Differential Metabolomic Fingerprinting of the Crude Extracts of Three Asteraceae Species with Assessment of Their In Vitro Antioxidant and Enzyme-Inhibitory Activities Supported by In Silico Investigations
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Preparation of Extracts
2.2. HPLC-ESI-MS/MS Analysis of the Methanol Extracts of Three Asteraceae Species
2.3. Antioxidant Assays
2.4. Enzyme Inhibitory Assays
2.5. Molecular Docking
2.6. Statistical Analysis
3. Results and Discussion
3.1. ESI-MS-MS Fingerprinting of the Three Asteraceae Crude Extracts under Three Different Extraction Methods
| Peak No. | Rt | [M−H]−/[M+H]+ | MS/MS | UV (λmax) | Compound Name | Phytochemical Class | Relative Amount (%) | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| HAE | MAC | UAE | ||||||||
| 1 | 0.77 | 377 | 341 | 223, 294 | Caffeic acid derivative | Phenolic acid | 6.98 | 10.5 | 11.0 | [34] |
| 2 | 1.29 | 315 | 152, 108 | 221 | Protocatechuic acid hexoside | Phenolic acid glycoside | 0.65 | 1.22 | 0.85 | [34] |
| 3 | 1.59 | 353 | 191 | 221, 317 | Neochlorogenic acid or Chlorogenic acid | Phenolic acid | 2.80 | 5.41 | 4.73 | [34] |
| 4 | 2.59 | 353 | 191 | 221, 317 | Neochlorogenic acid or Chlorogenic acid | Phenolic acid | 8.36 | 4.45 | 4.25 | [34] |
| 5 | 3.02 | 353 | 191 | 221, 317 | Neochlorogenic acid or Chlorogenic acid | Phenolic acid | 2.78 | - | - | [34] |
| 6 | 6.15 | 197 | 197, 169, 124 | 210, 225 | Syringic acid | Phenolic acid | 0.94 | 0.76 | 1.28 | |
| 7 | 7.03 | 463/465 | 300, 463, 271, 255, 151 | 210, 317 | Quercetin -O-hexoside or hesperitin hexoside | Flavonoid glycoside | 3.78 | 0.23 | 0.21 | [35,36,37] |
| 8 | 7.37 | 515 | 353, 173, 179, 135 | 223, 294 | Dicaffeoylquinic acid | Phenolic acid | 2.63 | 12.6 | 14.5 | [34] |
| 9 | 7.49 | 515 | 353, 173, 179, 135 | 223, 294 | Dicaffeoylquinic acid | Phenolic acid | 6.92 | 13.2 | 14.3 | [34] |
| 10 | 7.68 | -/447 | 271 | 204, 325 | Apigenin-O-hexouronide | Flavonoid glycoside | 0.42 | 0.66 | 0.97 | [38] |
| 11 | 7.70 | 515 | 353, 173, 179, 135 | 223, 294 | Dicaffeoylquinic acid | Phenolic acid | 9.02 | 0.77 | 0.95 | [34] |
| 12 | 8.73 | 435 | 297, 315, 163, 152, 137, 108 | 217, 324 | Shimobashiraside C | Phenolic acid ester glycoside | 2.20 | 3.86 | 2.89 | [34] |
| 13 | 9.0 | 582/584 | 462, 342, 299, 292, 119 | 222, 289 | N′,N′′,N′′′-Tris-p-coumaroyl spermidine | Amine derivative | 1.34 | 2.04 | 1.66 | [39] |
| 14 | 9.47 | 327 | 171, 183, 211, 229, 291, 199 | n.d. | Trihydroxy-octadecadienoic acid | Fatty acid | 0.71 | 0.72 | 1.15 | [34] |
| 15 | 9.64 | 577 | 269, 145, 431, 117 | 206, 318 | Apigenin-(p-coumaroyl)-hexoside isomer or rhoifolin | Flavonoid glycoside | 0.70 | 1.37 | 1.86 | [34,40] |
| 16 | 9.80 | 579 | 271, 145, 119, 163, 295 | 221, 317 | Naringenin-coumaroyl- hexoside | Flavonoid glycoside | - | 0.65 | - | [34] |
| 17 | 10.02 | 329 | 211, 229, 171, 139, 99, 155 | n.d. | Trihydroxyoctadecenoic acid or pinellic acid | Fatty acid | 2.21 | 2.81 | 4.06 | [34,40] |
| 18 | 12.35 | 293 | 265, 275 | n.d. | Octadecadienoic acid | Fatty acid | 0.43 | 1.29 | 0.92 | [40] |
| 19 | 14.93 | 293 | 265, 275 | n.d. | Octadecadienoic acid | Fatty acid | 0.44 | 0.52 | 0.48 | [40] |
| 20 | 15.11 | 293 | 265, 275 | n.d. | Octadecadienoic acid | Fatty acid | 0.24 | 0.24 | 1.05 | [40] |
| 21 | 16.13 | 295 | 277, 171, 195, 183 | n.d. | Hydroxyoctadecadieoic acid | Fatty acid | 1.68 | 1.61 | 2.69 | [34,40] |
| 22 | 19.64 | 455 | 455 | n.d. | Betulinic acid | Triterpene | - | 10.08 | 2.12 | [41] |
| 23 | 21.31 | -/256 | 116, 102, 88 | n.d. | Palmitamide | Fatty acid amide | 13.8 | - | 10.4 | [42,43] |
| 24 | 21.95 | -/282 | 97, 69, 149, 163 | n.d. | Oleamide | Fatty acid amide | 49.4 | - | 36.2 | [42,43] |
| 25 | 22.60 | -/282 | 97, 69, 149, 163 | n.d. | Oleamide | Fatty acid amide | 0.57 | - | - | [42,43] |
| Peak No. | Rt | [M−H]−/[M+H]+ | MS/MS | UV (λmax) | Compound Name | Phytochemical Class | Relative Amount (%) | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| HAE | MAC | UAE | ||||||||
| 1. | 0.75 | 191 | - | 265 | Quinic acid | Organic acid | 4.74 | 3.96 | 4.11 | [44] |
| 2. | 1.65 | 343 | 267, 203, 177, 135 | 275, 330 | Eupatorin | Flavonoid | - | 1.16 | - | |
| 3. | 2.05 | 417 | 285, 249, 199, 144 | 265, 360 | Kaempferol pentoside | Flavonoid glycoside | 1.95 | 1.33 | 1.93 | [45] |
| 4. | 2.45 | 353 | 191 | 339 | Chlorogenic acid/ Neochlorogenic acid | Phenolic acid | 5.41 | 4.70 | 4.81 | [46] |
| 5. | 3.40 | 341 | 193, 175 | 265 | Caffeoyl hexoside | Phenolic acid glycoside | 10.28 | 9.00 | 10.18 | [47] |
| 6. | 4.44 | 337 | 191, 163 | 265 | Coumaroyl quinic acid | Phenolic acid | - | 0.73 | 0.68 | [46] |
| 7. | 5.25 | 593 | 473, 395, 383, 353, 297 | 271, 333 | Apigenin-di-C-hexoside (Vicenin-2) | Flavonoid glycoside | 5.72 | 5.66 | 4.98 | [48,49,50] |
| 8. | 5.75 | 579 | 459, 399, 369 | 271, 330 | Naringenin-O-neohesperidoside (Naringin) | Flavonoid glycoside | 2.13 | 2.34 | 1.85 | [51] |
| 9. | 6.02 | 197 | 169, 124 | 271 | Syringic acid | Phenolic acid | 4.71 | 4.01 | 4.95 | [52] |
| 10. | 6.09 | 337 | 191, 163 | 265 | Coumaroylquinic acid | Phenolic acid | 3.23 | 3.53 | 2.97 | [44] |
| 11. | 6.20 | 563 | 503, 473, 443, 383, 353 | 271, 333 | Apigenin-C-hexoside-C-pentoside (Schaftoside) | Flavonoid glycoside | 3.23 | 3.72 | 2.97 | [47] |
| 12. | 7.42 | 515 | 285, 191, 179, 173, 135 | 234, 294 | Dicaffeoylquinic acid | Phenolic acid | 2.73 | - | 1.92 | [46] |
| 13. | 7.52 | 447 | 285 | 252, 340 | Luteolin-O-hexoside | Flavonoid glycoside | 1.42 | 3.15 | 2.77 | [47] |
| 14. | 8.35 | 579 | 371 | 255, 278 | Arctiin | Lignan | 8.86 | 7.90 | 7.77 | [44] |
| 15. | 8.67 | 285 | - | 252, 340 | Luteolin | Flavonoid | 2.56 | 1.91 | 2.67 | [53] |
| 16. | 9.40 | 327 | 229, 211, 171, 139 | n.d. | Trihydroxyoctadecadienoic acid | Fatty acid | 1.46 | 1.70 | 1.63 | [44] |
| 17. | 9.57 | 785 | 639, 545, 399 | 269, 327 | Jaceosidin di-O-hexoside-deoxyhexoside | Flavonoid glycoside | 2.06 | 2.14 | 1.85 | [54] |
| 18. | 9.61 | 299 | 299, 284, 256 | 269, 327 | Trihydroxymethoxyflavone (Hispidulin) | Flavonoid | 2.06 | - | - | [46] |
| 19. | 9.97 | 329 | 229, 211, 183, 171 | n.d. | Trihydroxyoctadecenoic acid | Fatty acid | 2.47 | 2.23 | 2.61 | [55,56] |
| 20. | 13.78 | 313 | 255, 225 | 276, 331 | Dihydroxydimethoxyflavone (Cirsimaritin) | Flavonoid | 0.27 | 0.40 | 0.38 | [57] |
| 21. | 15.00 | 293 | 275, 211, 183, 171 | n.d. | Octadecadienoic acid | Fatty acid | 2.63 | 1.25 | 2.15 | [55,56] |
| 22. | 16.10 | 295/297 | 277, 171 | n.d. | Hydroxyoctadecadienoic acid | Fatty acid | 3.01 | 4.15 | 3.67 | [55,56] |
| 23. | 16.60 | 311 | 293, 183, 171, 153, 137, 131 | n.d. | Dihydroxyoctadecadienoic acid | Fatty acid | 1.09 | - | 0.33 | [55,56] |
| 24. | 17.43 | 293 | 275, 211, 183, 171 | n.d. | Octadecadienoic acid | Fatty acid | - | 1.58 | 1.04 | [54,56] |
| 25. | 20.77 | 271 | 225 | n.d. | Hydroxyhexadecanoic acid | Fatty acid | 2.50 | 2.51 | 2.19 | [55,56] |
| 26. | 21.28 | -/256.25 | 116, 102, 88, 71 | n.d. | Palmitamide | Fatty acid amide | 3.39 | 3.27 | 3.70 | [42,43] |
| 27. | 21.90 | -/282.30 | 265, 247, 149, 135, 121, 111, 97, 83 | n.d. | Oleamide | Fatty acid amide | 10.03 | 12.26 | 11.28 | [42,43] |
| 28. | 21.83 | 343 | 315, 299, 285, 253, 225 | 276, 331 | Dihydroxytrimethoxyflavone | Flavonoid | 2.08 | 1.47 | 2.61 | [57] |
| 29. | 24.80 | 327 | - | 270, 331 | Hydroxytrimethoxyflavone (Salvigenin) | Flavonoid | -- | 9.15 | - | [47] |
| Peak No. | Rt | [M−H]−/[M+H]+ | MS/MS | UV (λmax) | Compound Name | Phytochemical Class | Relative Amount (%) | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| HAE | MAC | UAE | ||||||||
| 1. | 0.76 | 149 | - | 225 | Tartaric acid | Organic acid | - | - | 3.63 | [58] |
| 2. | 1.21 | 191 | - | 265 | Quinic acid | Organic acid | 1.55 | 2.04 | 0.96 | [44] |
| 3. | 1.52 | 353 | 191 | 325 | Chlorogenic acid/ Neochlorogenic acid | Phenolic acid | 0.38 | 0.43 | 7.35 | [34] |
| 4. | 2.38 | 353 | 191 | 325 | Chlorogenic acid/ Neochlorogenic acid | Phenolic acid | 7.78 | 9.66 | - | [34] |
| 5. | 2.73 | 353 | 191 | 325 | Chlorogenic acid/ Neochlorogenic acid | Phenolic acid | 1.47 | - | - | [34] |
| 6. | 3.46 | 341 | 193, 161 | 265 | Caffeoyl hexoside | Phenolic acid glycoside | 1.22 | - | - | [47] |
| 7. | 3.97 | 387 | 207, 163, 119, 89 | n.d. | Medioresinol | Lignan | 8.80 | 11.19 | 6.23 | [59] |
| 8. | 4.83 | 311 | 179, 149, 135 | 325 | Caftaric acid | Phenolic acid | 4.25 | 5.61 | 3.46 | [60,61] |
| 9. | 5.63 | 225/227 | 207/209, 179/181, 135/137, 97/99 | 272 | 2-benzoylbenzoic acid | Phenolic acid | 2.10 | 2.13 | 2.58 | [62] |
| 10. | 6.01 | 197 | 169, 124 | 271 | Syringic acid | Phenolic acid | 0.99 | - | - | [52] |
| 11. | 6.28 | 479 | 317 | 271, 316 | Myricetin-O-hexoside | Flavonoid glycoside | 1.02 | 0.84 | 0.77 | [63] |
| 12. | 6.52 | 525 | 481, 207, 301, 119 | n.d. | Butanoyl betulinic acid | Triterpene | - | 0.28 | - | [64] |
| 13. | 6,76 | 325 | 193, 161, 149, 134 | 322 | Fertaric acid | Phenolic acid | 0.98 | 1.99 | 0.62 | [65] |
| 14. | 7.06 | 493 | 331 | 360 | Patuletin-O-hexoside | Flavonoid glycoside | 1.32 | 1.05 | 1.03 | [66,67] |
| 15. | 7.45 | 515 | 353, 191, 173, 179 | 234, 294 | Dicaffeoylquinic acid | Phenolic acid | 8.54 | 8.25 | 6.09 | [68] |
| 16. | 7.66 | 515 | 353, 191, 173, 179 | 234, 294 | Dicaffeoylquinic acid | Phenolic acid | 9.99 | 10.13 | 7.67 | [68] |
| 17. | 7.98 | 491/493 | 332, -/317 | 250, 362 | Isorhamnetin-O-hexouronoide | Flavonoid glycoside | - | - | 0.93 | [69] |
| 18. | 8.33 | 579 | 271, 145, 119, 163, 295 | 221, 317 | Naringenin-coumaroyl- hexoside | Flavonoid glycoside | 1.63 | - | - | [34] |
| 19. | 8.66 | 315 | 300, 151 | 260, 342 | Isorhamnetin | Flavonoid | 2.33 | 2.52 | 2.16 | [68] |
| 20. | 9.00 | 345 | 330, 315, 287 | 275, 344 | Quercetagetin-dimethyl ether | Flavonoid | 0.64 | 0.91 | 1.23 | [66] |
| 21. | 9.43 | 327 | 291, 229, 211, 183, 171, 147 | n.d. | Trihydroxy-octadecadienoic acid | Fatty acid | 0.40 | 0.58 | 0.96 | [44] |
| 22. | 9.60 | 785 | 665, 545, 399 | 269, 327 | Jaceosidin di-O-hexoside-deoxyhexoside | Flavonoid glycoside | 1.48 | 2.13 | 3.56 | [55] |
| 23. | 9.81 | 315 | 300, 151 | 260, 342 | Isorhamnetin | Flavonoid | 3.64 | 4.98 | 5.71 | [68] |
| 24. | 9.98 | 329 | 299, 229, 211, 171 | n.d. | Trihydroxyoctadecenoic acid | Fatty acid | 1.57 | 2.45 | 3.15 | [55,56] |
| 25. | 11.46 | 307 | 217, 185, 99 | n.d. | Eicosadienoic acid | Fatty acid | 0.68 | 1.58 | 1.98 | [70] |
| 26. | 12.19 | 251 | 207 | n.d. | Hexadecadienoic acid | Fatty acid | 0.43 | 1.63 | 2.24 | [55,56] |
| 27. | 13.50 | 313 | 255, 225 | 276, 331 | Dihydroxydimethoxyflavone (Cirsimaritin) | Flavonoid | - | - | 0.13 | [57] |
| 28. | 13.78 | 313 | 255, 225 | 276, 331 | Dihydroxydimethoxyflavone (Cirsimaritin) | Flavonoid | - | - | 0.20 | [57] |
| 29. | 14.92 | 293 | 275, 235, 183, 171 | n.d. | Octadecadienoic acid | Fatty acid | 1.73 | 3.99 | 2.93 | [55,56] |
| 30. | 15.08 | 293 | 275, 235, 183, 171 | n.d. | Octadecadienoic acid | Fatty acid | - | - | 1.54 | [55,56] |
| 31. | 15.44 | 309 | 291, 183, 71 | n.d. | Eicosaenoic acid | Fatty acid | 0.75 | - | 1.08 | [70] |
| 32. | 16.04 | 295 | 277, 195, 183, 171 | n.d. | Hydroxyoctadecadienoic acid | Fatty acid | - | 3.37 | 3.76 | [55,56] |
| 33. | 16.08 | 295 | 277, 195, 183, 171 | n.d. | Hydroxyoctadecadienoic acid | Fatty acid | 2.04 | - | - | [55,56] |
| 34. | 16.41 | 311 | 183, 171, 153, 137, 131 | n.d. | Dihydroxyoctadecadienoic acid | Fatty acid | 0.38 | - | 0.31 | [55,56] |
| 35. | 16.59 | 311 | 183, 171, 153, 137, 131 | n.d. | Dihydroxyoctadecadienoic acid | Fatty acid | 1.33 | - | 1.33 | [55,56] |
| 36. | 16.88 | 293 | 275, 235, 183, 171 | n.d. | Octadecadienoic acid | Fatty acid | - | - | 0.19 | [55,56] |
| 37. | 17.20 | 293 | 275, 235, 183, 171 | n.d. | Octadecadienoic acid | Fatty acid | - | - | 0.23 | [55,56] |
| 38. | 18.16 | 295 | 277, 195, 183, 171 | n.d. | Hydroxyoctadecadienoic acid | Fatty acid | 1.32 | 2.21 | 3.74 | [55,56] |
| 39. | 20.22 | 297 | 253, 239, 183 | n.d. | Hydroxy octadecenoic acid | Fatty acid | 1.70 | 1.49 | 1.91 | [55,56] |
| 40. | 20.72 | 271 | 225 | n.d. | Hydroxyhexadecanoic acid | Fatty acid | 0.89 | - | 0.29 | [55,56] |
3.2. In Vitro Assessment of the Antioxidant Activities in the Extracts
3.3. Enzyme Inhibitory Effects
3.4. Comparison of the Biological Activities of the Three Species Samples
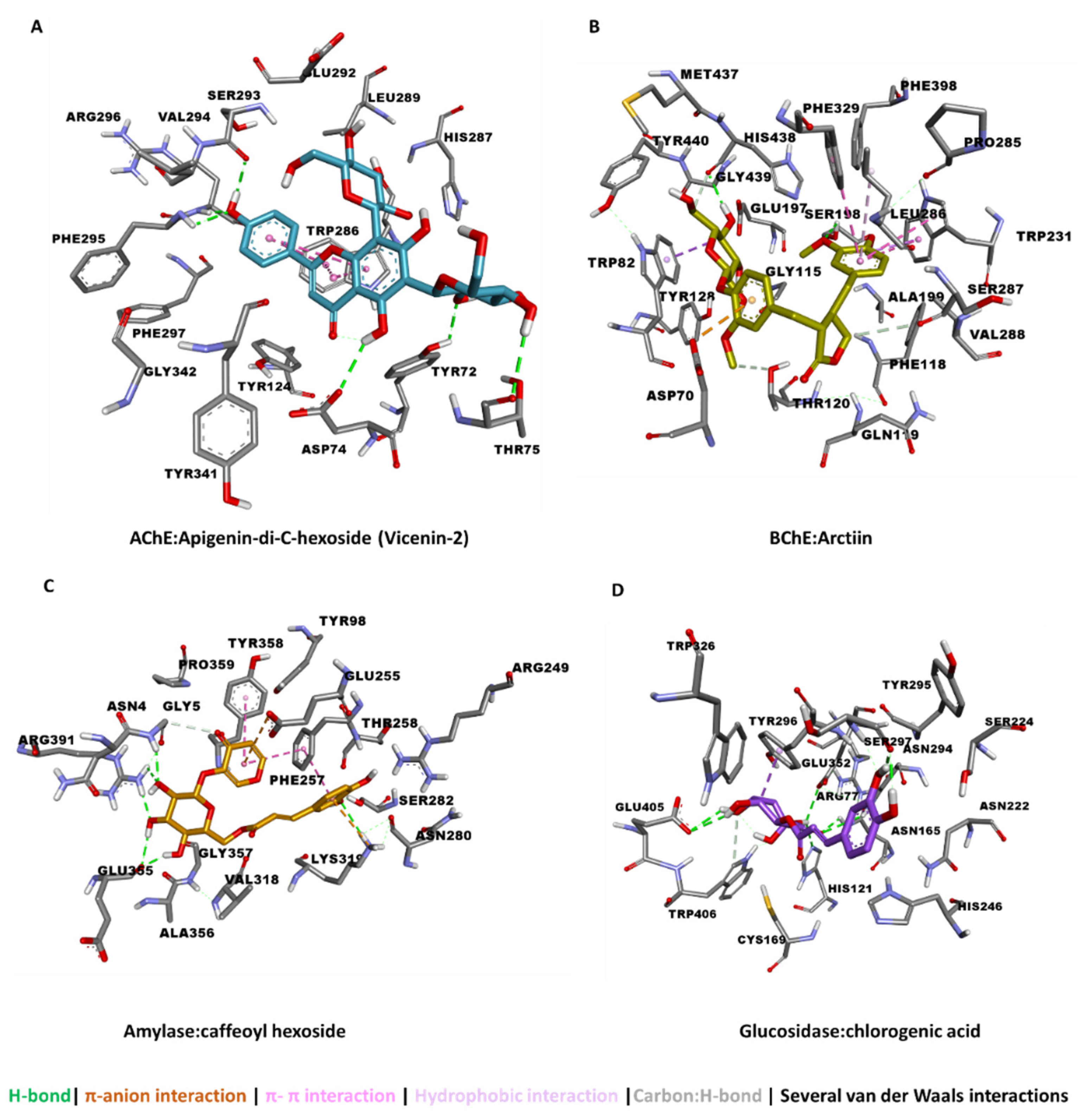
3.5. Molecular Docking
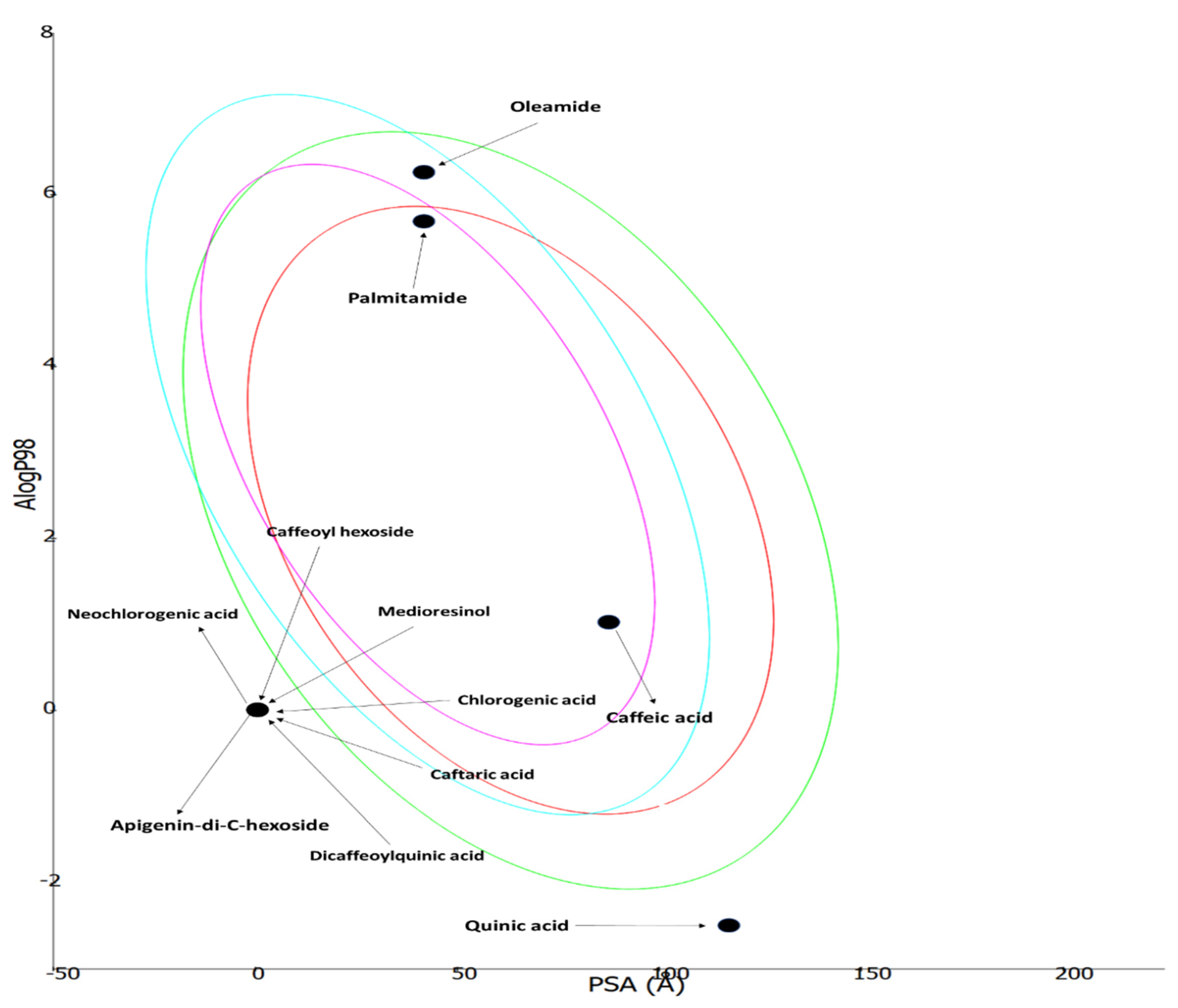
3.6. ADMET Prediction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Brinza, I.; Abd-Alkhalek, A.M.; El-Raey, M.A.; Boiangiu, R.S.; Eldahshan, O.A.; Hritcu, L. Ameliorative Effects of Rhoifolin in Scopolamine-Induced Amnesic Zebrafish (Danio rerio) Model. Antioxidants 2020, 9, 580. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Ghffar, E.A.; Al-Sayed, E.; Shehata, S.M.; Eldahshan, O.A.; Efferth, T. The protective role of Ocimum basilicum L. (Basil) against aspirin-induced gastric ulcer in mice: Impact on oxidative stress, inflammation, motor deficits and anxiety-like behavior. Food Funct. 2018, 9, 4457–4468. [Google Scholar] [CrossRef]
- Abd El-Ghffar, E.A.; Eldahshan, O.A.; Barakat, A.; Efferth, T. The prophylactic effect of a Eugenia aquea extract against oxidative stress and inflammation associated with the development of arthritis in an adjuvant-induced arthritis rat model. Food Funct. 2018, 9, 6643–6651. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- El-Nashar, H.A.S.; El-Din, M.I.G.; Hritcu, L.; Eldahshan, O.A. Insights on the Inhibitory Power of Flavonoids on Tyrosinase Activity: A Survey from 2016 to 2021. Molecules 2021, 26, 7546. [Google Scholar] [CrossRef]
- Younis, M.M.; Ayoub, I.M.; Mostafa, N.M.; El Hassab, M.A.; Eldehna, W.M.; Al-Rashood, S.T.; Eldahshan, O.A. GC/MS Profiling, Anti-Collagenase, Anti-Elastase, Anti-Tyrosinase and Anti-Hyaluronidase Activities of a Stenocarpus sinuatus Leaves Extract. Plants 2022, 11, 918. [Google Scholar] [CrossRef]
- Ashmawy, A.; Mostafa, N.; Eldahshan, O. GC/MS Analysis and Molecular Profiling of Lemon Volatile Oil against Breast Cancer. J. Essent. Oil Bear. Plants 2019, 22, 903–916. [Google Scholar] [CrossRef]
- Mostafa, N.M.; Ashour, M.L.; Eldahshan, O.A.; Singab, A.N. Cytotoxic activity and molecular docking of a novel biflavonoid isolated from Jacaranda acutifolia (Bignoniaceae). Nat. Prod. Res. 2016, 30, 2093–2100. [Google Scholar] [CrossRef]
- Singab, A.N.B.; Mostafa, N.M.; Eldahshan, O.A.; Ashour, M.L.; Wink, M. Profile of Volatile Components of Hydrodistilled and Extracted Leaves of Jacaranda acutifolia and their Antimicrobial Activity Against Foodborne Pathogens. Nat. Prod. Commun. 2014, 9, 1934578X1400900731. [Google Scholar] [CrossRef]
- Al-Madhagy, S.A.; Mostafa, N.M.; Youssef, F.S.; Awad, G.E.A.; Eldahshan, O.A.; Singab, A.N.B. Metabolic profiling of a polyphenolic-rich fraction of Coccinia grandis leaves using LC-ESI-MS/MS and in vivo validation of its antimicrobial and wound healing activities. Food Funct. 2019, 10, 6267–6275. [Google Scholar] [CrossRef] [PubMed]
- El-Nashar, H.A.S.; Mostafa, N.M.; Eldahshan, O.A.; Singab, A.N.B. A new antidiabetic and anti-inflammatory biflavonoid from Schinus polygama (Cav.) Cabrera leaves. Nat. Prod. Res. 2022, 36, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Güneş, A.; Kordali, Ş.; Turan, M.; Usanmaz Bozhüyük, A. Determination of antioxidant enzyme activity and phenolic contents of some species of the Asteraceae family from medicanal plants. Ind. Crops Prod. 2019, 137, 208–213. [Google Scholar] [CrossRef]
- Koc, S.; Isgor, B.S.; Isgor, Y.G.; Shomali Moghaddam, N.; Yildirim, O. The potential medicinal value of plants from Asteraceae family with antioxidant defense enzymes as biological targets. Pharm. Biol. 2015, 53, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Eruygur, N.; Koçyiğit, U.M.; Taslimi, P.; Ataş, M.; Tekin, M.; Gülçin, İ. Screening the in vitro antioxidant, antimicrobial, anticholinesterase, antidiabetic activities of endemic Achillea cucullata (Asteraceae) ethanol extract. S. Afr. J. Bot. 2019, 120, 141–145. [Google Scholar] [CrossRef]
- Aghraz, A.; Gonçalves, S.; Rodríguez-Solana, R.; Dra, L.A.; Di Stefano, V.; Dugo, G.; Cicero, N.; Larhsini, M.; Markouk, M.; Romano, A. Antioxidant activity and enzymes inhibitory properties of several extracts from two Moroccan Asteraceae species. S. Afr. J. Bot. 2018, 118, 58–64. [Google Scholar] [CrossRef]
- Li, L.-B.; Xiao, G.-D.; Xiang, W.; Yang, X.; Cao, K.-X.; Huang, R.-S. Novel Substituted Thiophenes and Sulf-Polyacetylene Ester from Echinops ritro L. Molecules 2019, 24, 805. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, F.; Liu, L.; Tian, S.; Li, W.; Yang, X.; Wu, Y.; Huang, Y.; Yi, J.; Yu, C.; et al. Antibacterial Activity and Action Mechanism of the Echinops ritro L. Essential Oil against Foodborne Pathogenic Bacteria. J. Essent. Oil Bear. Plants 2017, 20, 1172–1183. [Google Scholar] [CrossRef]
- Fokialakis, N.; Cantrell, C.L.; Duke, S.O.; Skaltsounis, A.L.; Wedge, D.E. Antifungal Activity of Thiophenes from Echinops ritro. J. Agric. Food Chem. 2006, 54, 1651–1655. [Google Scholar] [CrossRef]
- Chicca, A.; Tebano, M.; Adinolfi, B.; Ertugrul, K.; Flamini, G.; Nieri, P. Anti-proliferative activity of aguerin B and a new rare nor-guaianolide lactone isolated from the aerial parts of Centaurea deflexa. Eur. J. Med. Chem. 2011, 46, 3066–3070. [Google Scholar] [CrossRef]
- Bahmani, F.; Esmaeili, S.; Bashash, D.; Dehghan-Nayeri, N.; Mashati, P.; Gharehbaghian, A. Centaurea albonitens extract enhances the therapeutic effects of Vincristine in leukemic cells by inducing apoptosis. Biomed. Pharmacother. 2018, 99, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, A.; Amini, E.; Asili, J.; Masullo, M.; Piacente, S.; Iranshahi, M. Screening of several biological activities induced by different sesquiterpene lactones isolated from Centaurea behen L. and Rhaponticum repens (L.) Hidalgo. Nat. Prod. Res. 2018, 32, 1436–1440. [Google Scholar] [CrossRef] [PubMed]
- Kurkcuoglu, M.; Tosun, F.; Inceer, H.; Baser, K.H.C. Volatile compounds of Tripleurospermum decipiens from different sites in Turkey. Planta Med. 2016, 82 (Suppl. 01), 718. [Google Scholar] [CrossRef]
- Servi, H.; Yücel, Y.Y.; Polatoğlu, K. Composition and Acetylcholinesterase inhibition properties of Tripleurospermum inodorum (L.) Sch. Bip. Essential Oil from Istanbul. Aurum J. Health Sci. 2018, 1, 23–38. [Google Scholar]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; De Luca, E.; Mollica, A.; Aktumsek, A. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Front. Pharmacol. 2017, 8, 290. [Google Scholar] [CrossRef] [PubMed]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]
- Gerlits, O.; Ho, K.-Y.; Cheng, X.; Blumenthal, D.; Taylor, P.; Kovalevsky, A.; Radić, Z. A new crystal form of human acetylcholinesterase for exploratory room-temperature crystallography studies. Chem.-Biol. Interact. 2019, 309, 108698. [Google Scholar] [CrossRef]
- Rosenberry, T.; Brazzolotto, X.; Macdonald, I.; Wandhammer, M.; Trovaslet-Leroy, M.; Darvesh, S.; Nachon, F. Comparison of the Binding of Reversible Inhibitors to Human Butyrylcholinesterase and Acetylcholinesterase: A Crystallographic, Kinetic and Calorimetric Study. Molecules 2017, 22, 2098. [Google Scholar] [CrossRef]
- Fujieda, N.; Umakoshi, K.; Ochi, Y.; Nishikawa, Y.; Yanagisawa, S.; Kubo, M.; Kurisu, G.; Itoh, S. Copper–Oxygen Dynamics in the Tyrosinase Mechanism. Angew. Chem. Int. Ed. 2020, 59, 13385–13390. [Google Scholar] [CrossRef]
- Božić, N.; Rozeboom, H.J.; Lončar, N.; Slavić, M.Š.; Janssen, D.B.; Vujčić, Z. Characterization of the starch surface binding site on Bacillus paralicheniformis α-amylase. Int. J. Biol. Macromol. 2020, 165, 1529–1539. [Google Scholar] [CrossRef]
- Karade, S.S.; Hill, M.L.; Kiappes, J.L.; Manne, R.; Aakula, B.; Zitzmann, N.; Warfield, K.L.; Treston, A.M.; Mariuzza, R.A. N-Substituted Valiolamine Derivatives as Potent Inhibitors of Endoplasmic Reticulum α-Glucosidases I and II with Antiviral Activity. J. Med. Chem. 2021, 64, 18010–18024. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Kurkcuoglu, M.; Tosun, F.; Inceer, H.; Baser, K.H.C. Volatile Compounds of Tripleurospermum decipiens from Two Natural Populations in Turkey. Chem. Nat. Compounds 2019, 55, 565–567. [Google Scholar] [CrossRef]
- Bouzabata, A.; Montoro, P.; Gil, K.A.; Piacente, S.; Youssef, F.S.; Al Musayeib, N.M.; Cordell, G.A.; Ashour, M.L.; Tuberoso, C.I. HR-LC-ESI-Orbitrap-MS-Based Metabolic Profiling Coupled with Chemometrics for the Discrimination of Different Echinops spinosus Organs and Evaluation of Their Antioxidant Activity. Antioxidants 2022, 11, 453. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Li, X.; Saleri, F.; Guo, M. Analysis of Flavonoids in Rhamnus davurica and Its Antiproliferative Activities. Molecules 2016, 21, 1275. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Guo, H.; Xu, W.B.; Ge, J.; Li, X.; Alimu, M.; He, D.J. Rapid Identification of Flavonoid Constituents Directly from PTP1B Inhibitive Extract of Raspberry (Rubus idaeus L.) Leaves by HPLC-ESI-QTOF-MS-MS. J. Chromatogr. Sci. 2016, 54, 805–810. [Google Scholar] [CrossRef]
- Stanoeva, J.P.; Stefova, M.; Andonovska, K.B.; Stafilov, T. LC/DAD/MSn and ICP-AES Assay and Correlations between Phenolic Compounds and Toxic Metals in Endemic Thymus alsarensis from the Thallium Enriched Allchar Locality. Nat. Prod. Commun. 2017, 12, 1934578X1701200206. [Google Scholar] [CrossRef]
- Iwashina, T.; Kokubugata, G. Flavonoids in the Leaves and Flowers of Myoporum bontioides Native to Northernmost Region in the Myoporaceae. Bull. Natl. Mus. Nat. Sci. Ser. B Bot. 2010, 36, 117–125. [Google Scholar]
- Negri, G.; Barreto, L.M.R.C.; Sper, F.L.; Carvalho, C.D.; Campos, M.G. Phytochemical analysis and botanical origin of Apis mellifera bee pollen from the municipality of Canavieiras, Bahia State, Brazil. Braz. J. Food Technol. 2018, 21, Page. [Google Scholar] [CrossRef]
- Jackson Seukep, A.; Zhang, Y.-L.; Xu, Y.-B.; Guo, M.-Q. In Vitro Antibacterial and Antiproliferative Potential of Echinops lanceolatus Mattf. (Asteraceae) and Identification of Potential Bioactive Compounds. Pharmaceuticals 2020, 13, 59. [Google Scholar] [CrossRef]
- Shin, Y.G.; Cho, K.H.; Chung, S.M.; Graham, J.; Das Gupta, T.K.; Pezzuto, J.M. Determination of betulinic acid in mouse blood, tumor and tissue homogenates by liquid chromatography–electrospray mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 1999, 732, 331–336. [Google Scholar] [CrossRef]
- Madl, T.; Mittelbach, M. Quantification of primary fatty acid amides in commercial tallow and tallow fatty acid methyl esters by HPLC-APCI-MS. Analyst 2005, 130, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Nichols, K.K.; Ham, B.M.; Nichols, J.J.; Ziegler, C.; Green-Church, K.B. Identification of Fatty Acids and Fatty Acid Amides in Human Meibomian Gland Secretions. Investig. Ophthalmol. Vis. Sci. 2007, 48, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Llorent-Martínez, E.J.; Sinan, K.I.; Yıldıztugay, E.; Picot-Allain, C.; Mahomoodally, M.F. Chemical profiling of Centaurea bornmuelleri Hausskn. aerial parts by HPLC-MS/MS and their pharmaceutical effects: From nature to novel perspectives. J. Pharm. Biomed. Anal. 2019, 174, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Edible flowers as sources of phenolic compounds with bioactive potential. Food Res. Int. 2018, 105, 580–588. [Google Scholar] [CrossRef]
- Zengin, G.; Zheleva-Dimitrova, D.; Gevrenova, R.; Nedialkov, P.; Mocan, A.; Ćirić, A.; Glamočlija, J.; Soković, M.; Aktumsek, A.; Mahomoodally, M.F. Identification of phenolic components via LC–MS analysis and biological activities of two Centaurea species: C. drabifolia subsp. drabifolia and C. lycopifolia. J. Pharm. Biomed. Anal. 2018, 149, 436–441. [Google Scholar] [CrossRef]
- Güzel, Y. Specialized natural product analysis and chemophenetics of some Turkish endemic Centaurea L. (Asteraceae) taxa by electrospray ionization mass spectrometry fingerprinting and liquid chromatography-tandem mass spectrometry. Biochem. Syst. Ecol. 2020, 91, 104079. [Google Scholar] [CrossRef]
- Brito, A.; Ramirez, J.E.; Areche, C.; Sepúlveda, B.; Simirgiotis, M.J. HPLC-UV-MS profiles of phenolic compounds and antioxidant activity of fruits from three citrus species consumed in Northern Chile. Molecules 2014, 19, 17400–17421. [Google Scholar] [CrossRef]
- Silva, D.B.; Turatti, I.C.C.; Gouveia, D.R.; Ernst, M.; Teixeira, S.P.; Lopes, N.P. Mass Spectrometry of Flavonoid Vicenin-2, Based Sunlight Barriers in Lychnophora species. Sci. Rep. 2014, 4, 4309. [Google Scholar] [CrossRef]
- Mishio, T.; Takeda, K.; Iwashina, T. Anthocyanins and other flavonoids as flower pigments from eleven Centaurea species. Nat. Prod. Commun. 2015, 10, 1934578X1501000318. [Google Scholar] [CrossRef]
- Sánchez-Rabaneda, F.; Jáuregui, O.; Casals, I.; Andrés-Lacueva, C.; Izquierdo-Pulido, M.; Lamuela-Raventós, R.M. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of the phenolic composition of cocoa (Theobroma cacao). J. Mass Spectrom. 2003, 38, 35–42. [Google Scholar] [CrossRef]
- Mawahib, C.; Nabila, Z.; Nabila, S.; Chawki, B.; Salah, A.J. LC-MS analysis, antioxidant and alpha-glucosidase inhibitory activities of Centaurea papposa extracts. Bangladesh J. Pharmacol. 2019, 14, 159–165. [Google Scholar] [CrossRef]
- Zengin, G.; Zheleva-Dimitrova, D.; Gevrenova, R.; Aktumsek, A.; Sinan, K.I.; Mahomoodally, M.F. A comparative assessment of the LC-MS profiles and cluster analysis of four Centaurea species from Turkey. Biocatal. Agric. Biotechnol. 2019, 20, 101189. [Google Scholar] [CrossRef]
- Hammoud, L.; Seghiri, R.; Benayache, S.; Mosset, P.; Lobstein, A.; Chaabi, M.; León, F.; Brouard, I.; Bermejo, J.; Benayache, F. A new flavonoid and other constituents from Centaurea nicaeensis All. var. walliana M. Nat. Prod. Res. 2012, 26, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Gad, H.A.; Heiss, A.G.; Wessjohann, L.A. Metabolomics driven analysis of six Nigella species seeds via UPLC-qTOF-MS and GC–MS coupled to chemometrics. Food Chem. 2014, 151, 333–342. [Google Scholar] [CrossRef]
- Ayoub, I.M.; Korinek, M.; El-Shazly, M.; Wetterauer, B.; El-Beshbishy, H.A.; Hwang, T.-L.; Chen, B.-H.; Chang, F.-R.; Wink, M.; Singab, A.N.B. Anti-Allergic, Anti-Inflammatory and Anti-Hyperglycemic Activity of Chasmanthe aethiopica Leaf Extract and Its Profiling Using LC/MS and GLC/MS. Plants 2021, 10, 1118. [Google Scholar] [CrossRef] [PubMed]
- Köse, Y.B.; İşcan, G.; Göger, F.; Akalın, G.; Demirci, B.; Başer, K.H.C. Chemical Composition and Biological Activity of Centaurea baseri: New Species from Turkey. Chem. Biodivers. 2016, 13, 1369–1379. [Google Scholar] [CrossRef]
- Clark, A.C. The production of yellow pigments from (+)-catechin and dihydroxyfumaric acid in a model wine system. Eur. Food Res. Technol. 2008, 226, 925–931. [Google Scholar] [CrossRef]
- Llorent-Martínez, E.J.; Spínola, V.; Gouveia, S.; Castilho, P. HPLC-ESI-MSn characterization of phenolic compounds, terpenoid saponins, and other minor compounds in Bituminaria bituminosa. Ind. Crops Prod. 2015, 69, 80–90. [Google Scholar] [CrossRef]
- Jaiswal, R.; Kiprotich, J.; Kuhnert, N. Determination of the hydroxycinnamate profile of 12 members of the Asteraceae family. Phytochemistry 2011, 72, 781–790. [Google Scholar] [CrossRef]
- Bahri, M.; Hance, P.; Grec, S.; Quillet, M.-C.; Trotin, F.; Hilbert, J.-L.; Hendriks, T. A “Novel” Protocol for the Analysis of Hydroxycinnamic Acids in Leaf Tissue of Chicory (Cichorium intybus) L. Asteraceae). Sci. World J. 2012, 2012, 142983. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Amundson, L.M.; Owen, B.C.; Gallardo, V.A.; Habicht, S.C.; Fu, M.; Shea, R.C.; Mossman, A.B.; Kenttämaa, H.I. Differentiation of Regioisomeric Aromatic Ketocarboxylic Acids by Positive Mode Atmospheric Pressure Chemical Ionization Collision-Activated Dissociation Tandem Mass Spectrometry in a Linear Quadrupole Ion Trap Mass Spectrometer. J. Am. Soc. Mass Spectrom. 2011, 22, 670–682. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saldanha, L.L.; Vilegas, W.; Dokkedal, A.L. Characterization of flavonoids and phenolic acids in Myrcia bella Cambess. using FIA-ESI-IT-MS(n) and HPLC-PAD-ESI-IT-MS combined with NMR. Molecules 2013, 18, 8402–8416. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.D.L.e.; David, J.P.; Silva, L.C.R.C.; Santos, R.A.F.; David, J.M.; Lima, L.S.; Reis, P.S.; Fontana, R. Bioactive Oleanane, Lupane and Ursane Triterpene Acid Derivatives. Molecules 2012, 17, 12197–12205. [Google Scholar] [CrossRef] [PubMed]
- Khoza, B.S.; Gbashi, S.; Steenkamp, P.A.; Njobeh, P.B.; Madala, N.E. Identification of hydroxylcinnamoyl tartaric acid esters in Bidens pilosa by UPLC-tandem mass spectrometry. S. Afr. J. Bot. 2016, 103, 95–100. [Google Scholar] [CrossRef]
- Parejo, I.; Jáuregui, O.; Viladomat, F.; Bastida, J.; Codina, C. Characterization of acylated flavonoid-O-glycosides and methoxylated flavonoids from Tagetes maxima by liquid chromatography coupled to electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 2801–2810. [Google Scholar] [CrossRef]
- Liberal, Â.; Calhelha, R.C.; Pereira, C.; Adega, F.; Barros, L.; Dueñas, M.; Santos-Buelga, C.; Abreu, R.M.; Ferreira, I.C. A comparison of the bioactivity and phytochemical profile of three different cultivars of globe amaranth: Red, white, and pink. Food Funct. 2016, 7, 679–688. [Google Scholar] [CrossRef]
- Chen, M.; He, X.; Sun, H.; Sun, Y.; Li, L.; Zhu, J.; Xia, G.; Guo, X.; Zang, H. Phytochemical analysis, UPLC-ESI-Orbitrap-MS analysis, biological activity, and toxicity of extracts from Tripleurospermum limosum (Maxim.) Pobed. Arab. J. Chem. 2022, 15, 103797. [Google Scholar] [CrossRef]
- Zhu, M.-Z.; Wu, W.; Jiao, L.-L.; Yang, P.-F.; Guo, M.-Q. Analysis of Flavonoids in Lotus (Nelumbo nucifera) Leaves and Their Antioxidant Activity Using Macroporous Resin Chromatography Coupled with LC-MS/MS and Antioxidant Biochemical Assays. Molecules 2015, 20, 10553–10565. [Google Scholar] [CrossRef]
- Della Corte, A.; Chitarrini, G.; Di Gangi, I.M.; Masuero, D.; Soini, E.; Mattivi, F.; Vrhovsek, U. A rapid LC–MS/MS method for quantitative profiling of fatty acids, sterols, glycerolipids, glycerophospholipids and sphingolipids in grapes. Talanta 2015, 140, 52–61. [Google Scholar] [CrossRef]
- AYDIN, Ç.; Özcan, G.T.; Turan, M.; Mammadov, R. Phenolic contents and antioxidant properties of Echinops ritro L. and E. tournefortii Jaup. Et. Spach extract. Int. J. Second. Metab. 2016, 3, 74–81. [Google Scholar]
- Aktumsek, A.; Zengin, G.; Guler, G.O.; Cakmak, Y.S.; Duran, A. Antioxidant potentials and anticholinesterase activities of methanolic and aqueous extracts of three endemic Centaurea L. species. Food Chem. Toxicol. 2013, 55, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Ćavar Zeljković, S.; Ayaz, F.A.; Inceer, H.; Hayirlioglu-Ayaz, S.; Colak, N. Evaluation of chemical profile and antioxidant activity of Tripleurospermum insularum, a new species from Turkey. Nat. Prod. Res. 2015, 29, 293–296. [Google Scholar] [CrossRef]
- Mandegary, A.; Soodi, M.; Sharififar, F.; Ahmadi, S. Anticholinesterase, antioxidant, and neuroprotective effects of Tripleurospermum disciforme and Dracocephalum multicaule. J. Ayurveda Integr. Med. 2014, 5, 162. [Google Scholar]
- Ge, L.; Zhu, M.-m.; Yang, J.-Y.; Wang, F.; Zhang, R.; Zhang, J.-H.; Shen, J.; Tian, H.-F.; Wu, C.-F. Differential proteomic analysis of the anti-depressive effects of oleamide in a rat chronic mild stress model of depression. Pharmacol. Biochem. Behav. 2015, 131, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Eruygur, N.; Dural, E. Determination of 1-deoxynojirimycin by a developed and validated HPLC-FLD method and assessment of in-vitro antioxidant, α-amylase and α-glucosidase inhibitory activity in mulberry varieties from Turkey. Phytomedicine 2019, 53, 234–242. [Google Scholar] [CrossRef]
- Bouayed, J.; Rammal, H.; Dicko, A.; Younos, C.; Soulimani, R. Chlorogenic acid, a polyphenol from Prunus domestica (Mirabelle), with coupled anxiolytic and antioxidant effects. J. Neurol. Sci. 2007, 262, 77–84. [Google Scholar] [CrossRef]
- Agunloye, O.M.; Oboh, G. Caffeic acid and chlorogenic acid: Evaluation of antioxidant effect and inhibition of key enzymes linked with hypertension. J. Food Biochem. 2018, 42, e12541. [Google Scholar] [CrossRef]
- Cevenini, E.; Invidia, L.; Lescai, F.; Salvioli, S.; Tieri, P.; Castellani, G.; Franceschi, C. Human models of aging and longevity. Expert Opin. Biol. Ther. 2008, 8, 1393–1405. [Google Scholar] [CrossRef]
- Mechchate, H.; El Allam, A.; El Omari, N.; El Hachlafi, N.; Shariati, M.A.; Wilairatana, P.; Mubarak, M.S.; Bouyahya, A. Vegetables and Their Bioactive Compounds as Anti-Aging Drugs. Molecules 2022, 27, 2316. [Google Scholar] [CrossRef]
- Göger, G.; Yavaş, İ.; Yur, S.; Köse, Y.B.; Özek, G. Volatiles and fatty acid analyzes of Tripleurospermum decipiens (Fisch & CA Mey) Bornm and investigation of the extracts for antimicrobial and enzyme inhibitory activities. J. Res. Pharm. 2021, 25, 429–440. [Google Scholar]
- Khammar, A.; Djeddi, S. Pharmacological and biological properties of some Centaurea species. Eur. J. Sci. Res. 2012, 84, 398–416. [Google Scholar]
- Sokovic, M.; Ciric, A.; Glamoclija, J.; Skaltsa, H. Biological activities of sesquiterpene lactones isolated from the genus Centaurea L. (Asteraceae). Curr. Pharm. Des. 2017, 23, 2767–2786. [Google Scholar] [CrossRef] [PubMed]
- Servi, H.; Sen, A.; Dogan, A. Chemical composition and biological activities of endemic Tripleurospermum conoclinium (Boiss. & Balansa) Hayek essential oils. Flavour Fragr. J. 2020, 35, 713–721. [Google Scholar]
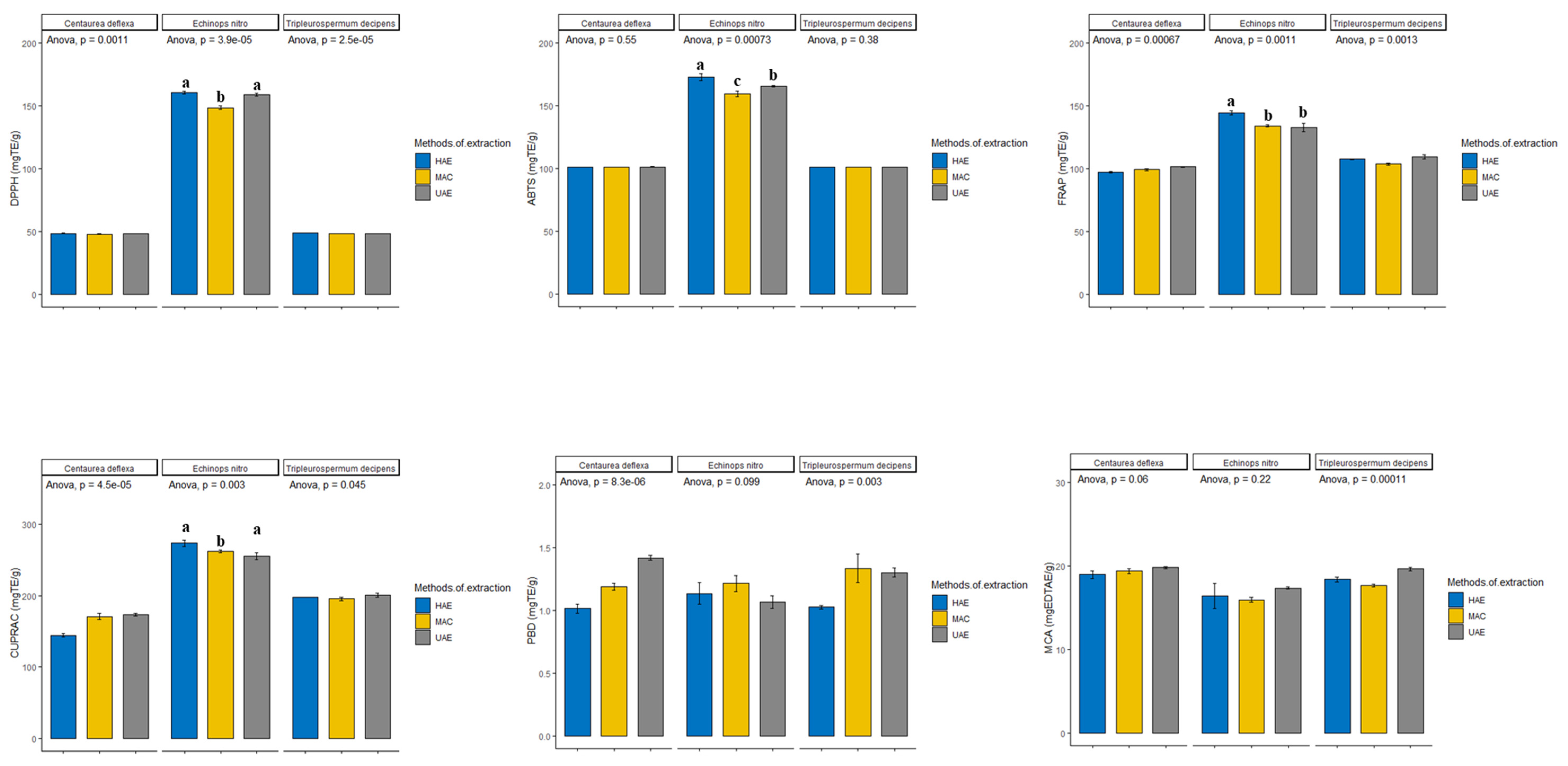
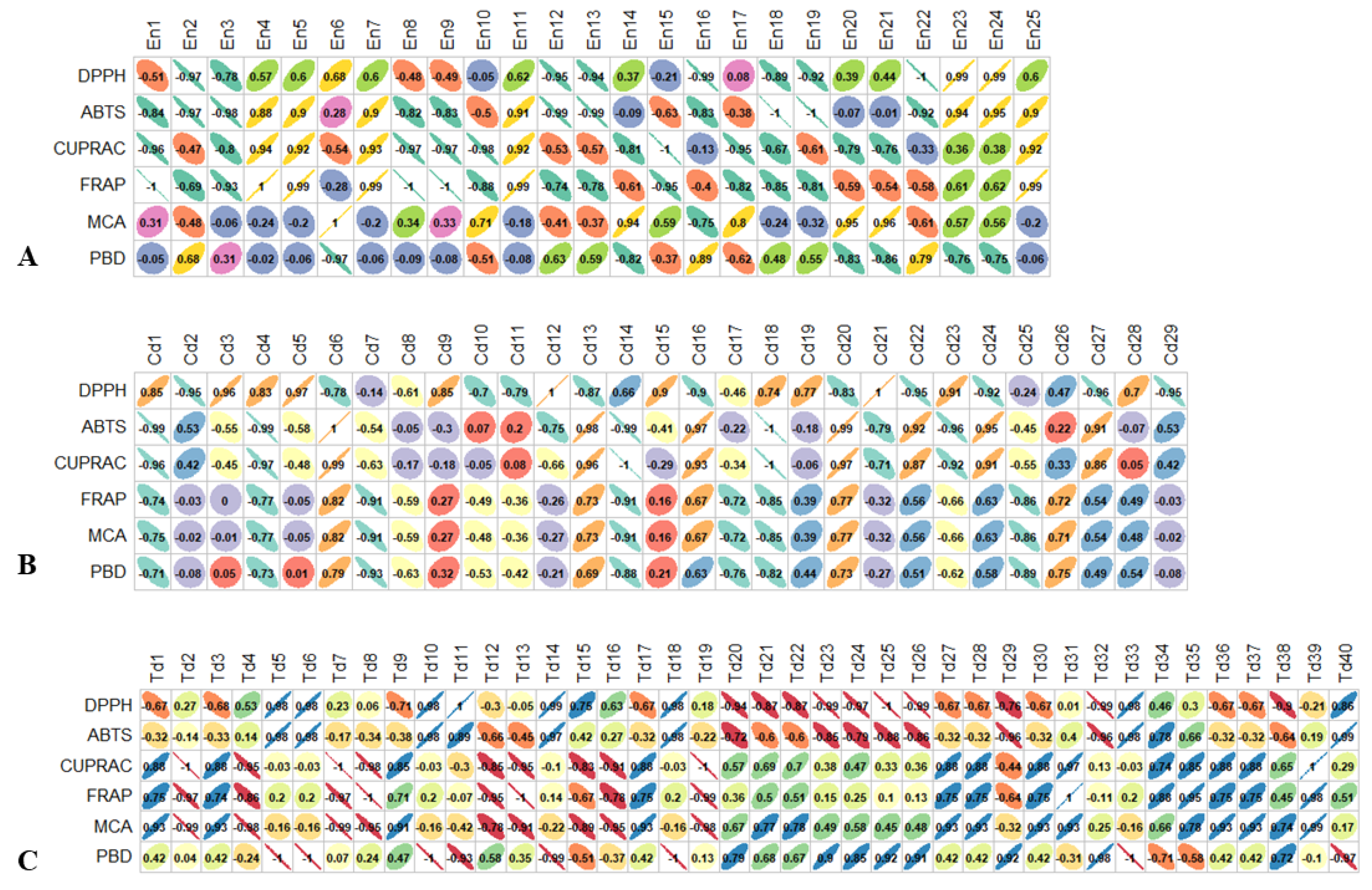


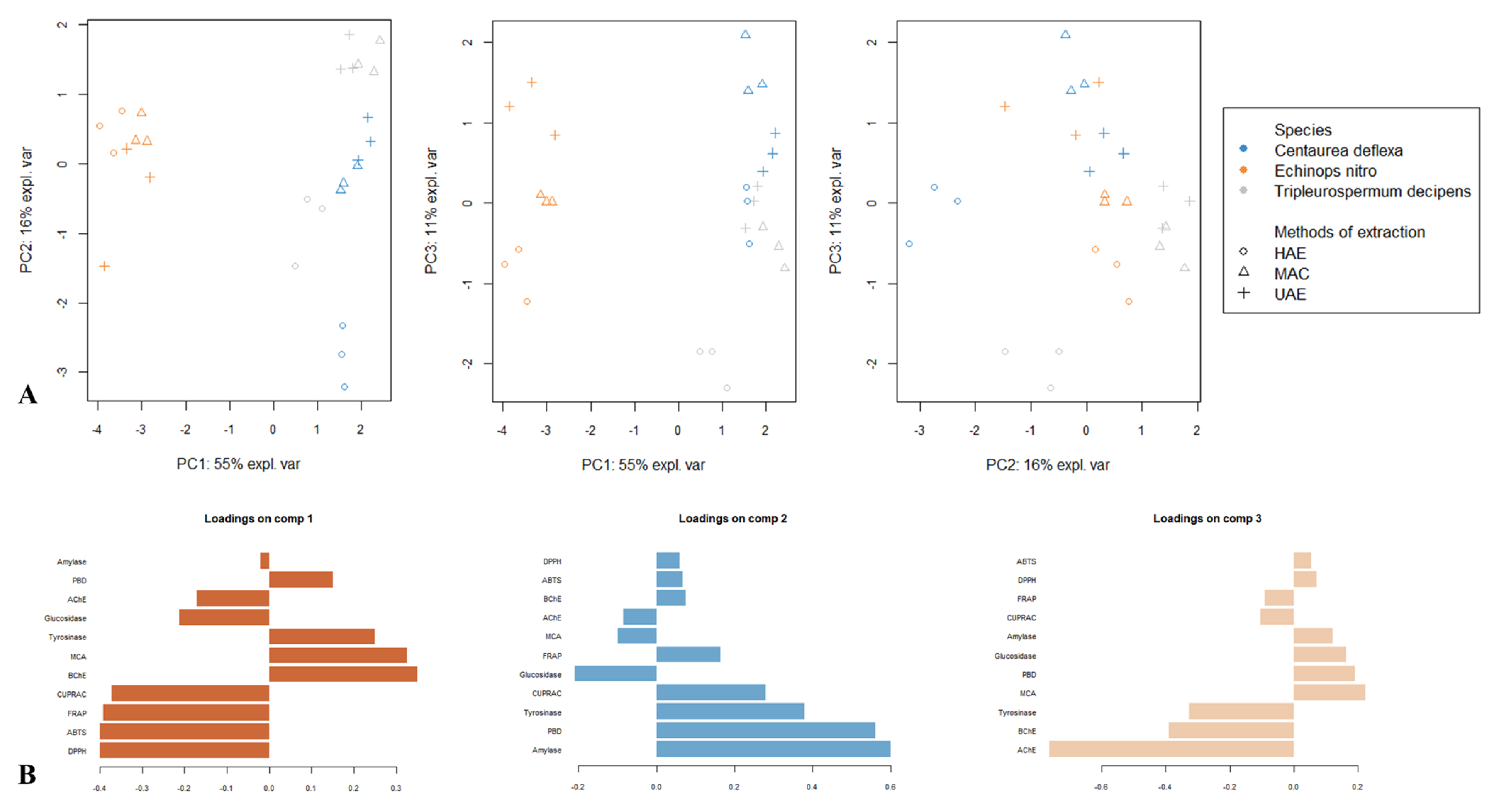
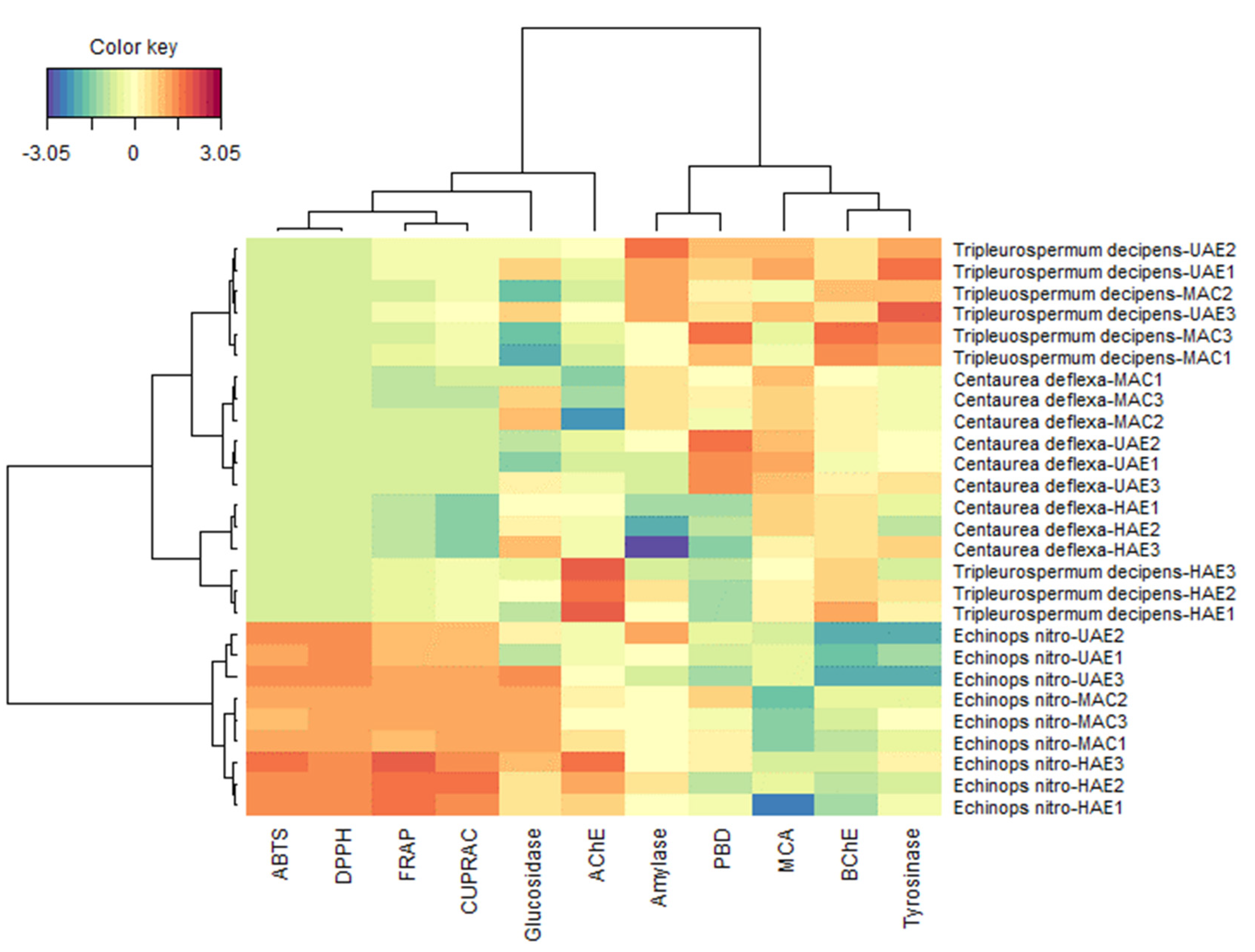
| Species | Extraction Method | DPPH (mg TE/g) | ABTS (mg TE/g) | CUPRAC (mg TE/g) | FRAP (mg TE/g) | MCA (mg EDTAE/g) | PBD (mmol TE/g) |
|---|---|---|---|---|---|---|---|
| Echinops ritro | HAE | 160.83 ± 1.28 a | 173.09 ± 2.73 a | 273.27 ± 4.32 a | 144.61 ± 1.75 a | 16.43 ± 1.49 a | 1.14 ± 0.12 a |
| MAC | 148.68 ± 1.25 b | 159.62 ± 2.10 c | 262.04 ± 2.08 b | 134.32 ± 1.03 b | 15.97 ± 0.29 a | 1.22 ± 0.10 a | |
| UAE | 159.21 ± 1.18 a | 165.56 ± 0.59 b | 254.98 ± 4.50 b | 132.92 ± 3.15 b | 17.38 ± 0.12 a | 1.07 ± 0.07 a | |
| Centaurea deflexa | HAE | 48.62 ± 0.14 a | 101.30 ± 0.06 a | 144.50 ± 2.23 b | 97.34 ± 0.77 a | 19.01 ± 0.46 a | 1.02 ± 0.05 c |
| MAC | 48.11 ± 0.04 b | 101.40 ± 0.07 a | 170.57 ± 4.33 a | 99.35 ± 0.68 b | 19.40 ± 0.30 a | 1.19 ± 0.04 b | |
| UAE | 48.46 ± 0.04 a | 101.39 ± 0.18 a | 173.45 ± 2.11 a | 101.54 ± 0.46 a | 19.82 ± 0.14 a | 1.42 ± 0.03 a | |
| Tripleurospermum decipens | HAE | 48.83 ± 0.06 a | 101.34 ± 0.09 a | 197.81 ± 0.12 b | 107.73 ± 0.33 a | 18.41 ± 0.29 b | 1.03 ± 0.02 b |
| MAC | 48.43 ± 0.03 b | 101.20 ± 0.09 a | 195.22 ± 2.17 a b | 103.81 ± 0.89 b | 17.68 ± 0.15 c | 1.34 ± 0.16 a | |
| UAE | 48.32 ± 0.05 c | 101.23 ± 0.17 a | 200.72 ± 2.79 a | 144.61 ± 1.75 a | 19.69 ± 0.21 a | 1.30 ± 0.05 a |
| Species | Extraction Methods | AChE (mg GALAE/g) | BChE (mg GALAE/g) | Tyrosinase (mg KAE/g) | Amylase (mmol ACAE/g) | Glucosidase (mmol ACAE/g) |
|---|---|---|---|---|---|---|
| Echinops ritro | HAE | 2.41 ± 0.04 a | 0.80 ± 0.10 a | 62.28 ± 0.59 a | 0.29 ± 0.01 a | 1.01 ± 0.03 a |
| MAC | 2.31 ± 0.02 a | 0.87 ± 0.11 a | 62.19 ± 0.38 a | 0.29 ± 0.01 a | 1.06 ± 0.01 a | |
| UAE | 2.27 ± 0.01 b | 0.36 ± 0.03 b | 60.64 ± 0.48 b | 0.29 ± 0.01 a | 0.97 ± 0.10 a | |
| Centaurea deflexa | HAE | 2.27 ± 0.01 a | 1.49 ± 0.01 a | 62.29 ± 0.99 a | 0.26 ± 0.01 b | 0.99 ± 0.04 a |
| MAC | 2.13 ± 0.05 b | 1.31 ± 0.06 a | 62.32 ± 0.03 a | 0.30 ± 0.01 a | 0.98 ± 0.08 a | |
| UAE | 2.25 ± 0.02 a | 1.34 ± 0.13 a | 62.80 ± 0.31 a | 0.28 ± 0.01 a | 0.90 ± 0.07 a | |
| Tripleurospermum decipens | HAE | 2.46 ± 0.01 a | 1.69 ± 0.11 a b | 62.56 ± 0.79 b | 0.29 ± 0.01 a | 0.91 ± 0.04 a |
| MAC | 2.22 ± 0.02 c | 1.96 ± 0.18 a | 63.86 ± 0.21 a b | 0.30 ± 0.01 a | 0.81 ± 0.01 b | |
| UAE | 2.27 ± 0.02 b | 1.52 ± 0.03 b | 64.30 ± 0.41 a | 0.31 ± 0.01 a | 0.98 ± 0.04 a |
| Compound | AChE | BChE | Tyrosinase | Amylase | Glucosidase |
|---|---|---|---|---|---|
| (Kcal/mol) | |||||
| Caffeic acid derivative | −6.77 | −5.56 | −4.54 | −4.08 | −5.03 |
| Neochlorogenic acid | −10.83 | −8.61 | −4.62 | −6.02 | −5.21 |
| Chlorogenic acid | −11.30 | −7.54 | −4.08 | −5.78 | −12.29 |
| Dicaffeoylquinic acid | −10.44 | −10.23 | −4.34 | −6.53 | −11.31 |
| Palmitamide | −6.02 | −5.68 | −3.45 | −2.84 | −3.17 |
| Oleamide | −7.61 | −5.86 | −2.14 | −2.89 | −3.16 |
| Quinic acid | −7.35 | −6.33 | −4.67 | −4.56 | −6.46 |
| Caffeoyl hexoside | −8.50 | −7.50 | −5.11 | −8.36 | −10.38 |
| Apigenin-di-C-hexoside (Vicenin-2) | −16.15 | −12.02 | −5.21 | −9.47 | −8.43 |
| Arctiin | −15.10 | −8.77 | −5.14 | −8.02 | −7.34 |
| Medioresinol | −11.22 | −5.99 | −4.12 | −4.86 | −7.27 |
| Caftaric acid | −11.81 | −8.96 | −5.40 | −7.24 | −11.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zengin, G.; Fahmy, N.M.; Sinan, K.I.; Uba, A.I.; Bouyahya, A.; Lorenzo, J.M.; Yildiztugay, E.; Eldahshan, O.A.; Fayez, S. Differential Metabolomic Fingerprinting of the Crude Extracts of Three Asteraceae Species with Assessment of Their In Vitro Antioxidant and Enzyme-Inhibitory Activities Supported by In Silico Investigations. Processes 2022, 10, 1911. https://doi.org/10.3390/pr10101911
Zengin G, Fahmy NM, Sinan KI, Uba AI, Bouyahya A, Lorenzo JM, Yildiztugay E, Eldahshan OA, Fayez S. Differential Metabolomic Fingerprinting of the Crude Extracts of Three Asteraceae Species with Assessment of Their In Vitro Antioxidant and Enzyme-Inhibitory Activities Supported by In Silico Investigations. Processes. 2022; 10(10):1911. https://doi.org/10.3390/pr10101911
Chicago/Turabian StyleZengin, Gokhan, Nouran M. Fahmy, Kouadio Ibrahime Sinan, Abdullahi Ibrahim Uba, Abdelhakim Bouyahya, José M. Lorenzo, Evren Yildiztugay, Omayma A. Eldahshan, and Shaimaa Fayez. 2022. "Differential Metabolomic Fingerprinting of the Crude Extracts of Three Asteraceae Species with Assessment of Their In Vitro Antioxidant and Enzyme-Inhibitory Activities Supported by In Silico Investigations" Processes 10, no. 10: 1911. https://doi.org/10.3390/pr10101911
APA StyleZengin, G., Fahmy, N. M., Sinan, K. I., Uba, A. I., Bouyahya, A., Lorenzo, J. M., Yildiztugay, E., Eldahshan, O. A., & Fayez, S. (2022). Differential Metabolomic Fingerprinting of the Crude Extracts of Three Asteraceae Species with Assessment of Their In Vitro Antioxidant and Enzyme-Inhibitory Activities Supported by In Silico Investigations. Processes, 10(10), 1911. https://doi.org/10.3390/pr10101911











