Probing Element Transfer Behavior during the Submerged Arc Welding Process for CaF2-SiO2-Na2O-Cr2O3 Agglomerated Fluxes: A Thermodynamic Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Flux Preparation
2.2. Welding Experiment
2.3. Chemical Composition Analysis
2.4. Thermodynamic Calculation
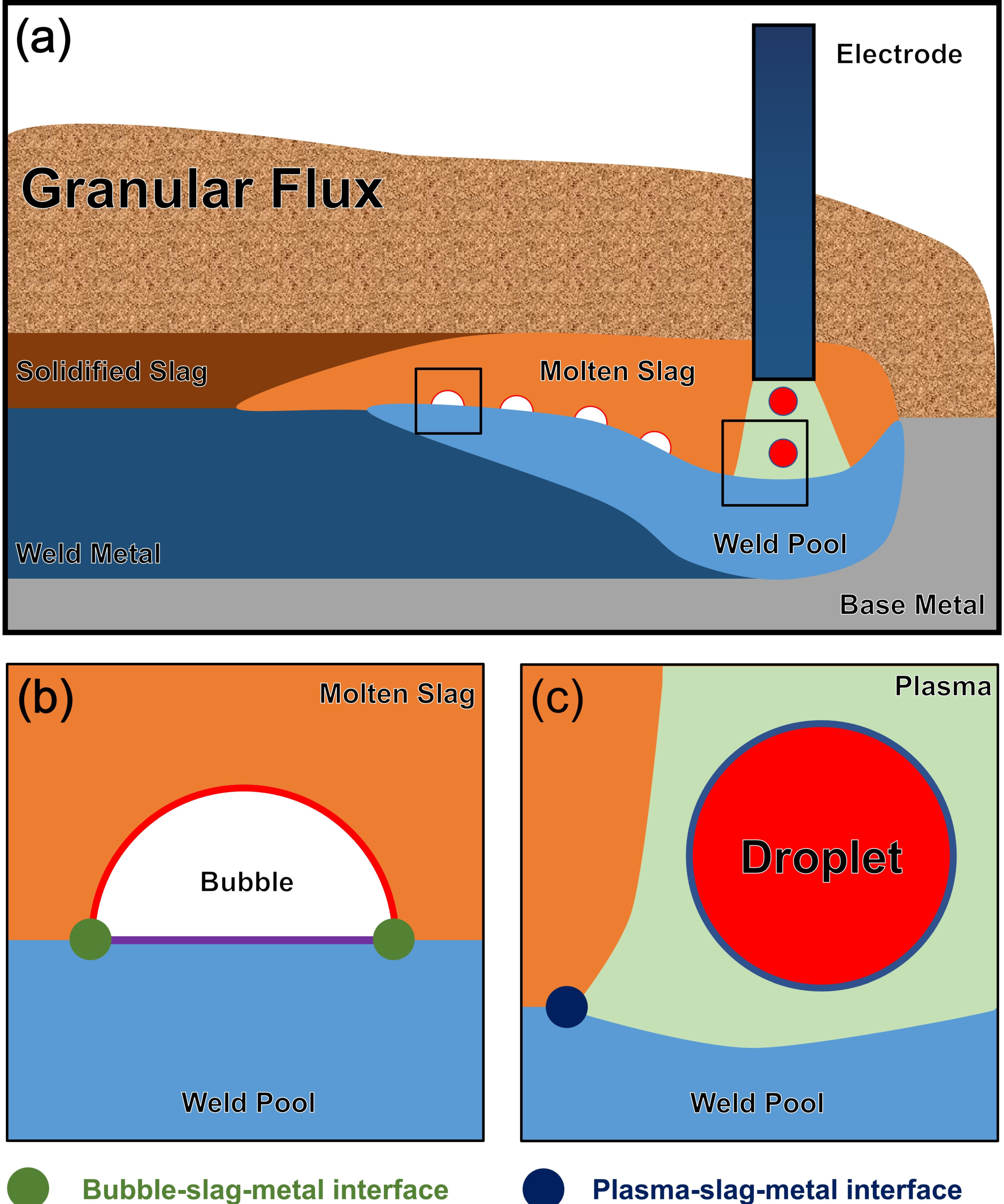
- It is impossible to capture the gases in the arc plasma and to sample the molten slag for analytical purposes since the arc plasma, molten slag, and weld pool are shielded under the flux during the SAW process.
- The effective equilibrium temperature of the SAW chemical system is as high as 2000 °C, under which the thermodynamic information remains scarce.
3. Results and Discussion
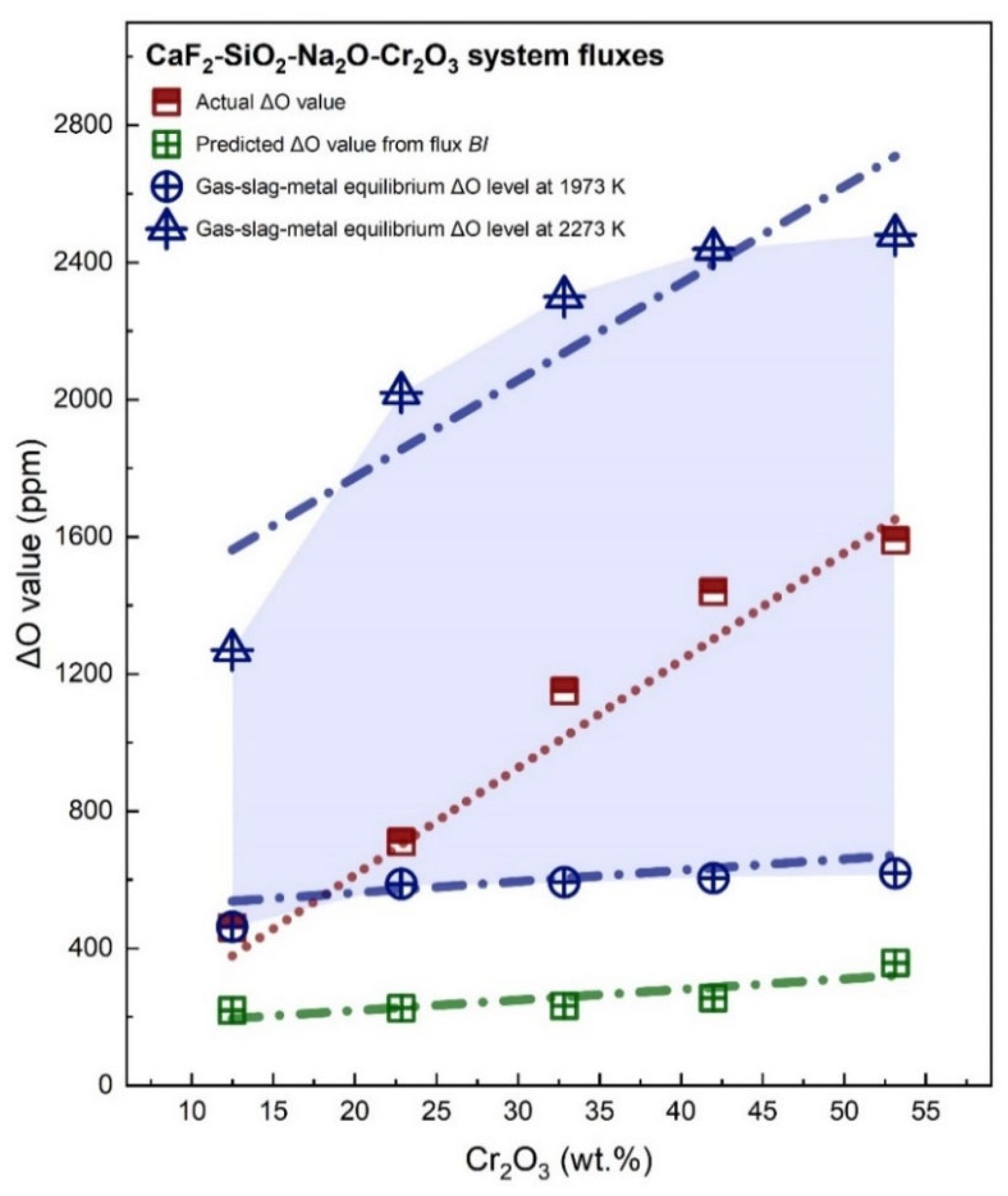
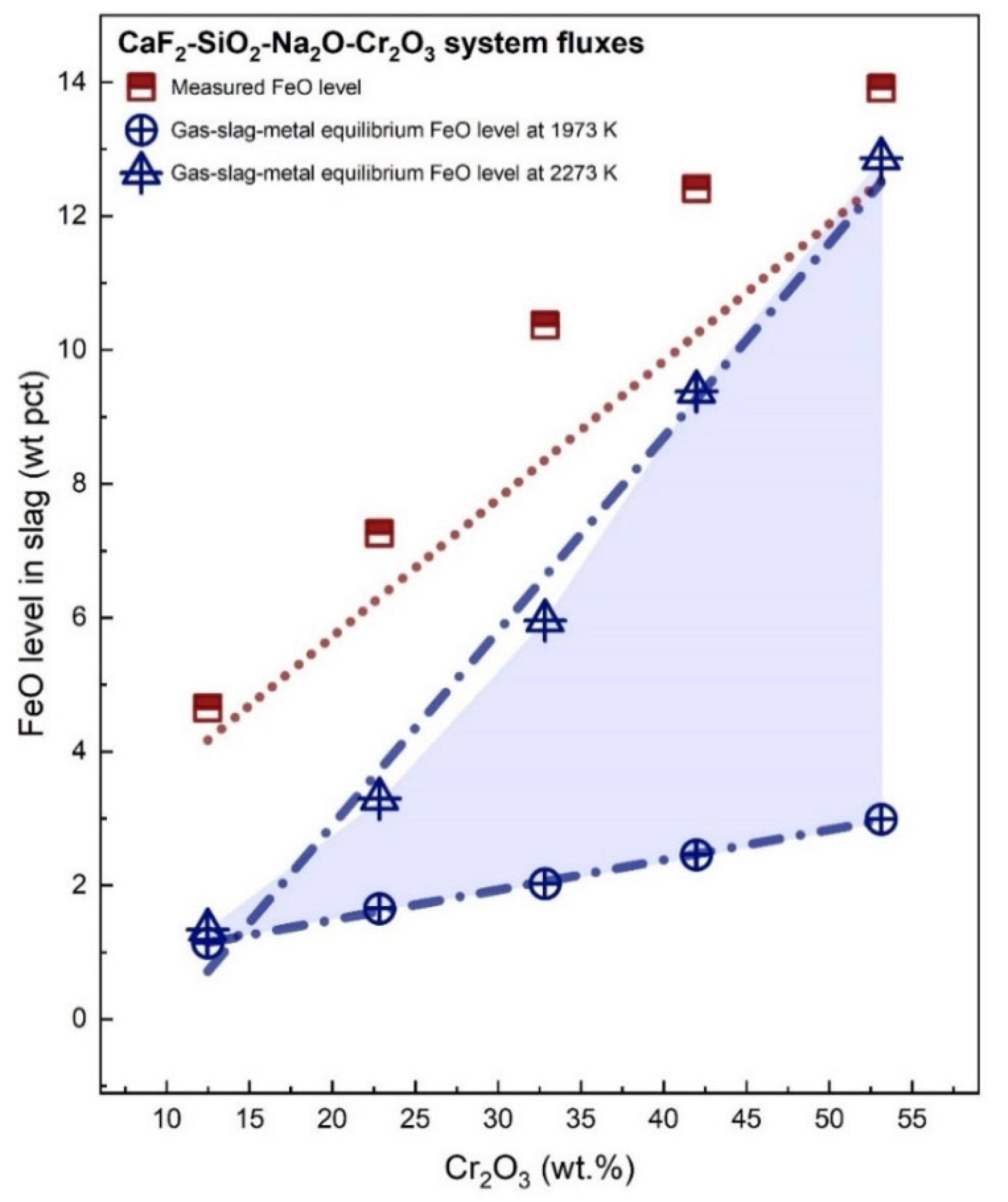
3.1. Transfer of O
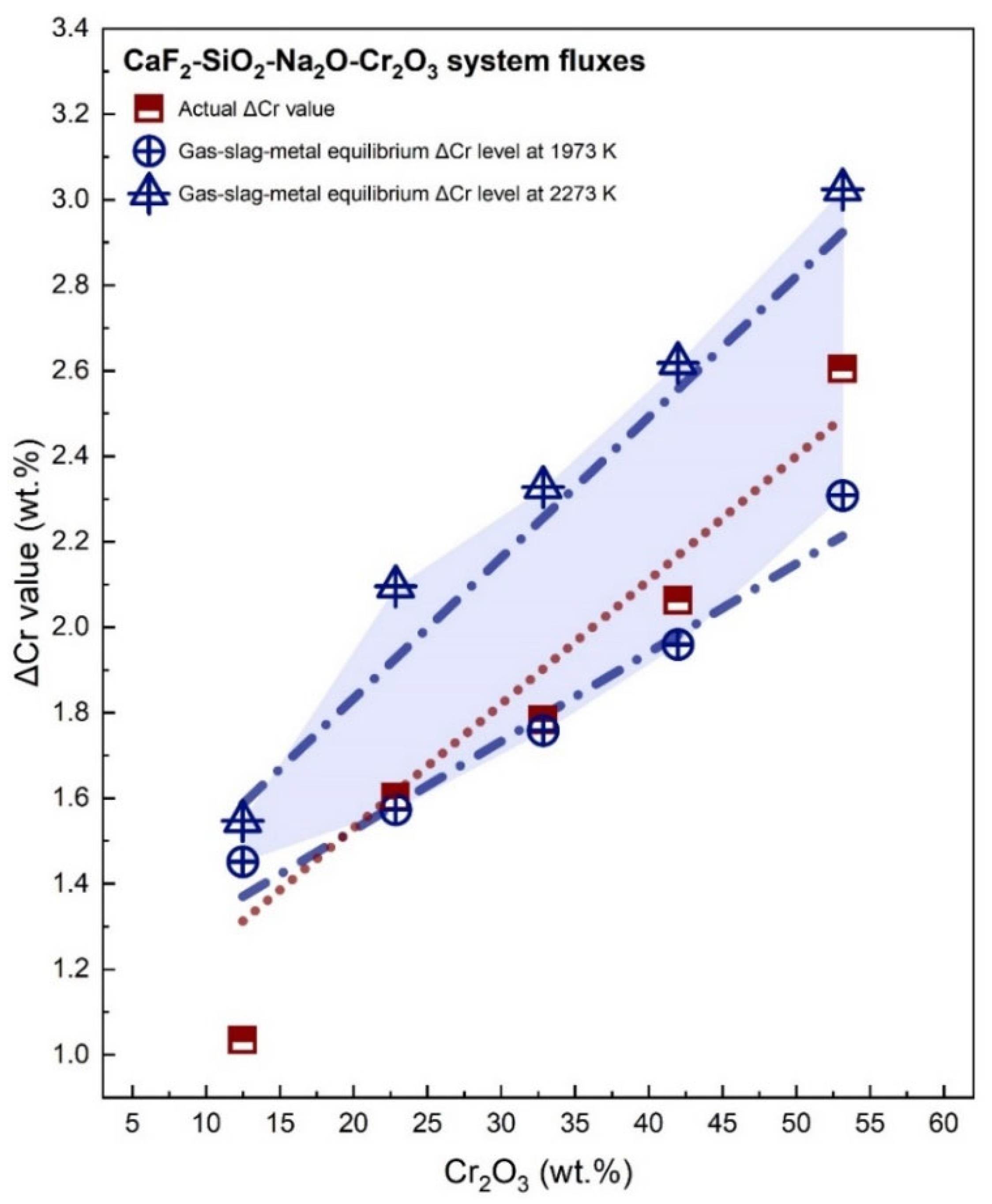
3.2. Transfer of Cr
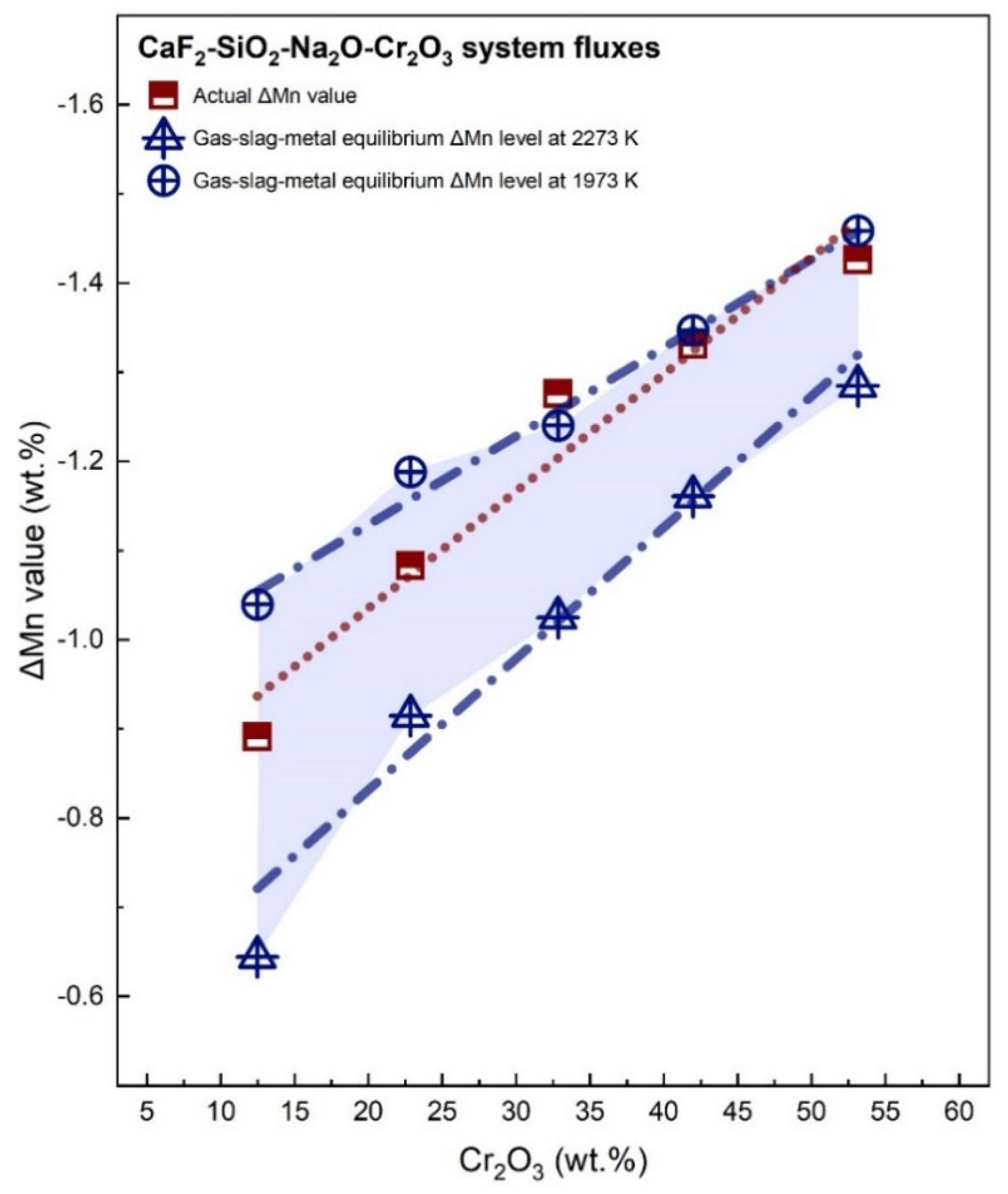
3.3. Transfer of Mn
4. Conclusions
- By performing a gas-slag-metal thermodynamic equilibrium calculation, the transfer direction of O, Cr, and Mn can be detected and the level of element transfer (ΔO, ΔCr, and ΔMn) can be constrained, which may pave a viable way for the prediction of element transfer behaviors when Cr2O3-bearing fluxes are applied.
- The measured slag compositions, coupled with thermodynamic data, demonstrated that the thermodynamic equilibrium for Fe transfer is not achieved.
- The loss of Mn from the weld pool is enhanced due to a higher level of Cr2O3 addition to the flux. The evidence regarding the loss of Cr and Mn from the SAW system to the gas phase was provided; such loss is predictable by using a gas-slag-metal equilibrium model.
- An electrode and/or a BM with higher Mn levels is recommended to match the Cr2O3-bearing flux, thereby compensating the possible Mn loss incurred by oxidation reactions.
- The content of Cr2O3 in the flux should be controlled under 30 wt.% since a WM with an O level higher than 1000 ppm may incur unexpected issues, such as enhanced porosity, reduced toughness, and depreciated hardenability.
- With a higher level of Cr2O3 addition to the flux, the Cr contents in the electrode and the BM should be restricted to avoid redundant Cr uptake from the flux.
- Since the high-temperature thermodynamic data are extended from the model, there must be an error with the real and predicted data.
- FactSage only considers the assumed thermodynamic equilibrium involved in SAW. However, kinetic factors should also be considered to improve the overall accuracy.
- More work on SAW experiments is required to determine the optimal calculation temperature.
Author Contributions
Funding
Conflicts of Interest
Appendix A. Gas-Slag-Metal Thermodynamic Equilibrium Calculation
- FToxid, Fstel, and FactPS databases were selected. The solution phases of ASlag-liq all oxides, S (FToxid-SLAGA), and LIQUID (FStel-Liqu) were selected to model the molten slag and steel phases.
- The equilibrium temperature in SAW was set to 1973 and 2273 K.
- The mass ratio of the flux to the electrode was set according to the measured data in Table A1. The measured composition of the flux in Table 2 was set as input flux chemistries. The predicted Δp value is calculated from Equation (A2), where MP indicates the predicted composition and MN indicates the nominal composition.
| Weld Metal | WM-1 | WM-2 | WM-3 | WM-4 | WM-5 |
|---|---|---|---|---|---|
| Flux | F-1 | F-2 | F-3 | F-4 | F-5 |
| dBM | 0.454 | 0.524 | 0.579 | 0.536 | 0.414 |
| Rslag/WM | 0.233 | 0.211 | 0.182 | 0.192 | 0.221 |
| WM-1 | WM-2 | WM-3 | WM-4 | WM-5 | |
|---|---|---|---|---|---|
| (C)N | 0.120 | 0.119 | 0.118 | 0.119 | 0.121 |
| (Si)N | 0.091 | 0.098 | 0.103 | 0.099 | 0.088 |
| (Mn)N | 1.600 | 1.592 | 1.586 | 1.591 | 1.604 |
| (Ti)N | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 |
| (Cr)N | 0.016 | 0.017 | 0.017 | 0.017 | 0.016 |
| (O)N | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 |
| Weld Metal | WM-1 | WM-2 | WM-3 | WM-4 | WM-5 | |
|---|---|---|---|---|---|---|
| Flux | F-1 | F-2 | F-3 | F-4 | F-5 | |
| CrF3 | Vol.% | 3.824 | 4.800 | 5.354 | 6.322 | 7.620 |
| MnF2 | 1.366 | 1.042 | 0.930 | 0.684 | 0.415 | |
| O2 | 10−10 atm. | 4.691 | 7.198 | 7.230 | 7.248 | 7.562 |
| CrO | Weight (g) | 0.215 | 0.302 | 0.276 | 0.322 | 0.411 |
| Cr2O3 | 0.018 | 0.032 | 0.027 | 0.025 | 0.026 | |
| FeO | 0.266 | 0.320 | 0.298 | 0.329 | 0.373 | |
| Weld Metal | WM-1 | WM-2 | WM-3 | WM-4 | WM-5 | |
|---|---|---|---|---|---|---|
| Flux | F-1 | F-2 | F-3 | F-4 | F-5 | |
| CrF3 | Vol.% | 8.269 | 11.990 | 11.883 | 11.710 | 10.479 |
| MnF2 | 5.770 | 4.291 | 3.309 | 2.342 | 1.489 | |
| O2 | 10−8 atm. | 3.520 | 8.240 | 10.420 | 11.400 | 12.280 |
| CrO | Weight (g) | 0.155 | 0.548 | 0.996 | 1.947 | 1.010 |
| Cr2O3 | 0.008 | 0.065 | 0.165 | 0.374 | 0.233 | |
| FeO | 0.301 | 0.679 | 1.070 | 1.837 | 0.967 | |
Appendix B
| Weld Metal | WM-1 | WM-2 | WM-3 | WM-4 | WM-5 |
|---|---|---|---|---|---|
| Flux | F-1 | F-2 | F-3 | F-4 | F-5 |
| Crloss | 0.074 | 0.077 | 0.056 | 0.205 | 0.531 |
| Mnloss | 0.252 | 0.389 | 0.501 | 0.543 | 0.286 |
Appendix C. Prediction of O Content from Flux BI
References
- Sengupta, V.; Havrylov, D.; Mendez, P. Physical Phenomena in the Weld Zone of Submerged Arc Welding-A Review. Weld. J. 2019, 98, 283–313. [Google Scholar] [CrossRef]
- Coetsee, T. The Role of Metallic Iron in Low Temperature Carbothermic Reduction of MnO: Phase Chemistry and Thermodynamic Analysis. Minerals 2021, 11, 1205. [Google Scholar] [CrossRef]
- Coetsee, T.; De Bruin, F. In Situ Modification of CaF2-SiO2-Al2O3-MgO Flux Applied in the Aluminium-Assisted Transfer of Titanium in the Submerged Arc Welding of Carbon Steel: Process Mineralogy and Thermochemical Analysis. Minerals 2022, 12, 604. [Google Scholar] [CrossRef]
- Coetsee, T.; De Bruin, F. Aluminium Assisted Nickel Alloying in Submerged Arc Welding of Carbon Steel: Application of Unconstrained Metal Powders. Appl. Sci. 2022, 12, 5392. [Google Scholar] [CrossRef]
- Coetsee, T.; De Bruin, F. Aluminium-Assisted Alloying of Carbon Steel in Submerged Arc Welding: Application of Al-Cr-Ti-Cu Unconstrained Metal Powders. Processes 2022, 10, 452. [Google Scholar] [CrossRef]
- Coetsee, T.; De Bruin, F. Chemical Interaction of Cr-Al-Cu Metal Powders in Aluminum-Assisted Transfer of Chromium in Submerged Arc Welding of Carbon Steel. Processes 2022, 10, 296. [Google Scholar] [CrossRef]
- Burck, P.; Indacochea, J.; Olson, D. Effects of Welding Flux Additions on 4340 Steel Weld Metal Composition. Weld. J. 1990, 3, 115–122. [Google Scholar]
- Indacochea, J.E.; Blander, M.; Christensen, N.; Olson, D.L. Chemical Reactions During Submerged Arc Welding with FeO-MnO-SiO2 Fluxes. Metall. Trans. B 1985, 16, 237–245. [Google Scholar] [CrossRef]
- Natalie, C.A.; Olson, D.L.; Blander, M. Physical and Chemical Behavior of Welding Fluxes. Annu. Rev. Mater. Sci. 1986, 16, 389–413. [Google Scholar] [CrossRef]
- Olson, D.; Liu, S.; Frost, R.; Edwards, G.; Fleming, D. Nature and Behavior of Fluxes Used for Welding. ASM Int. ASM Handb. 1993, 6, 55–63. [Google Scholar] [CrossRef]
- Zhang, J.; Coetsee, T.; Dong, H.; Wang, C. Element Transfer Behaviors of Fused CaF2-TiO2 Fluxes in EH36 Shipbuilding Steel during High Heat Input Submerged Arc Welding. Metall. Mater. Trans. B 2020, 51, 1953–1957. [Google Scholar] [CrossRef]
- Zhang, J.; Coetsee, T.; Dong, H.; Wang, C. Element Transfer Behaviors of Fused CaF2-SiO2-MnO Fluxes under High Heat Input Submerged Arc Welding. Metall. Mater. Trans. B 2020, 51, 885–890. [Google Scholar] [CrossRef]
- Zhang, J.; Coetsee, T.; Dong, H.; Wang, C. Fine-Tuned Element Transfer Strategies for Ternary CaF2-SiO2-CaO Fluxes in Submerged Arc Welding: An Environmentally Friendly Approach. Metall. Mater. Trans. B 2020, 51, 1350–1354. [Google Scholar] [CrossRef]
- Zhang, J.; Coetsee, T.; Wang, C. Element Transfer Behaviors of Fused CaF2-SiO2 Fluxes Subject to High Heat Input Submerged Arc Welding. Metall. Mater. Trans. B 2020, 51, 16–21. [Google Scholar] [CrossRef]
- Kanjilal, P.; Pal, T.; Majumdar, S. Prediction of Element Transfer in Submerged Arc Welding. Weld. J. 2007, 10, 40. [Google Scholar]
- Mitra, U.; Eagar, T. Slag Metal Reactions During Submerged Arc Welding of Alloy Steels. Metall. Trans. A 1984, 15, 217–227. [Google Scholar] [CrossRef]
- Li, Y.; Tang, H.; Lai, R. Microstructure and Mechanical Performance of Resistance Spot Welded Martensitic Advanced High Strength Steel. Processes 2021, 9, 1021. [Google Scholar] [CrossRef]
- Pfennig, A.; Wolf, M.; Kranzmann, A. Corrosion and Corrosion Fatigue of Steels in Downhole CCS Environment—A Summary. Processes 2021, 9, 594. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y. Influence of 12Cr1MoV Material on Tissue Properties at High Temperature and Long Operating Time. Processes 2022, 10, 192. [Google Scholar] [CrossRef]
- Czapla, A.; Ganesapillai, M.; Drewnowski, J. Composite as a Material of the Future in the Era of Green Deal Implementation Strategies. Processes 2021, 9, 2238. [Google Scholar] [CrossRef]
- Chai, C.; Eagar, T. Slag Metal Reactions in Binary CaF2-Metal Oxide Welding Fluxes. Weld. J. 1982, 61, 229–232. [Google Scholar]
- Chai, C.; Eagar, T. Slag-Metal Equilibrium during Submerged Arc Welding. Metall. Trans. B 1981, 12, 539–547. [Google Scholar] [CrossRef]
- Tuliani, S.; Boniszewski, T.; Eaton, N. Notch Toughness of Commercial Submerged Arc Weld metal. Weld. Met. Fabr. 1969, 37, 327–339. [Google Scholar]
- Chai, C.; Eagar, T. Prediction of Weld-metal Composition during Flux-shielded Welding. J. Mater. Energy Syst. 1983, 5, 160–164. [Google Scholar] [CrossRef]
- Chai, C.-S. Slag-Metal Reactions during Flux Shielded Arc Welding. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 1980. [Google Scholar]
- Zhang, J.; Coetsee, T.; Dong, H.; Wang, C. Elucidating the Roles of SiO2 and MnO upon Decarburization during Submerged Arc Welding: A Thermodynamic Study into EH36 Shipbuilding Steel. Metall. Mater. Trans. B 2020, 51, 1805–1812. [Google Scholar] [CrossRef]
- Cong, W.; Zhang, J. Fine-tuning Weld Metal Compositions via Flux Optimization in Submerged Arc Welding: An Overview. Acta Metall. Sin. 2022, 57, 1126–1140. [Google Scholar] [CrossRef]
- Bale, C.W.; Chartrand, P.; Degterov, S.; Eriksson, G.; Hack, K.; Mahfoud, R.B.; Melançon, J.; Pelton, A.; Petersen, S. FactSage Thermochemical Software and Databases. Calphad 2002, 26, 189–228. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.; Eriksson, G.; Gheribi, A.; Hack, K.; Jung, I.-H.; Kang, Y.-B.; Melançon, J. Reprint of: FactSage Thermochemical Software and Databases, 2010–2016. Calphad 2016, 55, 1–19. [Google Scholar] [CrossRef]
- Jung, I.-H. Overview of the Applications of Thermodynamic Databases to Steelmaking Processes. Calphad 2010, 34, 332–362. [Google Scholar] [CrossRef]
- Zhang, J.; Coetsee, T.; Basu, S.; Wang, C. Impact of Gas Formation on the Transfer of Ti and O From TiO2-bearing Basic-fluoride Fluxes to Submerged Arc Welded Metals: A Thermodynamic Approach. Calphad 2020, 71, 102195. [Google Scholar] [CrossRef]
- Chaveriat, P.; Kim, G.; Shah, S.; Indacochea, J. Low Carbon Steel Weld Metal Microstructures: The Role of Oxygen and Manganese. J. Mater. Eng. 1987, 9, 253–267. [Google Scholar] [CrossRef]
- Dallam, C.; Liu, S.; Olson, D. Flux Composition Dependence of Microstructure and Toughness of Submerged Arc HSLA Weldments. Weld. J. 1985, 64, 140–151. [Google Scholar]
- Ito, J.; Nakanishi, M. Study on Charpy Impact Properties of Weld Metal with Submerged-arc Welding. Weld. J. 1976, 15, 42–62. [Google Scholar]
- Kou, S. Welding Metallurgy, 3rd ed.; JohnWiley & Sons, Inc.: Hoboken, NJ, USA, 2003; pp. 22–122. [Google Scholar]
- Lau, T.; Weatherly, G.; McLean, A. The Sources of Oxygen and Nitrogen Contamination in Submerged Arc Welding Using CaO-Al2O3 Based Fluxes. Weld. J. 1985, 64, 343–347. [Google Scholar]
- Mitra, U.; Eagar, T. Slag-metal Reactions during Welding: Part II. Theory. Metall. Trans. B 1991, 22, 73–81. [Google Scholar] [CrossRef]
- Mitra, U.; Eagar, T. Slag-metal Reactions during Welding: Part III. Verification of the Theory. Metall. Trans. B 1991, 22, 83–100. [Google Scholar] [CrossRef]
- Coetsee, T. Phase Chemistry of Submerged Arc Welding (SAW) Fluoride Based Slags. J. Mater. Res. Technol. 2020, 9, 9766–9776. [Google Scholar] [CrossRef]
- Glasser, F.; Osborn, E. Phase Equilibrium Studies in the System CaO-Cr2O3-SiO2. J. Am. Ceram. Soc. 1958, 41, 358–367. [Google Scholar] [CrossRef]
- Coetsee, T.; Mostert, R.J.; Pistorius, P.G.H.; Pistorius, P.C. The Effect of Flux Chemistry on Element Transfer in Submerged Arc Welding: Application of Thermochemical Modelling. J. Mater. Res. Technol. 2021, 11, 2021–2036. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Coetsee, T. Assessment of Weld Metal Compositional Prediction Models Geared Towards Submerged Arc Welding: Case Studies Involving CaF2-SiO2-MnO and CaO-SiO2-MnO Fluxes. Mater. Trans. B 2021, 52, 2404–2415. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Coetsee, T. Thermodynamic Evaluation of Element Transfer Behaviors for Fused CaO-SiO2-MnO Fluxes Subjected to High Heat Input Submerged Arc Welding. Metall. Mater. Trans. B 2021, 52, 1937–1944. [Google Scholar] [CrossRef]
- Zhang, J.; Shao, G.; Guo, Y.; Xu, Q.; Liu, Z. Facilitating flux design process geared towards submerged arc welding via thermodynamic approach: Case study into CaF2–SiO2–Na2O–Al2O3–TiO2 agglomerated flux. Calphad 2022, 79, 102483. [Google Scholar]

| Flux | Cr2O3 | CaF2 |
|---|---|---|
| F-1 | 10 | 90 |
| F-2 | 20 | 80 |
| F-3 | 30 | 70 |
| F-4 | 40 | 60 |
| F-5 | 50 | 50 |
| Flux | Cr2O3 | SiO2 | Na2O | CaF2 | BI |
|---|---|---|---|---|---|
| F-1 | 12.58 | 7.99 | 0.52 | 78.91 | 5.56 |
| F-2 | 22.89 | 7.56 | 0.48 | 69.07 | 3.66 |
| F-3 | 32.88 | 7.63 | 0.55 | 58.94 | 2.47 |
| F-4 | 41.99 | 7.88 | 0.61 | 49.52 | 1.74 |
| F-5 | 53.19 | 7.66 | 0.49 | 38.66 | 1.14 |
| Flux | Cr2O3 | SiO2 | Na2O | FeO | MnO | Al2O3 | TiO2 | CaF2 |
|---|---|---|---|---|---|---|---|---|
| F-1 | 5.63 | 8.1 | 0.25 | 4.65 | 3.54 | 0.18 | 0.11 | 76.75 |
| F-2 | 11.25 | 8.52 | 0.21 | 7.26 | 4.25 | 0.17 | 0.10 | 67.41 |
| F-3 | 18.11 | 8.22 | 0.28 | 10.37 | 5.50 | 0.20 | 0.10 | 56.46 |
| F-4 | 24.72 | 8.58 | 0.21 | 12.41 | 5.30 | 0.21 | 0.13 | 47.41 |
| F-5 | 32.45 | 10.11 | 0.14 | 13.91 | 6.66 | 0.2 | 0.11 | 35.63 |
| C | Si | Mn | Ti | Cr | O | |
|---|---|---|---|---|---|---|
| Q345A | 0.112 | 0.142 | 1.540 | 0.015 | 0.018 | 0.003 |
| Electrode | 0.127 | 0.049 | 1.650 | 0.015 | 0.015 | 0.003 |
| Weld Metal | WM-1 | WM-2 | WM-3 | WM-4 | WM-5 |
|---|---|---|---|---|---|
| Flux | F-1 | F-2 | F-3 | F-4 | F-5 |
| (O)A | 0.049 | 0.074 | 0.118 | 0.147 | 0.162 |
| (O)N | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 |
| ΔO | 0.046 | 0.071 | 0.115 | 0.144 | 0.159 |
| (Cr)A | 1.050 | 1.620 | 1.800 | 2.080 | 2.620 |
| (Cr)N | 0.016 | 0.017 | 0.017 | 0.017 | 0.016 |
| ΔCr | 1.034 | 1.603 | 1.783 | 2.063 | 2.604 |
| (Mn)A | 0.710 | 0.510 | 0.310 | 0.260 | 0.190 |
| (Mn)N | 1.601 | 1.593 | 1.586 | 1.591 | 1.616 |
| ΔMn | −0.891 | −1.083 | −1.276 | −1.331 | −1.426 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Xu, Q. Probing Element Transfer Behavior during the Submerged Arc Welding Process for CaF2-SiO2-Na2O-Cr2O3 Agglomerated Fluxes: A Thermodynamic Approach. Processes 2022, 10, 1900. https://doi.org/10.3390/pr10101900
Zhang J, Xu Q. Probing Element Transfer Behavior during the Submerged Arc Welding Process for CaF2-SiO2-Na2O-Cr2O3 Agglomerated Fluxes: A Thermodynamic Approach. Processes. 2022; 10(10):1900. https://doi.org/10.3390/pr10101900
Chicago/Turabian StyleZhang, Jin, and Qiong Xu. 2022. "Probing Element Transfer Behavior during the Submerged Arc Welding Process for CaF2-SiO2-Na2O-Cr2O3 Agglomerated Fluxes: A Thermodynamic Approach" Processes 10, no. 10: 1900. https://doi.org/10.3390/pr10101900
APA StyleZhang, J., & Xu, Q. (2022). Probing Element Transfer Behavior during the Submerged Arc Welding Process for CaF2-SiO2-Na2O-Cr2O3 Agglomerated Fluxes: A Thermodynamic Approach. Processes, 10(10), 1900. https://doi.org/10.3390/pr10101900







