Abstract
Zika virus (ZIKV) infection in pregnancy is associated with birth and developmental alterations in infants. In this study, clinical records of 47 infants whose mothers had Zika during pregnancy or clinical manifestations compatible with Zika were reviewed. A description of the infants’ anomalies was established, and a neurodevelopmental assessment was performed on 18 infants, using the Evaluation of Infant Development (EDI for its initialism in Spanish) and DDST-II (Denver Developmental Screening Test II) tests. From his sample, 74.5% of the infants evaluated had major anomalies and 51.9% had minor anomalies. The incidence of major anomalies, related to trimester of pregnancy, was 84.2% for the first trimester, 77.8% for the second trimester, and 37.5% in the third trimester. A similar trend was observed in the frequency of infants without anomalies and was less evident in the incidence of minor anomalies (p = 0.016). Through neurodevelopmental assessments, EDI identified 27.8% of infants as having normal development, while 55.5% of affected infants had developmental delay, and 16.7% were at risk for developmental delay. The DDSST-II showed that 77.7% infants had delay in the gross motor and language area, 88.8% in the fine-adaptative motor area, and 72.2% in the personal–social area. In this work, children of mothers with ZIKV infection during pregnancy may have major or minor anomalies regardless of the trimester of pregnancy in which the infection occurred. The neurodevelopmental assessment shows that ZIKV can cause a developmental delay in infants with the fine-adaptative motor area being the most affected.
Keywords:
Zika; ZIKV; congenital Zika syndrome; major anomalies; minor anomalies; neurodevelopmental; EDI; DDSST-II 1. Introduction
Zika virus (ZIKV) is a virus different from other arboviruses. One of these particularities is that mosquito bite is not its only route of transmission, it can be transmitted sexually [1], from mother to child during pregnancy [2], and through blood transfusion [3]. ZIKV infection can cause Zika disease, characterized by rash, pruritus, arthralgia, headache, myalgia, and fever, among others [4]. However, it is estimated that ZIKV infection may be asymptomatic in 80% of the infected population [5].
Outbreaks of Zika virus ZIKV in humans were sporadic until early 2015, when the first cases were confirmed in Brazil [6]. ZIKV spread rapidly in the Americas, with active virus transmission reported in 38 countries on the American continent [7]. The importance of ZIKV made it a worldwide public-health emergency decreed by the World Health Organization (WHO) in 2016 [8]. In Mexico, ZIKV was detected for the first time in 2015 and since then, and until the end of 2021, 55.1% of the reported cases had been in pregnant women [9]. It is estimated that 14% of children exposed to ZIKV during gestation may develop health problems associated with infection, including neurodevelopmental disorders [10]. The high incidence of cases in pregnant women is important due to the fact that ZIKV infection during pregnancy has been associated with the development of anomalies in newborns [11], such as morphological–cranial [12], cerebral [13,14,15], ophthalmic [16], neurological [17], and joint contractures [18,19], which have been grouped into a new syndrome called Congenital Zika Syndrome (CZS) [11,20]. Some anomalies caused by intrauterine ZIKV infection can manifest postnatally, such as postnatal microcephaly [21] and neurodevelopmental disorders, that could affect cognitive, motor, and social functions [22], even in healthy infants [23]. Neurodevelopmental assessment in infants exposed to ZIKV during pregnancy is of great importance because it allows the characterization of the effects of gestational ZIKV infection at birth and in the long term, due to the possible impairment of cognitive, motor, and social skills in infants. Characterization of neurodevelopmental disorders should be performed by standardized tests according to the age of the infant [24].
ZIKV infection has been important to Mexico since some studies have found cases of infants exposed to ZIKV during gestation with signs of CZS [25,26]. In normocephalic Mexican children, it has also been observed that exposure to ZIKV could affect neurodevelopment by decreasing the infant’s fine and gross motor skills, such as visual reception, and receptive language up to 6 months of age [27]. The aim of this study is to evaluate abnormalities and neurodevelopment of infants with a mean age of 33 months born to mothers infected with ZIKV during pregnancy. The sample was taken in a public institution in the state of Veracruz, Mexico, which provides care to low-income children with different disabilities.
2. Materials and Methods
2.1. Study Design
This study was approved by the Research Ethics Committee of the Institute of Public Health Research Center (CEI-ISP-R02/2020), the Ministry of Health (SEIC-004-18), and authorities of the Center for Infant Rehabilitation of Veracruz (CRIVER for its acronym in Spanish). A retrospective cohort study including 47 children whose mothers presented symptoms compatible with Zika during pregnancy, was analyzed. Participants were grouped into confirmed cases considering any probable case of ZIKV infection confirmed by laboratory techniques approved by health authorities, and probable cases considering any pregnant woman presenting two or more symptoms compatible with Zika and with a history of visiting or residing in areas endemic for the ZIKV. This was performed according to definitions used by the Mexican Ministry of Health (SSA) [28], similar to other studies [18,29].
2.2. Clinical Data Collection
The variables of interest were collected using an instrument developed by the research team. This instrument comprises anthropometric measures and descriptive variables of the mother and the infant that are part of the study.
The medical records of the infants were reviewed to determine the possible anomalies that occurred at birth, taking into consideration: (a) low weight: newborns weighing < 2500 g, (b) low size: newborns with growth patterns below gestational age according to the parameters of the Fenton growth chart provided by the Pan American Health Organization (PAHO), (c) prematurity: newborns born at <37 weeks pregnancy, and (d) decreased head circumference: determined according to gestational age following the parameters of the Fenton growth chart provided by PAHO [30]. The anomalies reported in the development of the infants were divided into six categories: cranial morphology, cerebral alterations, ophthalmological alterations, joint contractures, neurological sequelae, and others, similar to other studies [11,20]. The anomalies were classified into major and minor anomalies based on the Centers for Disease Control and Prevention (CDC) definitions of congenital anomalies, indicated as: major anomalies to those anomalies of body structure or function that account for the majority of deaths, morbidity, and disability related to congenital anomalies; and minor anomalies to those anomalies of structure or function that pose no significant health problems in the neonatal period and tend to have limited social or cosmetic consequences for the affected individual [31]. For the obstetric risk maternal age variable, risk ages were considered: adolescent pregnancy ≤ 19 years [32] and geriatric pregnancy ≥ 35 years [33].
2.3. Neurodevelopmental Assessments
Two neurodevelopmental assessments were applied to 18 infants: (1) EDI [34], developed by the National Institute of Perinatology of Mexico, to be used for using in Mexican infants under 5 years of age to detect neurodevelopmental problems, and (2) DDST-II, used to evaluate the development of infants from 0 to 6 years in the gross-motor, fine-adaptive motor, language, and personal–social areas [35]. The tests were applied by a pediatrician and a neuropediatrician.
2.4. Statistical Analysis
The categorical variables were described as absolute frequencies and percentages, while age was described as the mean and standard deviation. Comparison between categorical variables was performed using the chi-square test for trends or Fisher’s exact test. The probability of infants having developmental abnormalities was expressed as cumulative incidence. Statistical significance was defined as a value of p ≤ 0.05. Statistical analysis was performed with SPSS (IBM SPSS Statistics for Mac Version 21.0. Armonk, NY) and StatCalc (Epi Info; CDC, Atlanta, GA, USA).
3. Results
3.1. Characteristics of Pregnant Women and Effects of ZIKV Infection at Birth
Forty-seven infants whose mothers presented signs and symptoms compatible with ZIKV during pregnancy were included. The mothers were classified into two groups, confirmed cases (n = 20) and probable cases (n = 27), with a mean age of 24.8 ± (5.4) and 23.4 ± (6.4) years, respectively. The sociodemographic characteristics of the mothers are described in Table 1. According to the trimester of pregnancy for confirmed cases, ZIKV infection was detected in 50% (n = 10) in pregnant women in their first trimester, while 25% (n = 5) was found in pregnant women in their second trimester and 25% (n = 5) were in the third trimester. In probable cases, 7.4% (n = 2), it was not possible to determine the trimester in which the infection occurred. Of the documented cases, 33.3% (n = 9) were in the first trimester, 48.1% (n = 13) were in the second, and 11.1% (n = 3) were in the third (Table 1).

Table 1.
General characteristics of mothers during pregnancy and of the infant at birth for confirmed and probable cases of ZIKV.
In the studied newborns, 20% (n = 4) of the confirmed cases presented low weight; 40% (n = 8) low size; 15% (n = 3) prematurity; and 4 % (n = 9) decreased head circumference. In probable cases, 18.5% (n = 5) had low weight; 51.9% (n = 14) low size; 14.8% (n = 4) prematurity; and 70.3% (n = 19) decreased head circumference (Table 1).
3.2. Presence of Major and Minor Anomalies in Infants
Information was collected on the anomalies reported in infants and classified into six categories: cranial morphology, cerebral alterations, ophthalmological alterations, joint contractures, neurological sequelae, and others, similar to other studies [20], to categorize them into major and minor anomalies [31].
The most frequent major anomalies in confirmed cases of ZIKV infection were spastic quadriplegia in 60% (n = 12); microcephaly in 50% (n = 10); and multiple joint contractures in 45% (n = 9). Similarly, the most frequent major anomalies in probable cases were microcephaly in 74.1% (n = 20); spastic quadriplegia in 66.7% (n = 18); and multiple joint contractures in 25.9% (n = 7) (Figure 1). The most frequent minor anomalies in the confirmed cases of ZIKV infection were delayed psychomotor development in 60% (n = 12); cognitive impairment in 25% (n = 5); and diffuse calcifications in 25% (n = 5). As for the probable cases, delayed psychomotor development in 88.9% (n = 24); cognitive impairment in 63% (n = 17); and seizures in 40.7% (n = 11) (Figure 1). Of the infants, 4.3% (n = 2) died and 5% (n = 1) of the infants of confirmed with ZIKV and 3.4% (n = 1) of probable cases developed postnatal microcephaly.
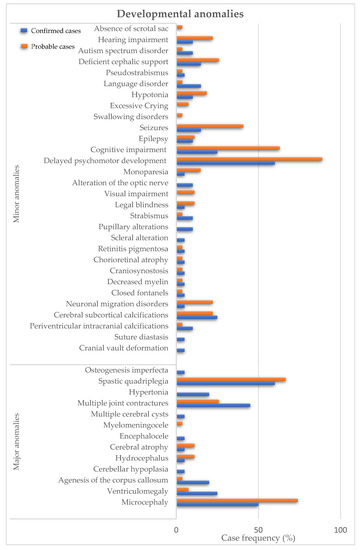
Figure 1.
Developmental anomalies in children of mothers infected with ZIKV during pregnancy. Major anomalies are described in confirmed cases (blue bars) and probable cases (orange bars).
An inverse relationship was found between the cumulative incidence of major anomalies and the trimester of pregnancy (Table 2). Mothers in the first trimester of pregnancy presented the highest incidence (84.2%), followed by those in the second trimester with (77.8%), and finally, an incidence of (37.5%) for those in the third trimester (p for trend = 0.016). Although this relationship was not as evident when comparing the incidences of minor anomalies. A similar trend was observed when the frequency of infants without anomalies increased in relation to the trimester of pregnancy: first trimester (0%), second trimester (16.7%), and third trimester (25.0%). Concerning maternal age as a factor for the development of major or minor anomalies in infants, no significant difference was found in the incidence of major or minor anomalies between women with and without obstetric risk age (91.7% vs. 88.6%, respectively) (p = 0.99) (Table 2).

Table 2.
Cumulative incidence of anomalies according to trimester of pregnancy and maternal age at the time of ZIKV infection.
3.3. Neurodevelopmental Assessment
Of the infants included in this study, 53.2% abandoned their care process on CRIVER; therefore, the neurodevelopmental evaluation was performed in 18 infants, with a mean age of 33.1 ± (6.4) months. The EDI and DSST-II tests were applied; with the DDSST-II test, the percentage of infants showing delay was determined according to the four areas evaluated (gross-motor, fine-adaptive motor, language, and personal–social) to observe the delay presented in the population (Table 3). Moreover, 27.8% of the infants presented normal neurodevelopment and 72.2% presented some risk or delay in one of the developmental areas evaluated: 77.7% (n = 14) showed delay in the gross-motor area; 88.8% (n = 16) delay in the fine-adaptative motor area; 77.7% (n = 14) delay in language area; and 72.2% (n = 13) delay in the personal–social area (Table 3). Through the EDI test, it was determined that 27.8% (n = 5) showed normal development while 55.5% (n = 10) and 16.7% (n = 3) of the infants evaluated presented risk of developmental delay and developmental delay, respectively (Table 3).

Table 3.
Neurodevelopmental assessment in children exposed to ZIKV infection during pregnancy.
4. Discussion
ZIKV is an arbovirus of recent circulation in Mexico [36]. In contrast to other arboviruses, ZIKV is teratogenic and its effects on infants were addressed in various research studies [37,38,39,40]. CZS is characterized by birth and developmental abnormalities that are currently not fully understood [40,41,42]. This study analyzed the characteristics in children of mothers who were infected with ZIKV during pregnancy, at birth, and during their development.
In our study population, 55% of the newborns of confirmed cases and 29.7% of probable cases were born healthy. Microcephaly was one of the most frequent alterations found at birth, as in other studies [37,41]. In addition, two cases of postnatal microcephaly were identified, a characteristic that has been previously described [21], which highlights the importance of following infants who were exposed to intrauterine ZIKV infection. The classification of major and minor anomalies in infants with intrauterine ZIKV infection allowed us to observe their possible negative impact during development. The most frequent major anomalies in infants of this study, in addition to microcephaly, were spastic quadriplegia and multiple joint contractures, previously described as part of the pattern of signs for CZS [11]. The most frequent minor anomalies in the infants in this study, both in confirmed and probable cases, were delayed psychomotor development and cognitive impairment, related to neurodevelopmental alterations. The description of motor impairments in infants with ZIKV exposure in utero has been reported [43]. The diagnosis of some of the minor anomalies, such as those observed in this study, needs to be characterized through neurodevelopmental assessment tests. Because of this, the inclusion of these tests should be considered important in the care of children exposed to ZIKV in pregnancy.
We also evaluated the incidence of anomalies according to the pregnancy trimester in which ZIKV infection occurred. As in other studies, we found that the highest incidence of major anomalies is found in the first trimester [44,45,46]. However, in this study, the incidence of developing major anomalies is latent throughout pregnancy. Regarding minor anomalies, the highest incidence was found in the third trimester group. It was also observed that the frequency of infants without anomalies increased as the pregnancy progressed, indicating that infants born to ZIKV-infected mothers in late pregnancy are less likely to develop anomalies. Maternal age was also analyzed, with the incidence of anomalies in newborns; however, no significant association was found, suggesting that the development of these anomalies could be intrinsic to gestational ZIKV infection, in agreement with what has been previously reported [41]. The development of major and minor anomalies is present throughout pregnancy, and although minor anomalies are not life-threatening for the infant during development [31], they may have an impact on the infant’s quality of life and family. Therefore, the early detection and characterization of these minor anomalies could allow the implementation of appropriate therapies and treatments to help improve the quality of life of the child. These data highlight the need for measures to prevent infection throughout pregnancy, regardless of the trimester in which the infection occurred.
On the other hand, SCZ is a new syndrome, so its characterization is important and for which neurodevelopmental assessments are a valuable tool. In previous reports of infants exposed to ZIKV during pregnancy, it was observed that the risk of developing neurodevelopmental disorders may be related to the presence of microcephaly [22,47]. The risk of neurodevelopmental delay can range from 13.8% to 20.2% in normocephalic infants, while this percentage increases from 99.1% to 100% when infants develop severe microcephaly or from 65% to 70% in cases of moderate microcephaly [48]. Our results show that 72.2% of the infants studied, with and without anomalies, may present developmental risk or delay. This percentage could be high considering that our population includes infants without microcephaly. More studies are needed to elucidate the role of structural and physical abnormalities in the functional impairment of the infant. The DDST-II test was used to evaluate infants exposed to intrauterine cytomegalovirus and rubella virus infections [49,50]. Intrauterine cytomegalovirus exposure caused cognitive/developmental impairment and motor delay in 16.4% and 7.3%, respectively [49]. The same test, in cases of infants exposed to rubella virus intrauterine, showed language delay in 69.2%, impairment of the gross-motor area in 46.2% of cases; of the fine-adaptive motor area in 30.8 %; and the personal–social area in 46.2% [50]. The infants in this study presented greater damage, since 88.8% of the infants presented impairment in the fine-adaptive motor area; 77.7% in gross motor and language delay; and 72.2% in the personal–social area. Our results suggest that ZIKV could be one of the most teratogenic viruses severely affecting neurodevelopment in infants.
Two neurodevelopmental assessments tests were used in our study: EDI and DDST-II. Although the tests evaluate different aspects, the results of both show correlation in the developmental damage of the infant. In Mexico, there is already a validated test for the national population that could be used in a similar way to the DDST-II and compared with the results of other international studies [51,52]. One of the limitations of our study is it only studied children of ZIKV-infected mothers who had some alteration and who attended a medical center for the care of children with disabilities which may make the frequency of anomalies higher than that reported in other studies [44,53]. Another limitation of our study was the small sample size, however, our findings suggest that the risk of developing anomalies in newborns with gestational Zika is present throughout pregnancy and propose the establishment of a management program for children of mothers infected with ZIKV during gestation similar to other teratogenic viruses, such as CMV [54] and rubella [55]. Further, prevention and control measures for ZIKV infection in pregnant women should be implemented throughout pregnancy.
5. Conclusions
Our study evaluates for the first time the abnormalities and neurodevelopment in infants, with and without microcephaly, exposed to ZIKV during pregnancy in Mexico at 33 months of age. This work highlights the importance of studying infants exposed to ZIKV during pregnancy to characterize CZS because, as a new syndrome, the clinical manifestations have not been fully described. Our study suggests that ZIKV could be teratogenic, regardless of the trimester of pregnancy in which the infant was exposed to the virus. However, future studies with a larger population are needed to corroborate these findings. If these findings are consistent with future studies, prenatal management guidelines should be modified to include ZIKV screening of pregnant women in ZIKV-endemic areas, as well as follow-up and neurodevelopmental evaluation of ZIKV-exposed infants.
Author Contributions
Conceptualization, M.V.-C., J.M.-R., R.Z.-C., C.L.S., C.S.O.-C. and H.M.; methodology, M.V.-C., V.O.P.-R., C.S.O.-C. and H.M.; software, J.M.-R.; validation, M.V.-C., J.M.-R., Á.R.-L. and H.M.; formal analysis, K.G., M.V.-C., V.O.P.-R., J.M.-R. and H.M.; investigation, K.G., M.V.-C., V.O.P.-R., O.G.-M., C.S.O.-C., R.P.-V., C.F.M.-T. and H.M.; resources, R.Z.-C. and H.M.; writing—review and editing, K.G., M.V.-C., V.O.P.-R., J.M.-R., R.Z.-C., Á.R.-L., O.G.-M., C.L.S., C.S.O.-C., R.P.-V., C.F.M.-T. and H.M.; supervision, J.M.-R., R.Z.-C., Á.R.-L., C.L.S. and H.M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received financial support for the publication fees of this article from Instituto de Salud Pública.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Research Ethics Committee of the Institute of Public Health (CEI-ISP-R02/2020), by the Secretary of Health of Veracruz (SEIC-004-18) and the authorities of the Center for Infant Rehabilitation of Veracruz (CRIVER).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgments
K.G. (#718185) was the recipient of a scholarship from the Consejo Nacional de Ciencia y Tecnología (CONACYT) and PROMUV. We thank Edith Rodríguez Romero, Abel Gutiérrez Ruiz, José Manuel Zarrabal García, and Ana Saraí Mirón Cruz for their support in conducting this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Calvet, G.A.; Kara, E.O.; Giozza, S.P.; Botto-Menezes, C.H.A.; Gaillard, P.; de Oliveira Franca, R.F.; de Lacerda, M.V.G.; da Costa Castilho, M.; Brasil, P.; de Sequeira, P.C.; et al. Study on the persistence of Zika virus (ZIKV) in body fluids of patients with ZIKV infection in Brazil. BMC Infect. Dis. 2018, 18, 49. [Google Scholar] [CrossRef] [PubMed]
- Villalobos-Sanchez, E.; Burciaga-Flores, M.; Zapata-Cuellar, L.; Camacho-Villegas, T.A.; Elizondo-Quiroga, D.E. Possible Routes for Zika Virus Vertical Transmission in Human Placenta: A Comprehensive Review. Viral Immunol. 2022, 35, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Nhan, T.; Robin, E.; Roche, C.; Bierlaire, D.; Zisou, K.; Shan Yan, A.; Cao-Lormeau, V.M.; Broult, J. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Eurosurveillance 2014, 19, 20761. [Google Scholar] [CrossRef]
- Guanche Garcell, H.; Gutierrez Garcia, F.; Ramirez Nodal, M.; Ruiz Lozano, A.; Perez Diaz, C.R.; Gonzalez Valdes, A.; Gonzalez Alvarez, L. Clinical relevance of Zika symptoms in the context of a Zika Dengue epidemic. J. Infect. Public Health 2020, 13, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Haby, M.M.; Pinart, M.; Elias, V.; Reveiz, L. Prevalence of asymptomatic Zika virus infection: A systematic review. Bull. World Health Organ. 2018, 96, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Zanluca, C.; Melo, V.C.; Mosimann, A.L.; Santos, G.I.; Santos, C.N.; Luz, K. First report of autochthonous transmission of Zika virus in Brazil. Memórias Do Inst. Oswaldo Cruz 2015, 110, 569–572. [Google Scholar] [CrossRef]
- PAHO. Zika a Year Later: How a New Virus Took the Americas by Surprise. Available online: https://www3.paho.org/hq/index.php?option=com_content&view=article&id=11989:zika-a-year-later&Itemid=135&lang=en#:~:text=Today%2C%20Aedes%20aegypti%20mosquitoes%20are,the%20vast%20majority%20in%20Brazil (accessed on 1 June 2022).
- Oladapo, O.T.; Souza, J.P.; De Mucio, B.; de Leon, R.G.; Perea, W.; Gulmezoglu, A.M.; WHO. WHO interim guidance on pregnancy management in the context of Zika virus infection. Lancet Glob. Health 2016, 4, e510–e511. [Google Scholar] [CrossRef]
- SINAVE. Casos Confirmados Autóctonos de Enfermedad por Virus del Zika; Transmisibles, D., Ed.; Secreatría de Salud: Cd. México, México, 2022.
- Rice, M.E.; Roth, N.M.; Llington, S.R.; Moore, C.A.V.-P.; Ellis, E.M.; Tufa, A.J.; Taulung, L.A.; Alfred, J.P.-P.; Delgado-López, C.A.; Zaki, S.R.; et al. Vital Signs: Zika-Associated Birth Defects and Neurodevelopmental Abnormalities Possibly Associated with Congenital Zika Virus Infection—U.S. Territories and Freely Associated States, 2018. Morb. Mortal. Wkly. Rep. 2018, 67, 858–867. [Google Scholar] [CrossRef]
- Moore, C.A.; Staples, J.E.; Dobyns, W.B.; Pessoa, A.; Ventura, C.V.; Fonseca, E.B.; Ribeiro, E.M.; Ventura, L.O.; Neto, N.N.; Arena, J.F.; et al. Characterizing the Pattern of Anomalies in Congenital Zika Syndrome for Pediatric Clinicians. JAMA Pediatr. 2017, 171, 288–295. [Google Scholar] [CrossRef]
- Dain Gandelman Horovitz, D.; da Silva Pone, M.V.; Moura Pone, S.; Dias Saad Salles, T.R.; Bastos Boechat, M.C. Cranial bone collapse in microcephalic infants prenatally exposed to Zika virus infection. Neurology 2016, 87, 118–119. [Google Scholar] [CrossRef]
- Brasil, P.; Pereira, J.P., Jr.; Moreira, M.E.; Ribeiro Nogueira, R.M.; Damasceno, L.; Wakimoto, M.; Rabello, R.S.; Valderramos, S.G.; Halai, U.A.; Salles, T.S.; et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro. N. Engl. J. Med. 2016, 375, 2321–2334. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Correa, J.E.; Pérez-Berenguer, J.L.; Correa-Rivas, M.S.; García, I.; Dávila-Castrodad, N.; de la Vega, A. Congenital Zika Syndrome in Puerto Rico: Neuropathological Findings and Review of the Medical Literature. Arch. Patol. 2021, 2, 51–57. [Google Scholar]
- He, Z.; An, S.; Chen, J.; Zhang, S.; Tan, C.; Yu, J.; Ye, H.; Wu, Y.; Yuan, J.; Wu, J.; et al. Neural progenitor cell pyroptosis contributes to Zika virus-induced brain atrophy and represents a therapeutic target. Proc. Natl. Acad. Sci. USA 2020, 117, 23869–23878. [Google Scholar] [CrossRef] [PubMed]
- Ventura, C.V.; Maia, M.; Bravo-Filho, V.; Gois, A.L.; Belfort, R., Jr. Zika virus in Brazil and macular atrophy in a child with microcephaly. Lancet 2016, 387, 228. [Google Scholar] [CrossRef]
- van der Linden, V.; Filho, E.L.; Lins, O.G.; van der Linden, A.; Aragao Mde, F.; Brainer-Lima, A.M.; Cruz, D.D.; Rocha, M.A.; Sobral da Silva, P.F.; Carvalho, M.D.; et al. Congenital Zika syndrome with arthrogryposis: Retrospective case series study. BMJ 2016, 354, i3899. [Google Scholar] [CrossRef]
- Sarno, M.; Aquino, M.; Pimentel, K.; Cabral, R.; Costa, G.; Bastos, F.; Brites, C. Progressive lesions of central nervous system in microcephalic fetuses with suspected congenital Zika virus syndrome. Ultrasound Obstet. Gynecol. 2017, 50, 717–722. [Google Scholar] [CrossRef]
- Martins-Filho, P.R.; Souza Tavares, C.S.; Araujo Carvalho, A.C.; Reis, M.; Santos, H.P., Jr.; Santos, V.S. Association Between Arthrogryposis and Mortality in Infants With Congenital Zika Syndrome: A Systematic Review and Meta-analysis. Pediatr. Neurol. 2020, 110, 20–24. [Google Scholar] [CrossRef]
- Matos-Alviso, L.J.; Santos-Calderón, L.A.; Reyes-Hernández, K.L.; Reyes-Gómez, U.; Santamaria-Arza, C.; Gerardo López-Cruz, G.; Reyes-Hernández, M.U.; López-Días, A.V.; Quero-Hernández, A.; de Lara-Huerta, J. Síndrome congénito por virus zika, conceptos basicos. Salud QR 2017, 10, 33–36. [Google Scholar]
- Shao, Q.; Herrlinger, S.; Yang, S.L.; Lai, F.; Moore, J.M.; Brindley, M.A.; Chen, J.F. Zika virus infection disrupts neurovascular development and results in postnatal microcephaly with brain damage. Development 2016, 143, 4127–4136. [Google Scholar] [CrossRef]
- Antoniou, E.; Orovou, E.; Andronikidi, P.E.; Orovas, C.; Rigas, N.; Palaska, E.; Sarella, A.; Iatrakis, G.; Voyiatzaki, C. Congenital Zika Infection and the Risk of Neurodevelopmental, Neurological, and Urinary Track Disorders in Early Childhood. A Systematic Review. Viruses 2021, 13, 1671. [Google Scholar] [CrossRef]
- Mulkey, S.B.; Arroyave-Wessel, M.; Peyton, C.; Bulas, D.I.; Fourzali, Y.; Jiang, J.; Russo, S.; McCarter, R.; Msall, M.E.; du Plessis, A.J.; et al. Neurodevelopmental Abnormalities in Children With In Utero Zika Virus Exposure Without Congenital Zika Syndrome. JAMA Pediatr. 2020, 3, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, K.N.; Eskenazi, B.; Schantz, S.; Yolton, K.; Rauh, V.A.; Johnson, C.B.; Alkon, A.; Canfield, R.L.; Pessah, I.N.; Berman, R.F. Principles and practices of neurodevelopmental assessment in children: Lessons learned from the Centers for Children’s Environmental Health and Disease Prevention Research. Environ. Health Perspect. 2005, 113, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Capetillo, S.N.; Palma-Baquedano, J.R.; Valadez-Gonzalez, N.; Manrique-Saide, P.; Barrera-Perez, H.A.M.; Pinto-Escalante, D.; Pavia-Ruz, N. Case Report: Congenital Arthrogryposis and Unilateral Absences of Distal Arm in Congenital Zika Syndrome. Front. Med. 2021, 8, 499016. [Google Scholar] [CrossRef]
- Contreras-Capetillo, S.N.; Valadez-Gonzalez, N.; Manrique-Saide, P.; Carcano-Castillo, R.E.; Pacheco-Tugores, F.; Barrera-Perez, H.A.M.; Pinto-Escalante, D.; Lliteras-Cardin, M.; Hoil-Parra, J.A.; Caceres-Solis, J.L.; et al. Birth Defects Associated With Congenital Zika Virus Infection in Mexico. Clin. Pediatr. 2018, 57, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Familiar, I.; Boivin, M.; Magen, J.; Azcorra, J.A.; Phippen, C.; Barrett, E.A.; Miller, S.; Ruisenor-Escudero, H. Neurodevelopment outcomes in infants born to women with Zika virus infection during pregnancy in Mexico. Child Care Health Dev. 2021, 47, 311–318. [Google Scholar] [CrossRef]
- DGE. Manual de Procedimientos Estandarizados Para la Vigilancia Epidemiológica de las Enfermedades Transmitidas por Vector (ETV); Secretaría de Salud: Cd. México, México, 2021; pp. 63–65.
- Mulkey, S.B.; Ansusinha, E.; Cristante, C.; Russo, S.M.; Biddle, C.; Kousa, Y.A.; Pesacreta, L.; Jantausch, B.; Hanisch, B.; Harik, N.; et al. Complexities of Zika Diagnosis and Evaluation in a U.S. Congenital Zika Program. Am. J. Trop. Med. Hyg. 2021, 104, 2210–2219. [Google Scholar] [CrossRef]
- OPS. Lineamientos Preliminares de Vigilancia de Microcefalia en Recién Nacidos en Entornos Con Riesgo de Circulación de Virus Zika; Organización Panamericana de Salud: Washington, DC, USA, 2016; pp. 17–19. [Google Scholar]
- Division of Birth Defects and Developmental Disabilities. Centers for Disease Control and Prevention. Congenital Anomalies-Definitions. Available online: https://www.cdc.gov/ncbddd/birthdefects/surveillancemanual/chapters/chapter-1/chapter1-4.html (accessed on 1 March 2022).
- WHO. El Embarazo en la Adolescencia. Available online: https://www.who.int/es/news-room/fact-sheets/detail/adolescent-pregnancy (accessed on 1 March 2022).
- México Health Secretary. Norma Oficial Mexicana Nom-007-SSA2-2016, Para la Atencion de la Mujer Durante el Embarazo, Parto Y Puerperio, Y de la Persona Recien Nacida; Jurídica, S.d.I., Ed.; Comisión Nacional de los Derechos Humanos, Dirección General de Epidemiología: Cd.México, México, 2016.
- México Health Secretary. Manual para la Aplicación de la Prueba Evaluación del Desarrollo Infantil “EDI”; Instituto Nacional de Perinatología: Cd. México, México, 2013.
- Frankenburg, W.K.; Dodds, J.; Archer, P.; Shapiro, H.; Bresnick, B. The Denver II: A major revision and restandardization of the Denver Developmental Screening Test. Pediatrics 1992, 89, 91–97. [Google Scholar] [CrossRef]
- Diaz-Quinonez, J.A.; Lopez-Martinez, I.; Torres-Longoria, B.; Vazquez-Pichardo, M.; Cruz-Ramirez, E.; Ramirez-Gonzalez, J.E.; Ruiz-Matus, C.; Kuri-Morales, P. Evidence of the presence of the Zika virus in Mexico since early 2015. Virus Genes 2016, 52, 855–857. [Google Scholar] [CrossRef]
- Honein, M.A.; Dawson, A.L.; Petersen, E.E.; Jones, A.M.; Lee, E.H.; Yazdy, M.M.; Ahmad, N.; Macdonald, J.; Evert, N.; Bingham, A.; et al. Birth Defects Among Fetuses and Infants of US Women With Evidence of Possible Zika Virus Infection During Pregnancy. JAMA 2017, 317, 59–68. [Google Scholar] [CrossRef]
- Orofino, D.H.G.; Passos, S.R.L.; de Oliveira, R.V.C.; Farias, C.V.B.; Leite, M.; Pone, S.M.; Pone, M.; Teixeira Mendes, H.A.R.; Moreira, M.E.L.; Nielsen-Saines, K. Cardiac findings in infants with in utero exposure to Zika virus- a cross sectional study. PLoS Negl. Trop. Dis. 2018, 12, e0006362. [Google Scholar] [CrossRef]
- Mulkey, S.B.; Bulas, D.I.; Vezina, G.; Fourzali, Y.; Morales, A.; Arroyave-Wessel, M.; Swisher, C.B.; Cristante, C.; Russo, S.M.; Encinales, L.; et al. Sequential Neuroimaging of the Fetus and Newborn With In Utero Zika Virus Exposure. JAMA Pediatr. 2019, 173, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Peloggia, A.; Ali, M.; Nanda, K.; Bahamondes, L. Zika virus exposure in pregnancy and its association with newborn visual anomalies and hearing loss. Int. J. Gynaecol. Obstet. 2018, 143, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Hcini, N.; Kugbe, Y.; Rafalimanana, Z.H.L.; Lambert, V.; Mathieu, M.; Carles, G.; Baud, D.; Panchaud, A.; Pomar, L. Association between confirmed congenital Zika infection at birth and outcomes up to 3 years of life. Nat. Commun. 2021, 12, 3270. [Google Scholar] [CrossRef] [PubMed]
- Ventura, C.V.; Ventura, L.O. Ophthalmologic Manifestations Associated With Zika Virus Infection. Pediatrics 2018, 141, S161–S166. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, A.; van der Linden, V.; Yeargin-Allsopp, M.; Carvalho, M.; Ribeiro, E.M.; Van Naarden Braun, K.; Durkin, M.S.; Pastula, D.M.; Moore, J.T.; Moore, C.A. Motor Abnormalities and Epilepsy in Infants and Children With Evidence of Congenital Zika Virus Infection. Pediatrics 2018, 141, S167–S179. [Google Scholar] [CrossRef]
- Coutinho, C.M.; Negrini, S.; Araujo, D.; Teixeira, S.R.; Amaral, F.R.; Moro, M.; Fernandes, J.; da Motta, M.; Negrini, B.; Caldas, C.; et al. Early maternal Zika infection predicts severe neonatal neurological damage: Results from the prospective Natural History of Zika Virus Infection in Gestation cohort study. BJOG 2021, 128, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Sanchez, L.A.; Becerra-Mojica, C.H.; Rojas, M.A.; Diaz-Martinez, L.A.; Perez Vera, L.A.; Contreras Garcia, G.A.; Pinilla Garcia, L.S. Fetal central nervous system anomalies according to RT-PCR and trimester of maternal infection with Zika virus: A prospective cohort study. Acta Obstet. Gynecol. Scand. 2022, 101, 221–231. [Google Scholar] [CrossRef]
- Jabrane-Ferrat, N.; Veas, F. Zika Virus Targets Multiple Tissues and Cell Types During the First Trimester of Pregnancy. Methods Mol. Biol. 2020, 2142, 235–249. [Google Scholar] [CrossRef]
- Aguilar Ticona, J.P.; Nery, N., Jr.; Doss-Gollin, S.; Gambrah, C.; Lessa, M.; Rastely-Junior, V.; Matos, A.; de Paula Freitas, B.; Borja, A.; Wunder, E.A., Jr.; et al. Heterogeneous development of children with Congenital Zika Syndrome-associated microcephaly. PLoS ONE 2021, 16, e0256444. [Google Scholar] [CrossRef]
- Sobral da Silva, P.F.; Eickmann, S.H.; Arraes de Alencar Ximenes, R.; Ramos Montarroyos, U.; de Carvalho Lima, M.; Turchi Martelli, C.M.; Velho Barreto de Araujo, T.; Brickley, E.B.; Cunha Rodrigues, L.; Lima da Silva Pastich Goncalves, F.C.; et al. Pediatric neurodevelopment by prenatal Zika virus exposure: A cross-sectional study of the Microcephaly Epidemic Research Group Cohort. BMC Pediatr. 2020, 20, 472. [Google Scholar] [CrossRef]
- Giannattasio, A.; Bruzzese, D.; Di Costanzo, P.; Capone, E.; Romano, A.; D’Amico, A.; Bravaccio, C.; Grande, C.; Capasso, L.; Raimondi, F. Neuroimaging Profiles and Neurodevelopmental Outcome in Infants With Congenital Cytomegalovirus Infection. Pediatr. Infect. Dis. J. 2018, 37, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Toizumi, M.; Nguyen, G.T.; Motomura, H.; Nguyen, T.H.; Pham, E.; Kaneko, K.I.; Uematsu, M.; Nguyen, H.A.; Dang, D.A.; Hashizume, M.; et al. Sensory defects and developmental delay among children with congenital rubella syndrome. Sci. Rep. 2017, 7, 46483. [Google Scholar] [CrossRef]
- Alves, L.V.; Paredes, C.E.; Silva, G.C.; Mello, J.G.; Alves, J.G. Neurodevelopment of 24 children born in Brazil with congenital Zika syndrome in 2015: A case series study. BMJ Open 2018, 8, e021304. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, R.; Khosla, S.; Rondon, J.; Pena, F.; Sullivan, G.; Perez, M.; Mehta, S.D.; Brito, M.O. Birth Defects and Long-Term Neurodevelopmental Abnormalities in Infants Born During the Zika Virus Epidemic in the Dominican Republic. Ann. Glob. Health 2021, 87, 4. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, V.; Pessoa, A.; Dobyns, W.; Barkovich, A.J.; Junior, H.V.; Filho, E.L.; Ribeiro, E.M.; Leal, M.C.; Coimbra, P.P.; Aragao, M.F.; et al. Description of 13 Infants Born During October 2015-January 2016 With Congenital Zika Virus Infection Without Microcephaly at Birth-Brazil. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 1343–1348. [Google Scholar] [CrossRef]
- National Center for Immunization and Respiratory Diseases. Cytomegalovirus (CMV) and Congenital CMV Infection. Available online: https://www.cdc.gov/cmv/congenital-infection.html (accessed on 3 March 2022).
- Cutts, F.T.; Best, J.; Siqueira, M.M.; Engstrom, K.; Robertson, S.E. Guidelines for Surveillance of Congenital Rubella Syndrome and Rubella; World Health Organization: Geneva, Switzerland, 1999. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).


