Methanol-Enhanced Fe(III) Oleate-Catalyzed Aquathermolysis of Heavy Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Catalyst
2.3. Catalyzed Aquathermolysis of Heavy Oil
2.4. Product Evaluation
3. Results and Discussion
3.1. Effect of Reaction Temperature
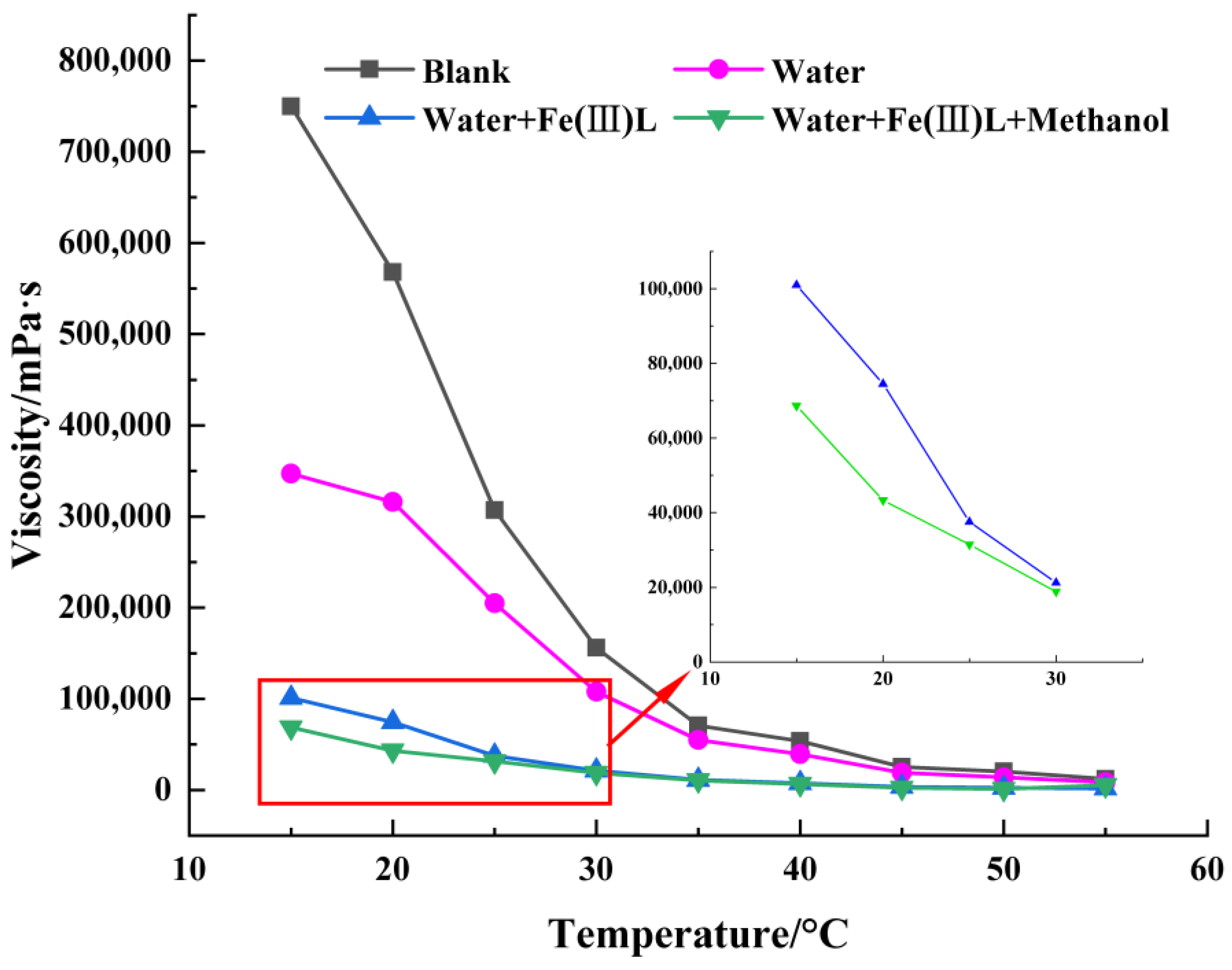
3.2. Effect of Water
3.3. Effect of Methanol as Hydrogen Donor
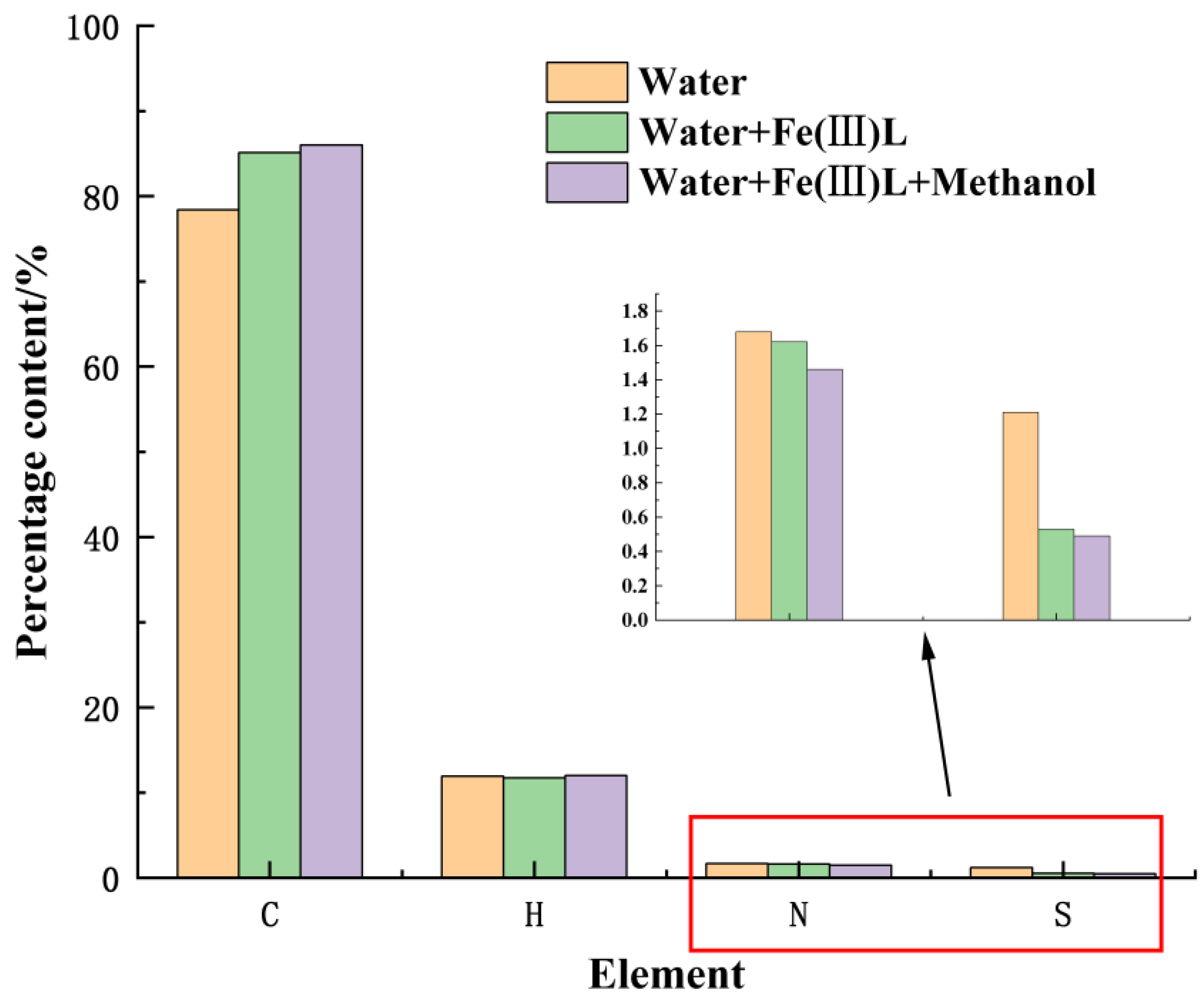
3.4. Elemental Analysis and Carbon Number Distribution (EA)
3.5. Thermogravimetric Analysis (TGA)
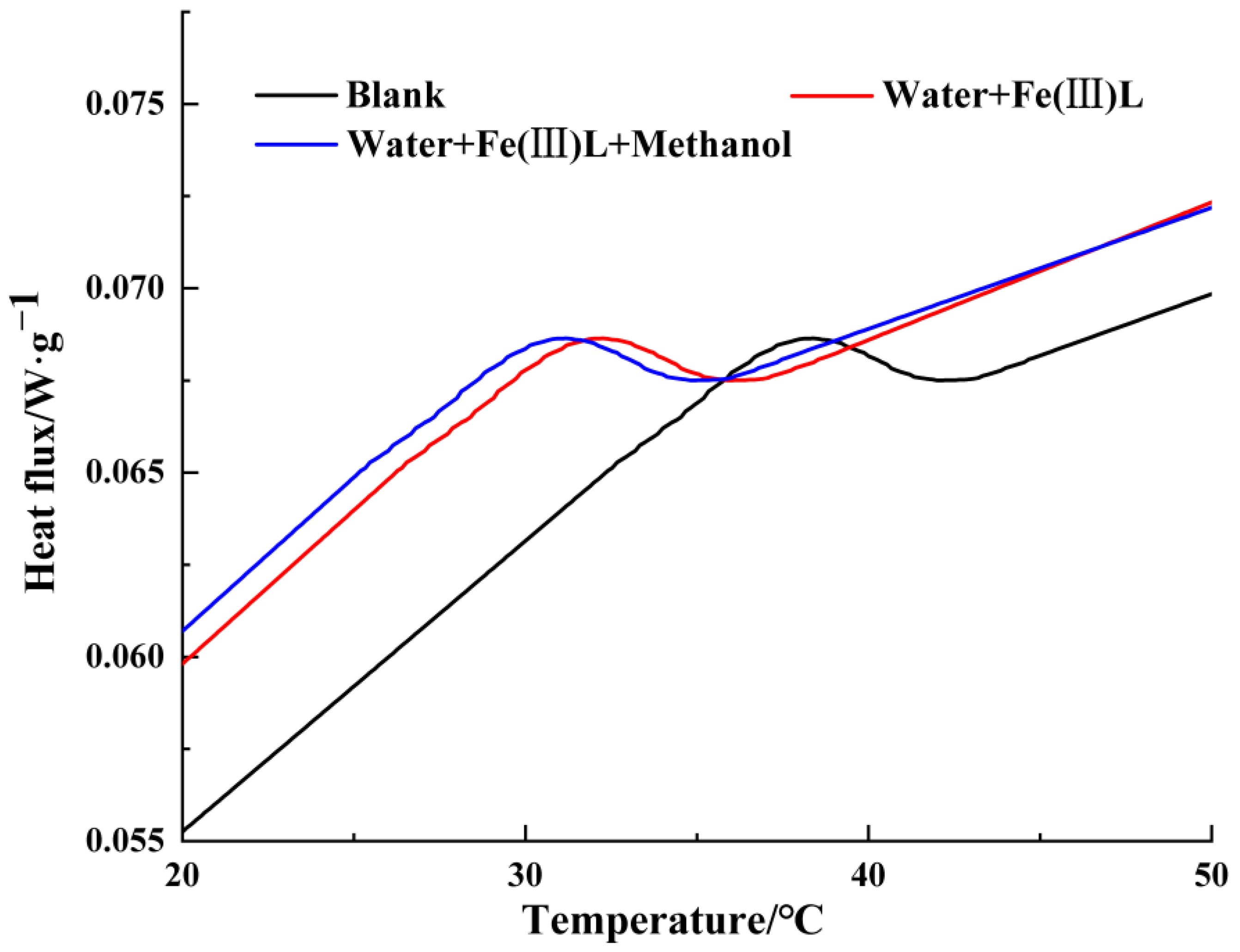
3.6. Differential Scanning Calorimetry Analysis (DSC)
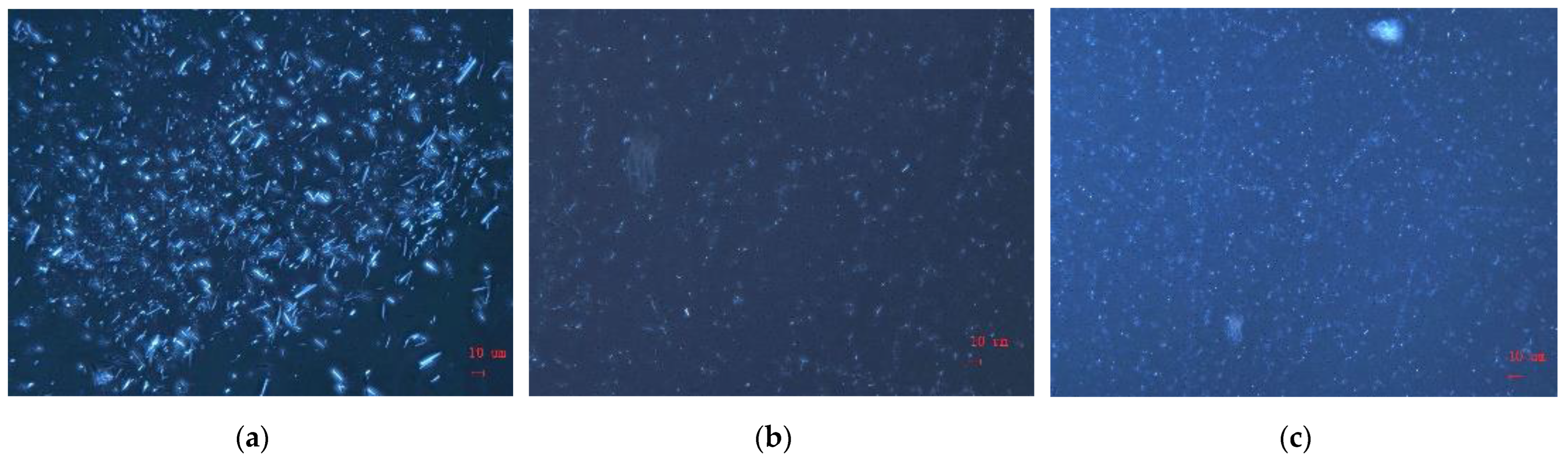
3.7. Paraffin Crystals
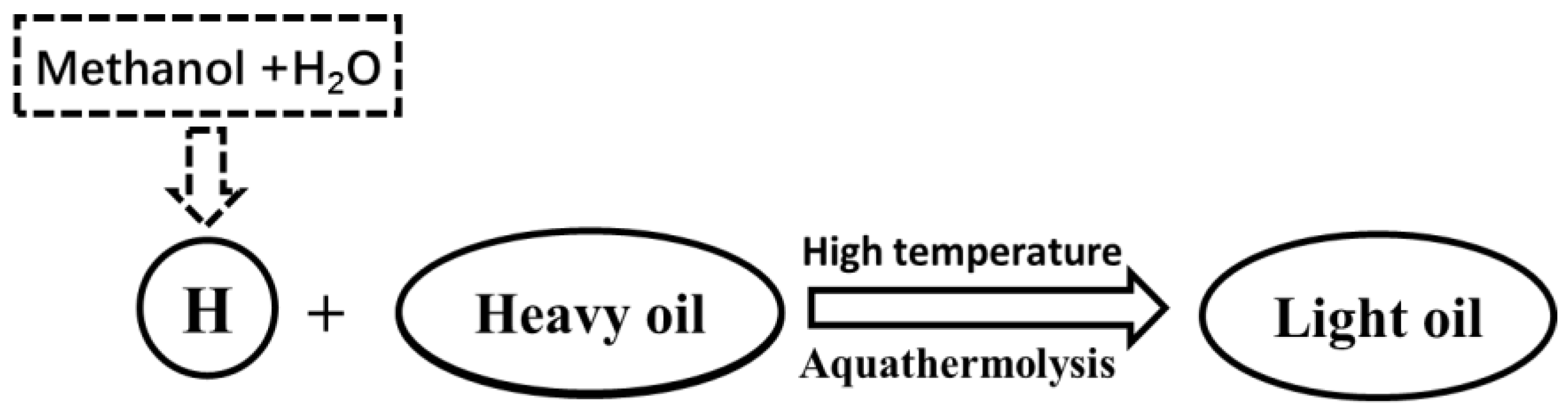
3.8. Mechanism
4. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiayu, N.; Jianyi, H. Formation and distribution of Heavy oil and tar sands in China. Mar. Pet. Geol. 1999, 16, 85–95. [Google Scholar] [CrossRef]
- Ali, S.M.F. Heavy oil—Evermore mobile. J. Pet. Sci. Eng. 2003, 37, 5–9. [Google Scholar] [CrossRef]
- Hein, F.J. Geology of bitumen and Heavy oil: An overview. J. Pet. Sci. Eng. 2017, 154, 551–563. [Google Scholar] [CrossRef]
- Speight, J.G. Heavy Oil Production Processes, 1st ed.; Gulf Professional Publishing: Oxford, UK, 2013. [Google Scholar]
- Demirbas, A.; Bafail, A.; Nizami, A.S. Heavy oil upgrading: Unlocking the future fuel supply. Pet. Sci. Technol. 2016, 34, 303–308. [Google Scholar] [CrossRef]
- Shaban, S.; Dessouky, S.; Badawi, A.E.F.; El Sabagh, A.; Zahran, A.; Mousa, M. Upgrading and viscosity reduction of Heavy oil by catalyst ionic liquid. Energy Fuel 2014, 28, 6545–6553. [Google Scholar] [CrossRef]
- Lv, S.; Peng, S.; Zhang, R.; Guo, Z.; Du, W.; Zhang, J.; Chen, G. Viscosity reduction of Heavy oil by ultrasonic. Pet. Chem. 2020, 60, 998–1002. [Google Scholar] [CrossRef]
- Chen, G.; Zhou, Z.; Shi, X.; Zhang, X.; Dong, S.; Zhang, J. Synthesis of alkylbenzenesulfonate and its behavior as flow improver in crude oil. Fuel 2021, 288, 119644. [Google Scholar] [CrossRef]
- Zhou, Z.; Dong, S.; Zhang, X.; Zhang, J.; Song, H.; Chen, G. Synthesis of multi-alkylpolyamine and performance in crude oil as flow improver. Tenside Surfactants Deterg. 2022, 59, 104–110. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, W.; Dong, S.; Zhang, J.; Chen, G. Synthesis of aluminum alkylbenzene sulfonate and its behavior as a flow improver for crude oil. Tenside Surfactants Deterg. 2022, 59, 353–361. [Google Scholar] [CrossRef]
- Xu, H.; Pu, C. Mechanism of underground Heavy oil catalytic aquathermolysis. Chem. Technol. Fuels Oils 2018, 53, 913–921. [Google Scholar] [CrossRef]
- Jeon, S.G.; Na, J.-G.; Ko, C.H.; Yi, K.B.; Rho, N.S.; Bin Park, S. Preparation and application of an oil-soluble CoMo bimetallic catalyst for the hydrocracking of oil sands bitumen. Energy Fuels 2011, 25, 4256–4260. [Google Scholar] [CrossRef]
- Li, C.; Huang, W.; Zhou, C.; Chen, Y. Advances on the transition-metal based catalysts for aquathermolysis upgrading of Heavy crude oil. Fuel 2019, 257, 115779. [Google Scholar] [CrossRef]
- Wen, S.; Zhao, Y.; Liu, Y.; Hu, S. A Study on Catalytic Aquathermolysis of Heavy Crude Oil during Steam Stimulation. In Proceedings of the International Symposium on Oilfield Chemistry, Houston, TX, USA, 28 February–2 March 2007. [Google Scholar]
- Feoktistov, D.A.; Kayukova, G.P.; Vakhin, A.V.; Sitnov, S.A. Catalytic aquathermolysis of high-viscosity oil using iron, cobalt, and copper tallates. Chem. Technol. Fuels Oils 2018, 53, 905–912. [Google Scholar] [CrossRef]
- Li, Y.-R.; Li, Q.-Y.; Wang, X.-D.; Yu, L.-G.; Yang, J.-J. Aquathermolysis of Heavy crude oil with ferric oleate catalyst. Pet. Sci. 2018, 15, 613–624. [Google Scholar] [CrossRef]
- Al-Muntaser, A.A.; Varfolomeev, M.A.; Suwaid, M.A.; Feoktistov, D.A.; Yuan, C.; Klimovitskii, A.E.; Gareev, B.I.; Djimasbe, R.; Nurgaliev, D.K.; Kudryashov, S.I.; et al. Hydrogen donating capacity of water in catalytic and non-catalytic aquathermolysis of extra-Heavy oil: Deuterium tracing study. Fuel 2021, 283, 118957. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, Y.; Fu, Z.; Zhao, X. Using hydrogen donor with oil-soluble catalysts for upgrading Heavy oil. Russ. J. Appl. Chem. 2014, 87, 1498–1506. [Google Scholar] [CrossRef]
- Zhou, Z.; Slaný, M.; Kuzielová, E.; Zhang, W.; Ma, L.; Dong, S.; Zhang, J.; Chen, G. Influence of reservoir minerals and ethanol on catalytic aquathermolysis of Heavy oil. Fuel 2022, 307, 121871. [Google Scholar] [CrossRef]
- Deng, Q.; Slaný, M.; Zhang, H.; Gu, X.; Li, Y.; Du, W.; Chen, G. Synthesis of Alkyl Aliphatic Hydrazine and Application in Crude Oil as Flow Improvers. Energies 2021, 14, 4703. [Google Scholar] [CrossRef]
- Núñez-Méndez, K.S.; Salas-Chia, L.M.; Molina, D.V.; Muñoz, S.F.; León, P.A.; León, A.Y. Effect of the Catalytic Aquathermolysis Process on the Physicochemical Properties of a Colombian Crude Oil. Energy Fuels 2021, 35, 5231–5240. [Google Scholar] [CrossRef]
- Gu, X.; Gao, L.; Li, Y.; Chen, S.; Zhang, J.; Du, W.; Qu, C.; Chen, G. Performance and mechanism of span surfactants as clean flow improvers for crude oil. Pet. Chem. 2020, 60, 140–145. [Google Scholar] [CrossRef]
- Tirado, A.; Yuan, C.; Varfolomeev, M.A.; Ancheyta, J. Kinetic modeling of aquathermolysis for upgrading of Heavy oils. Fuel 2022, 310, 122286. [Google Scholar] [CrossRef]
- Li, G.-R.; Chen, Y.; An, Y.; Chen, Y.-L. Catalytic aquathermolysis of super-Heavy oil: Cleavage of CS bonds and separation of light organosulfurs. Fuel Processing Technol. 2016, 153, 94–100. [Google Scholar] [CrossRef]
- Mukhamatdinov, I.; Salih, I.; Rakhmatullin, I.; Sviridenko, N.; Pevneva, G.; Sharma, R.; Vakhin, A. Transformation of Resinous Components of the Ashalcha Field Oil during Catalytic Aquathermolysis in the Presence of a Cobalt-Containing Catalyst Precursor. Catalysts 2021, 11, 745. [Google Scholar] [CrossRef]
- Aliev, F.A.; Mukhamatdinov, I.I.; Sitnov, S.A.; Ziganshina, M.R.; Onishchenko, Y.V.; Sharifullin, A.V.; Vakhin, A.V. In-situ Heavy oil aquathermolysis in the presence of nanodispersed catalysts based on transition metals. Processes 2021, 9, 127. [Google Scholar] [CrossRef]
- Wang, P.; Gu, X.; Xue, M.; Li, Y.; Dong, S.; Chen, G.; Zhang, J. Resource utilization of medical waste under COVID-19: Waste mask used as crude oil fluidity improver. J. Clean. Prod. 2022, 358, 131903. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, S.; Zhang, X.; Dong, S.; Yu, T.; Slaný, M.; Chen, G. Enhanced aquathermolysis of Heavy oil catalysed by bentonite supported Fe (III) complex in the present of ethanol. J. Chem. Technol. Biotechnol. 2022, 97, 1128–1137. [Google Scholar] [CrossRef]
- Deng, Q.; Li, Y.; Chen, G.; Yan, J.; Zhang, J.; Meng, M.; Qu, C.; Jeje, A. Water-soluble complexes catalyzed coupling aquathermolysis of water-Heavy oil-methanol at low temperature. Pet. Chem. 2018, 58, 727–732. [Google Scholar] [CrossRef]
- Petrukhina, N.N.; Kayukova, G.; Romanov, G.; Tumanyan, B.P.; Foss, L.E.; Kosachev, I.P.; Musin, R.Z.; Ramazanova, A.I.; Vakhin, A.V. Conversion processes for high-viscosity Heavy crude oil in catalytic and noncatalytic aquathermolysis. Chem. Technol. Fuels Oils 2014, 50, 315–326. [Google Scholar] [CrossRef]
- Al-Muntaser, A.A.; Varfolomeev, M.A.; Suwaid, M.A.; Saleh, M.M.; Djimasbe, R.; Yuan, C.; Zairov, R.R.; Ancheyta, J. Effect of decalin as hydrogen-donor for in-situ upgrading of Heavy crude oil in presence of nickel-based catalyst. Fuel 2022, 313, 122652. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Nourgaliev, D.; Mukhamatdinov, I.I.; Sitnov, S.A.; Sharifullin, A.V.; Kudryashov, S.I.; Afanasiev, I.S.; Petrashov, O.V.; Nurgaliev, D.K. Catalytic aquathermolysis of boca de jaruco heavy oil with nickel-based oil-soluble catalyst. Processes 2020, 8, 532. [Google Scholar] [CrossRef]





| Freezing Point/°C | Moisture Content/% | Asphaltene, % | Saturated HC, % | Aromatic HC, % | Resin, % |
|---|---|---|---|---|---|
| 5.3 | 11.41 | 0.02 | 48.50 | 21.00 | 19.07 |
| Carbon Number Distribution | Blank/% | Fe(III)L/% | Fe(III)L + Methanol/% |
|---|---|---|---|
| <C10 | 49.39 | 73.59 | 75.06 |
| C10~C15 | 38.21 | 19.91 | 22.12 |
| C15~C20 | 11.71 | 6.50 | 2.82 |
| >C20 | 0.69 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, R.; Fu, W.; Qu, L.; Li, Y.; Yuan, W.; Chen, G. Methanol-Enhanced Fe(III) Oleate-Catalyzed Aquathermolysis of Heavy Oil. Processes 2022, 10, 1956. https://doi.org/10.3390/pr10101956
Guo R, Fu W, Qu L, Li Y, Yuan W, Chen G. Methanol-Enhanced Fe(III) Oleate-Catalyzed Aquathermolysis of Heavy Oil. Processes. 2022; 10(10):1956. https://doi.org/10.3390/pr10101956
Chicago/Turabian StyleGuo, Rui, Wei Fu, Le Qu, Yongfei Li, Weihua Yuan, and Gang Chen. 2022. "Methanol-Enhanced Fe(III) Oleate-Catalyzed Aquathermolysis of Heavy Oil" Processes 10, no. 10: 1956. https://doi.org/10.3390/pr10101956
APA StyleGuo, R., Fu, W., Qu, L., Li, Y., Yuan, W., & Chen, G. (2022). Methanol-Enhanced Fe(III) Oleate-Catalyzed Aquathermolysis of Heavy Oil. Processes, 10(10), 1956. https://doi.org/10.3390/pr10101956





