidant capacity of coffee beverage. J. Food Process. Preserv. 2022, 46, e16626. [Google Scholar] [CrossRef]
- Demir, E.; Silah, H.; Aydogdu, N. Electrochemical Applications for the Antioxidant Sensing in Food Samples Such as Citrus and Its Derivatives, Soft Drinks, Supplementary Food and Nutrients. In Citrus-Research, Development and Biotechnology; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Yildirim, S.; Demir, E.; Gök, I. Determination of Total Antioxidant Capacities in Turkish and Filter Coffee Samples by Electrochemical Methods. BSEU J. Sci. 2020, 7, 382–393. [Google Scholar] [CrossRef]
- Escarpa, A. Food electroanalysis: Sense and simplicity. Chem. Rec. 2011, 12, 72–91. [Google Scholar] [CrossRef] [PubMed]

| Coffee Samples | Reduction Potential (V) | Oxidation Potential (V) | E1/2 (V) | Reduction Current (A) | Oxidation Current (A) |
|---|---|---|---|---|---|
| Cold Brew | |||||
| Kenya | 0.424 | 0.391 | 0.408 | 9.71 × 10−7 | −5.94 × 10−7 |
| Papua New Guinea | 0.426 | 0.391 | 0.409 | 1.06 × 10−6 | −7.70 × 10−7 |
| Sumatra | 0.424 | 0.385 | 0.405 | 9.56 × 10−7 | −7.42 × 10−7 |
| Tanzania | 0.427 | 0.385 | 0.406 | 1.09 × 10−6 | −7.25 × 10−7 |
| Hot Brew | |||||
| Kenya | 0.436 | 0.389 | 0.413 | 1.24 × 10−6 | −8.28 × 10−7 |
| Papua New Guinea | 0.426 | 0.397 | 0.412 | 8.95 × 10−7 | −7.78 × 10−7 |
| Sumatra | 0.420 | 0.385 | 0.403 | 1.06 × 10−6 | −7.99 × 10−7 |
| Tanzania | 0.412 | 0.382 | 0.397 | 1.00 × 10−6 | −7.85 × 10−7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwarzmann, E.T.; Washington, M.P.; Rao, N.Z. Physicochemical Analysis of Cold Brew and Hot Brew Peaberry Coffee. Processes 2022, 10, 1989. https://doi.org/10.3390/pr10101989
Schwarzmann ET, Washington MP, Rao NZ. Physicochemical Analysis of Cold Brew and Hot Brew Peaberry Coffee. Processes. 2022; 10(10):1989. https://doi.org/10.3390/pr10101989
Chicago/Turabian StyleSchwarzmann, Evan T., Marlena P. Washington, and Niny Z. Rao. 2022. "Physicochemical Analysis of Cold Brew and Hot Brew Peaberry Coffee" Processes 10, no. 10: 1989. https://doi.org/10.3390/pr10101989
APA StyleSchwarzmann, E. T., Washington, M. P., & Rao, N. Z. (2022). Physicochemical Analysis of Cold Brew and Hot Brew Peaberry Coffee. Processes, 10(10), 1989. https://doi.org/10.3390/pr10101989








