Gastroprotective, Biochemical and Acute Toxicity Effects of Papaver decaisnei against Ethanol-Induced Gastric Ulcers in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Collection
2.2. Plant Extraction
2.3. Chemicals and Reagents
2.4. Moral Declaration and the Animal Study
2.5. Acute Toxicity and Rat Trials
2.6. Gastric Ulcer Experiment
- (1)
- Group 1 and 2 received carboxymethylcellulose (CMC, 0.5%), orally (normal and negative control).
- (2)
- Group 3 was orally administered 20 mg/kg of omeprazole (reference).
- (3)
- Groups 4 and 5 were administered 200 mg and 400 mg/kg of the PDE, respectively.
2.7. Gastric Gross Study
2.8. Measurement of the Stomach Mucus Content
2.9. Formulating the Stomach Tissue Homogenate
2.10. Evaluation of the Antioxidants
2.11. Lipid Peroxidation (MDA) Status of the Tissue Homogenates
2.12. Histological Analysis
2.13. Hematoxylin and Eosin Stains
2.14. Immunohistochemical Staining
2.15. Evaluation of the Cytokines
2.16. Statistical Analysis
3. Results
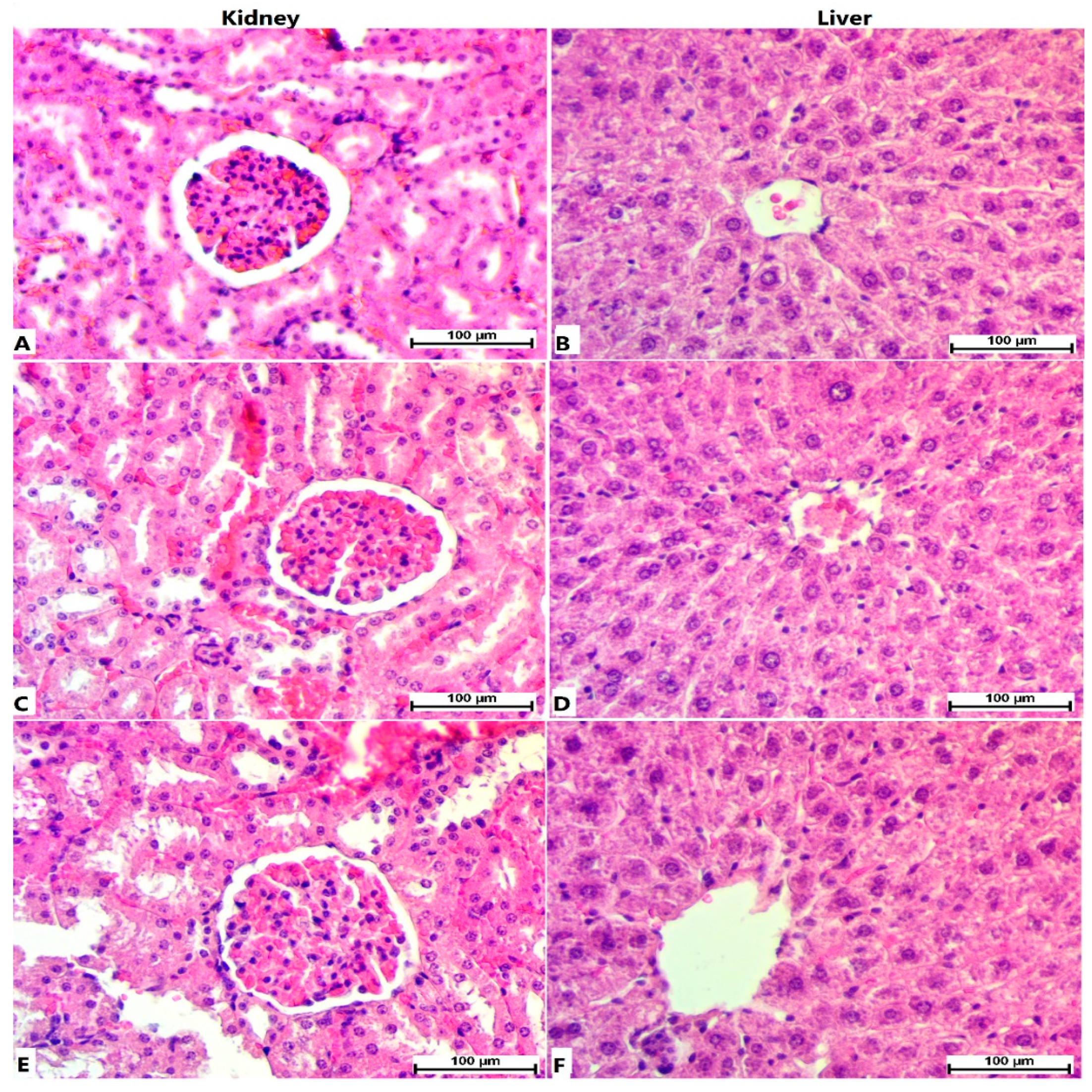
3.1. Acute Toxicity
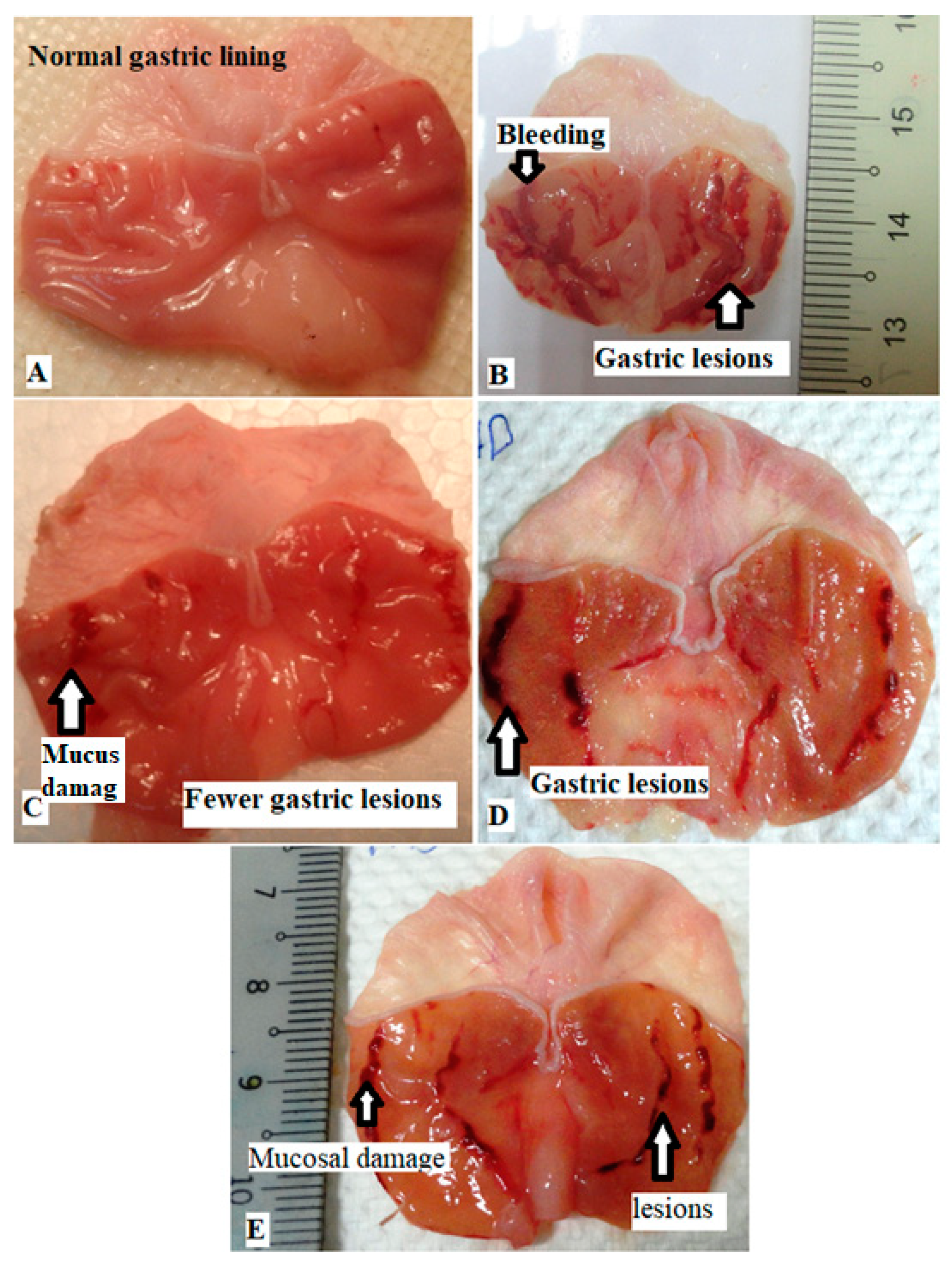
3.2. The Stomach Gross Analysis
3.3. Gastric Mucus Level
3.4. P. decaisnei Effects on the Stomach pH
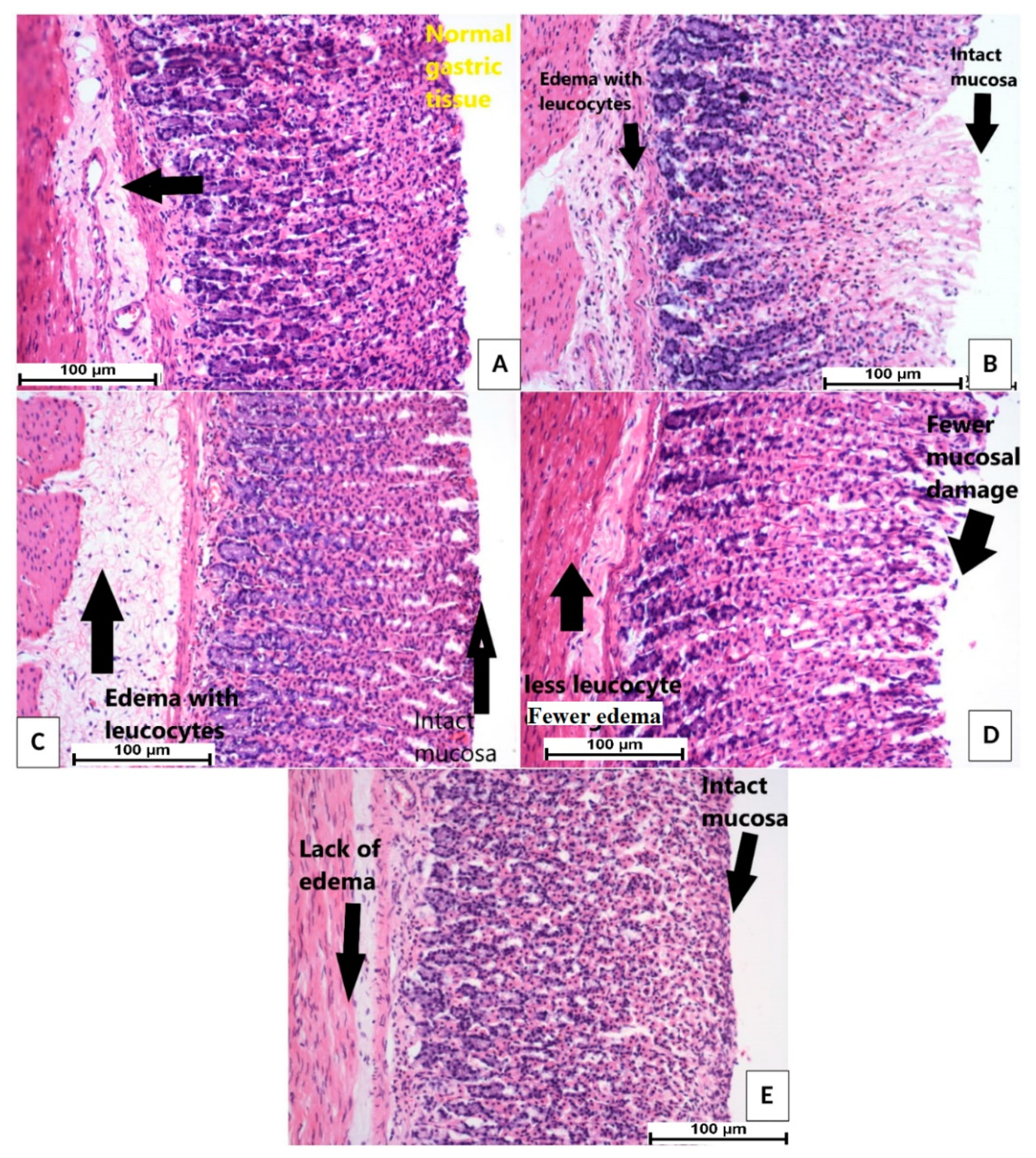
3.5. H & E Stain
3.6. PAS Stain
3.7. Immunohistochemical Staining
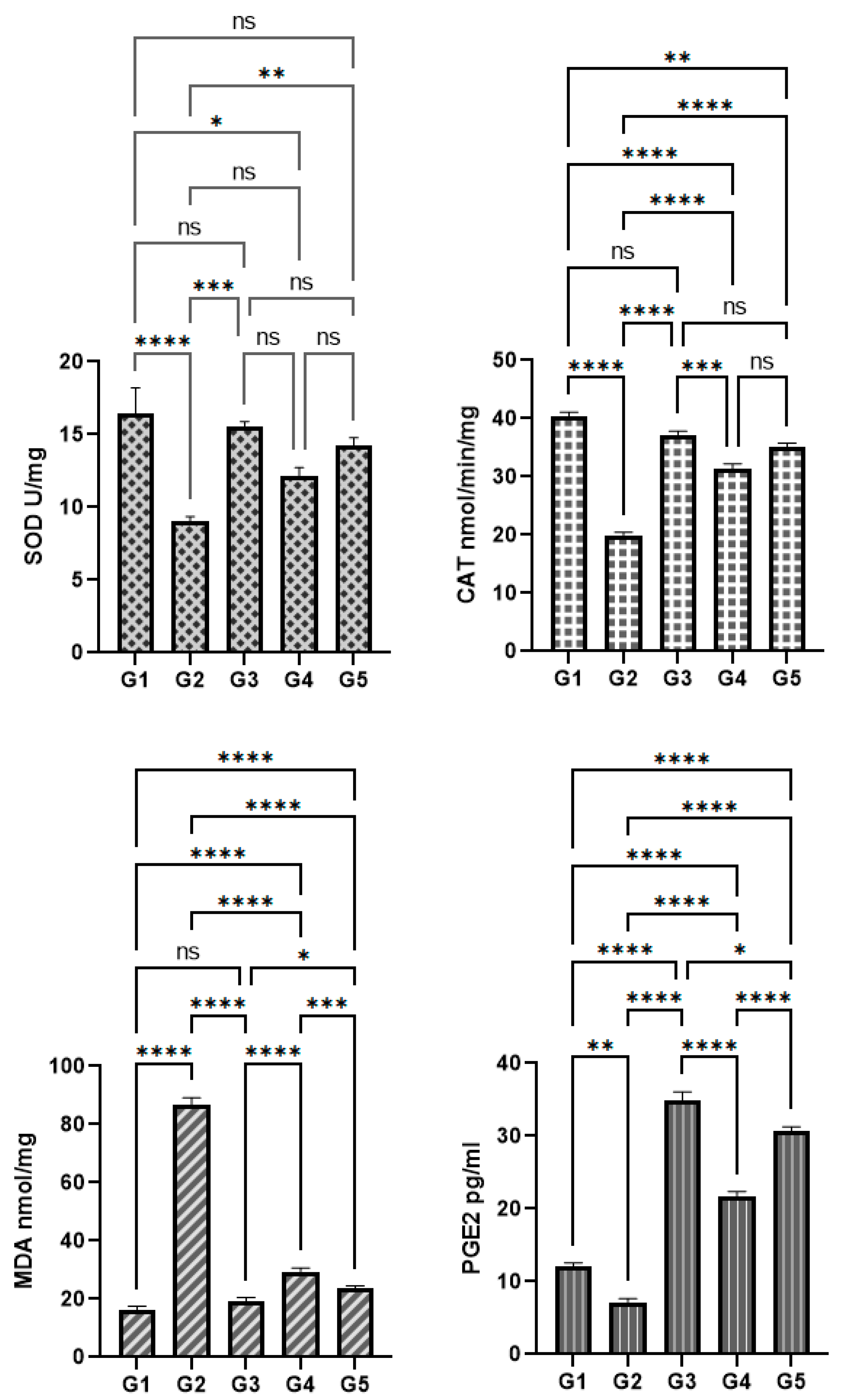
3.8. P. decaisnei Effects on the Gastric Antioxidants
3.9. P. decaisnei Effects on Malondialdehyde (MDA) in Gastric Homogenates
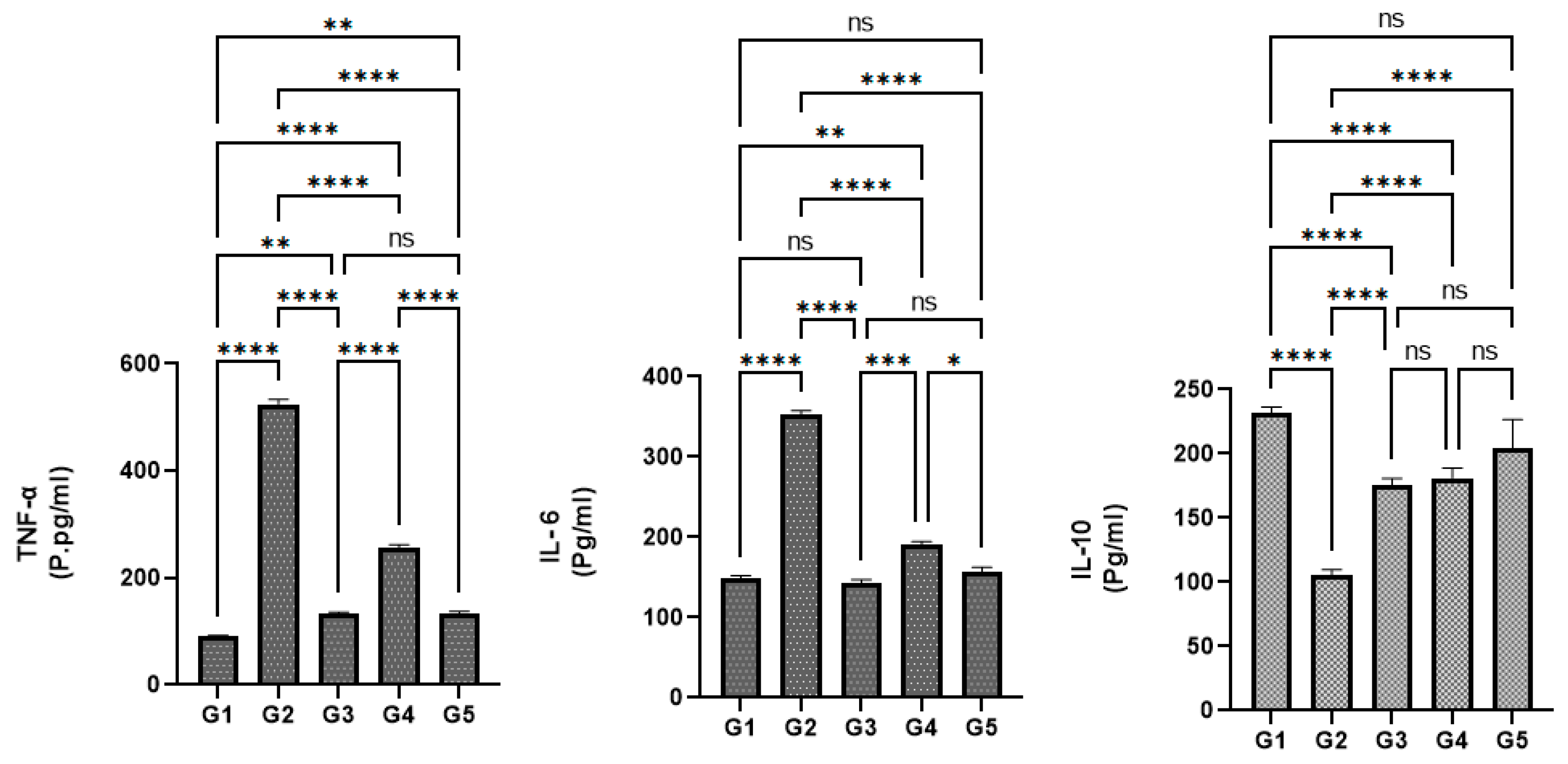
3.10. Effects of P. decaisnei extracts on the Serum Cytokine Enzymes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Wei, Y.; An, L.; Wang, K.; Hong, D.; Shi, Y.; Zang, A.; Su, S.; Li, W. SEMA3D Plays a Critical Role in Peptic Ulcer Disease-Related Carcinogenesis Induced by H. Pylori Infection. Int. J. Gen. Med. 2022, 15, 1239–1260. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.; Park, M.; Jeong, J.E.; Lee, J.Y.; Kim, M.G. Frequently Reported Adverse Events of Rebamipide Compared to Other Drugs for Peptic Ulcer and Gastroesophageal Reflux Disease. Sci. Rep. 2022, 12, 7839. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.F.G.; Allam, F.A.F.A. Potential Stem Cell—Conditioned Medium and Their Derived Exosomes versus Omeprazole in Treatment of Experimental Model of Gastric Ulcer. Acta Histochem. 2022, 124, 151896. [Google Scholar] [CrossRef] [PubMed]
- Yaghoobi, M.; Armstrong, D. Peptic Ulcer Disease. In Yamada’s Textbook of Gastroenterology; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 924–976. [Google Scholar]
- Haber, P.S.; Kortt, N.C. Alcohol Use Disorder and the Gut. Addiction 2021, 116, 658–667. [Google Scholar] [CrossRef]
- Guzmán-Gómez, O.; García-Rodríguez, R.V.; Quevedo-Corona, L.; Pérez-Pastén-Borja, R.; Rivero-Ramírez, N.L.; Ríos-Castro, E.; Pérez-Gutiérrez, S.; Pérez-Ramos, J.; Chamorro-Cevallos, G.A. Amelioration of Ethanol-Induced Gastric Ulcers in Rats Pretreated with Phycobiliproteins of Arthrospira (Spirulina) Maxima. Nutrients 2018, 10, 763. [Google Scholar] [CrossRef]
- Manu, P.; Rogozea, L.M.; Sandor, V.; Dumitraşcu, D.L. Pharmacological Management of Peptic Ulcer: A Century of Expert Opinions in Cecil Textbook of Medicine. Am. J. Ther. 2021, 28, e552–e559. [Google Scholar] [CrossRef]
- Abdullah, F.O.; Hussain, F.H.S.; Sardar, A.S.; Vita-Finzi, P.; Vidari, G. Phytochemistry and ethnopharmacology of medicinal plants used on Safeen Mountain in the Kurdistan region of Iraq. Nat. Prod. Commun. 2016, 11, 1923–1927. [Google Scholar] [CrossRef]
- Jabbar, A.A. Gastroprotective and Immuno-Supportive Role of Alcea Kurdica against Stress Induced Lesion in Japanese Quails. Baghdad Sci. J. 2022, 19, 716–724. [Google Scholar]
- Li, C.; Wang, L.; Zhao, J.; Wei, Y.; Zhai, S.; Tan, M.; Guan, K.; Huang, Z.; Chen, C. Lonicera Rupicola Hook.f.et Thoms Flavonoids Ameliorated Dysregulated Inflammatory Responses, Intestinal Barrier, and Gut Microbiome in Ulcerative Colitis via PI3K/AKT Pathway. Phytomedicine 2022, 104, 154284. [Google Scholar] [CrossRef]
- Jabbar, A.A.; Abdullah, F.O.; Abdulrahman, K.K.; Galali, Y.; Sardar, A.S. GC-MS Analysis of Bioactive Compounds in Methanolic Extracts of Papaver Decaisnei and Determination of Its Antioxidants and Anticancer Activities. J. Food Qual. 2022, 2022, 1405157. [Google Scholar] [CrossRef]
- Shaghaghi, A.; Alirezalu, A.; Nazarianpour, E.; Sonboli, A.; Nejad-Ebrahimi, S. Opioid Alkaloids Profiling and Antioxidant Capacity of Papaver Species from Iran. Ind. Crops Prod. 2019, 142, 111870. [Google Scholar] [CrossRef]
- Jabbar, A.A.; Saeed, C.H.; Abdulaziz, S.M.; Mahmood, B.J. Chemical Differentiation and Antimicrobial Potential of Four Brassica napus L. Seed Oils. Iraqi J. Sci. 2021, 62, 4597–4613. [Google Scholar] [CrossRef]
- Hajrezaie, M.; Golbabapour, S.; Hassandarvish, P.; Gwaram, N.S.; A. Hadi, A.H.; Mohd Ali, H.; Majid, N.; Abdulla, M.A. Acute Toxicity and Gastroprotection Studies of a New Schiff Base Derived Copper (II) Complex against Ethanol-Induced Acute Gastric Lesions in Rats. PLoS ONE 2012, 7, e51537. [Google Scholar] [CrossRef] [PubMed]
- Sisay Zewdu, W.; Jemere Aragaw, T. Evaluation of the Anti-Ulcer Activity of Hydromethanolic Crude Extract and Solvent Fractions of the Root of Rumex nepalensis in Rats. J. Exp. Pharmacol. 2020, 12, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, A.N.; Asrade Atnafie, S.; Wahab Atta, M.A. Evaluation of Antiulcer Activity of 80% Methanol Extract and Solvent Fractions of the Root of Croton macrostachyus Hocsht: Ex Del. (Euphorbiaceae) in Rodents. Evid.-Based Complement. Altern. Med. 2020, 2020, 2809270. [Google Scholar] [CrossRef]
- Quintans-Júnior, L.J.; Gandhi, S.R.; Passos, F.R.S.; Heimfarth, L.; Pereira, E.W.M.; Monteiro, B.S.; dos Santos, K.S.; Duarte, M.C.; Abreu, L.S.; Nascimento, Y.M.; et al. Dereplication and Quantification of the Ethanol Extract of Miconia albicans (Melastomaceae) by HPLC-DAD-ESI-/MS/MS, and Assessment of Its Anti-Hyperalgesic and Anti-Inflammatory Profiles in a Mice Arthritis-like Model: Evidence for Involvement of TNF-α, I. J. Ethnopharmacol. 2020, 258, 112938. [Google Scholar] [CrossRef]
- Hajjaj, G. Analgesic and Anti-Inflammatory Effects of Papaver rhoeas L. a Traditional Medicinal Plant of Morocco. J. Nat. Ayurvedic Med. 2018, 2, 1–8. [Google Scholar] [CrossRef]
- Soulimani, R.; Younos, C.; Jarmouni-Idrissi, S.; Bousta, D.; Khalouki, F.; Laila, A. Behavioral and Pharmaco-Toxicological Study of Papaver rhoeas L. in Mice. J. Ethnopharmacol. 2001, 74, 265–274. [Google Scholar] [CrossRef]
- Bülbül, T.; Gür, E.; Bozkurt, F.; Eryavuz, A.; Bülbül, A. Biochemical, Hematological and Histopathological Evaluation of the Food-Safety of the Leaf Extract of Papaver somniferum in Rats. Fresenius Environ. Bull. 2021, 30, 9515–9522. [Google Scholar]
- Zhou, D.; Yang, Q.; Tian, T.; Chang, Y.; Li, Y.; Duan, L.R.; Li, H.; Wang, S.W. Gastroprotective Effect of Gallic Acid against Ethanol-Induced Gastric Ulcer in Rats: Involvement of the Nrf2/HO-1 Signaling and Anti-Apoptosis Role. Biomed. Pharmacother. 2020, 126, 110075. [Google Scholar] [CrossRef]
- Mansour, R.B.; Beji, R.S.; Wasli, H.; Zekri, S.; Ksouri, R.; Megdiche-Ksouri, W.; Cardoso, S.M. Gastroprotective Effect of Microencapsulated Myrtus Communis Essential Oil against Ethanol/HCl-Induced Acute Gastric Lesions. Molecules 2022, 27, 1566. [Google Scholar] [CrossRef]
- Li, W.-S.; Lin, S.-C.; Chu, C.-H.; Chang, Y.-K.; Zhang, X.; Lin, C.-C.; Tung, Y.-T. The Gastroprotective Effect of Naringenin against Ethanol-Induced Gastric Ulcers in Mice through Inhibiting Oxidative and Inflammatory Responses. Int. J. Mol. Sci. 2021, 22, 11985. [Google Scholar] [CrossRef]
- Hama Amin, R.R.; Aziz, T.A. Gastroprotective Effect of Azilsartan Through Ameliorating Oxidative Stress, Inflammation, and Restoring Hydroxyproline, and Gastrin Levels in Ethanol-Induced Gastric Ulcer. J. Inflamm. Res. 2022, 15, 2911–2923. [Google Scholar] [CrossRef] [PubMed]
- Andargie, Y.; Sisay, W.; Molla, M.; Norahun, A.; Singh, P. Evaluation of the Antiulcer Activity of Methanolic Extract and Solvent Fractions of the Leaves of Calpurnia aurea (Ait.) Benth. (Fabaceae) in Rats. Evid.-Based Complement. Altern. Med. 2022, 2022, 4199284. [Google Scholar] [CrossRef]
- Gürbüz, İ.; Üstün, O.; Yesilada, E.; Sezik, E.; Kutsal, O. Anti-Ulcerogenic Activity of Some Plants Used as Folk Remedy in Turkey. J. Ethnopharmacol. 2003, 88, 93–97. [Google Scholar] [CrossRef]
- Li, W.-F.; Hao, D.-J.; Fan, T.; Huang, H.-M.; Yao, H.; Niu, X.-F. Protective Effect of Chelerythrine against Ethanol-Induced Gastric Ulcer in Mice. Chem. Biol. Interact. 2014, 208, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, R.F.; De Sales, I.R.; De Oliveira Formiga, R.; Barbosa-Filho, J.M.; Sobral, M.V.; Tavares, J.F.; Diniz, M.D.; Batista, L.M. Activity of Alkaloids on Peptic Ulcer: What’s New? Molecules 2015, 20, 929–950. [Google Scholar] [CrossRef]
- Oh, J.-H.; Yun, M.; Park, D.; Ha, I.J.; Kim, C.-K.; Kim, D.-W.; Kim, E.-O.; Lee, S.-G. Papaver nudicaule (Iceland Poppy) Alleviates Lipopolysaccharide-Induced Inflammation through Inactivating NF-ΚB and STAT3. BMC Complement. Altern. Med. 2019, 19, 90. [Google Scholar] [CrossRef] [PubMed]
- Khursheed, A.; Ahmad, S.; Khan, K.-R.; Tousif, M.I.; Aati, H.Y.; Ovatlarnporn, C.; Rao, H.; Khurshid, U.; Ghalloo, B.A.; Tabassum, S.; et al. Efficacy of Phytochemicals Derived from Roots of Rondeletia odorata as Antioxidant, Antiulcer, Diuretic, Skin Brightening and Hemolytic Agents—A Comprehensive Biochemical and In Silico Study. Molecules 2022, 27, 4204. [Google Scholar] [CrossRef]
- Wuniqiemu, T.; Teng, F.; Qin, J.; Lv, Y.; Nabijan, M.; Luo, Q.; Zhou, Y.; Cui, J.; Yi, L.; Tang, W.; et al. Iristectorigenin A Exerts Novel Protective Properties against Airway Inflammation and Mucus Hypersecretion in OVA-Induced Asthmatic Mice: Iristectorigenin A Ameliorates Asthma Phenotype. Phytomedicine 2022, 104, 154252. [Google Scholar] [CrossRef]
- Lokman, M.S.; Zaafar, D.; Althagafi, H.A.; Abdel Daim, M.M.; Theyab, A.; Hasan Mufti, A.; Algahtani, M.; Habotta, O.A.; Alghamdi, A.A.A.; Alsharif, K.F.; et al. Antiulcer Activity of Proanthocyanidins Is Mediated via Suppression of Oxidative, Inflammatory, and Apoptotic Machineries. J. Food Biochem. 2022, 46, e14070. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, H.M.; Ahmed, S.M.; Welson, N.N.; Abdelzaher, W.Y. Rivastigmine Ameliorates Indomethacin Experimentally Induced Gastric Mucosal Injury via Activating A7nAChR with Inhibiting Oxidative Stress and Apoptosis. J. Biochem. Mol. Toxicol. 2022, 15, e23147. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, A.A. Onosma Mutabilis: Phytochemical Composition, Antioxidant, Cytotoxicity, and Acute Oral Toxicity. Food Sci. Nutr. 2021, 9, 5755–5764. [Google Scholar] [CrossRef] [PubMed]
- Abduljabbar, A.A.; Abdoulrahman, K.K. Onion (Allium cepa) and Garlic (Allium sativa L.) Oil Effects on Blood Glucose Levels and Body Weight of Local Quails in Erbil Province. Zanco J. Pure Appl. Sci. 2018, 30, 158–167. [Google Scholar]
- Alharbi, K.S.; Afzal, O.; Kazmi, I.; Shaikh, M.A.J.; Thangavelu, L.; Gulati, M.; Singh, S.K.; Jha, N.K.; Gupta, P.K.; Chellappan, D.K. Nuclear Factor-Kappa B (NF-ΚB) Inhibition as a Therapeutic Target for Plant Nutraceuticals in Mitigating Inflammatory Lung Diseases. Chem. Biol. Interact. 2022, 354, 109842. [Google Scholar] [CrossRef]
- Pandrangi, S.L.; Chalumuri, S.S.; Garimella, S. Emerging Therapeutic Efficacy of Alkaloids as Anticancer Agents. Ann. Rom. Soc. Cell Biol. 2022, 26, 64–74. [Google Scholar]
- Hasanvand, D.; Amiri, I.; Soleimani Asl, S.; Saidijam, M.; Shabab, N.; Artimani, T. Effects of CeO(2) Nanoparticles on the HO-1, NQO1, and GCLC Expression in the Testes of Diabetic Rats. Can. J. Physiol. Pharmacol. 2018, 96, 963–969. [Google Scholar] [CrossRef]
- Kim, H.; Han, S.; Song, K.; Lee, M.Y.; Park, B.; Ha, I.J.; Lee, S.-G. Ethyl Acetate Fractions of Papaver rhoeas L. and Papaver nudicaule L. Exert Antioxidant and Anti-Inflammatory Activities. Antioxidants 2021, 10, 1895. [Google Scholar] [CrossRef]
- Weng, Q.; Hu, T.; Shen, X.; Han, J.; Zhang, Y.; Luo, J. Ezetimibe Prevents IL-1β-Induced Inflammatory Reaction in Mouse Chon-Drocytes via Modulating NF-ΚB and Nrf2/HO-1 Signaling Crosstalk. Curr. Pharm. Biotechnol. 2022, 23, 1772–1780. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Cardile, V.; Graziano, A.C.E.; Avola, R.; Bruno, M.; Rigano, D. Involvement of Bax and Bcl-2 in Induction of Apoptosis by Essential Oils of Three Lebanese Salvia Species in Human Prostate Cancer Cells. Int. J. Mol. Sci. 2018, 19, 292. [Google Scholar] [CrossRef]
- Walensky, L.D. Targeting BAX to Drug Death Directly. Nat. Chem. Biol. 2019, 15, 657–665. [Google Scholar] [CrossRef]
- Babaei, F.; Moafizad, A.; Darvishvand, Z.; Mirzababaei, M.; Hosseinzadeh, H.; Nassiri-Asl, M. Review of the Effects of Vitexin in Oxidative Stress-Related Diseases. Food Sci. Nutr. 2020, 8, 2569–2580. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Ashraf, M.; Wijaya, L.; Ahmad, P. The Putative Role of Endogenous Nitric Oxide in Brassinosteroid-Induced Antioxidant Defence System in Pepper (Capsicum annuum L.) Plants under Water Stress. Plant Physiol. Biochem. 2019, 143, 119–128. [Google Scholar]
- Kapoor, D.; Singh, S.; Kumar, V.; Romero, R.; Prasad, R.; Singh, J. Antioxidant Enzymes Regulation in Plants in Reference to Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS). Plant Gene 2019, 19, 100182. [Google Scholar] [CrossRef]
- Zhao, H.; Jiang, Z.; Chang, X.; Xue, H.; Yahefu, W.; Zhang, X. 4-Hydroxyphenylacetic Acid Prevents Acute APAP-Induced Liver Injury by Increasing Phase II and Antioxidant Enzymes in Mice. Front. Pharmacol. 2018, 9, 653. [Google Scholar] [CrossRef]
- Istifli, E.S. Chemical Composition, Antioxidant and Enzyme Inhibitory Activities of Onosma bourgaei and Onosma trachytricha and in Silico Molecular Docking Analysis of Dominant Compounds. Molecules 2021, 26, 2981. [Google Scholar] [CrossRef]
- Jabbar, A.A.; Abdulrahman, K.K.; Abdulsamad, P.; Mojarrad, S.; Mehmetçik, G.; Sardar, A.S. Phytochemical Profile, Antioxidant, Enzyme Inhibitory and Acute Toxicity Activity of Astragalus bruguieri. Baghdad Sci. J. 2022, 20, 157–165. [Google Scholar]
- Sharopov, F.; Valiev, A.; Gulmurodov, I.; Sobeh, M.; Satyal, P.; Wink, M. Alkaloid Content, Antioxidant and Cytotoxic Activities of Various Parts of Papaver somniferum. Pharm. Chem. J. 2018, 52, 459–463. [Google Scholar] [CrossRef]
- Ciciliato, M.P.; de Souza, M.C.; Tarran, C.M.; de Castilho, A.L.; Vieira, A.J.; Rozza, A.L. Anti-Inflammatory Effect of Vanillin Protects the Stomach against Ulcer Formation. Pharmaceutics 2022, 14, 755. [Google Scholar] [CrossRef] [PubMed]
- Drishya, S.; Dhanisha, S.S.; Guruvayoorappan, C. Antioxidant-Rich Fraction of Amomum Subulatum Fruits Mitigates Experimental Methotrexate-Induced Oxidative Stress by Regulating TNF-α, IL-1β, and IL-6 Proinflammatory Cytokines. J. Food Biochem. 2022, 46, e13855. [Google Scholar] [CrossRef]
- Altyar, A.E.; Munir, A.; Ishtiaq, S.; Rizwan, M.; Abbas, K.; Kensara, O.; Elhady, S.S.; Rizg, W.Y.; Youssef, F.S.; Ashour, M.L. Malva parviflora Leaves and Fruits Mucilage as Natural Sources of Anti-Inflammatory, Antitussive and Gastro-Protective Agents: A Comparative Study Using Rat Models and Gas Chromatography. Pharmaceutics 2022, 15, 427. [Google Scholar] [CrossRef] [PubMed]




| Animal Groups | Pre-Feeding (5 mL/kg) | Mucus Weight (g) | pH | Ulcer Area (mm2) | Inhibition (%) |
|---|---|---|---|---|---|
| G1 | 0.5% CMC | 1.75 ± 0.65 a | 6.10 ± 0.55 a | - | - |
| G2 | 0.5% CMC | 0.67 ± 0.32 b | 2.9 ± 0.8 d | 655.78 ± 8.55 d | - |
| G3 | 20 mg/kg omeprazole | 1.77 ± 0.48 a | 6.2 ± 0.60 a | 98.33 ± 5.43 a | 85.0% a |
| G4 | 200 mg/kg PDE | 1.52 ± 0.33 c | 5.22 ± 0.5 c | 152.66 ± 7.30 c | 76.72% b |
| G5 | 400 mg/kg PDE | 1.76 ± 0.58 a | 5.8 ± 0.6 b | 108.16 ± 4.89 b | 83.5% a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabbar, A.A.; Abdullah, F.O.; Abdoulrahman, K.; Galali, Y.; Ibrahim, I.A.A.; Alzahrani, A.R.; Hassan, R.R. Gastroprotective, Biochemical and Acute Toxicity Effects of Papaver decaisnei against Ethanol-Induced Gastric Ulcers in Rats. Processes 2022, 10, 1985. https://doi.org/10.3390/pr10101985
Jabbar AA, Abdullah FO, Abdoulrahman K, Galali Y, Ibrahim IAA, Alzahrani AR, Hassan RR. Gastroprotective, Biochemical and Acute Toxicity Effects of Papaver decaisnei against Ethanol-Induced Gastric Ulcers in Rats. Processes. 2022; 10(10):1985. https://doi.org/10.3390/pr10101985
Chicago/Turabian StyleJabbar, Ahmed Aj., Fuad O. Abdullah, Kamaran Abdoulrahman, Yaseen Galali, Ibrahim Abdel Aziz Ibrahim, Abdullah R. Alzahrani, and Rawaz Rizgar Hassan. 2022. "Gastroprotective, Biochemical and Acute Toxicity Effects of Papaver decaisnei against Ethanol-Induced Gastric Ulcers in Rats" Processes 10, no. 10: 1985. https://doi.org/10.3390/pr10101985
APA StyleJabbar, A. A., Abdullah, F. O., Abdoulrahman, K., Galali, Y., Ibrahim, I. A. A., Alzahrani, A. R., & Hassan, R. R. (2022). Gastroprotective, Biochemical and Acute Toxicity Effects of Papaver decaisnei against Ethanol-Induced Gastric Ulcers in Rats. Processes, 10(10), 1985. https://doi.org/10.3390/pr10101985