Predicting the Risk of Alzheimer’s Disease and Related Dementia in Patients with Mild Cognitive Impairment Using a Semi-Competing Risk Approach
Abstract
1. Introduction
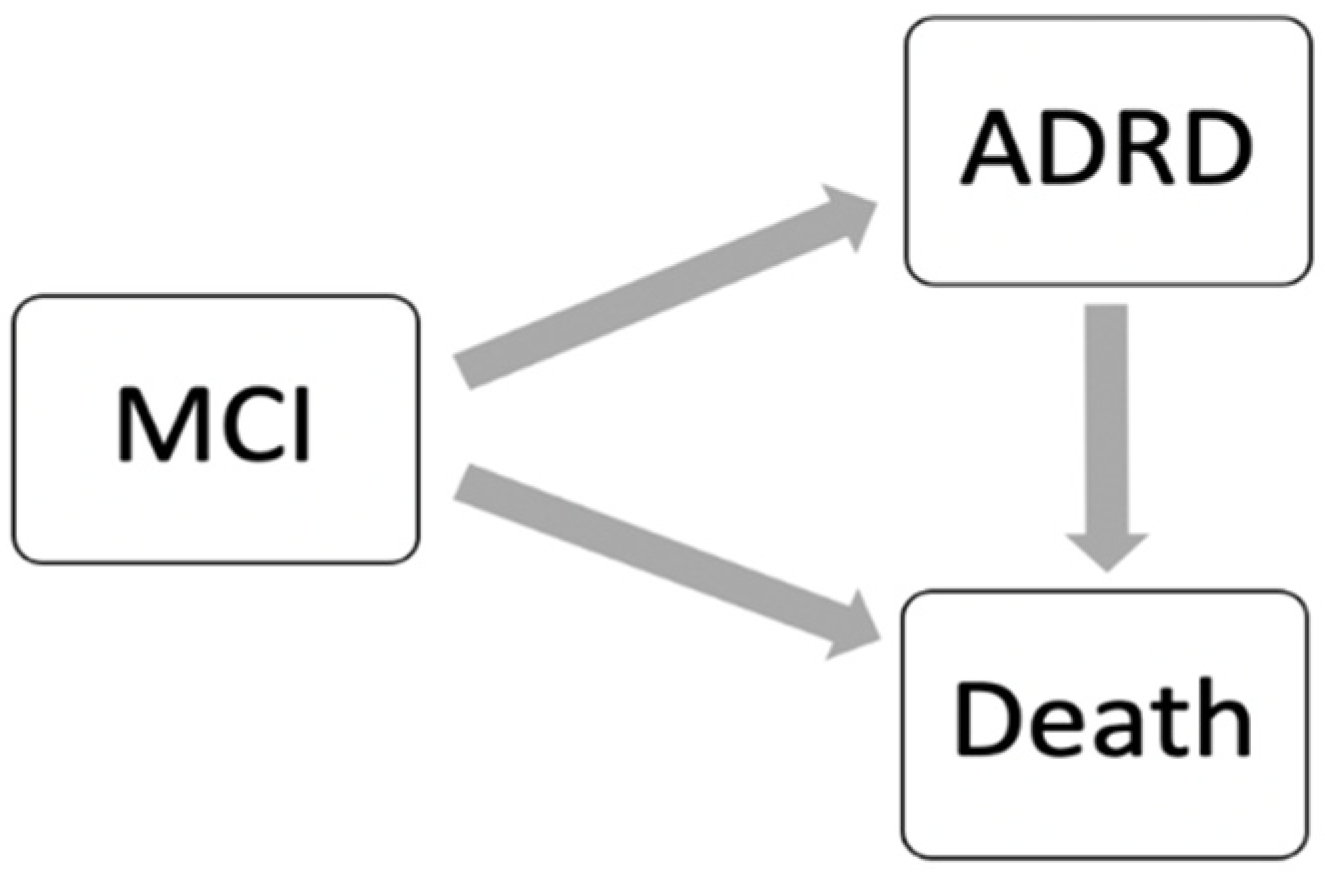
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alzheimer’s Association. 2022 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2022, 18, 700–789. [Google Scholar] [CrossRef]
- Wong, W. Economic Burden of Alzheimer Disease and Managed Care Considerations. Suppl. Featured Publ. 2020, 26, S177–S183. Available online: https://cdn.sanity.io/files/0vv8moc6/ajmc/732db911e3e68ecd4cdb59c9b2ed2556c903e975.pdf/AJMC_ACE0178_Alzheimer__Web.pdf (accessed on 20 April 2022).
- Lam, B.; Masellis, M.; Freedman, M.; Stuss, D.T.; Black, S.E. Clinical, imaging, and pathological heterogeneity of the Alzheimer’s disease syndrome. Alzheimer’s Res. Ther. 2013, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Goyal, D.; Tjandra, D.; Migrino, R.Q.; Giordani, B.; Syed, Z.; Wiens, J. Characterizing heterogeneity in the progression of Alzheimer’s disease using longitudinal clinical and neuroimaging biomarkers. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2018, 10, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Smith, G.E.; Waring, S.C.; Ivnik, R.J.; Tangalos, E.G.; Kokmen, E. Mild cognitive impairment: Clinical characterization and outcome. Arch. Neurol. 1999, 56, 303–308. [Google Scholar] [CrossRef]
- Tábuas-Pereira, M.; Baldeiras, I.; Duro, D.; Santiago, B.; Ribeiro, M.H.; Leitão, M.J.; Oliveira, C.; Santana, I. Prognosis of Early-Onset vs. Late-Onset Mild Cognitive Impairment: Comparison of Conversion Rates and Its Predictors. Geriatrics 2016, 1, 11. [Google Scholar] [CrossRef]
- Davis, M.; Connell, O.T.; Johnson, S.; Cline, S.; Merikle, E.; Martenyi, F.; Simpson, K. Estimating Alzheimer’s Disease Progression Rates from Normal Cognition Through Mild Cognitive Impairment and Stages of Dementia. Curr. Alzheimer Res. 2018, 15, 777–788. [Google Scholar] [CrossRef]
- Farias, S.T.; Mungas, D.; Reed, B.R.; Harvey, D.; DeCarli, C. Progression of mild cognitive impairment to dementia in clinic- vs community-based cohorts. Arch. Neurol. 2009, 66, 1151–1157. [Google Scholar] [CrossRef]
- Bozoki, A.; Giordani, B.; Heidebrink, J.L.; Berent, S.; Foster, N.L. Mild cognitive impairments predict dementia in nondemented elderly patients with memory loss. Arch. Neurol. 2001, 58, 411–416. [Google Scholar] [CrossRef]
- Plassman, B.L.; Langa, K.M.; Fisher, G.G.; Heeringa, S.G.; Weir, D.R.; Ofstedal, M.B.; Burke, J.R.; Hurd, M.D.; Potter, G.G.; Rodgers, W.L. Prevalence of cognitive impairment without dementia in the United States. Ann. Intern. Med. 2008, 148, 427–434. [Google Scholar] [CrossRef]
- Kumar, S.; Oh, I.; Schindler, S.; Lai, A.M.; Payne, P.R.O.; Gupta, A. Machine learning for modeling the progression of Alzheimer disease dementia using clinical data: A systematic literature review. JAMIA Open 2021, 4, ooab052. [Google Scholar] [CrossRef] [PubMed]
- Grueso, S.; Viejo-Sobera, R. Machine learning methods for predicting progression from mild cognitive impairment to Alzheimer’s disease dementia: A systematic review. Alzheimer’s Res. Ther. 2021, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Rowe, T.W.; Katzourou, I.K.; Stevenson-Hoare, J.O.; Bracher-Smith, M.R.; Ivanov, D.K.; Escott-Price, V. Machine learning for the life-time risk prediction of Alzheimer’s disease: A systematic review. Brain Commun. 2021, 3, fcab246. [Google Scholar] [CrossRef] [PubMed]
- Sherman, R.E.; Anderson, S.A.; Dal Pan, G.J.; Gray, G.W.; Gross, T.; Hunter, N.L.; LaVange, L.; Marinac-Dabic, D.; Marks, P.W.; Robb, M.A.; et al. Real-world evidence—What is it and what can it tell us? N. Engl. J. Med. 2016, 375, 2293–2297. [Google Scholar] [CrossRef]
- Rajkomar, A.; Oren, E.; Chen, K.; Dai, A.M.; Hajaj, N.; Hardt, M.; Liu, P.J.; Liu, X.; Marcus, J.; Sun, M. Scalable and accurate deep learning with electronic health records. NPJ Digit. Med. 2018, 1, 18. [Google Scholar] [CrossRef] [PubMed]
- Tomašev, N.; Glorot, X.; Rae, J.W.; Zielinski, M.; Askham, H.; Saraiva, A.; Mottram, A.; Meyer, C.; Ravuri, S.; Protsyuk, I.; et al. A clinically applicable approach to continuous prediction of future acute kidney injury. Nature 2019, 572, 116–119. [Google Scholar] [CrossRef]
- Xu, Z.; Chou, J.; Zhang, X.S.; Luo, Y.; Isakova, T.; Adekkanattu, P.; Ancker, J.S.; Jiang, G.; Kiefer, R.C.; Pacheco, J.A.; et al. Identifying sub-phenotypes of acute kidney injury using structured and unstructured electronic health record data with memory networks. J. Biomed. Inform. 2020, 102, 103361. [Google Scholar] [CrossRef]
- Zhang, X.; Chou, J.; Liang, J.; Xiao, C.; Zhao, Y.; Sarva, H.; Henchcliffe, C.; Wang, F. Data-Driven Subtyping of Parkinson’s Disease Using Longitudinal Clinical Records: A Cohort Study. Sci. Rep. 2019, 9, 797. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, F.; Hu, J.; Sorrentino, R. Towards personalized medicine: Leveraging patient similarity and drug similarity analytics. AMIA Summits Transl. Sci. Proc. 2014, 2014, 132–136. [Google Scholar]
- Wang, F.; Preininger, A. AI in Health: State of the Art, Challenges, and Future Directions. Yearb. Med. Inform. 2019, 28, 16–26. [Google Scholar] [CrossRef]
- Fine, J.P.; Gray, R.J. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- World Report on Ageing and Health. 2015. Available online: https://www.who.int/publications/i/item/9789241565042 (accessed on 21 April 2023).
- Christensen, K.; Doblhammer, G.; Rau, R.; Vaupel, J.W. Ageing populations: The challenges ahead. Lancet 2009, 374, 1196–1208. [Google Scholar] [CrossRef] [PubMed]
- Costantino, S.; Paneni, F.; Cosentino, F. Ageing, metabolism and cardiovascular disease. J. Physiol. 2016, 594, 2061–2073. [Google Scholar] [CrossRef]
- Fine, J.P.; Jiang, H.; Chappell, R. On semi-competing risks data. Biometrika 2001, 88, 907–919. [Google Scholar] [CrossRef]
- Hogan, W.R.; Shenkman, E.A.; Robinson, T.; Carasquillo, O.; Robinson, P.S.; Essner, R.Z.; Bian, J.; Lipori, G.; Harle, C.; Magoc, T. The OneFlorida Data Trust: A centralized, translational research data infrastructure of statewide scope. J. Am. Med. Inform. Assoc. 2021, 29, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Bian, J.; Loiacono, A.; Sura, A.; Mendoza Viramontes, T.; Lipori, G.; Guo, Y.; Shenkman, E.; Hogan, W. Implementing a hash-based privacy-preserving record linkage tool in the OneFlorida clinical research network. JAMIA Open 2019, 2, 562–569. [Google Scholar] [CrossRef]
- van der Flier, W.M.; Scheltens, P. Epidemiology and risk factors of dementia. J. Neurol. Neurosurg. Psychiatry 2005, 76 (Suppl. S5), v2–v7. [Google Scholar] [CrossRef]
- Azad, N.A.; Al Bugami, M.; Loy-English, I. Gender differences in dementia risk factors. Gend. Med. 2007, 4, 120–129. [Google Scholar] [CrossRef]
- Tariq, S.; Barber, P.A. Dementia risk and prevention by targeting modifiable vascular risk factors. J. Neurochem. 2018, 144, 565–581. [Google Scholar] [CrossRef]
- Lindsay, J.; Laurin, D.; Verreault, R.; Hébert, R.; Helliwell, B.; Hill, G.B.; McDowell, I. Risk factors for Alzheimer’s disease: A prospective analysis from the Canadian Study of Health and Aging. Am. J. Epidemiol. 2002, 156, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Imtiaz, B.; Tolppanen, A.M.; Kivipelto, M.; Soininen, H. Future directions in Alzheimer’s disease from risk factors to prevention. Biochem. Pharmacol. 2014, 88, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Kalbfleisch, J.D.; Tai, B. Statistical analysis of illness-death processes and semicompeting risks data. Biometrics 2010, 66, 716–725. [Google Scholar] [CrossRef]
- Lee, K.H.; Haneuse, S.; Schrag, D.; Dominici, F. Bayesian Semi-parametric Analysis of Semi-competing Risks Data: Investigating Hospital Readmission after a Pancreatic Cancer Diagnosis. J. R. Stat. Society Ser. C Appl. Stat. 2015, 64, 253–273. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Dominici, F.; Schrag, D.; Haneuse, S. Hierarchical models for semi-competing risks data with application to quality of end-of-life care for pancreatic cancer. J. Am. Stat. Assoc. 2016, 111, 1075–1095. [Google Scholar] [CrossRef]
- Alvares, D.; Haneuse, S.; Lee, C.; Lee, K.H. SemiCompRisks: An R Package for the Analysis of Independent and Cluster-correlated Semi-competing Risks Data. R J. 2019, 11, 376–400. [Google Scholar] [CrossRef]
- Baumgart, M.; Snyder, H.M.; Carrillo, M.C.; Fazio, S.; Kim, H.; Johns, H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimer’s Dement. 2015, 11, 718–726. [Google Scholar] [CrossRef]
- Iadecola, C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat. Rev. Neurosci. 2004, 5, 347–360. [Google Scholar] [CrossRef]
- Biessels, G.J.; Kappelle, L.J.; Utrecht Diabetic Encephalopathy Study Group. Increased risk of Alzheimer’s disease in Type II diabetes: Insulin resistance of the brain or insulin-induced amyloid pathology? Biochem. Soc. Trans. 2005, 33, 1041–1044. [Google Scholar] [CrossRef]
- Chatterjee, S.; Peters, S.A.; Woodward, M.; Mejia Arango, S.; Batty, G.D.; Beckett, N.; Beiser, A.; Borenstein, A.R.; Crane, P.K.; Haan, M.; et al. Type 2 Diabetes as a Risk Factor for Dementia in Women Compared With Men: A Pooled Analysis of 2.3 Million People Comprising More Than 100,000 Cases of Dementia. Diabetes Care 2016, 39, 300–307. [Google Scholar] [CrossRef]
- Baglietto-Vargas, D.; Shi, J.; Yaeger, D.M.; Ager, R.; LaFerla, F.M. Diabetes and Alzheimer’s disease crosstalk. Neurosci. Biobehav. Rev. 2016, 64, 272–287. [Google Scholar] [CrossRef] [PubMed]
- Vega, I.E.; Cabrera, L.Y.; Wygant, C.M.; Velez-Ortiz, D.; Counts, S.E. Alzheimer’s Disease in the Latino Community: Intersection of Genetics and Social Determinants of Health. J. Alzheimer’s Dis. 2017, 58, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Stickel, A.; McKinnon, A.; Ruiz, J.; Grilli, M.D.; Ryan, L. The impact of cardiovascular risk factors on cognition in Hispanics and non-Hispanic whites. Learn. Mem. 2019, 26, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.P.; Moeschberger, M.L. Survival Analysis; Springer: New York, NY, USA, 1997. [Google Scholar]
- Grambsch, P.M.; Therneau, T.M. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994, 81, 515–526. [Google Scholar] [CrossRef]
- Clayton, D.G. A model for association in bivariate life tables and its application in epidemiological studies of familial tendency in chronic disease incidence. Biometrika 1978, 65, 141–151. [Google Scholar] [CrossRef]
- Oakes, D. Semiparametric inference in a model for association in bivanate survival data. Biometrika 1986, 73, 353–361. [Google Scholar] [CrossRef]
- He, Z.; Bian, J.; Carretta, H.J.; Lee, J.; Hogan, W.R.; Shenkman, E.; Charness, N. Prevalence of multiple chronic conditions among older adults in Florida and the United States: Comparative analysis of the OneFlorida data trust and National Inpatient Sample. J. Med. Internet Res. 2018, 20, e137. [Google Scholar] [CrossRef]
- Dignam, J.J.; Zhang, Q.; Kocherginsky, M. The use and interpretation of competing risks regression models. Clin. Cancer Res. 2012, 18, 2301–2308. [Google Scholar] [CrossRef]
- Zhang, Z. Survival analysis in the presence of competing risks. Ann. Transl. Med. 2017, 5, 47. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, H.; Yang, X.; Wu, S.; He, X.; Xu, J.; Guo, J.; Prosperi, M.; Wang, F.; Xu, H.; et al. Assess the documentation of cognitive tests and biomarkers in electronic health records via natural language processing for Alzheimer’s disease and related dementias. Int. J. Med. Inform. 2023, 170, 104973. [Google Scholar] [CrossRef]


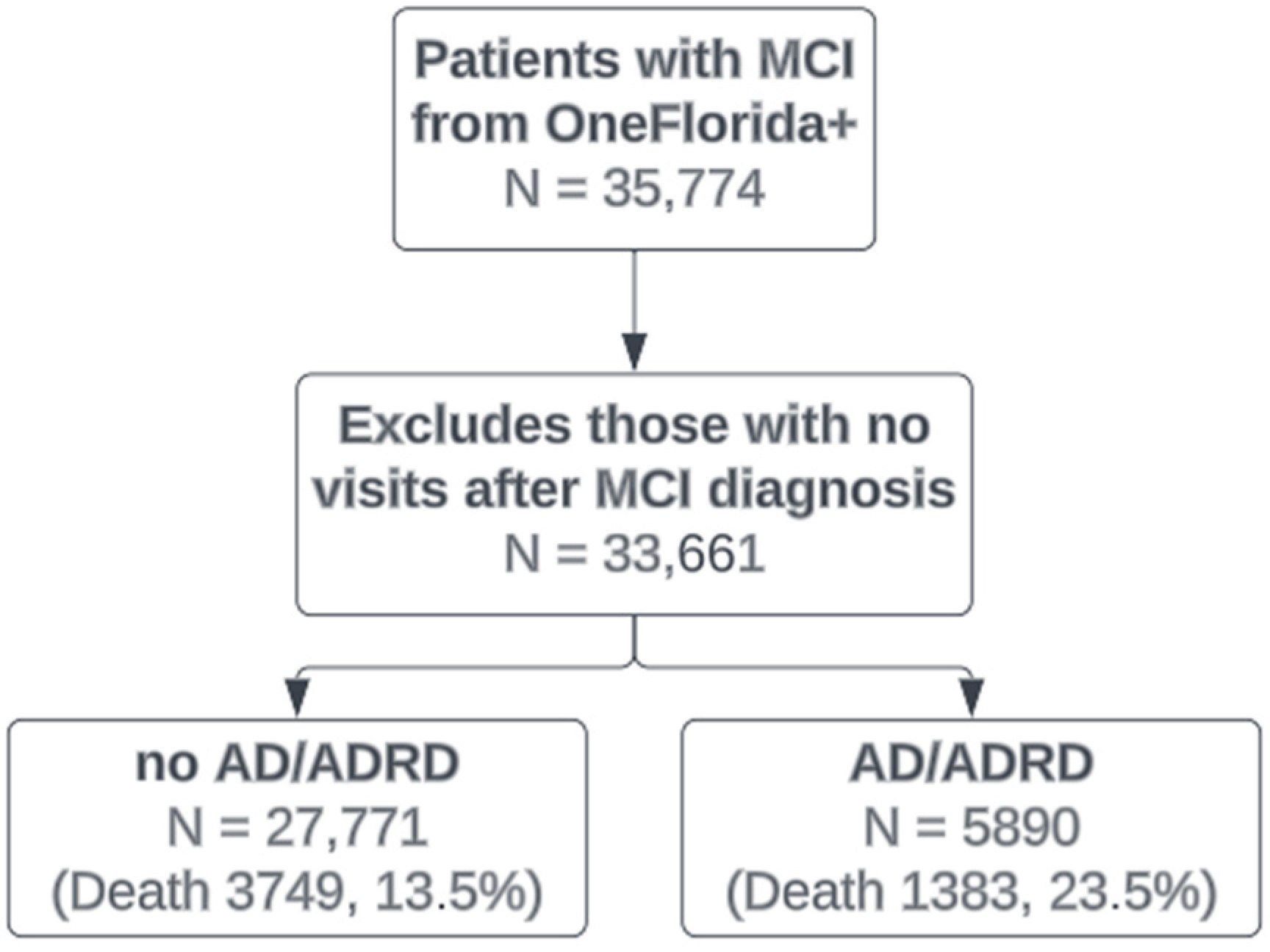
| No AD/ADRD | Developed AD/ADRD | |
|---|---|---|
| (N = 27,771) | (N = 5890) | |
| Sex | ||
| Female | 14,654 (52.8%) | 3538 (60.1%) |
| Male | 13,117 (47.2%) | 2352 (39.9%) |
| Race/ethnicity | ||
| Hispanic | 5065 (18.2%) | 1515 (25.7%) |
| NHB | 4328 (15.6%) | 776 (13.2%) |
| NHW | 12,008 (43.2%) | 2577 (43.8%) |
| Other | 1266 (4.6%) | 200 (3.4%) |
| Unknown | 5104 (18.4%) | 822 (14.0%) |
| Age | ||
| Mean (SD) | 59.4 (21.2) | 74.4 (12.2) |
| Smoking | ||
| Current smoker | 4007 (14.4%) | 569 (9.7%) |
| Former smoker | 4995 (18.0%) | 1103 (18.7%) |
| Never smoker | 3221 (11.6%) | 615 (10.4%) |
| Unknown | 15,548 (56.0%) | 3603 (61.2%) |
| BMI | ||
| Mean (SD) | 27.4 (6.72) | 26.9 (5.46) |
| Death | ||
| Mean (SD) | 3749 (13.5%) | 1383 (23.5%) |
| Anxiety | 9673 (34.8%) | 1895 (32.2%) |
| Apathy | 32 (0.1%) | 6 (0.1%) |
| Depression | 12,163 (43.8%) | 2653 (45.0%) |
| Hypertension | 17,907 (64.5%) | 4588 (77.9%) |
| Diabetes | 9115 (32.8%) | 2386 (40.5%) |
| Cerebrovascular diseases | 8088 (29.1%) | 2313 (39.3%) |
| Cardiovascular diseases | 22,025 (79.3%) | 5103 (86.6%) |
| Atrial fibrillation | 3266 (11.8%) | 982 (16.7%) |
| Hypercholesterolemia | 4214 (15.2%) | 1081 (18.4%) |
| Myocardial infarction | 2303 (8.3%) | 601 (10.2%) |
| Congestive heart failure | 4559 (16.4%) | 1212 (20.6%) |
| Peripheral vascular disease | 5280 (19.0%) | 1446 (24.6%) |
| Cerebrovascular disease | 6964 (25.1%) | 2047 (34.8%) |
| Chronic pulmonary disease | 8540 (30.8%) | 1825 (31.0%) |
| Rheumatic disease | 1263 (4.5%) | 241 (4.1%) |
| Peptic ulcer disease | 1060 (3.8%) | 234 (4.0%) |
| Mild liver disease | 3348 (12.1%) | 503 (8.5%) |
| Diabetes without chronic complication | 8170 (29.4%) | 2131 (36.2%) |
| Diabetes with chronic complication | 3608 (13.0%) | 936 (15.9%) |
| Hemiplegia or paraplegia | 2166 (7.8%) | 351 (6.0%) |
| Renal disease | 4363 (15.7%) | 1189 (20.2%) |
| Any malignancy | 3158 (11.4%) | 553 (9.4%) |
| Moderate or severe liver disease | 513 (1.8%) | 64 (1.1%) |
| Metastatic solid tumor | 750 (2.7%) | 81 (1.4%) |
| AIDS/HIV | 562 (2.0%) | 33 (0.6%) |
| Obesity | 7961 (28.7%) | 1235 (21.0%) |
| hyperlipidemia | 12,375 (44.6%) | 3134 (53.2%) |
| Stroke | 13,570 (48.9%) | 3376 (57.3%) |
| Traumatic brain injury | 6088 (21.9%) | 1881 (31.9%) |
| Sleep disorder | 3153 (11.4%) | 583 (9.9%) |
| Periodontitis | 6323 (22.8%) | 1177 (20.0%) |
| Alcohol use disorder | 225 (0.8%) | 32 (0.5%) |
| Exercise | 2554 (9.2%) | 383 (6.5%) |
| Visual impairment | 754 (2.7%) | 89 (1.5%) |
| Hearing impairment | 453 (1.6%) | 112 (1.9%) |
| Variable | Hazard Ratio (HR) | |
|---|---|---|
| Treating Death as Random Censoring | Considering Death as a Semi-Competing Risk | |
| Age | 1.054 (1.052, 1.057) * | 1.049 (1.047, 1.054) * |
| Sex (ref = Male) | 0.958 (0.907, 1.012) | 0.969 (0.908, 1.014) |
| Race/ethnicity (ref = NHW) | ||
| Hispanic | 1.257 (1.178, 1.341) * | 1.233 (1.154, 1.317) * |
| NHB | 1.040 (0.957, 1.113) | 1.014 (0.934, 1.109) |
| Other | 0.702 (0.606, 0.814) * | 0.721 (0.625, 0.827) * |
| Unknown | 0.826 (0.761, 0.896) * | 0.838 (0.774, 0.916) * |
| Anxiety | 1.027 (0.964, 1.093) | 0.965 (0.903, 1.016) |
| Depression | 1.205 (1.136, 1.278) * | 1.096 (1.027, 1.165) * |
| Hypertension | 1.071 (0.975, 1.177) | 1.047 (0.941, 1.139) |
| Diabetes | 1.146 (1.006, 1.305) * | 1.170 (1.034, 1.329) * |
| Cerebrovascular diseases | 1.187 (1.068, 1.322) * | 1.287 (1.158, 1.450) * |
| Cardiovascular diseases | 0.928 (0.829, 1.040) | 0.938 (0.850, 1.026) |
| Atrial fibrillation | 0.972 (0.901, 1.048) | 0.979 (0.906, 1.074) |
| Hypercholesterolemia | 0.987 (0.918, 1.061) | 1.000 (0.930, 1.087) |
| Myocardial infarction | 1.008 (0.919, 1.106) | 1.056 (0.966, 1.156) |
| Congestive heart failure | 0.995 (0.922, 1.074) | 0.941 (0.869, 1.012) |
| Peripheral vascular disease | 1.008 (0.941, 1.079) | 0.984 (0.923, 1.066) |
| Cerebrovascular disease | 1.044 (0.923, 1.182) | 0.909 (0.899, 1.020) |
| Chronic pulmonary disease | 0.980 (0.921, 1.044) | 0.950 (0.899, 1.020) |
| Rheumatic disease | 0.809 (0.710, 0.923) * | 0.817 (0.725, 0.938) * |
| Peptic ulcer disease | 1.045 (0.912. 1.204) | 1.045 (0.912. 1.204) |
| Mild liver disease | 0.875 (0.792, 0.967) * | 0.894 (0.804, 0.990) * |
| Diabetes without chronic complication | 0.974 (0.852, 1.113) | 0.935 (0.824, 1.060) |
| Diabetes with chronic complication | 1.012 (0.927, 1.104) | 0.995 (0.908, 1.084) |
| Hemiplegia or paraplegia | 1.041 (0.929, 1.166) | 1.022 (0.907, 1.143) |
| Renal disease | 1.096 (1.020, 1.178) * | 1.031 (0.907, 1.143) |
| Any malignancy | 0.815 (0.742, 0.894) * | 0.822 (0.748, 0.897) * |
| Moderate or severe liver disease | 0.985 (0.761, 1.275) | 0.963 (0.742, 0.897) * |
| Metastatic solid tumor | 0.865 (0.687, 1.090) | 0.894 (0.730, 1.138) |
| AIDS/HIV | 0.502 (0.356, 0.709) * | 0.471 (0.335, 0.660) * |
| Obesity | 0.811 (0.756, 0.871) * | 0.819 (0.763, 0.886) * |
| hyperlipidemia | 0.961 (0.900, 1.026) | 0.941 (0.866, 1.015) |
| Stroke | 1.084 (1.011, 1.162) * | 1.184 (1.085, 1.310) * |
| Traumatic brain injury | 1.110 (1.015, 1.214) * | 0.998 (0.891, 1.084) |
| Sleep disorder | 0.908 (0.847, 0.973) * | 0.897 (0.838, 0.964) * |
| Periodontitis | 1.095 (0.773, 1.555) | 1.195 (0.825, 1.675) |
| Alcohol use | 1.106 (0.988, 1.238) | 1.099 (0.973, 1.224) |
| Exercise | 0.995 (0.806, 1.229) | 1.035 (0.860, 1.345) |
| Visual impairment | 1.211 (1.002, 1.463) * | 1.128 (0.934, 1.329) |
| Hearing impairment | 0.861 (0.785, 0.944) * | 0.827 (0.752, 0.909) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Yang, Y.; Zhang, D.; Guo, J.; Guo, Y.; Hu, X.; Chen, Y.; Bian, J. Predicting the Risk of Alzheimer’s Disease and Related Dementia in Patients with Mild Cognitive Impairment Using a Semi-Competing Risk Approach. Informatics 2023, 10, 46. https://doi.org/10.3390/informatics10020046
Chen Z, Yang Y, Zhang D, Guo J, Guo Y, Hu X, Chen Y, Bian J. Predicting the Risk of Alzheimer’s Disease and Related Dementia in Patients with Mild Cognitive Impairment Using a Semi-Competing Risk Approach. Informatics. 2023; 10(2):46. https://doi.org/10.3390/informatics10020046
Chicago/Turabian StyleChen, Zhaoyi, Yuchen Yang, Dazheng Zhang, Jingchuan Guo, Yi Guo, Xia Hu, Yong Chen, and Jiang Bian. 2023. "Predicting the Risk of Alzheimer’s Disease and Related Dementia in Patients with Mild Cognitive Impairment Using a Semi-Competing Risk Approach" Informatics 10, no. 2: 46. https://doi.org/10.3390/informatics10020046
APA StyleChen, Z., Yang, Y., Zhang, D., Guo, J., Guo, Y., Hu, X., Chen, Y., & Bian, J. (2023). Predicting the Risk of Alzheimer’s Disease and Related Dementia in Patients with Mild Cognitive Impairment Using a Semi-Competing Risk Approach. Informatics, 10(2), 46. https://doi.org/10.3390/informatics10020046








