Investigation on the Usefulness of Sulfamethoxazole Trimethoprim Combination Small Tablets in Pediatric Pharmacotherapy: A Single Center Observational Study Using a Questionnaire
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting and Participants
2.2. Research Procedures
2.3. Questionnaire Structure and Evaluation
2.4. Sample Size and Statistical Analysis
2.5. Data Handling
3. Results
3.1. Patients’ Background and Dosing Status
3.2. Administration Status
3.3. Patient’s Reactions before and after Switching to sTab
3.4. Dosing Methods
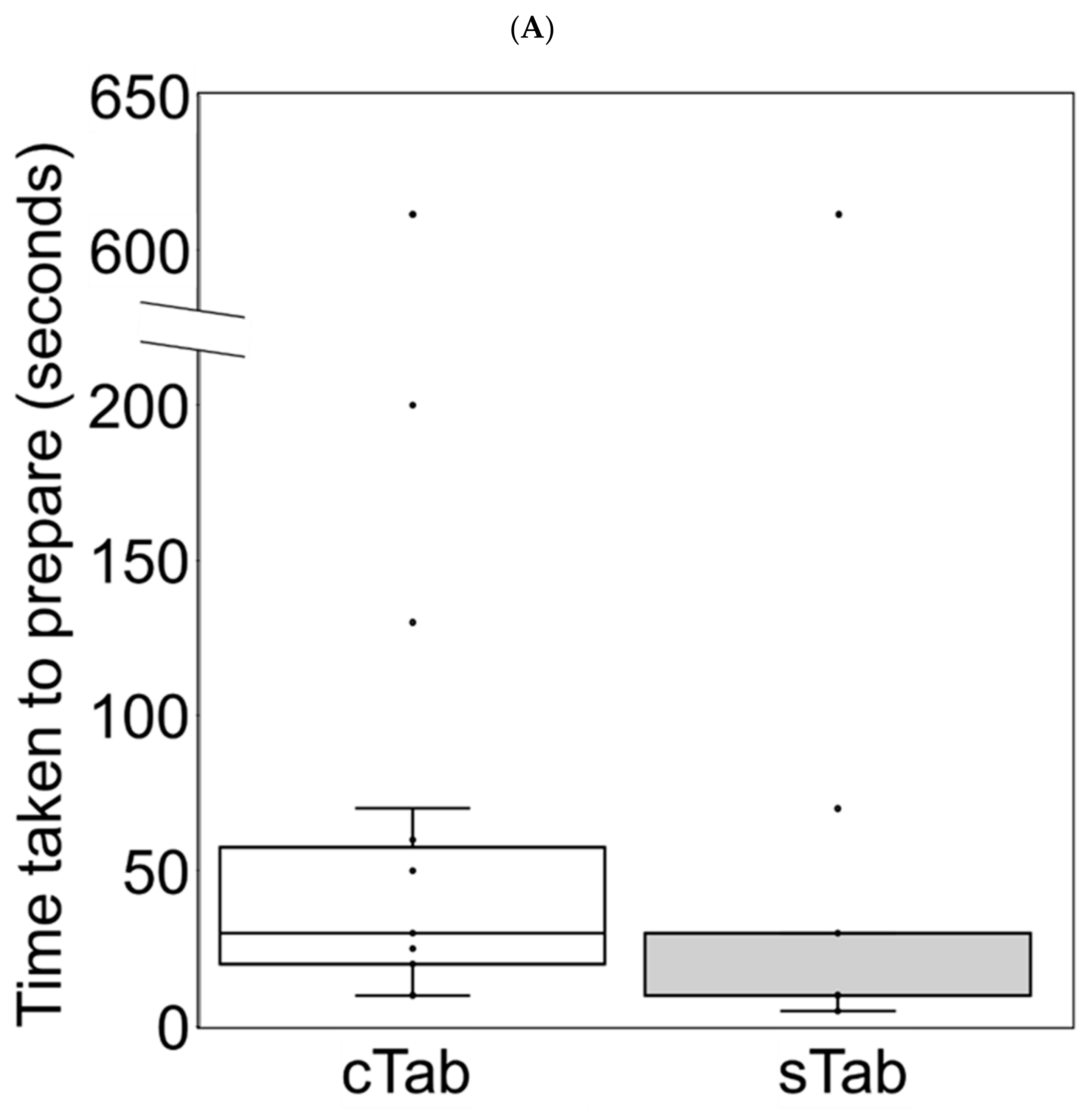
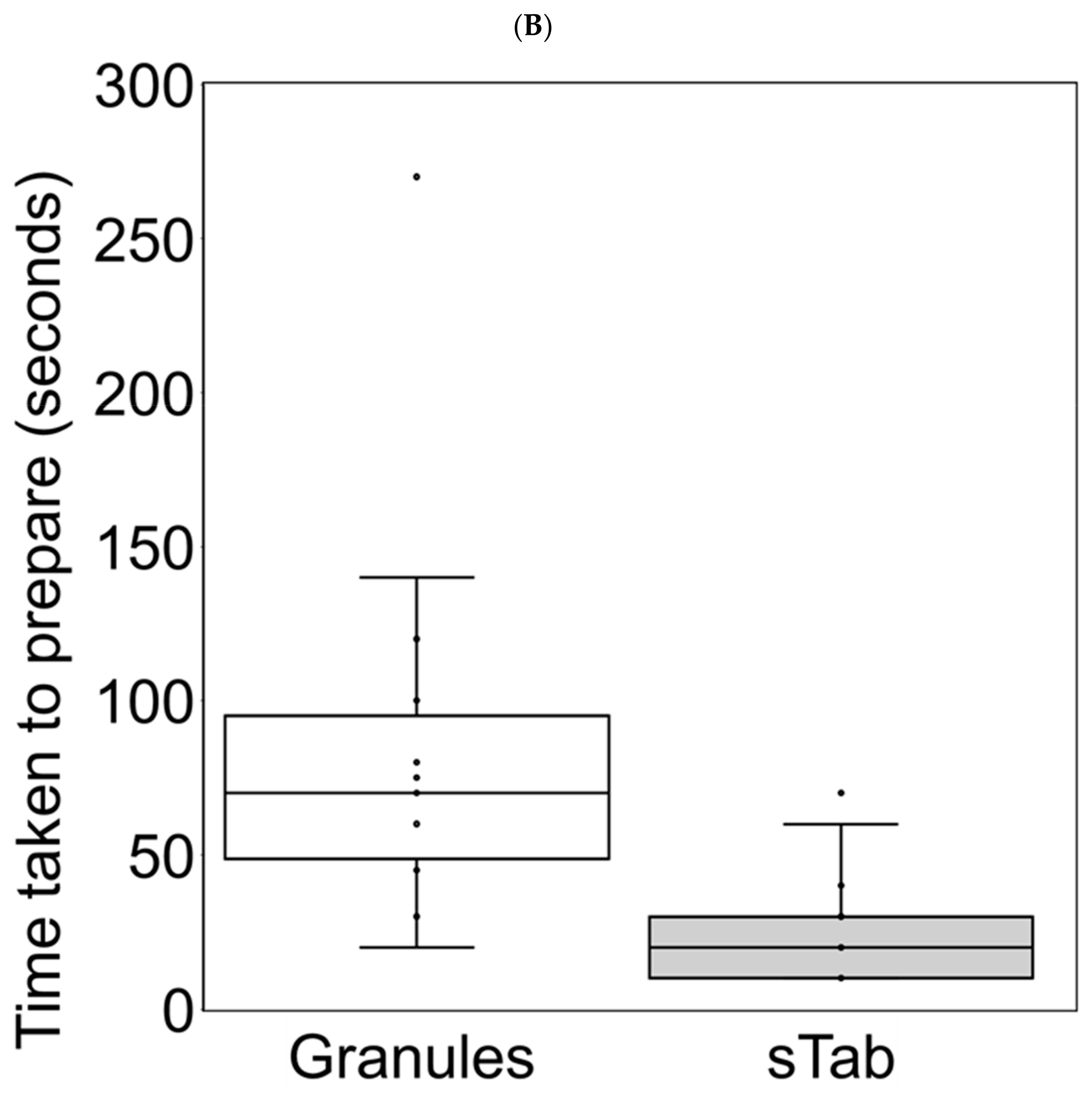
3.5. Changes in Preparation Time
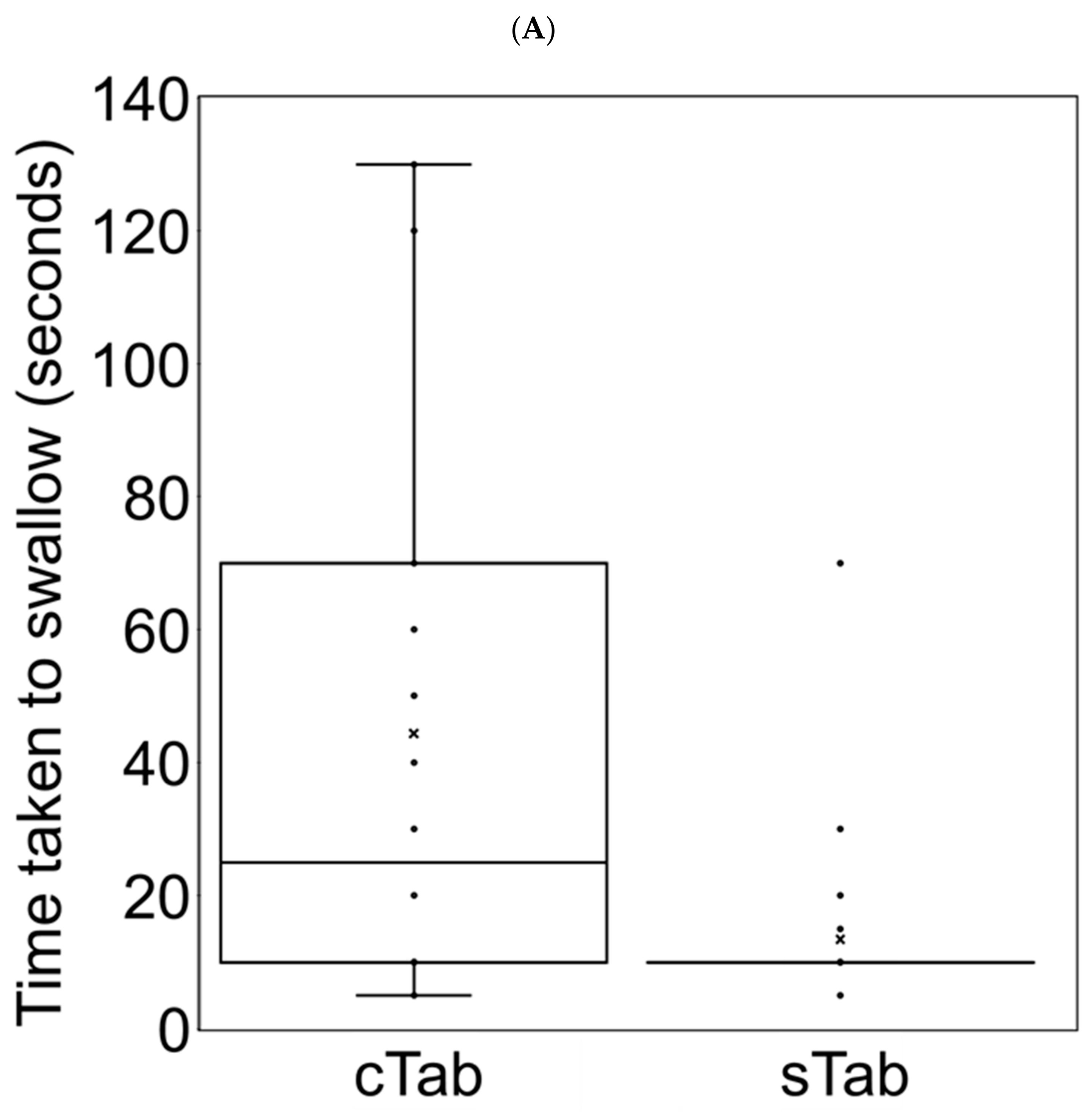
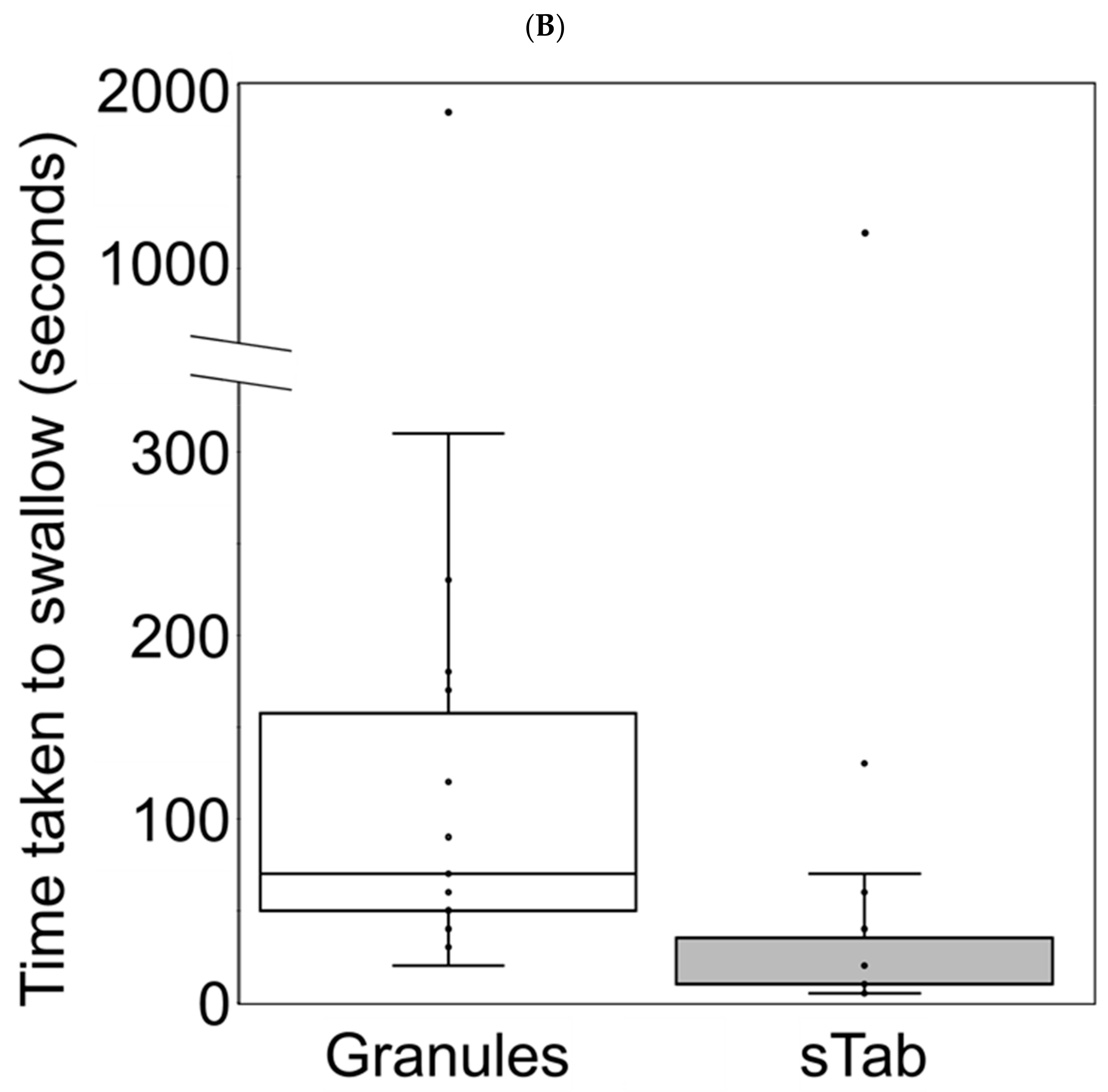
3.6. Changes in Administration Time
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EMEA/CHMP/PEG/194810/2005; Committee for Medicinal Products for Human Use (CHMP) EMA Reflection Paper: Formulations of Choice for the Paediatric Population. European Medicines Agency EMA: London, UK, 2006.
- Fishman, J.A.; Gans, H. AST Infectious Diseases Community of Practice. Pneumocystis jiroveci in solid organ transplantation: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13587. [Google Scholar] [CrossRef] [PubMed]
- Ohata, Y.; Ohta, H.; Hashii, Y.; Tokimasa, S.; Ozono, K.; Hara, J. Intermittent oral trimethoprim/sulfamethoxazole on two non-consecutive days per week is effective as Pneumocystis jiroveci pneumonia prophylaxis in pediatric patients receiving chemotherapy or hematopoietic stem cell transplantation. Pediatr. Blood Cancer 2009, 52, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Takafuji, Y.; Saito, J.; Akabane, M.; Komura, M.; Yamatani, A.; Ishikawa, Y. A questionnaire on the acceptability of Sulfamethoxazole/Trimethoprim oral dosage form. Jpn. J. Dev. Pharmacol. Ther. 2019, 32, 114–119. [Google Scholar]
- Product Information, BAKTAR Mini Combination Tablets; Shionogai Pharma Co., Ltd.: Osaka, Japan, 2021. Available online: https://www.info.pmda.go.jp/go/pack/6290100D1088_2_05 (accessed on 4 October 2022).
- Lopez, F.L.; Bowles, A.; Gul, M.O.; Clapham, D.; Ernest, T.B.; Tuleu, C. Effect of formulation variables on oral grittiness and preferences of multiparticulate formulations in adult volunteers. Eur. J. Pharm. Sci. 2016, 92, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Uchida, S.; Kanada, K.; Namiki, N. Effect of granule properties on rough mouth feel and palatability of orally disintegrating tablets. Int. J. Pharm. 2015, 484, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.S.; Parker, R.M.; Sanders, L.M.; Dreyer, B.P.; Mendelsohn, A.L.; Bailey, S.; Patel, D.A.; Jimenez, J.J.; Kim, K.A.; Jacobson, K.; et al. Liquid Medication Errors and Dosing Tools: A Randomized Controlled Experiment. Pediatrics 2016, 138, e20160357. [Google Scholar] [CrossRef]
- Rouaz, K.; Chiclana-Rodríguez, B.; Nardi-Ricart, A.; Suñé-Pou, M.; Mercadé-Frutos, D.; Suñé-Negre, J.M.; Pérez-Lozano, P.; García-Montoya, E. Excipients in the Paediatric Population: A Review. Pharmaceutics 2021, 13, 387. [Google Scholar] [CrossRef] [PubMed]
- Orlu, M.; Ranmal, S.R.; Sheng, Y.; Tuleu, C.; Seddon, P. Acceptability of orodispersible films for delivery of medicines to infants and preschool children. Drug Deliv. 2017, 24, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Rabel, S.; Bukhtar, Q.; Qadir, M.I.; Jabeen, F.; Khan, A. Orally disintegrating films: A modern expansion in drug delivery system. Saudi Pharm. J. SPJ 2016, 24, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Lee, J.J.; Kim, M.G.; Kim, K.T.; Cho, C.W.; Kim, D.D.; Lee, J.Y. Sprinkle formulations-A review of commercially available products. Asian J. Pharm. Sci. 2020, 15, 292–310. [Google Scholar] [CrossRef] [PubMed]
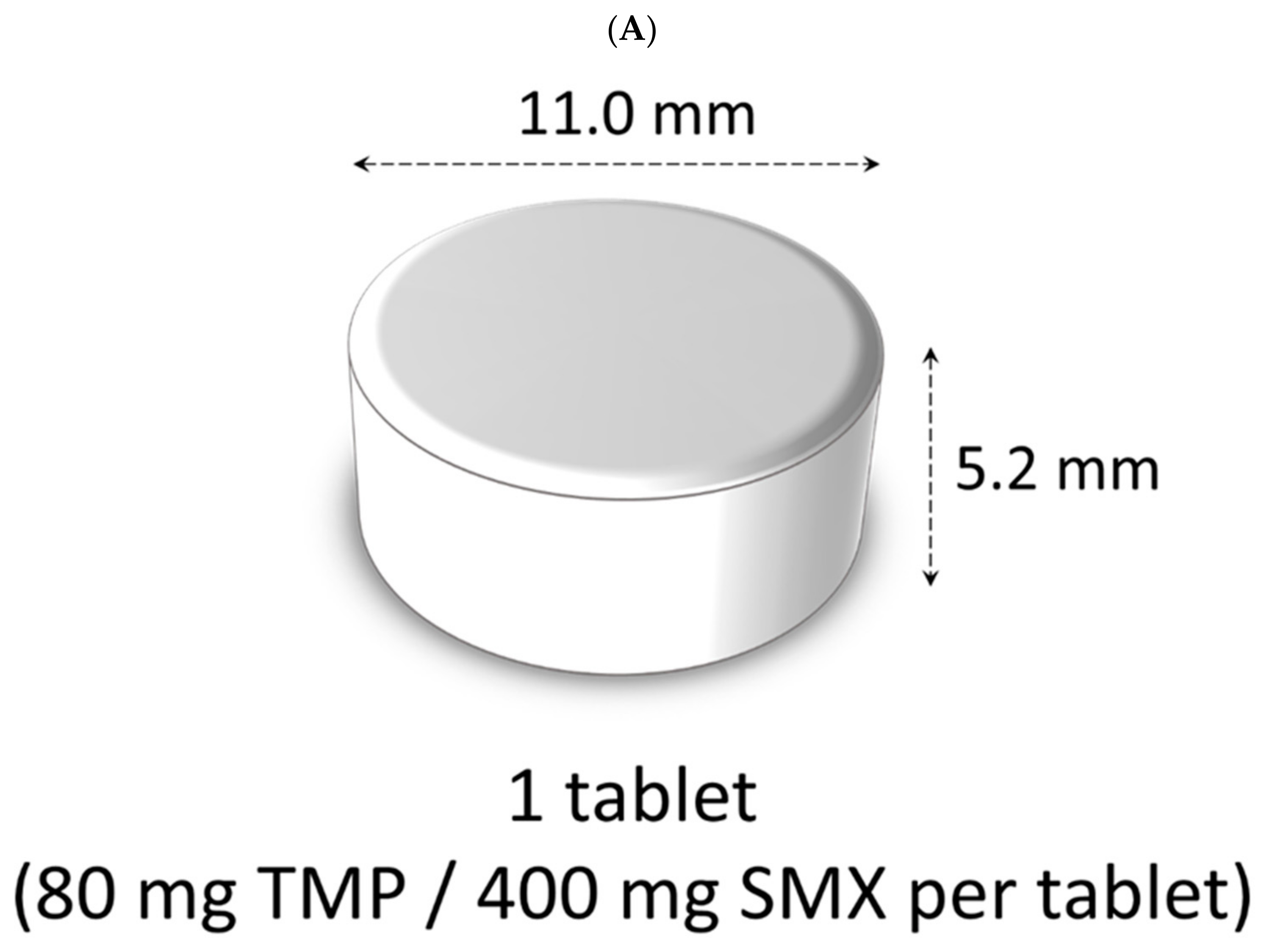
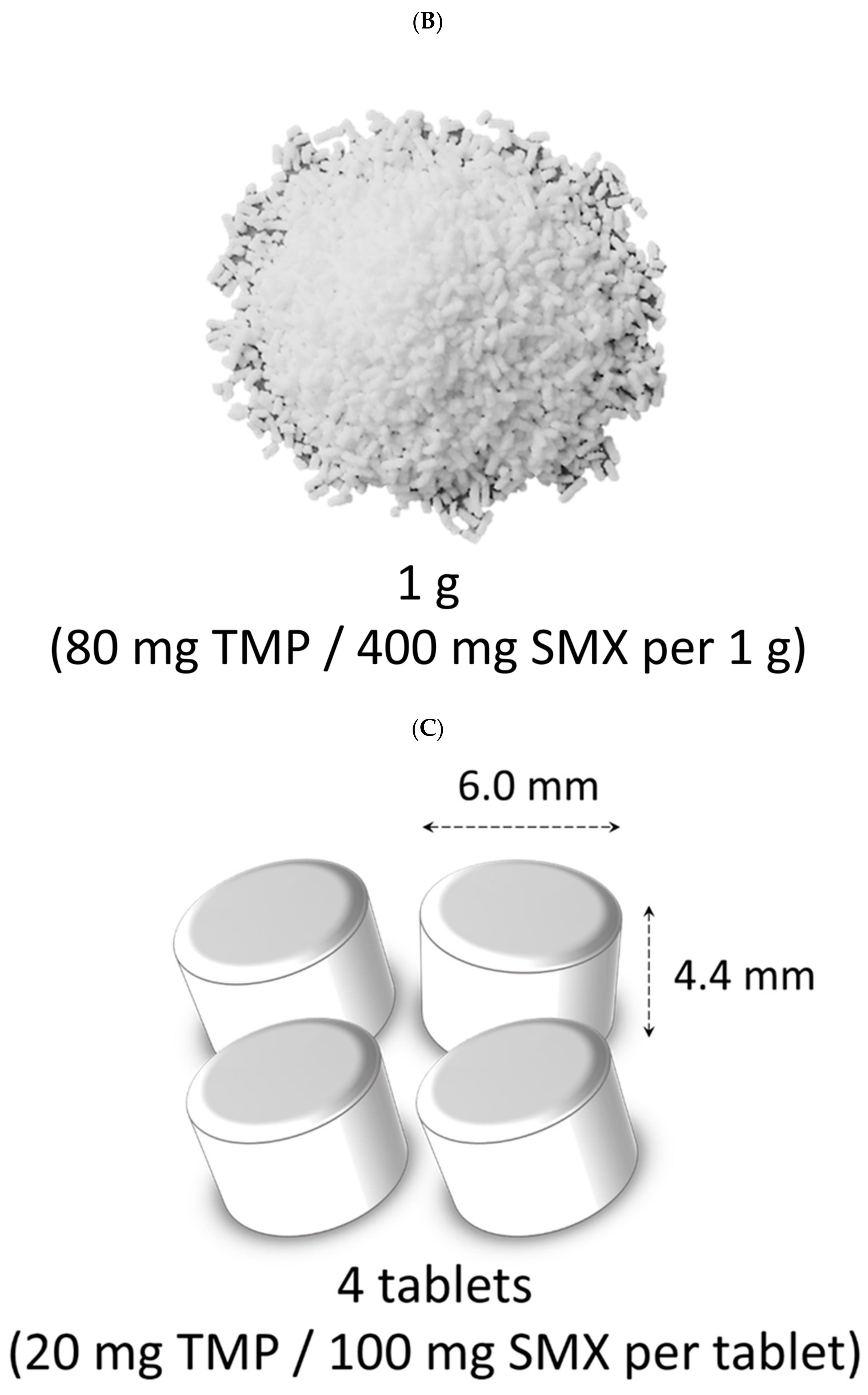
| 1. I agree to participate in this survey | ||
| □ Yes | ||
| □ No | ||
| 2. The observer in charge of the reports: | ||
| □ A caregiver | ||
| □ A healthcare professional | ||
| □ A patient | ||
| 3. The patients | ||
| 3-1. What is the gender of the patient? | ||
| □ Girl | ||
| □ Boy | ||
| 3-2. What is the age of the patient? | ||
| 3-3. What is the patient’s weight? | ||
| 4. Dosages for ST combination drugs | ||
| 4-1. Which ST combination drug is the patient taking? | ||
| □ Conventional ST combination tablet | ||
| □ ST combination granules | ||
| □ Small ST combination tablets | ||
| 4-2. What is the dosage for one dose of ST combination drug? | ||
| 4-3. How often should this medication be taken? | ||
| 4-4. What is the duration of administration? | ||
| □ For less than 1 month | ||
| □ For 1 month or more | ||
| □ For 3 months or more | ||
| 5. The context of use | ||
| 5-1. Where was the medication taken? | ||
| □ At home | ||
| □ In the hospital | ||
| □ Other | ||
| 5-2. At what time of day was the medication taken? | ||
| 6. Observations | ||
| 6-1. What was the patient’s reaction when taking the medication? | ||
 |  |  |
| 6-2. How much of the medication had been taken? | ||
| □ All of the prescribed dose has been taken | ||
| □ A part of the prescribed dose has been taken | ||
| □ The prescribed dose hasn’t been taken | ||
| 6-3. How long did it take to prepare the prescribed dose of medication? | ||
| (starting from the opening of the medication package) | ||
| 6-4. What was the administration time of the prescribed dose of medication? | ||
| (starting from the moment it is ready to use) | ||
| 6-5. Before dosing, the prescribed dose of the medication had to be... | ||
| □ … modified before administration | ||
| (e.g., prescribed dose of tablet divided or crushed into powder) | ||
| □ … mixed with drink or food | ||
| 6-6. When the patient puts it in the mouth, the patient had to… | ||
| □ … take a drink or food to mask the taste or for easier swallowing | ||
| □ … be promised a reward | ||
| □ Other | ||
| (A) cTab to sTab | (B) Granules to sTab | |
|---|---|---|
| Subject number (boy, %) | 22 (11, 50.0%) | 20 (11, 55.0%) |
| Median age (range) | 11.0 (5.0–17.0) | 5.0 (1.0–13.0) |
| Median body weight (kg, range) | 25.7 (14.5–65.1) | 19.2 (8.4–42.2) |
| Duration of conventional ST combination treatment | ||
| Less than 1 month | 0 (0.0%) | 0 (0.0%) |
| 1 to 3 months | 2 (9.1%) | 5 (25.0%) |
| More than 3 months | 20 (90.9%) | 15 (75.0%) |
| Duration of sTab treatment | ||
| Less than 1 month | 15 (68.2%) | 16 (80.0%) |
| 1 to 3 months | 7 (31.8%) | 4 (20.0%) |
| More than 3 months | 0 (0.0%) | 0 (0.0%) |
| Median amount of cTab or granules per dose (range) | 1.0 (0.5–1.5) tablets | 0.75 (0.2–2.0) gram |
| Median amount of sTab per dose (range) | 4.0 (1.0–4.0) tablets | 2.0 (1.0–4.0) tablets |
| cTab to sTab | Granules to sTab | |||
|---|---|---|---|---|
| cTab (n = 22) | sTab (n = 22) | Granules (n = 20) | sTab (n = 20) | |
| (A) Dosing location | ||||
| Home | 8 (36.4%) | 8 (36.4%) | 8 (40.0%) | 8 (40.0%) |
| Hospital | 14 (63.6%) | 14 (63.6%) | 16 (60.0%) | 16 (60.0%) |
| (B) Dosing timing | ||||
| Morning | 20 (90.9%) | 20 (90.9%) | 19 (95.0%) | 19 (95.0%) |
| Evening | 2 (9.1%) | 2 (9.1%) | 1 (5.0%) | 1 (5.0%) |
| (C) Dosing status | ||||
| Fully taken | 22 (100.0%) | 22 (100.0%) | 19 (95.0%) | 19 (95.0%) |
| Partly taken | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (5.0%) |
| Not taken | 0 (0.0%) | 0 (0.0%) | 1 (5.0%) | 0 (0.0%) |
| (D) Patient reaction | ||||
| Positive | 5 (22.7%) | 9 (40.9%) | 1 (5.0%) | 10 (50.0%) |
| Neutral | 7 (31.8%) | 13 (59.1%) | 7 (35.0%) | 9 (45.0%) |
| Negative | 10 (45.5%) | 0 (0.0%) | 12 (60.0%) | 1 (5.0%) |
| cTab to sTab | Granules to sTab | |||
|---|---|---|---|---|
| cTab (n = 22) | sTab (n = 22) | Granules (n = 20) | sTab (n = 20) | |
| (A) Dosing split and manipulation for intake | ||||
| No (taken all at one time) | 16 (72.7%) | 20 (90.9%) | 11 (55.0%) | 10 (50.0%) |
| Divided into two or more | 0 (0.0%) | 2 (9.1%) | 9 (45.0%) | 9 (45.0%) |
| Split into two or more | 3 (13.6%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Crushed into powder | 3 (13.6%) | 0 (0.0%) | 0 (0.0%) | 1 (5.0%) |
| (B) Mixed with food/drink/other masking agents | ||||
| No | 22 (100.0%) | 22 (100.0%) | 3 (15.0%) | 19 (95.0%) |
| Dissolved and suspended in water | 0 (0.0%) | 0 (0.0%) | 11 (55.0%) | 1(5.0%) |
| Dissolved and suspended in sucrose syrup | 0 (0.0%) | 0 (0.0%) | 5 (25.0%) | 0 (0.0%) |
| Dissolved and suspended in carbonated water | 0 (0.0%) | 0 (0.0%) | 1 (5%) | 0 (0.0%) |
| (C) Taken with food/drink/other masking agents | ||||
| No additional concomitant intake or taken with water only | 14 (63.6%) | 19 (86.3%) | 17 (85.0%) | 19 (95.0%) |
| Taken with sucrose syrup | 6 (27.3%) | 2 (9.1%) | 2 (10.0%) | 1 (5.0%) |
| Taken with ice cream | 1 (4.5%) | 0 (0.0%) | 1 (5.0%) | 0 (0.0%) |
| Taken with apple juice | 1 (4.5%) | 1 (4.5%) | 0 (0.0%) | 0 (0.0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, J.; Yamaguchi, M.; Shimizu, S.; Chiba, K.; Utano, T.; Fukuda, A.; Sakamoto, S.; Kasahara, M.; Yamatani, A. Investigation on the Usefulness of Sulfamethoxazole Trimethoprim Combination Small Tablets in Pediatric Pharmacotherapy: A Single Center Observational Study Using a Questionnaire. Children 2022, 9, 1598. https://doi.org/10.3390/children9101598
Saito J, Yamaguchi M, Shimizu S, Chiba K, Utano T, Fukuda A, Sakamoto S, Kasahara M, Yamatani A. Investigation on the Usefulness of Sulfamethoxazole Trimethoprim Combination Small Tablets in Pediatric Pharmacotherapy: A Single Center Observational Study Using a Questionnaire. Children. 2022; 9(10):1598. https://doi.org/10.3390/children9101598
Chicago/Turabian StyleSaito, Jumpei, Miho Yamaguchi, Seiichi Shimizu, Kyoko Chiba, Tomoyuki Utano, Akinari Fukuda, Seisuke Sakamoto, Mureo Kasahara, and Akimasa Yamatani. 2022. "Investigation on the Usefulness of Sulfamethoxazole Trimethoprim Combination Small Tablets in Pediatric Pharmacotherapy: A Single Center Observational Study Using a Questionnaire" Children 9, no. 10: 1598. https://doi.org/10.3390/children9101598
APA StyleSaito, J., Yamaguchi, M., Shimizu, S., Chiba, K., Utano, T., Fukuda, A., Sakamoto, S., Kasahara, M., & Yamatani, A. (2022). Investigation on the Usefulness of Sulfamethoxazole Trimethoprim Combination Small Tablets in Pediatric Pharmacotherapy: A Single Center Observational Study Using a Questionnaire. Children, 9(10), 1598. https://doi.org/10.3390/children9101598






