Valorization of Tomato Residues by Supercritical Fluid Extraction
Abstract
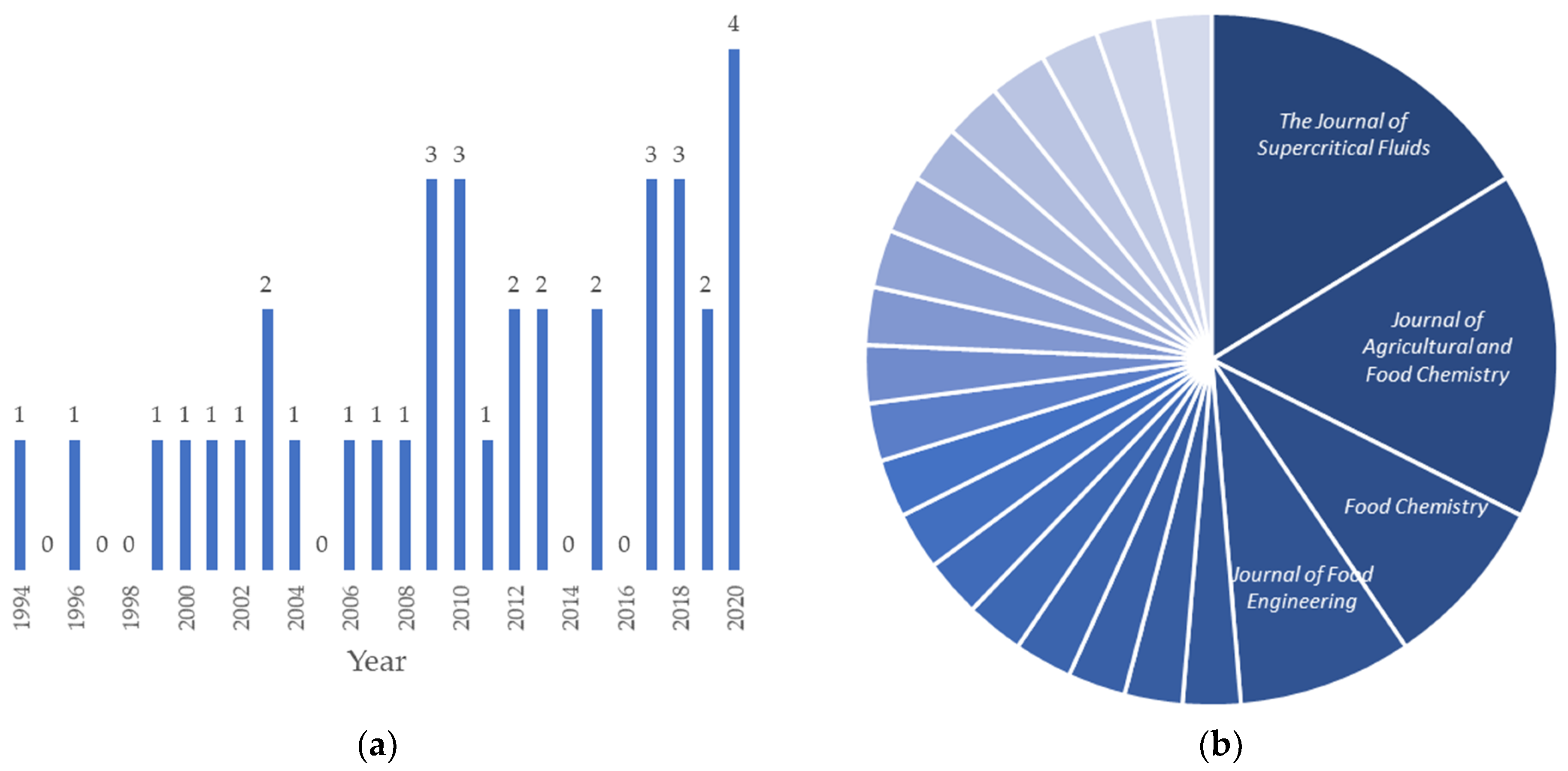
1. Introduction
2. Literature Overview
| Tomato Part | Pre-Treatment | Particle Size | Cosolvent | Pressure (Bar) | Temperature (°C) | SCF | SFE System (Model, Company) | Targets | Best Yield or Recovery | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| seeds | dried, milled, sieved | 0.25–0.46 mm | No | 245 | 40 | 50–392 kg/kgbio | - | oil | 0.4 kg/kg oil free sample | [60] |
| seeds | dried, milled | 0.27 mm | No | 108–245 | 40–70 | 295 kg/kgbio | - | oil | 0.3 kg/kg sample | [61] |
| pulp or skins of ripe tomatoes | dried, ground | - | No | 172–275 | 40–80 | 6000 L/kgbio | SFE-400, Supelco | lycopene and β-carotene | 64.41 (lyc) mg/100 g 34.88 (beta) mg/100 g | [62] |
| paste waste | dried, ground, sieved | 3 mm | 5–15% EtOH | 200–300 | 35–65 | 33–400 kg/kgbio | Extraction setup, Sitec Sieber | lycopene and β-carotene | 51% (lyc) 50% (beta) | [47] |
| skins | dried, powdered | - | No | 400 | 60–110 | 400 L/kgbio | SFX-3560, Isco | lycopene | 100% | [63] |
| seeds and skins | none | - | No | 138–483 | 32–86 | 16–100 L/kgbio | SFX-210, Isco | lycopene and tocopherols | 7.19 ug/g, 61% (lyc), 0.15 ug/g, 86% (toc) | [64] |
| skin and pulp | dried, ground | - | No | 77–281 | 40 | 240 L/kgbio | 7680A, Hewlett-Packard | all-trans-lycopene | 88% (% of total lyc) | [65] |
| seeds and skins | dried, ground | 80 and 345 μm | No | 250–300 | 60–80 | 120 kg/kgbio | TOC-27-40, HIP | lycopene and β-carotene | 80% (lyc), 88% (beta) | [66] |
| sun-dried tomato | dried, ground | 1 mm | 1–20% vegetable oil | 335–450 | 45–70 | 15–53 kg/kgbio | - | lycopene | 60% | [50] |
| skins | dried | - | No | 200–500 | 40–100 | 1.5–4.5 mL/min; 330 min d | 10 mL, Thar Designs | lycopene | 1.18 mg/g (lyc) | [67] |
| pomace | none | 0.3–0.6 | No | 380–460 | 40–80 | 0.05 L/kgbio c | - | carotenes and tocopherols | 9.04 mg/g (carotenoids), 5.96 mg/g (tocopherols) | [68] |
| skin and seeds | ground, pre-treatment with modifier | 5–15% EtOH | 250–450 | 40–70 | 29,167 L/kgbio c | Spe-ed SFE NP 7013,Applied Separations | lycopene | 23.9 ug/g (lyc) | [48] | |
| pulp and skins | dried, ground, mixed with hazelnuts | 1 mm | No | 400–450 | 60–70 | 21–53 kg/kgbio | - | lycopene | 72.5% | [69] |
| skins | freeze-dried, ground | 1 mm | 5–15% EtOH, water, canola oil | 250–350 | 45–75 | 3.5 L/min d | Spe-ed SFE NP 7013,Applied Separations | lycopene | 73.3% (lyc) (49:3 trans:cis) | [49] |
| skins and seeds | dried, ground | 0.15–0.72 mm | No | 200–300 | 40–80 | 0.01–0.07 kg/h d | - | trans-lycopene | 93% (lyc) | [70] |
| seeds | dried, ground, mixed with grape seeds | No | 335–450 | 45–70 | 21–53 kg/kgbio | - | oleoresins | - | [71] | |
| juice | removed serum | - | No | 200–350 | 40–80 | 0.01–0.04 kg/kgbio | - | lycopene | 76.9% | [72] |
| ripe tomato puree | dried, processed to puree | - | No | 450 | 65–70 | 41–46 kg/kgbio | - | lycopene | 80% 9.31 mg/g | [73] |
| pomace | freeze-dried, powdered | 0.2–0.45 mm | No | 350–450 | 40–60 | d | SFX-220, Isco | lycopene | 32.5 g/100 g dw | [74] |
| peel and seed | ground, sieved | 1 mm | No | 200–400 | 70–90 | 90–180 L/kgbio | 10 mL vessel, Thar Tech | lycopene and β-carotene | 56% (lyc), 68% (beta) | [3] |
| skins and seeds | dried, ground | 0.15–0.56 mm | No | 120–300 | 40–80 | 19–131 kg/kgbio a | - | lycopene | 80% | [46] |
| pomace and byproducts of SFE | freeze-dried, milled | 500 μm | No | 450 | 65–70 | 41–46 kg/kgbio | - | carbohydrate | - | [75] |
| pomace | wet | - | No | 100–300 | 30–50 | 5–15 kg/h d | Pilot plant, Muller Extract | lycopene | 1.58 mg/L | [76] |
| pomace | dried, ground | - | No | 69–275 | 40–80 | 9.6–28.8 L/kgbio | SFT-100, SupercriticalFluid Technologies | lycopene | 82% | [77] |
| ripe tomato puree | freeze-dried, puree, enzyme digestion | 1 mm | No | 500 | 86 | 10 L/kgbio c | Spe-ed SFE, Applied Separations | lycopene | 27.6 mg/g dw | [78] |
| pomace | freeze dried, milled, mixed with avocado | avocado oil b | 200–400 | 40–60 | 71 kg/kgbio | - | lycopene | 80% | [51] | |
| pomace | sun-dried, ground | 300 μm | No | 300–500 | 50–80 | 31–63 kg/kgbio | Spe-ed SFE-2/4,Applied Separations | lycopene and β-carotene | 60.8% (lyc), 58.8% (beta) | [79] |
| pomace | freeze-dried, ground | - | No | 200–550 | 40–80 | d | SFT-150, SupercriticalFluid Technologies | cis-lycopene | 62% of total lyc (cis-lyc) 251.15 g/kg dw (oleoresin) | [80] |
| puree | dried, sieved | - | No | 350 | 60 | 4.8 L/kgbio | Spe-ed SFE, Applied Separations | lycopene and β-carotene | 2.92 g/kg (all-trans-lyc), 1.12 g/kg (beta) | [81] |
| pulp | dried | 0.25 mm | 5% hazelnut oil | 300–500 | 50–80 | 225 L/kgbio | 10 mL vessel, Thar Tech | lycopene | 22% | [52] |
| peel and seed | dried, milled | 0.1/1 mm | No | 300–500 | 40–80 | 12 L/kgbio | SFT 110, Supercritical Fluids | lycopene | 246 g/kg (oleoresin) | [82] |
| peel and seed | dried, milled | - | No | 400 | 60 | 20 L/kgbio | SFT 110, Supercritical Fluids | lycopene | 1.32 mg/kg | [83] |
| skin | dried | 350 μm | No | 350–550 | 60 | 2.2 L/kgbio | Spe-ed SFE, Applied Separations | lycopene and β-carotene | 79% (oil) 0.86 (lyc), 1.5 (beta) mg/100 g | [84] |
| pomace | wet | - | No | 380 | 80 | 100 kg/kgbio | Separeco | lycopene | 619 mg/kg (lyc) | [85] |
| powder | freeze-dried, ground | - | No | 400 | 50 | 10.8–18 kg/kgbio | - | high antioxidant activity | 3.91 g/100 g dw (total), 64.9% (lyc), 34.9% (beta) | [86] |
| pomace | dried, milled or powdered | 0.2–1.5 mm | No | 380 | 80 | 35 kg/kgbio | Separeco | lycopene | 165.3 g/kg (total), 1.34 mg/g (lyc) | [87] |
| peels | dried, ground | - | No | 400 | 70–80 | 300 kg/kgbio | Natex | lycopene and β-carotene | 39.1 mg/g dw (lyc), 68.2 mg/g dw (beta) | [88] |
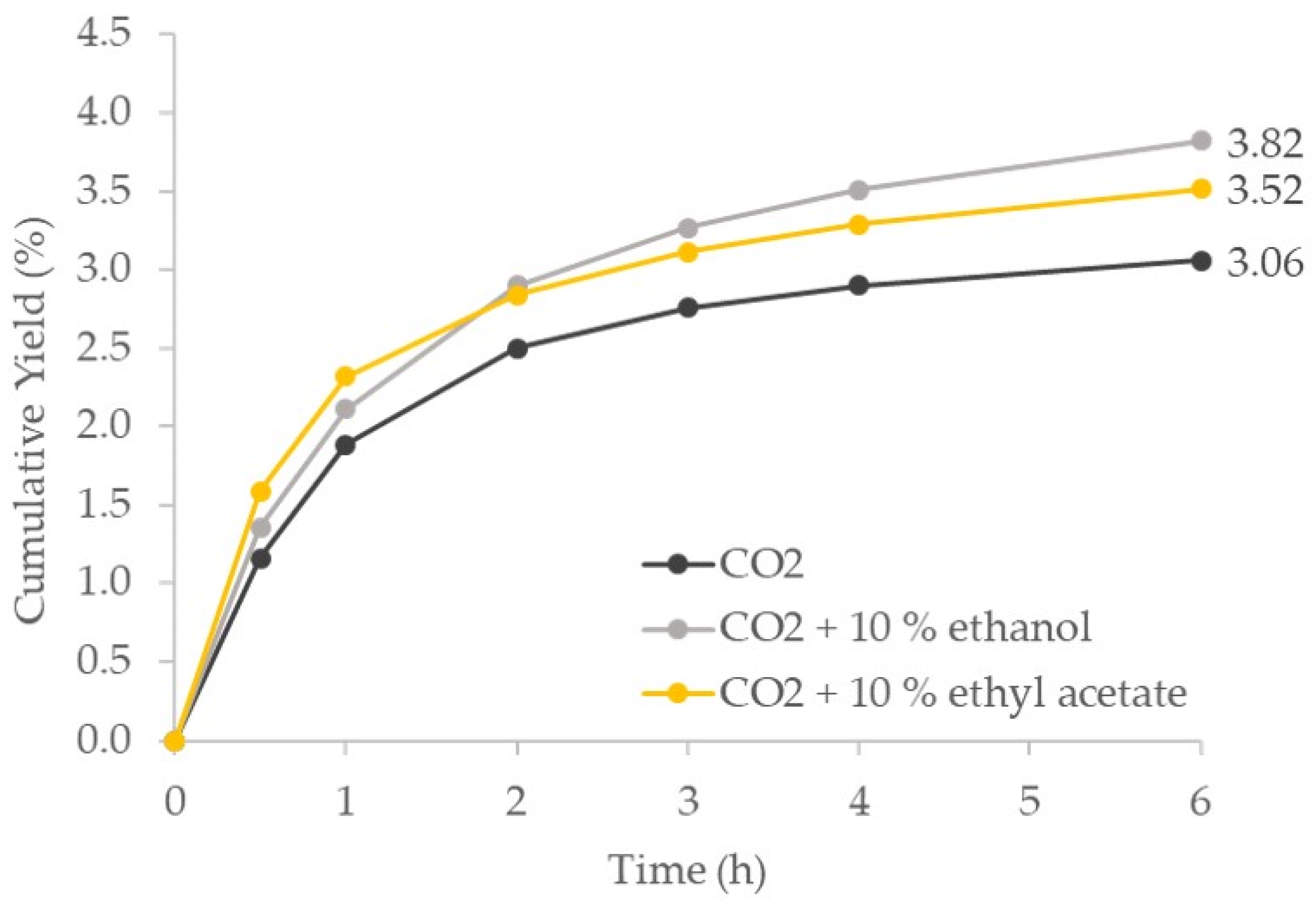
| pomace | dried | As is | EtOH and EtAc | 300 | 60 | 216 kg/kgbio | Spe-ed SFE Helix, Applied Separations | oil | 30.56 g/kg | Unpublished (see S.M.) |
3. Target Compounds
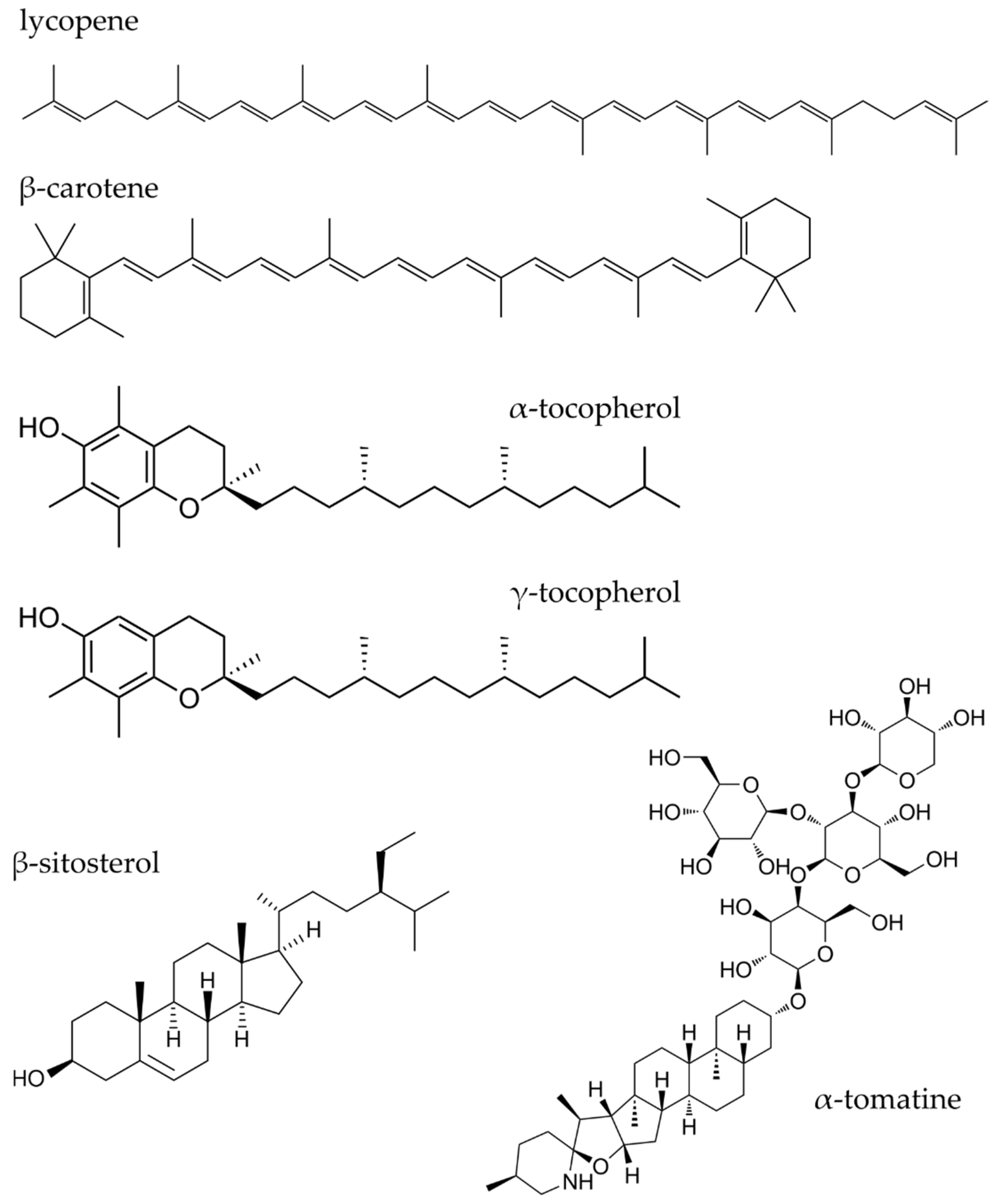
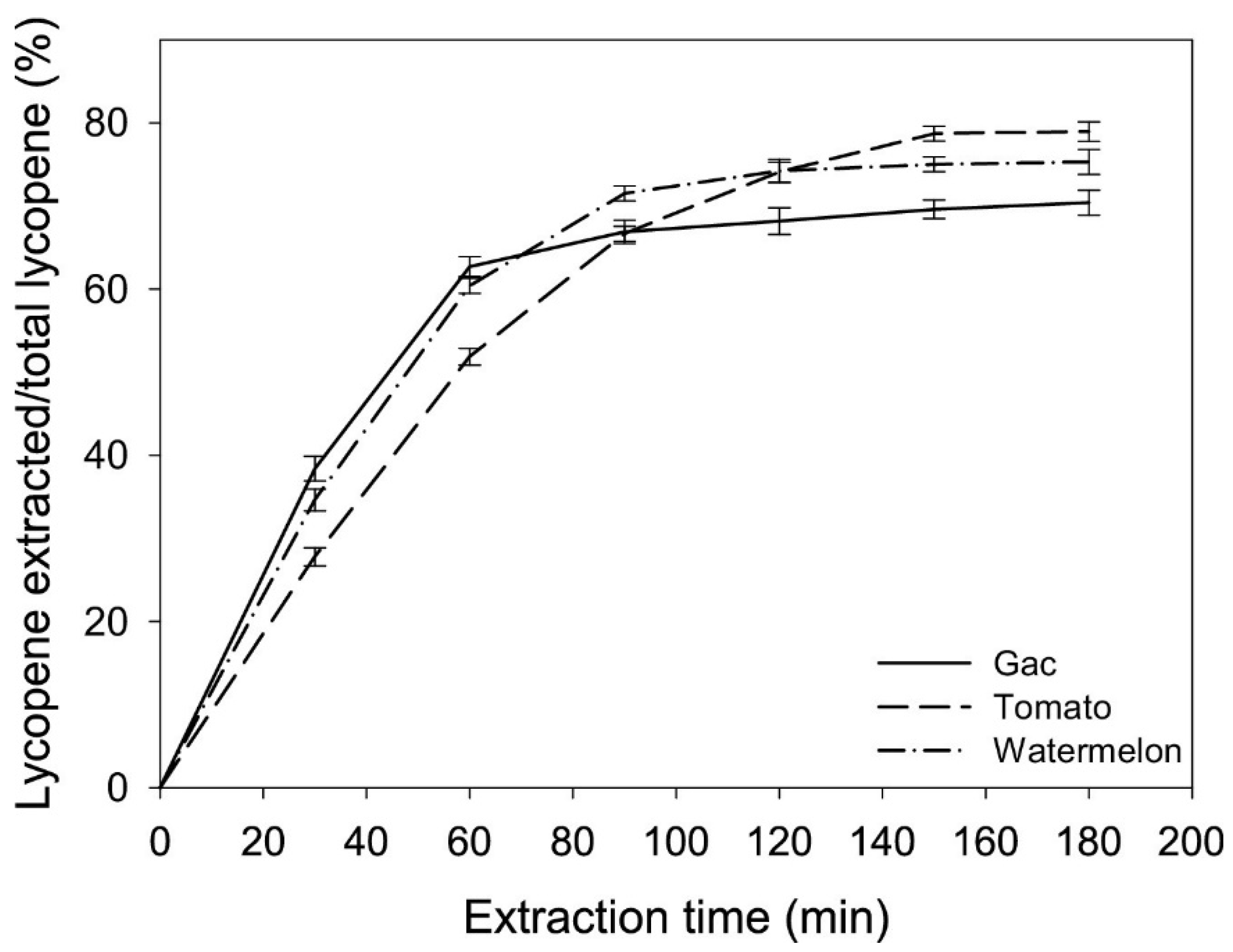
3.1. Carotenoids (Lycopene and β-Carotene)
3.2. Tocopherols and Sitosterols
3.3. Other Compounds
4. SFE of Tomato
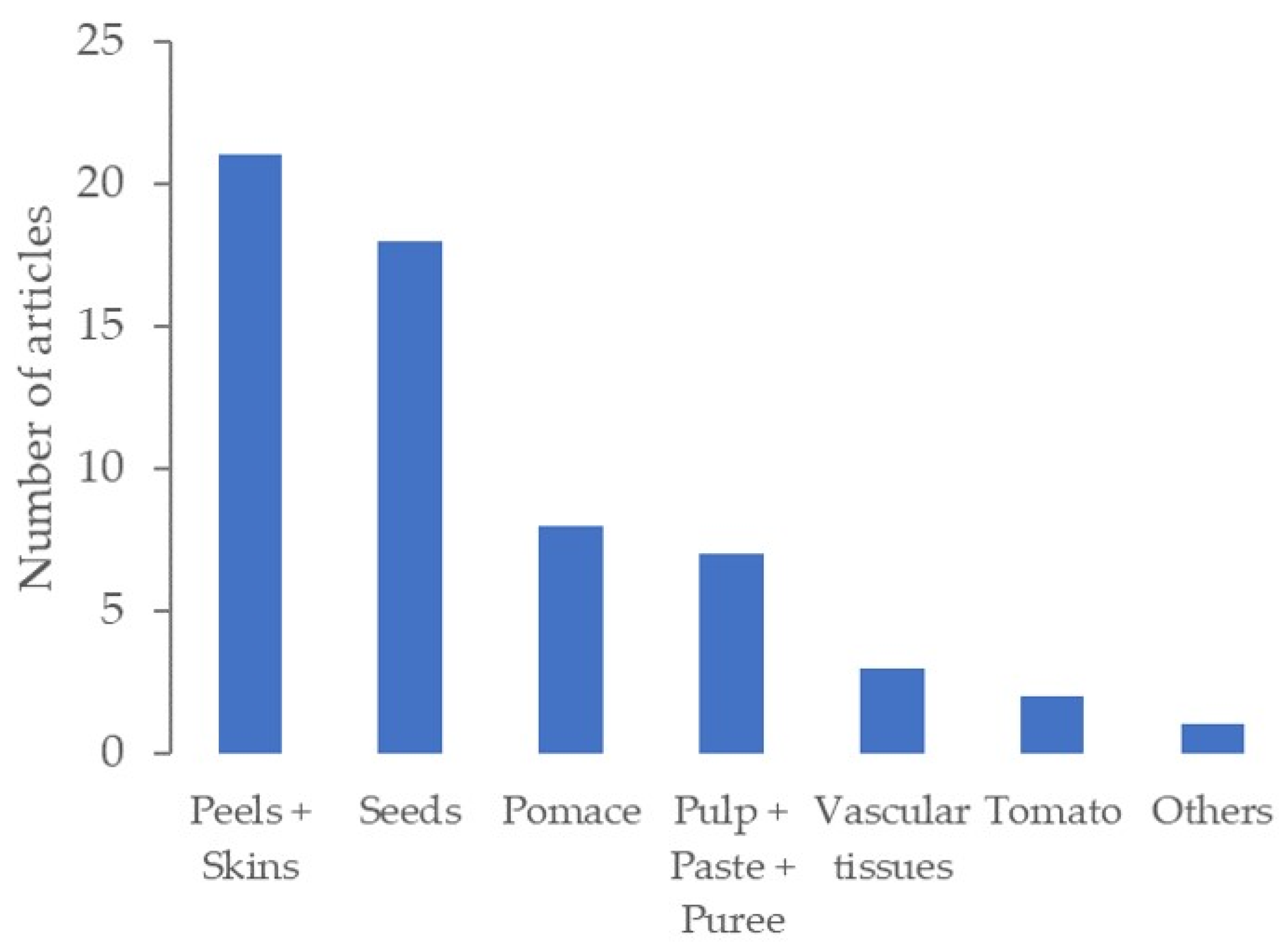
4.1. Tomato Sources
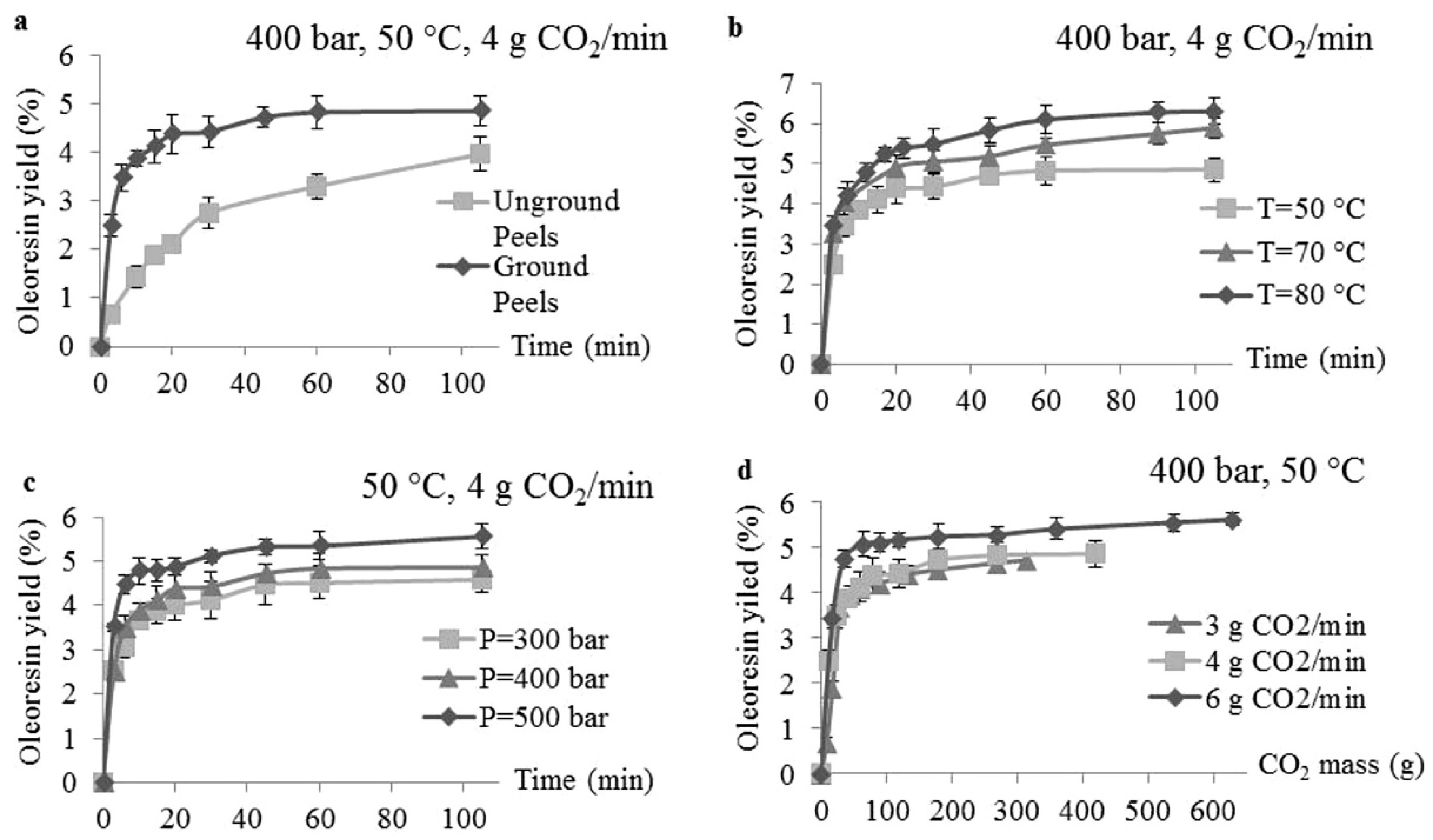
4.2. Pretreatments
4.3. Supercritical Fluid Extraction Conditions
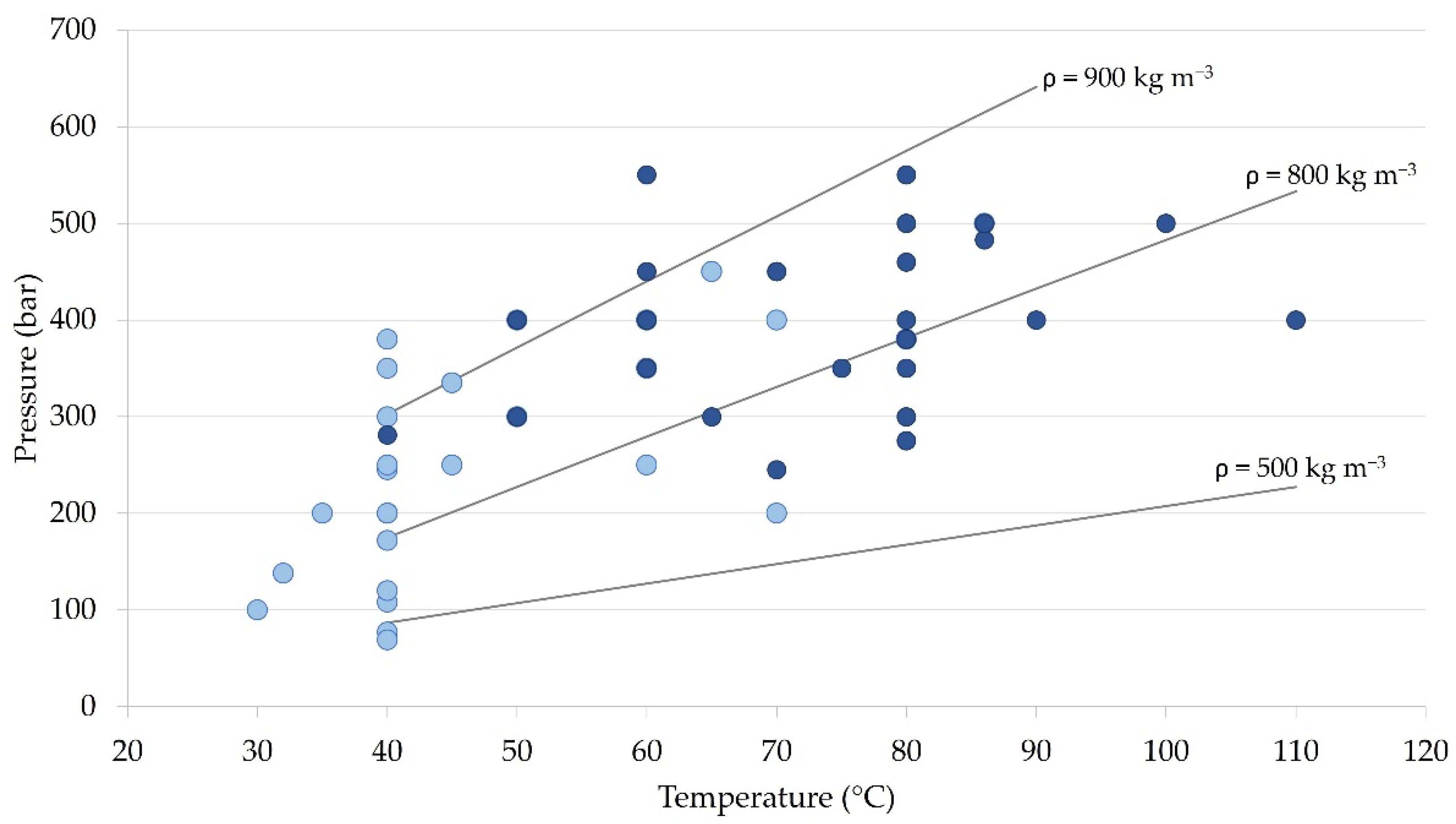
4.3.1. Pressure
4.3.2. Temperature
4.3.3. Supercritical Solvent Modifier (Cosolvent)
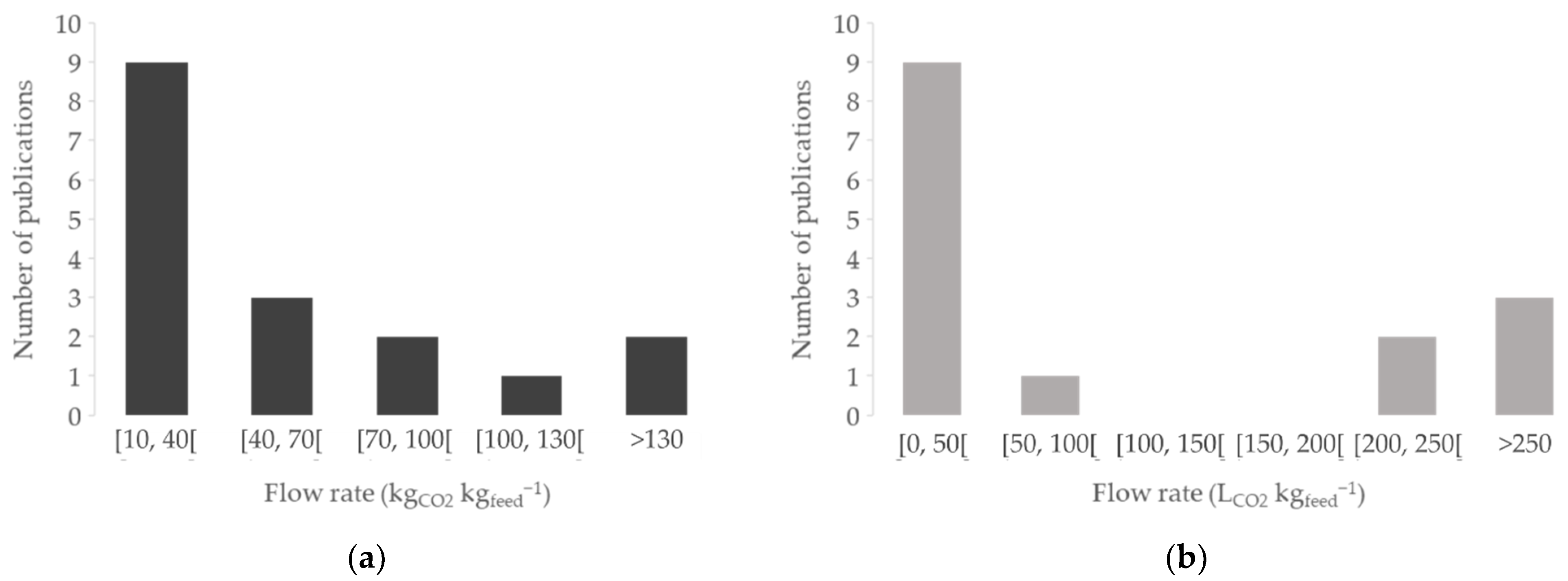
4.3.4. Flow Rate and Extraction Time
5. Final Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Tayyib, S. Value of Agricultural Production. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 14 September 2021).
- MacHmudah, S.; Zakaria; Winardi, S.; Sasaki, M.; Goto, M.; Kusumoto, N.; Hayakawa, K. Lycopene extraction from tomato peel by-product containing tomato seed using supercritical carbon dioxide. J. Food Eng. 2012, 108, 290–296. [Google Scholar] [CrossRef]
- Shi, J.; Le Maguer, M. Lycopene in tomatoes: Chemical and physical properties affected by food processing. Crit. Rev. Food Sci. Nutr. 2000, 40, 1–42. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- de Melo, M.M.R.; Portugal, I.; Silvestre, A.J.D.; Silva, C.M. Environmentally Benign Supercritical Fluid Extraction. In The Application of Green Solvents in Separation Processes; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 325–348. ISBN 9780128054437. [Google Scholar]
- Strati, I.F.; Oreopoulou, V. Recovery of carotenoids from tomato processing by-products—A review. Food Res. Int. 2014, 65, 311–321. [Google Scholar] [CrossRef]
- Hoang, T.H.; Sharma, R.; Susanto, D.; Di Maso, M.; Kwong, E. Microwave-assisted extraction of active pharmaceutical ingredient from solid dosage forms. J. Chromatogr. A 2007, 1156, 149–153. [Google Scholar] [CrossRef]
- Ho, K.K.H.Y.; Ferruzzi, M.G.; Liceaga, A.M.; San Martín-González, M.F. Microwave-assisted extraction of lycopene in tomato peels: Effect of extraction conditions on all-trans and cis-isomer yields. LWT—Food Sci. Technol. 2015, 62, 160–168. [Google Scholar] [CrossRef]
- Pinela, J.; Prieto, M.A.; Carvalho, A.M.; Barreiro, M.F.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Microwave-assisted extraction of phenolic acids and flavonoids and production of antioxidant ingredients from tomato: A nutraceutical-oriented optimization study. Sep. Purif. Technol. 2016, 164, 114–124. [Google Scholar] [CrossRef]
- Proestos, C.; Komaitis, M. Application of microwave-assisted extraction to the fast extraction of plant phenolic compounds. LWT—Food Sci. Technol. 2008, 41, 652–659. [Google Scholar] [CrossRef]
- Li, H.; Deng, Z.; Wu, T.; Liu, R.; Loewen, S.; Tsao, R. Microwave-assisted extraction of phenolics with maximal antioxidant activities in tomatoes. Food Chem. 2012, 130, 928–936. [Google Scholar] [CrossRef]
- Eh, A.L.S.; Teoh, S.G. Novel modified ultrasonication technique for the extraction of lycopene from tomatoes. Ultrason. Sonochem. 2012, 19, 151–159. [Google Scholar] [CrossRef]
- Kumcuoglu, S.; Yilmaz, T.; Tavman, S. Ultrasound assisted extraction of lycopene from tomato processing wastes. J. Food Sci. Technol. 2014, 51, 4102–4107. [Google Scholar] [CrossRef]
- Grassino, A.N.; Brnčić, M.; Vikić-Topić, D.; Roca, S.; Dent, M.; Brnčić, S.R. Ultrasound assisted extraction and characterization of pectin from tomato waste. Food Chem. 2016, 198, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Luengo, E.; Condón-Abanto, S.; Condón, S.; Álvarez, I.; Raso, J. Improving the extraction of carotenoids from tomato waste by application of ultrasound under pressure. Sep. Purif. Technol. 2014, 136, 130–136. [Google Scholar] [CrossRef]
- Sengar, A.S.; Rawson, A.; Muthiah, M.; Kalakandan, S.K. Comparison of different ultrasound assisted extraction techniques for pectin from tomato processing waste. Ultrason. Sonochem. 2020, 61, 104812. [Google Scholar] [CrossRef]
- Ranveer, R.C.; Patil, S.N.; Sahoo, A.K. Effect of different parameters on enzyme-assisted extraction of lycopene from tomato processing waste. Food Bioprod. Process. 2013, 91, 370–375. [Google Scholar] [CrossRef]
- Strati, I.F.; Gogou, E.; Oreopoulou, V. Enzyme and high pressure assisted extraction of carotenoids from tomato waste. Food Bioprod. Process. 2015, 94, 668–674. [Google Scholar] [CrossRef]
- Qiu, W.; Jiang, H.; Wang, H.; Gao, Y. Effect of high hydrostatic pressure on lycopene stability. Food Chem. 2006, 97, 516–523. [Google Scholar] [CrossRef]
- Xi, J. Effect of high pressure processing on the extraction of lycopene in tomato paste waste. Chem. Eng. Technol. 2006, 29, 736–739. [Google Scholar] [CrossRef]
- Lianfu, Z.; Zelong, L. Optimization and comparison of ultrasound/microwave assisted extraction (UMAE) and ultrasonic assisted extraction (UAE) of lycopene from tomatoes. Ultrason. Sonochem. 2008, 15, 731–737. [Google Scholar] [CrossRef]
- Konwarh, R.; Pramanik, S.; Kalita, D.; Mahanta, C.L.; Karak, N. Ultrasonication—A complementary “green chemistry” tool to biocatalysis: A laboratory-scale study of lycopene extraction. Ultrason. Sonochem. 2012, 19, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Chemat-Djenni, Z.; Ferhat, M.A.; Tomao, V.; Chemat, F. Carotenoid extraction from tomato using a green solvent resulting from orange processing waste. J. Essent. Oil-Bear. Plants 2010, 13, 139–147. [Google Scholar] [CrossRef]
- Span, R.; Wagner, W. A new equation of state for carbon dioxide covering the fluid region from the triple-point temperature to 1100 K at pressures up to 800 MPa. J. Phys. Chem. Ref. Data 1996, 25, 1509–1596. [Google Scholar] [CrossRef]
- Shi, J.; Jun Xue, S.; Jiang, Y.; Ye, X. Supercritical-fluid extraction of lycopene from tomatoes. In Separation, Extraction and Concentration Processes in the Food, Beverage and Nutraceutical Industries; Elsevier Ltd.: Amsterdam, The Netherlands, 2010; pp. 619–645. ISBN 9781845696450. [Google Scholar]
- Barbosa, H.M.; De Melo, M.M.R.; Coimbra, M.; Passos, C.P.; Silva, C.M. Optimization of the supercritical fluid coextraction of oil and diterpenes from spent coffee grounds using experimental design and response surface methodology. J. Supercrit. Fluids 2014, 85, 165–172. [Google Scholar] [CrossRef]
- de Melo, M.M.R.; Silva, R.P.; Silvestre, A.J.D.; Silva, C.M. Valorization of water hyacinth through supercritical CO2 extraction of stigmasterol. Ind. Crops Prod. 2016, 80, 177–185. [Google Scholar] [CrossRef]
- del Pilar Garcia-Mendoza, M.; Espinosa-Pardo, F.A.; Baseggio, A.M.; Barbero, G.F.; Maróstica Junior, M.R.; Rostagno, M.A.; Martínez, J. Extraction of phenolic compounds and anthocyanins from juçara (Euterpe edulis Mart.) residues using pressurized liquids and supercritical fluids. J. Supercrit. Fluids 2017, 119, 9–16. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Iovine, A.; Larocca, V.; Di Sanzo, G.; Martino, M.; Casella, P.; Chianese, S.; Musmarra, D. Extraction of Astaxanthin and Lutein from Microalga Haematococcus pluvialis in the Red Phase Using CO₂ Supercritical Fluid Extraction Technology with Ethanol as Co-Solvent. Mar. Drugs 2018, 16, 432. [Google Scholar] [CrossRef]
- Valadez-Carmona, L.; Ortiz-Moreno, A.; Ceballos-Reyes, G.; Mendiola, J.A.; Ibáñez, E. Valorization of cacao pod husk through supercritical fluid extraction of phenolic compounds. J. Supercrit. Fluids 2018, 131, 99–105. [Google Scholar] [CrossRef]
- de Melo, M.M.R.; Vieira, P.G.; Şen, A.; Pereira, H.; Portugal, I.; Silva, C.M. Optimization of the supercritical fluid extraction of Quercus cerris cork towards extraction yield and selectivity to friedelin. Sep. Purif. Technol. 2020, 238, 116395. [Google Scholar] [CrossRef]
- de Melo, M.M.R.; Carius, B.; Simões, M.M.Q.; Portugal, I.; Saraiva, J.; Silva, C.M. Supercritical CO2 extraction of V. vinifera leaves: Influence of cosolvents and particle size on removal kinetics and selectivity to target compounds. J. Supercrit. Fluids 2020, 165, 104959. [Google Scholar] [CrossRef]
- Rodrigues, V.H.; de Melo, M.M.R.; Portugal, I.; Silva, C.M. Supercritical fluid extraction of Eucalyptus globulus leaves. Experimental and modelling studies of the influence of operating conditions and biomass pretreatment upon yields and kinetics. Sep. Purif. Technol. 2018, 191, 173–181. [Google Scholar] [CrossRef]
- Rodrigues, V.H.; De Melo, M.M.R.; Portugal, I.; Silva, C.M. Extraction of Added-Value Triterpenoids from Acacia dealbata Leaves Using Supercritical Fluid Extraction. Processes 2021, 9, 1159. [Google Scholar] [CrossRef]
- Razgonova, M.; Zakharenko, A.; Shin, T.S.; Chung, G.; Golokhvast, K. Supercritical CO2 Extraction and Identification of Ginsenosides in Russian and North Korean Ginseng by HPLC with Tandem Mass Spectrometry. Molecules 2020, 25, 1407. [Google Scholar] [CrossRef] [PubMed]
- De Melo, M.M.R.R.; Silvestre, A.J.D.D.; Silva, C.M. Supercritical fluid extraction of vegetable matrices: Applications, trends and future perspectives of a convincing green technology. J. Supercrit. Fluids 2014, 92, 115–176. [Google Scholar] [CrossRef]
- Saini, R.K.; Moon, S.H.; Keum, Y.S. An updated review on use of tomato pomace and crustacean processing waste to recover commercially vital carotenoids. Food Res. Int. 2018, 108, 516–529. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, J.; Gao, R.; Ye, F.; Zhao, G. Sustainable valorisation of tomato pomace: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 172–187. [Google Scholar] [CrossRef]
- Ahangari, H.; King, J.W.; Ehsani, A.; Yousefi, M. Supercritical fluid extraction of seed oils—A short review of current trends. Trends Food Sci. Technol. 2021, 111, 249–260. [Google Scholar] [CrossRef]
- Fritsch, C.; Staebler, A.; Happel, A.; Márquez, M.A.C.; Aguiló-Aguayo, I.; Abadias, M.; Gallur, M.; Cigognini, I.M.; Montanari, A.; López, M.J.; et al. Processing, valorization and application of bio-waste derived compounds from potato, tomato, olive and cereals: A review. Sustainability 2017, 9, 1492. [Google Scholar] [CrossRef]
- Sovová, H. Mathematical model for supercritical fluid extraction of natural products and extraction curve evaluation. J. Supercrit. Fluids 2005, 33, 35–52. [Google Scholar] [CrossRef]
- Sovová, H. Rate of the vegetable oil extraction with supercritical CO2—I. Modelling of extraction curves. Chem. Eng. Sci. 1994, 49, 409–414. [Google Scholar] [CrossRef]
- Brunner, G. Mass Transfer from Solid Material in Gas Extraction. Ber. Bunsenges. Phys. Chem. 1984, 88, 887–891. [Google Scholar] [CrossRef]
- Chrastil, J. Solubility of solids and liquids in supercritical gases. J. Phys. Chem. 2002, 86, 3016–3021. [Google Scholar] [CrossRef]
- Nobre, B.P.; Gouveia, L.; Matos, P.G.S.; Cristino, A.F.; Palavra, A.F.; Mendes, R.L. Supercritical Extraction of Lycopene from Tomato Industrial Wastes with Ethane. Molecules 2012, 17, 8397–8407. [Google Scholar] [CrossRef] [PubMed]
- Baysal, T.; Ersus, S.; Starmans, D.A.J. Supercritical CO2 extraction of β-carotene and lycopene from tomato paste waste. J. Agric. Food Chem. 2000, 48, 5507–5511. [Google Scholar] [CrossRef]
- Kassama, L.S.; Shi, J.; Mittal, G.S. Optimization of supercritical fluid extraction of lycopene from tomato skin with central composite rotatable design model. Sep. Purif. Technol. 2008, 60, 278–284. [Google Scholar] [CrossRef]
- Shi, J.; Yi, C.; Xue, S.J.; Jiang, Y.; Ma, Y.; Li, D. Effects of modifiers on the profile of lycopene extracted from tomato skins by supercritical CO2. J. Food Eng. 2009, 93, 431–436. [Google Scholar] [CrossRef]
- Vasapollo, G.; Longo, L.; Rescio, L.; Ciurlia, L. Innovative supercritical CO2 extraction of lycopene from tomato in the presence of vegetable oil as co-solvent. J. Supercrit. Fluids 2004, 29, 87–96. [Google Scholar] [CrossRef]
- Barros, H.D.F.Q.; Grimaldi, R.; Cabral, F.A. Lycopene-rich avocado oil obtained by simultaneous supercritical extraction from avocado pulp and tomato pomace. J. Supercrit. Fluids 2017, 120, 1–6. [Google Scholar] [CrossRef]
- Watanabe, Y.; Honda, M.; Higashiura, T.; Fukaya, T.; Machmudah, S.; Wahyudiono; Kanda, H.; Goto, M. Rapid and Selective Concentration of Lycopene Z-isomers from Tomato Pulp by Supercritical CO2 with Co-solvents. Solvent Extr. Res. Dev. Jpn. 2018, 25, 47–57. [Google Scholar] [CrossRef]
- Del Castillo, M.L.R.; Gómez-Prieto, M.S.; Herraiz, M.; Santa-María, G. Lipid composition in tomato skin supercritical fluid extracts with high lycopene content. JAOCS J. Am. Oil Chem. Soc. 2003, 80, 271–274. [Google Scholar] [CrossRef]
- Cortés, J.M.; Vázquez, A.; Santa-María, G.; Blanch, G.P.; Villén, J. Pesticide residue analysis by RPLC-GC in lycopene and other carotenoids obtained from tomatoes by supercritical fluid extraction. Food Chem. 2009, 113, 280–284. [Google Scholar] [CrossRef]
- Honda, M.; Higashiura, T.; Fukaya, T. Safety assessment of a natural tomato oleoresin containing high amounts of Z-isomers of lycopene prepared with supercritical carbon dioxide. J. Sci. Food Agric. 2017, 97, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Romano, R.; Aiello, A.; Pizzolongo, F.; Rispoli, A.; De Luca, L.; Masi, P. Characterisation of oleoresins extracted from tomato waste by liquid and supercritical carbon dioxide. Int. J. Food Sci. Technol. 2020, 55, 3334–3342. [Google Scholar] [CrossRef]
- Gómez-Prieto, M.S.; Caja, M.M.; Santa-María, G. Solubility in supercritical carbon dioxide of the predominant carotenes of tomato skin. J. Am. Oil Chem. Soc. 2002, 79, 897–902. [Google Scholar] [CrossRef]
- Saldaña, M.D.A.; Temelli, F.; Guigard, S.E.; Tomberli, B.; Gray, C.G. Apparent solubility of lycopene and β-carotene in supercritical CO2, CO2 + ethanol and CO2 + canola oil using dynamic extraction of tomatoes. J. Food Eng. 2010, 99, 1–8. [Google Scholar] [CrossRef]
- Silva, A.F.; de Melo, M.M.R.; Silva, C.M. Supercritical solvent selection (CO2 versus ethane) and optimization of operating conditions of the extraction of lycopene from tomato residues: Innovative analysis of extraction curves by a response surface methodology and cost of manufacturin. J. Supercrit. Fluids 2014, 95, 618–627. [Google Scholar] [CrossRef]
- Roy, B.C.; Goto, M.; Hirose, T.; Navaro, O.; Hortacsu, O. Extraction Rates of Oil from Tomato Seeds with Supercritical Carbon Dioxide. J. Chem. Eng. Jpn. 1994, 27, 768–772. [Google Scholar] [CrossRef]
- Roy, B.C.; Goto, M.; Hirose, T. Temperature and pressure effects on supercritical CO2 extraction of tomato seed oil. Int. J. Food Sci. Technol. 1996, 31, 137–141. [Google Scholar] [CrossRef]
- Cadoni, E.; Rita De Giorgi, M.; Medda, E.; Poma, G. Supercritical CO2 extraction of lycopene and β-carotene from ripe tomatoes. Dye. Pigment. 1999, 44, 27–32. [Google Scholar] [CrossRef]
- Ollanketo, M.; Hartonen, K.; Riekkola, M.L.; Holm, Y.; Hiltunen, R. Supercritical carbon dioxide extraction of lycopene in tomato skins. Eur. Food Res. Technol. 2001, 212, 561–565. [Google Scholar] [CrossRef]
- Rozzi, N.L.; Singh, R.K.; Vierling, R.A.; Watkins, B.A. Supercritical fluid extraction of lycopene from tomato processing byproducts. J. Agric. Food Chem. 2002, 50, 2638–2643. [Google Scholar] [CrossRef]
- Gómez-Prieto, M.S.; Caja, M.M.; Herraiz, M.; Santa-María, G. Supercritical fluid extraction of all-trans-lycopene from tomato. J. Agric. Food Chem. 2003, 51, 3–7. [Google Scholar] [CrossRef]
- Sabio, E.; Lozano, M.; Montero De Espinosa, V.; Mendes, R.L.; Pereira, A.P.; Palavra, A.F.; Coelho, J.A. Lycopene and β-Carotene Extraction from Tomato Processing Waste Using Supercritical CO2. Ind. Eng. Chem. Res. 2003, 42, 6641–6646. [Google Scholar] [CrossRef]
- Topal, U.; Sasaki, M.; Goto, M.; Hayakawa, K. Extraction of lycopene from tomato skin with supercritical carbon dioxide: Effect of operating conditions and solubility analysis. J. Agric. Food Chem. 2006, 54, 5604–5610. [Google Scholar] [CrossRef] [PubMed]
- Vági, E.; Simándi, B.; Vásárhelyiné, K.P.; Daood, H.; Kéry, Á.; Doleschall, F.; Nagy, B. Supercritical carbon dioxide extraction of carotenoids, tocopherols and sitosterols from industrial tomato by-products. J. Supercrit. Fluids 2007, 40, 218–226. [Google Scholar] [CrossRef]
- Ciurlia, L.; Bleve, M.; Rescio, L. Supercritical carbon dioxide co-extraction of tomatoes (Lycopersicum esculentum L.) and hazelnuts (Corylus avellana L.): A new procedure in obtaining a source of natural lycopene. J. Supercrit. Fluids 2009, 49, 338–344. [Google Scholar] [CrossRef]
- Nobre, B.P.; Palavra, A.F.; Pessoa, F.L.P.; Mendes, R.L. Supercritical CO2 extraction of trans-lycopene from Portuguese tomato industrial waste. Food Chem. 2009, 116, 680–685. [Google Scholar] [CrossRef]
- Leone, A.; Zefferino, R.; Longo, C.; Leo, L.; Zacheo, G. Supercritical CO2-extracted tomato oleoresins enhance gap junction intercellular communications and recover from mercury chloride inhibition in keratinocytes. J. Agric. Food Chem. 2010, 58, 4769–4778. [Google Scholar] [CrossRef]
- Egydio, J.A.; Moraes, Â.M.; Rosa, P.T.V. Supercritical fluid extraction of lycopene from tomato juice and characterization of its antioxidation activity. J. Supercrit. Fluids 2010, 54, 159–164. [Google Scholar] [CrossRef]
- Lenucci, M.S.; Caccioppola, A.; Durante, M.; Serrone, L.; Leonardo, R.; Piro, G.; Dalessandro, G. Optimisation of biological and physical parameters for lycopene supercritical CO2 extraction from ordinary and high-pigment tomato cultivars. J. Sci. Food Agric. 2010, 90, 1709–1718. [Google Scholar] [CrossRef]
- Zhang, K.S.; Jiang, H.; Ren, Y.X. The effect of technical parameters on lycopene extraction in supercritical fluid extraction from freeze-dried tomato pomace (peels and seeds). In Advanced Materials Research; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2011; Volume 236–238, pp. 2868–2871. [Google Scholar]
- Lenucci, M.S.; Durante, M.; Anna, M.; Dalessandro, G.; Piro, G. Possible use of the carbohydrates present in tomato pomace and in byproducts of the supercritical carbon dioxide lycopene extraction process as biomass for bioethanol production. J. Agric. Food Chem. 2013, 61, 3683–3692. [Google Scholar] [CrossRef]
- Perretti, G.; Troilo, A.; Bravi, E.; Marconi, O.; Galgano, F.; Fantozzi, P. Production of a lycopene-enriched fraction from tomato pomace using supercritical carbon dioxide. J. Supercrit. Fluids 2013, 82, 177–182. [Google Scholar] [CrossRef]
- Haddadin, M.S.Y.; Haddadin, J.S. Lycopene extraction from tomato pomace with Supercritical carbon dioxide: Effect of pressures, temperatures and CO2 flow rates and evaluation of antioxidant activity and stability of lycopene. Pak. J. Nutr. 2015, 14, 942–956. [Google Scholar] [CrossRef]
- Lenucci, M.S.; De Caroli, M.; Marrese, P.P.; Iurlaro, A.; Rescio, L.; Böhm, V.; Dalessandro, G.; Piro, G. Enzyme-aided extraction of lycopene from high-pigment tomato cultivars by supercritical carbon dioxide. Food Chem. 2015, 170, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Kehili, M.; Kammlott, M.; Choura, S.; Zammel, A.; Zetzl, C.; Smirnova, I.; Allouche, N.; Sayadi, S. Supercritical CO2 extraction and antioxidant activity of lycopene and β-carotene-enriched oleoresin from tomato (Lycopersicum esculentum L.) peels by-product of a Tunisian industry. Food Bioprod. Process. 2017, 102, 340–349. [Google Scholar] [CrossRef]
- Urbonaviciene, D.; Viskelis, P. The cis-lycopene isomers composition in supercritical CO2 extracted tomato by-products. LWT—Food Sci. Technol. 2017, 85, 517–523. [Google Scholar] [CrossRef]
- Bruno, A.; Durante, M.; Marrese, P.P.; Migoni, D.; Laus, M.N.; Pace, E.; Pastore, D.; Mita, G.; Piro, G.; Lenucci, M.S. Shades of red: Comparative study on supercritical CO2 extraction of lycopene-rich oleoresins from gac, tomato and watermelon fruits and effect of the α-cyclodextrin clathrated extracts on cultured lung adenocarcinoma cells’ viability. J. Food Compos. Anal. 2018, 65, 23–32. [Google Scholar] [CrossRef]
- Vallecilla-Yepez, L.; Ciftci, O.N. Increasing cis-lycopene content of the oleoresin from tomato processing byproducts using supercritical carbon dioxide. LWT 2018, 95, 354–360. [Google Scholar] [CrossRef]
- Hatami, T.; Meireles, M.A.A.; Ciftci, O.N. Supercritical carbon dioxide extraction of lycopene from tomato processing by-products: Mathematical modeling and optimization. J. Food Eng. 2019, 241, 18–25. [Google Scholar] [CrossRef]
- Pellicanò, T.M.; Sicari, V.; Loizzo, M.R.; Leporini, M.; Falco, T.; Poiana, M. Optimizing the supercritical fluid extraction process of bioactive compounds from processed tomato skin by-products. Food Sci. Technol. 2020, 40, 692–697. [Google Scholar] [CrossRef]
- Scaglia, B.; D’Incecco, P.; Squillace, P.; Dell’Orto, M.; De Nisi, P.; Pellegrino, L.; Botto, A.; Cavicchi, C.; Adani, F. Development of a tomato pomace biorefinery based on a CO2-supercritical extraction process for the production of a high value lycopene product, bioenergy and digestate. J. Clean. Prod. 2020, 243, 118650. [Google Scholar] [CrossRef]
- Lee, K.; Rahman, M.S.; Kim, A.; Gul, K.; Lee, M.; Kim, J.I.; Ha, T.J.; Kwak, D.; Shin, E.; Kim, H.; et al. Supercritical fluid tomato extract for stabilization of perilla oil subjected to thermal treatment. J. Food Process. Preserv. 2020, 44, e14367. [Google Scholar] [CrossRef]
- Squillace, P.; Adani, F.; Scaglia, B. Supercritical CO2 extraction of tomato pomace: Evaluation of the solubility of lycopene in tomato oil as limiting factor of the process performance. Food Chem. 2020, 315, 126224. [Google Scholar] [CrossRef] [PubMed]
- Mihalcea, L.; Crăciunescu, O.; Gheonea (Dima), I.; Prelipcean, A.-M.; Enachi, E.; Barbu, V.; Bahrim, G.E.; Râpeanu, G.; Oancea, A.; Stănciuc, N. Supercritical CO2 Extraction and Microencapsulation of Lycopene-Enriched Oleoresins from Tomato Peels: Evidence on Antiproliferative and Cytocompatibility Activities. Antioxidants 2021, 10, 222. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Goupy, P.; Fröhlich, K.; Dangles, O.; Caris-Veyrat, C.; Böhm, V. Comparative study on antioxidant activity of lycopene (Z)-isomers in different assays. J. Agric. Food Chem. 2011, 59, 4504–4511. [Google Scholar] [CrossRef]
- Breinholt, V.; Lauridsen, S.T.; Daneshvar, B.; Jakobsen, J. Dose-response effects of lycopene on selected drug-metabolizing and antioxidant enzymes in the rat. Cancer Lett. 2000, 154, 201–210. [Google Scholar] [CrossRef]
- Hadley, C.W.; Clinton, S.K.; Schwartz, S.J. The consumption of processed tomato products enhances plasma lycopene concentrations in association with a reduced lipoprotein sensitivity to oxidative damage. J. Nutr. 2003, 133, 727–732. [Google Scholar] [CrossRef][Green Version]
- Ishida, B.K.; Bartley, G.E. Carotenoids: Chemistry, Sources, and Physiology. In Encyclopedia of Human Nutrition; Elsevier: Amsterdam, The Netherlands, 2005; pp. 330–338. [Google Scholar]
- Arab, L.; Steck, S. Lycopene and cardiovascular disease. Am. J. Clin. Nutr. 2000, 71, 1691S–1695S. [Google Scholar] [CrossRef] [PubMed]
- Clinton, S.K. Lycopene: Chemistry, Biology, and Implications for Human Health and Disease. Nutr. Rev. 2009, 56, 35–51. [Google Scholar] [CrossRef]
- Rao, A.V.; Agarwal, S. Role of lycopene as antioxidant carotenoid in the prevention of chronic diseases: A review. Nutr. Res. 1999, 19, 305–323. [Google Scholar] [CrossRef]
- Ahsan, H.; Ahad, A.; Iqbal, J.; Siddiqui, W.A. Pharmacological potential of tocotrienols: A review. Nutr. Metab. 2014, 11, 1–22. [Google Scholar] [CrossRef]
- Ju, J.; Picinich, S.C.; Yang, Z.; Zhao, Y.; Suh, N.; Kong, A.N.; Yang, C.S. Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis 2009, 31, 533–542. [Google Scholar] [CrossRef]
- Szewczyk, K.; Chojnacka, A.; Górnicka, M. Tocopherols and tocotrienols—bioactive dietary compounds; what is certain, what is doubt? Int. J. Mol. Sci. 2021, 22, 6222. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.; Jayaraman, S. An update on β-sitosterol: A potential herbal nutraceutical for diabetic management. Biomed. Pharmacother. 2020, 131, 110702. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Anticarcinogenic, cardioprotective, and other health benefits of tomato compounds lycopene, α-tomatine, and tomatidine in pure form and in fresh and processed tomatoes. J. Agric. Food Chem. 2013, 61, 9534–9550. [Google Scholar] [CrossRef] [PubMed]
- Kozukue, N.; Friedman, M. Tomatine, chlorophyll, β-carotene and lycopene content in tomatoes during growth and maturation. J. Sci. Food Agric. 2003, 83, 195–200. [Google Scholar] [CrossRef]
- Kozukue, N.; Han, J.S.; Lee, K.R.; Friedman, M. Dehydrotomatine and α-Tomatine Content in Tomato Fruits and Vegetative Plant Tissues. J. Agric. Food Chem. 2004, 52, 2079–2083. [Google Scholar] [CrossRef]
- Eller, F.J.; Moser, J.K.; Kenar, J.A.; Taylor, S.L. Extraction and Analysis of Tomato Seed Oil. J. Am. Oil Chem. Soc. 2010, 87, 755–762. [Google Scholar] [CrossRef]
- Krishna, J.G.; Chandrasekaran, M. Chandrasekaran Biochemical and Nutritional Aspects of Food Processing By-Products. In Valorization of Food Processing By-Products; Chandrasekaran, M., Ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781138199422. [Google Scholar]
- Johannsen, M.; Brunner, G. Solubilities of the Fat-Soluble Vitamins A, D, E, and K in Supercritical Carbon Dioxide. J. Chem. Eng. Data 1997, 42, 106–111. [Google Scholar] [CrossRef]
- Tonthubthimthong, P.; Chuaprasert, S.; Douglas, P.; Luewisutthichat, W. Supercritical CO2 extraction of nimbin from need seeds—An experimental study. J. Food Eng. 2001, 47, 289–293. [Google Scholar] [CrossRef]
- Gupta, R.B.; Shim, J.-J. Solubility in Supercritical Carbon Dioxide, 1st ed.; CRC Press: Boca Raton, FL, USA, 2006; ISBN 9780849342400. [Google Scholar]
- Brunner, G.; Peter, S. On the Solubility of Glycerides and Fatty Acids in Compressed Gases in the Presence of an Entrainer. Sep. Sci. Technol. 2006, 17, 199–214. [Google Scholar] [CrossRef]
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Robert McElroy, C.; Sherwood, J. Tools and techniques for solvent selection: Green solvent selection guides. Sustain. Chem. Process. 2016, 4, 7. [Google Scholar] [CrossRef]
- Sovová, H.; Stateva, R.P.; Galushko, A.A. Solubility of β-carotene in supercritical CO2 and the effect of entrainers. J. Supercrit. Fluids 2001, 21, 195–203. [Google Scholar] [CrossRef]
- Yeo, S.-D.; Park, S.-J.; Kim, J.-W.; Kim, J.-C. Critical Properties of Carbon Dioxide + Methanol, + Ethanol, + 1-Propanol, and + 1-Butanol. J. Chem. Eng. Data 2000, 45, 932–935. [Google Scholar] [CrossRef]
- Casas, L.; Mantell, C.; Rodríguez, M.; Torres, A.; Macías, F.A.; Martínez de la Ossa, E. Effect of the addition of cosolvent on the supercritical fluid extraction of bioactive compounds from Helianthus annuus L. J. Supercrit. Fluids 2007, 41, 43–49. [Google Scholar] [CrossRef]
- de Melo, M.M.R.; Oliveira, E.L.G.; Silvestre, A.J.D.; Silva, C.M. Supercritical Fluid Extraction of Triterpenic Acids from Eucalyptus globulus Bark. J. Supercrit. Fluids 2012, 70, 137–145. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aniceto, J.P.S.; Rodrigues, V.H.; Portugal, I.; Silva, C.M. Valorization of Tomato Residues by Supercritical Fluid Extraction. Processes 2022, 10, 28. https://doi.org/10.3390/pr10010028
Aniceto JPS, Rodrigues VH, Portugal I, Silva CM. Valorization of Tomato Residues by Supercritical Fluid Extraction. Processes. 2022; 10(1):28. https://doi.org/10.3390/pr10010028
Chicago/Turabian StyleAniceto, José P. S., Vítor H. Rodrigues, Inês Portugal, and Carlos M. Silva. 2022. "Valorization of Tomato Residues by Supercritical Fluid Extraction" Processes 10, no. 1: 28. https://doi.org/10.3390/pr10010028
APA StyleAniceto, J. P. S., Rodrigues, V. H., Portugal, I., & Silva, C. M. (2022). Valorization of Tomato Residues by Supercritical Fluid Extraction. Processes, 10(1), 28. https://doi.org/10.3390/pr10010028









