Stability Study of Baclofen in an Oral Powder Form Compounded for Pediatric Patients in Japan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Test Solution Preparation
2.2. Baclofen Powder Compounding
2.3. Drug Stability Study
2.3.1. Experimental Design
2.3.2. Instrumentation and Chromatographic Conditions
2.3.3. Assay for Known Baclofen Impurities (A and B)
2.3.4. Validation of Quantitative Analysis
Calibration Standards and Quality Control
Limit of Detection (LOD) and Lower Limit of Quantification (LLOQ)
Precision and Accuracy
2.4. Drug Release Study
2.5. Differential Scanning Calorimetry (DSC)
2.6. Powder X-ray Diffraction Analysis
2.7. Color Evaluation
3. Results
3.1. Liquid Chromatography Method and Validation
3.2. Stability Study
3.3. Impurity Study
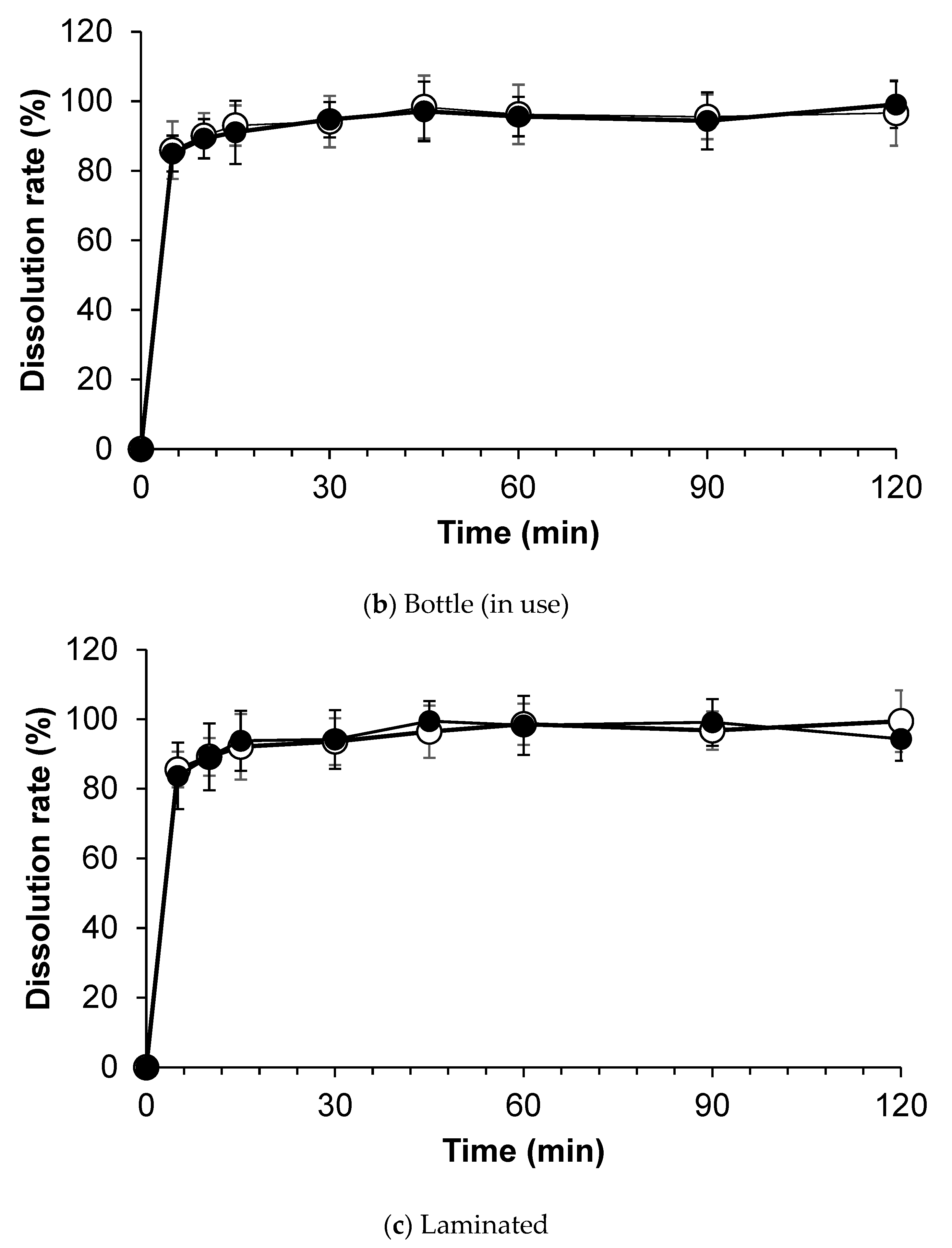
3.4. Dissolution Test
3.5. The Thermal Behavior of the Various Samples
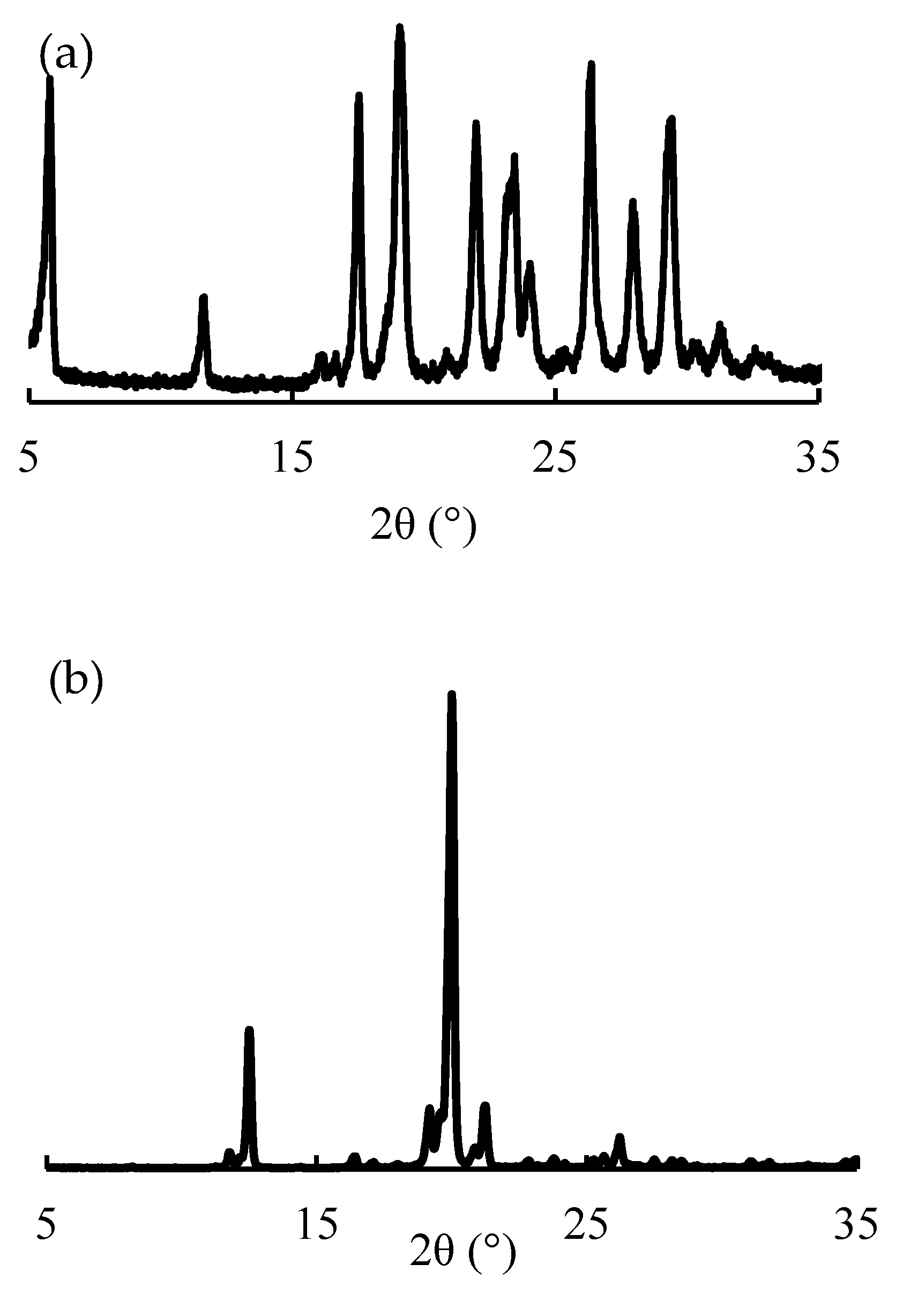
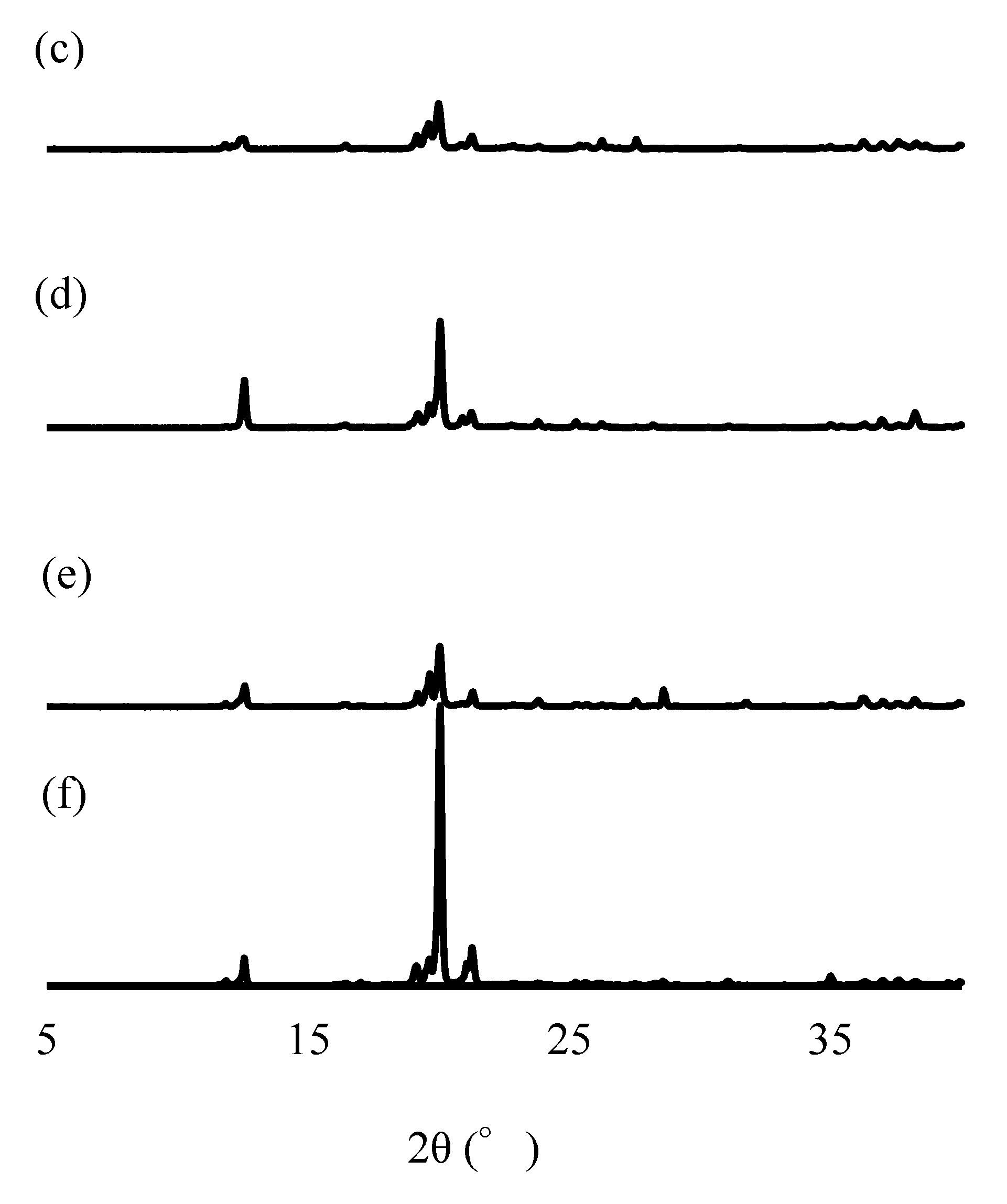
3.6. PXRD Analysis
3.7. Color Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taketomo, C.K.; Hodding, J.H.; Kraus, D.M. Pediatric Dosage Handbook, 13th ed.; Lexi-Comp: Hudson, OH, USA, 2006; pp. 395–396, 432–435. [Google Scholar]
- McLaughlin, M.J.; He, Y.; Brunstrom-Hernandez, J.; Thio, L.L.; Carleton, B.C.; Ross, C.J.D.; Gaedigk, A.; Lewandowski, A.; Dai, H.; Jusko, W.J.; et al. Pharmacogenomic variability of oral baclofen clearance and clinical response in children with cerebral palsy. PM&R 2018, 10, 235–243. [Google Scholar] [CrossRef]
- Krach, L.E. Pharmacotherapy of spasticity: Oral medications and intrathecal baclofen. J. Child Neurol. 2001, 16, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Verrotti, A.; Greco, R.; Spalice, A.; Chiarelli, F.; Iannetti, P. Pharmacotherapy of spasticity in children with cerebral palsy. Pediatr. Neurol. 2006, 34, 1–6. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.J.; Abdel-Rahman, S.; Leeder, J.S. Examining the role of precision medicine with oral baclofen in pediatric patients with cerebral palsy. Curr. Phys. Med. Rehabil. Rep. 2019, 7, 40–45. [Google Scholar] [CrossRef]
- McLaughlin, M.J.; Ratnasingam, D.; McGhee, E. Variability of steady state oral baclofen prescribing practices in pediatric patients with cerebral palsy. Am. J. Phys. Med. Rehabil. 2020, 99, 441–443. [Google Scholar] [CrossRef]
- Harvey, A.; Reddihough, D.; Scheinberg, A.; Williams, K. Oral medication prescription practices of tertiary-based specialists for dystonia in children with cerebral palsy. J. Paediatr Child Health 2018, 54, 401–404. [Google Scholar] [CrossRef]
- Gabalon® Tablets 10 mg [Prescribing Information]; Alfresa Pharma Co., Ltd.: Osaka, Japan, 2020.
- He, Y.; Brunstrom-Hernandez, J.E.; Thio, L.L.; Lackey, S.; Gaebler-Spira, D.; Kuroda, M.M.; Stashinko, E.; Hoon, A.H.; Vargus-Adams, J.; Stevenson, R.D.; et al. Population pharmacokinetics of oral baclofen in pediatric patients with cerebral palsy. J. Pediatr. 2014, 164, 1181–1188.e8. [Google Scholar] [CrossRef]
- Moran, L.R.; Cincotta, T.; Krishnamoorthy, K.; Insoft, R.M. The use of baclofen in full-term neonates with hypertonia. J. Perinatol. 2005, 25, 66–68. [Google Scholar] [CrossRef]
- Schulz, E.; Mathew, O.P. Is oral baclofen effective in neonatal hypertonia? J. Child Neurol. 2012, 27, 197–199. [Google Scholar] [CrossRef]
- Guglani, L.; Lodha, R. Enteral baclofen in the management of tetanus-related spasms: Case report and review of literature. J. Trop. Pediatr. 2007, 53, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, I.; Hussain, A. Intrathecal baclofen withdrawal syndrome-a life-threatening complication of baclofen pump: A case report. BMC Clin. Pharmacol. 2004, 4, 6. [Google Scholar] [CrossRef]
- Saito, J.; Akabane, M.; Ishikawa, Y.; Iwahashi, K.; Nakamura, H.; Yamatani, A. Retrospective survey of compounded medications for children in Japan. Eur. J. Pharm. Biopharm. 2020, 155, 122–127. [Google Scholar] [CrossRef] [PubMed]
- ASHP Compounded Oral Liquid Version 1.01 Finalized List July 2017. Available online: https://www.ashp.org/-/media/assets/pharmacy-practice/s4s/docs/s4s-ashp-oral-compound-liquids.ashx (accessed on 10 July 2022).
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use [ICH]. Q1A-Q1F Stability. Available online: https://www.ich.org/page/quality-guidelines (accessed on 10 August 2022).
- Elagawany, M.; Farid, N.F.; Elgendy, B.; Abdelmomen, E.H.; Abdelwahab, N.S. Baclofen impurities: Facile synthesis and novel environmentally benign chromatographic method for their simultaneous determination in baclofen. Biomed. Chromatogr. 2019, 33, e4579. [Google Scholar] [CrossRef] [PubMed]
- The Ministry of Health, Labour and Welfare, The Japanese Pharmacopoeia Seventeenth Edition. 2016. Available online: https://www.pmda.go.jp/files/000217650.pdf (accessed on 10 July 2022).
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use [ICH]. Stability Testing of Active Pharmaceutical Ingredients and Finished Pharmaceutical Products. 2009. Available online: https://database.ich.org/sites/default/files/Q1F_Stability_Guideline_WHO_2018.pdf (accessed on 10 July 2022).
- European Medicines Agency. Note for Guidance on in-Use Stability Testing of Human Medicinal Products. 2001. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/note-guidance-use-stability-testing-human-medicinal-products_en.pdf (accessed on 10 July 2022).
- Allen, L.V. Chapter 2: Compounding Ingredient Considerations. In The Art, Science, and Technology of Pharmaceutical Compounding, 6th ed.; The American Pharmacists Association: Washington, DC, USA, 2020. [Google Scholar] [CrossRef]
- Suntornsuk, L.; Ployngam, S. Simultaneous determination of R-(−)-, S-(+)-baclofen and impurity A by electrokinetic chromatography. J. Pharm. Biomed. Anal. 2010, 51, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Shibata, H.; Izutsu, K.I.; Goda, Y. Comparison of Dissolution Similarity Assessment Methods for Products with Large Variations: F2 Statistics and Model-Independent Multivariate Confidence Region Procedure for Dissolution Profiles of Multiple Oral Products. Biol. Pharm. Bull. 2017, 40, 722–725. [Google Scholar] [CrossRef]
- Abbas, D.; Kalousian, J.; Orneto, C.; Piccerelle, P.; Portugal, H.; Nicolay, A. DSC and physico-chemical properties of substrtuted pyridoquinoline and its interaction study with excipients. J. Ther. Anal. Color. 2008, 92, 353–360. [Google Scholar] [CrossRef]
- Berlin, E.; Kliman, G.P.; Anderson, A.B.; Pallansch, J.M. Calorimetric measurement of the heat of desorption of water vapor from amorphous and crystalline lactose. Thermochim. Acta 1971, 2, 143–152. [Google Scholar] [CrossRef]
- Alzoubi, T.; Martin, P.G.; Barlow, J.D.; Royall, G.P. Stability of a-lactose monohydrate: The discovery of dehydration triggered solid-state epimerization. Int. J. Pharmaceut. 2021, 604, 120715. [Google Scholar] [CrossRef]
- Couvrat, N.; Sanselme, M.; Poupard, M.; Bensakoun, C.; Drouin, H.S.; Schneider, J.M.; Coquerel, G. Solid-state overview of R-Baclofen: Relative stability of forms A, B and C and characterization of a new heterosolvate. J. Pharm. Sci. 2021, 110, 3457–3463. [Google Scholar] [CrossRef]
- Gerrard, S.E.; Walsh, J.; Bowers, N.; Salunke, S.; Hershenson, S. Innovations in pediatric drug formulations and administration technologies for low resource settings. Pharmaceutics 2019, 11, 518. [Google Scholar] [CrossRef]
- Gadge, P.M.; Kenjale, P.P.; Pokharkar, V.B.; Gaikwad, V.L. Global pediatric regulations: An overview. Ther. Innov. Regul. Sci. 2019, 54, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Hepburn, C.M.; Gilpin, A.; Autmizguine, J.; Denburg, A.; Dupuis, L.L.; Finkelstein, Y.; Gruenwoldt, E.; Ito, S.; Jong, G.; Lacaze-Masmonteil, T.; et al. Improving paediatric medications: A prescription for Canadian children and youth. Paediatr. Child Health 2019, 24, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Ivanovska, V.; Rademaker, C.M.; van Dijk, L.; Mantel-Teeuwisse, A.K. Pediatric drug formulations: A review of challenges and progress. Pediatrics 2014, 134, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, H.; Salunke, S.; Tuleu, C. Formulating better medicines for children-reflections. Int. J. Pharm. 2015, 492, 301–303. [Google Scholar] [CrossRef]
- Conroy, S. Extemporaneous (magistral) preparation of oral medicines for children in European hospitals. Acta Paediatr. 2003, 92, 408–410. [Google Scholar] [CrossRef]
- Rood, J.M.; Engels, M.J.; Ciarkowski, S.L.; Wagenknecht, L.D.; Dickinson, C.J.; Stevenson, J.G. Variability in compounding of oral liquids for pediatric patients: A patient safety concern. J. Am. Pharm. Assoc. 2014, 54, 383–389. [Google Scholar] [CrossRef]
- Polonini, H.; da Silva, S.L.; Brandão, M.A.F.; Bauters, T.; de Moerloose, B.; Ferreira, A.O. Compatibility of baclofen, carvedilol, hydrochlorothiazide, mercaptopurine, methadone hydrochloride, oseltamivir phosphate, phenobarbital, propranolol hydrochloride, pyrazinamide, sotalol hydrochloride, spironolactone, tacrolimus monohydrate, ursodeoxycholic acid, and vancomycin hydrochloride oral suspensions compounded with SyrSpend SF pH4. Int. J. Pharm. Compd. 2018, 22, 516–526. [Google Scholar]
- Johnson, C.E.; Hart, S.M. Stability of an extemporaneously compounded baclofen oral liquid. Am. J. Hosp. Pharm. 1993, 50, 2353–2355. [Google Scholar] [CrossRef]
- Allen, L.V., Jr.; Erickson, M.A., 3rd. Stability of baclofen, captopril, diltiazem hydrochloride, dipyridamole, and flecainide acetate in extemporaneously compounded oral liquids. Am. J. Health Syst. Pharm. 1996, 53, 2179–2184. [Google Scholar] [CrossRef]
- Committee on Medical Products for Human Use (CHMP) and Paediatric Committee (PDCO), Guideline on the Pharmaceutical Development of Medicines for Paediatric Use. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-pharmaceutical-development-medicines-paediatric-use_en.pdf (accessed on 10 July 2022).
- FDA, Pediatric Study Plans: Content of and Process for Submitting Initial Pediatric Study Plans and Amended Initial Pediatric Study Plans, Guidance for Industry. 2013. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/pediatric-study-plans-content-and-process-submitting-initial-pediatric-study-plans-and-amended (accessed on 10 July 2022).
- Jackson, M.; Lowey, A. Handbook of Extemporaneous Formulation: A Guide to Pharmaceutical Compounding, 1st ed.; Pharmaceutical Pr.: London, UK, 2010; pp. 1–480. [Google Scholar]
- EMA, European Medicines Agency Gives Second Positive Opinion for A Paediatric-Use Marketing Authorization. 2014. Available online: https://www.ema.europa.eu/en/documents/press-release/european-medicines-agency-gives-second-positive-opinion-paediatric-use-marketing-authorisation_en.pdf (accessed on 10 July 2022).
- The Hospital for Sick Children (SickKids), Online Database. 2014. Available online: http://www.sickkids.ca/Pharmacy/Compounding-Service/index.html (accessed on 10 July 2022).
- EMA, European Directorate for the Quality of Medicines and HealthCare (EDQM) of the Council of Europe. Available online: https://www.ema.europa.eu/en/partners-networks/international-activities/multilateral-organisations-initiatives/european-directorate-quality-medicines-healthcare-edqm-council-europe (accessed on 10 July 2022).






| Study Methods | Storage Conditions | Storage Container | Test Periods (Days) | ||||
|---|---|---|---|---|---|---|---|
| 0 | 30 | 60 | 90 | 120 | |||
| Baclofen Concentrations * | |||||||
| Bottle (closed) | 25 °C ± 2 °C/60% ± 5% relative humidity | Amber/PC bottle | 100.0% | 100.2 ± 3.2% | 99.8 ± 2.8% | 99.0 ± 1.6% | 99.6 ± 2.7% |
| Bottle (in use) | Amber/PC bottle | 100.0% | 99.9 ± 2.9% | 98.9 ± 4.1% | 99.1 ± 2.5% | 98.7 ± 3.1% | |
| Laminated paper | Amber/CP laminated paper | 100.0% | 101.0 ± 2.2% | 99.7 ± 1.3% | 100.3 ± 2.7% | 99.6 ± 1.2% | |
| Storage Periods | Color Difference (ΔEab) |
|---|---|
| 0 days | 16.75 ± 0.102 |
| 60 days | 16.72 ± 0.208 |
| 90 days | 18.26 ± 0.046 |
| 120 days | 16.29 ± 0.128 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, J.; Hanawa, T.; Ozawa, A.; Matsumoto, T.; Yoshikawa, N.; Harada, T.; Iwahashi, K.; Yamatani, A. Stability Study of Baclofen in an Oral Powder Form Compounded for Pediatric Patients in Japan. Children 2022, 9, 1313. https://doi.org/10.3390/children9091313
Saito J, Hanawa T, Ozawa A, Matsumoto T, Yoshikawa N, Harada T, Iwahashi K, Yamatani A. Stability Study of Baclofen in an Oral Powder Form Compounded for Pediatric Patients in Japan. Children. 2022; 9(9):1313. https://doi.org/10.3390/children9091313
Chicago/Turabian StyleSaito, Jumpei, Takehisa Hanawa, Ayuna Ozawa, Takahiro Matsumoto, Nozomi Yoshikawa, Tsutomu Harada, Kana Iwahashi, and Akimasa Yamatani. 2022. "Stability Study of Baclofen in an Oral Powder Form Compounded for Pediatric Patients in Japan" Children 9, no. 9: 1313. https://doi.org/10.3390/children9091313
APA StyleSaito, J., Hanawa, T., Ozawa, A., Matsumoto, T., Yoshikawa, N., Harada, T., Iwahashi, K., & Yamatani, A. (2022). Stability Study of Baclofen in an Oral Powder Form Compounded for Pediatric Patients in Japan. Children, 9(9), 1313. https://doi.org/10.3390/children9091313







