Implementation of Surfactant Administration through Laryngeal or Supraglottic Airways (SALSA): A Jordanian NICU’s Journey to Improve Surfactant Administration
Abstract
:1. Introduction
1.1. Problem Description
1.2. Setting and Rationale
1.3. Aim
2. Materials and Methods
2.1. Implementation
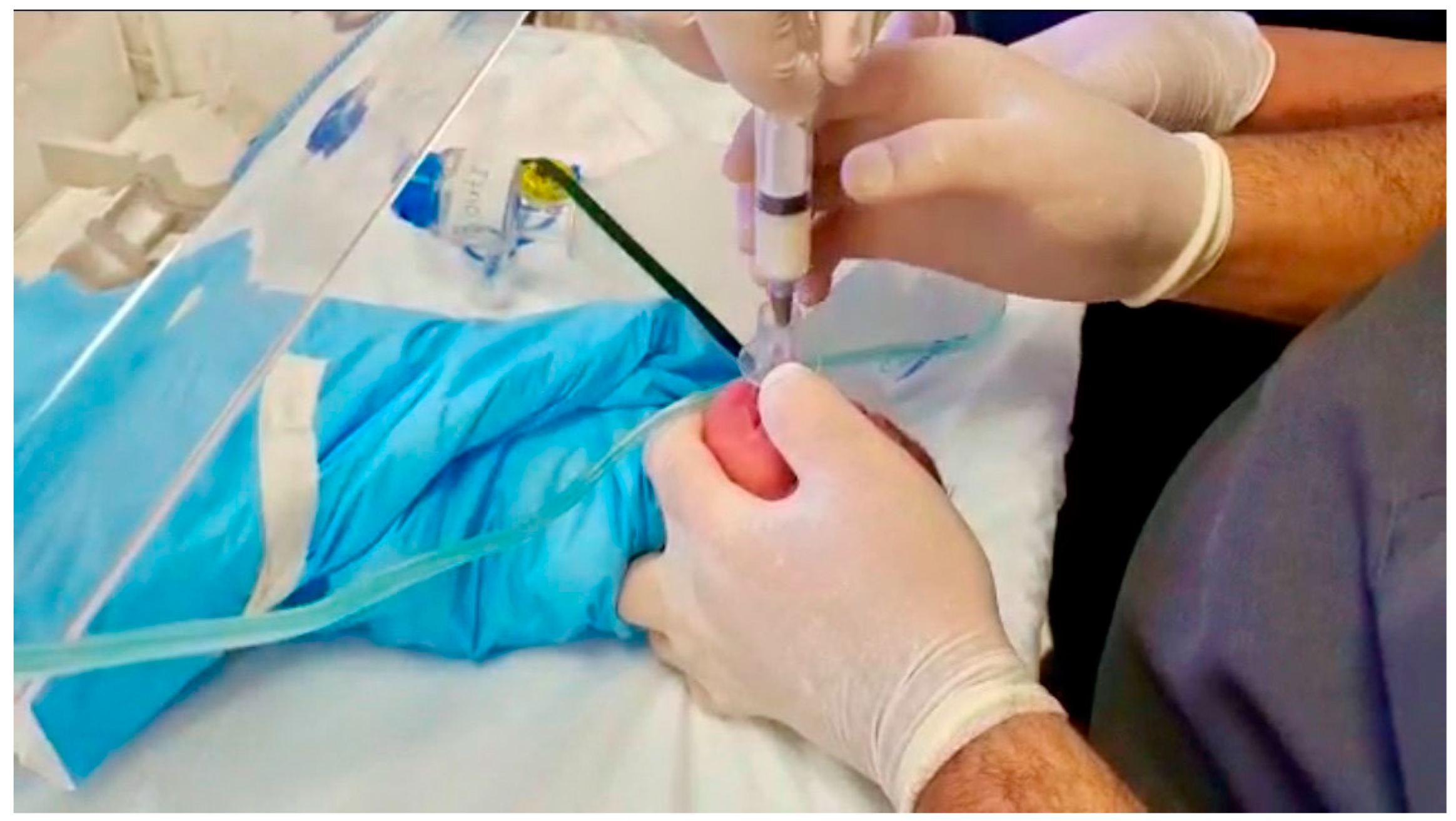
2.2. Intervention
2.3. Study of the Intervention
2.4. Measures
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. Summary
4.2. Interpretation
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McPherson, C.; Wambach, J.A. Prevention and Treatment of Respiratory Distress Syndrome in Preterm Neonates. Neonatal. Netw. 2018, 37, 169–177. [Google Scholar] [CrossRef]
- Avery, M.E.; Mead, J. Surface properties in relation to atelectasis and hyaline membrane disease. AMA J. Dis. Child. 1959, 97 Pt 1, 517–523. [Google Scholar] [CrossRef]
- Nkadi, P.O.; Merritt, T.A.; Pillers, D.A. An overview of pulmonary surfactant in the neonate: Genetics, metabolism, and the role of surfactant in health and disease. Mol. Genet. Metab. 2009, 97, 95–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parkash, A.; Haider, N.; Khoso, Z.A.; Shaikh, A.S. Frequency, causes and outcome of neonates with respiratory distress admitted to Neonatal Intensive Care Unit, National Institute of Child Health, Karachi. J. Pak. Med. Assoc. 2015, 65, 771–775. [Google Scholar]
- Khader, Y.S.; Alyahya, M.; Batieha, A. Perinatal and neonatal mortality in Jordan. In Handbook of Healthcare in the Arab World; Springer: Cham, Switzerland, 2019; pp. 1–22. [Google Scholar]
- Halliday, H.L. History of surfactant from 1980. Neonatology 2005, 87, 317–322. [Google Scholar] [CrossRef]
- Finan, E.; Bismilla, Z.; Campbell, C.; Leblanc, V.; Jefferies, A.; Whyte, H.E. Improved procedural performance following a simulation training session may not be transferable to the clinical environment. J. Perinatol. 2012, 32, 539–544. [Google Scholar] [CrossRef] [Green Version]
- Ehrlich, P.F.; Seidman, P.S.; Atallah, O.; Haque, A.; Helmkamp, J. Endotracheal intubations in rural pediatric trauma patients. J. Pediatr. Surg. 2004, 39, 1376–1380. [Google Scholar] [CrossRef]
- Foglia, E.E.; Ades, A.; Sawyer, T.; Glass, K.M.; Singh, N.; Jung, P.; Quek, B.H.; Johnston, L.C.; Barry, J.; Zenge, J.; et al. Neonatal intubation practice and outcomes: An international registry study. Pediatrics 2019, 143, e20180902. [Google Scholar] [CrossRef] [Green Version]
- Hatch, L.D.; Grubb, P.H.; Lea, A.S.; Walsh, W.F.; Markham, M.H.; Whitney, G.M.; Slaughter, J.C.; Stark, A.R.; Ely, E.W. Endotracheal intubation in neonates: A prospective study of adverse safety events in 162 infants. J. Pediatr. 2016, 168, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Khatami, S.F.; Parvaresh, P.; Behjati, S. Common complications of endotracheal intubation in newborns. Iran. J. Neonatol. IJN 2011, 2, 12–17. [Google Scholar] [CrossRef]
- Erdeve, Ö.; Okulu, E.; Roberts, K.D.; Guthrie, S.O.; Fort, P.; Kanmaz Kutman, H.G.; Dargaville, P.A. Alternative methods of surfactant administration in preterm infants with respiratory distress syndrome: State of the art. Turk. Arch. Pediatr. 2021, 56, 553–562. [Google Scholar] [CrossRef]
- Verder, H.; Robertson, B.; Greisen, G.; Ebbesen, F.; Albertsen, P.; Lundstrøm, K.; Jacobsen, T. Surfactant therapy and nasal continuous positive airway pressure for newborns with respiratory distress syndrome. Danish-Swedish Multicenter Study Group. N. Engl. J. Med. 1994, 331, 1051–1055. [Google Scholar] [CrossRef]
- Jobe, A.H. Mechanisms of Lung Injury and Bronchopulmonary Dysplasia. Am. J. Perinatol. 2016, 33, 1076–1078. [Google Scholar] [CrossRef]
- De Bisschop, B.; Derriks, M.F.; Cools, F. Early predictors for INtubation-SURfactant-Extubation failure in preterm infants with neonatal respiratory distress syndrome: A systematic review. Neonatology 2020, 117, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Kribs, A.; Pillekamp, F.; Hu€nseler, C.; Vierzig, A.; Roth, B. Early administration of surfactant in spontaneous breathing with nCPAP: Feasibility and outcome in extremely premature infants (postmenstrual age </=27 weeks). Paediatr. Anaesth. 2007, 17, 364–369. [Google Scholar] [CrossRef]
- Dargaville, P.A.; Aiyappan, A.; Cornelius, A.; Williams, C.; De Paoli, A.G. Preliminary evaluation of a new technique of minimally invasive surfactant therapy. Arch. Dis. Child. Fetal Neonatal Ed. 2011, 96, F243–F248. [Google Scholar] [CrossRef]
- Guthrie, S.O.; Fort, P.; Roberts, K.D. Surfactant administration through laryngeal or supraglottic airways. Neoreviews 2021, 22, e673–e688. [Google Scholar] [CrossRef]
- Bansal, S.C.; Caoci, S.; Dempsey, E.; Trevisanuto, D.; Roehr, C.C. The laryngeal mask airway and its use in neonatal resuscitation: A critical review of where we are in 2017/2018. Neonatology 2018, 113, 152–161. [Google Scholar] [CrossRef]
- Pejovic, N.J.; Trevisanuto, D.; Lubulwa, C.; Myrnerts Höök, S.; Cavallin, F.; Byamugisha, J.; Nankunda, J.; Tylleskär, T. Neonatal resuscitation using a laryngeal mask airway: A randomised trial in Uganda. Arch. Dis. Child. 2018, 103, 255–260. [Google Scholar] [CrossRef] [Green Version]
- Trevisanuto, D.; Micaglio, M.; Ferrarese, P.; Zanardo, V. The laryngeal mask airway: Potential applications in neonates. Arch. Dis. Child. Fetal Neonatal Ed. 2004, 89, F485–F489. [Google Scholar] [CrossRef]
- Wanous, A.A.; Wey, A.; Rudser, K.D.; Roberts, K.D. Feasibility of laryngeal mask airway device placement in neonates. Neonatology 2017, 111, 222–227. [Google Scholar] [CrossRef] [Green Version]
- Roberts, K.D.; Brown, R.; Lampland, A.L.; Leone, T.A.; Rudser, K.D.; Finer, N.N.; Rich, W.D.; Merritt, T.A.; Czynski, A.J.; Kessel, J.M.; et al. Laryngeal mask airway for surfactant administration in neonates: A randomized, controlled trial. J. Pediatr. 2018, 193, 40–46.e1. [Google Scholar] [CrossRef]
- Sadeghnia, A.; Tanhaei, M.; Mohammadizadeh, M.; Nemati, M. A comparison of surfactant administration through i-gel and ET-tube in the treatment of respiratory distress syndrome in newborns weighing more than 2000 grams. Adv. Biomed. Res. 2014, 3, 160. [Google Scholar] [CrossRef]
- Amini, E.; Sheikh, M.; Shariat, M.; Dalili, H.; Azadi, N.; Nourollahi, S. Surfactant administration in preterm neonates using laryngeal mask airway: A randomized clinical trial. Acta Med. Iran. 2019, 57, 348–354. [Google Scholar] [CrossRef]
- Pinheiro, J.; Santana-Rivas, Q.; Pezzano, C. Randomized trial of laryngeal mask airway versus endotracheal intubation for surfactant delivery. J. Perinatol. 2016, 36, 196–201. [Google Scholar] [CrossRef]
- Zapata, H.A.; Fort, P.; Roberts, K.D.; Kaluarachchi, D.C.; Guthrie, S.O. Surfactant administration through laryngeal or supraglottic airways (SALSA): A viable method for low-income and middle-income countries. Front. Pediatr. 2022, 10, 853831. [Google Scholar] [CrossRef]
- The World Bank. 2019. Available online: https://data.worldbank.org/indicator/SH.DYN.NMRT?locations=PE (accessed on 17 December 2021).
- Iyer, N.P.; Mhanna, M.J. Non-invasively derived respiratory severity score and oxygenation index in ventilated newborn infants. Pediatr. Pulmonol. 2013, 48, 364–369. [Google Scholar] [CrossRef]
- Jehier, A.; El-Naggar, W.; Baier, J.; Hatfield, T.R.; Narvey, M.R. Atropine versus placebo for neonatal intubation: A randomized clinical trial. In Proceedings of the Pediatric Academic Societies Meeting, Denver, CO, USA, 21–25 April 2022. [Google Scholar]
| Sample Characteristic | SALSA (n = 110) | InSurE (n = 110) | p-Value |
|---|---|---|---|
| Birth weight (kg) | 1.8 (0.5) | 1.8 (0.5) | 0.39 |
| Gestational age (weeks) | 32.5 (2.3) | 32.6 (2.8) | 0.69 |
| Sex (Female) | 53 (48.2) | 64 (58.1) | 0.18 |
| RSS at time of surfactant administration | 2.7 (0.4) | 2.9 (0.4) | 0.024 |
| HR during procedure | 113 (10) | 105 (12) | <0.0001 |
| Premedication with atropine | 108 (98.2) | 0 (0) | <0.0001 |
| Outcome | SALSA (n = 110) | InSurE (n = 110) | p-Value |
|---|---|---|---|
| Required second dose of surfactant | 6 (5.5) | 17 (15.5) | 0.026 |
| Required intubation for mechanical ventilation | 10 (9.1) | 23 (20.9) | 0.022 |
| Treated for pneumothorax | 2 (1.8) | 9 (8.2) | 0.059 |
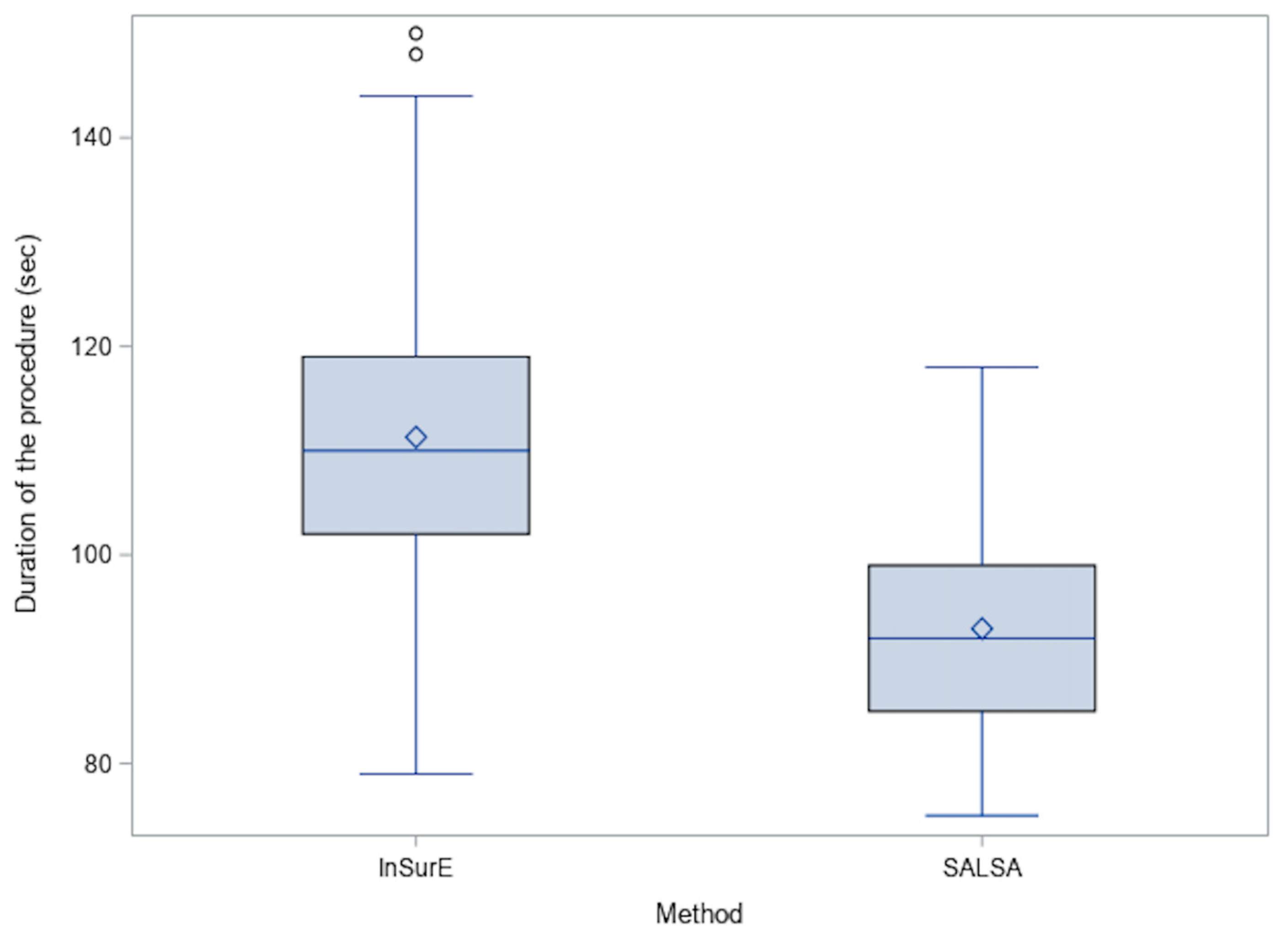
| Duration of procedure | 92.9 (10.5) | 111.3 (13.2) | <0.0001 |
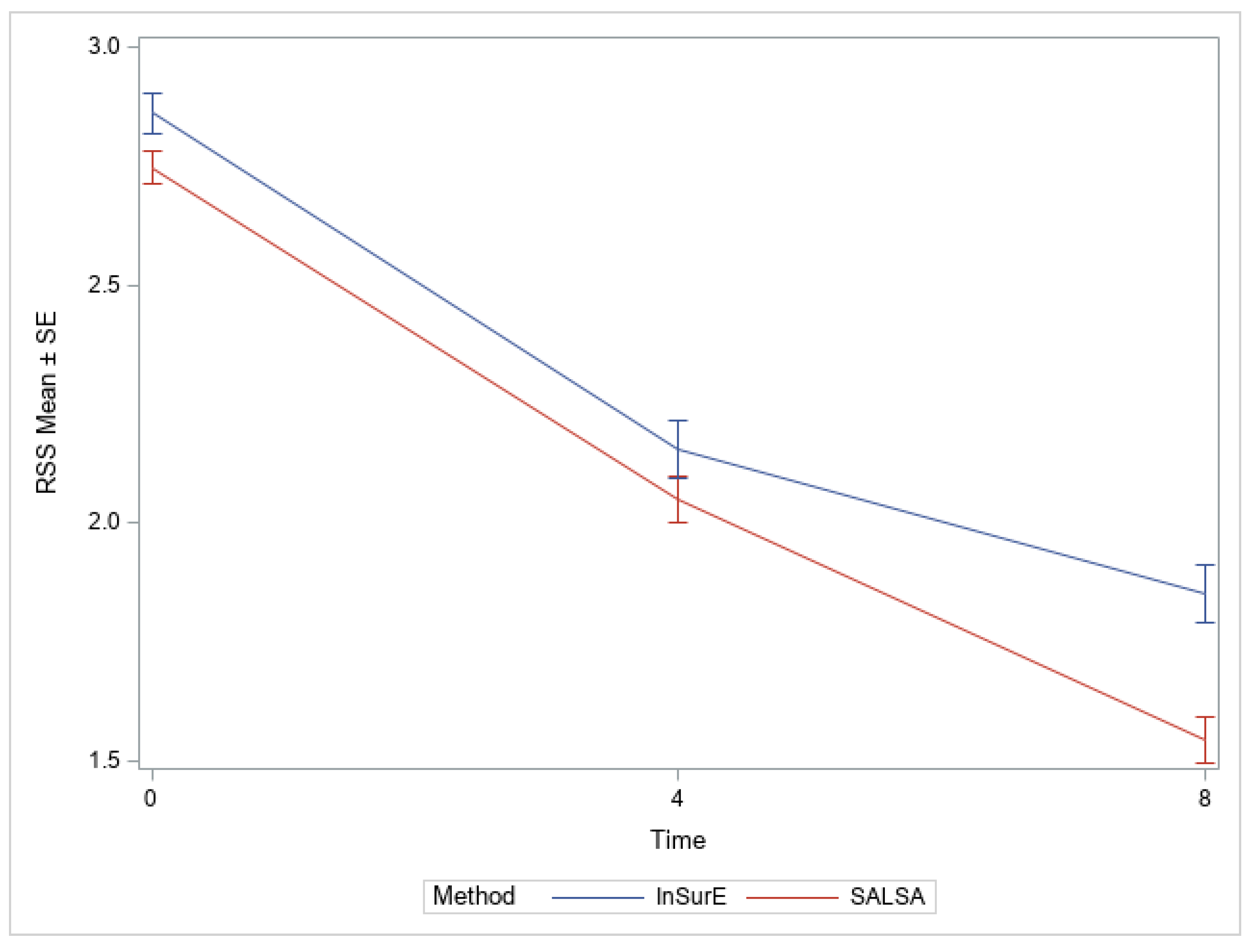
| RSS | |||
| 4 h | 2.0 (0.5) | 2.2 (0.6) | 0.1919 |
| 8 h | 1.5 (0.5) | 1.9 (0.6) | 0.0004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu Leyah, N.A.A.; Hasan, A.A.; Juneau, J.N.; Al Jammal, M.A.; Jaber, G.A.; Wilding, G.E.; Roberts, K.D.; Guthrie, S.O. Implementation of Surfactant Administration through Laryngeal or Supraglottic Airways (SALSA): A Jordanian NICU’s Journey to Improve Surfactant Administration. Children 2022, 9, 1147. https://doi.org/10.3390/children9081147
Abu Leyah NAA, Hasan AA, Juneau JN, Al Jammal MA, Jaber GA, Wilding GE, Roberts KD, Guthrie SO. Implementation of Surfactant Administration through Laryngeal or Supraglottic Airways (SALSA): A Jordanian NICU’s Journey to Improve Surfactant Administration. Children. 2022; 9(8):1147. https://doi.org/10.3390/children9081147
Chicago/Turabian StyleAbu Leyah, Naser Aldain A., Abeer A. Hasan, John N. Juneau, Maryam Ali Al Jammal, Ghada A. Jaber, Gregory E. Wilding, Kari D. Roberts, and Scott O. Guthrie. 2022. "Implementation of Surfactant Administration through Laryngeal or Supraglottic Airways (SALSA): A Jordanian NICU’s Journey to Improve Surfactant Administration" Children 9, no. 8: 1147. https://doi.org/10.3390/children9081147
APA StyleAbu Leyah, N. A. A., Hasan, A. A., Juneau, J. N., Al Jammal, M. A., Jaber, G. A., Wilding, G. E., Roberts, K. D., & Guthrie, S. O. (2022). Implementation of Surfactant Administration through Laryngeal or Supraglottic Airways (SALSA): A Jordanian NICU’s Journey to Improve Surfactant Administration. Children, 9(8), 1147. https://doi.org/10.3390/children9081147






